Abstract
Background
The usefulness of various prognostic factors for pancreatic cancer (PC) has been reported, but the number of elderly patients in these studies is disproportionately fewer compared with those in everyday practice. The purpose of this study was to investigate the prognostic factors for unresectable PC in elderly patients.
Methods
We retrospectively analyzed 67 elderly (age ≥75 years) patients with unresectable PC who underwent chemotherapy between January 2006 and December 2014 at our hospital. Univariate and multivariate Cox regression models were applied to investigate independent prognostic factors.
Results
Multivariate analysis revealed that an increased neutrophil-lymphocyte ratio (NLR) [hazard ratio (HR) 1.91, P=0.03] and Eastern Cooperative Oncology Group (ECOG) performance status (PS) 2 (HR 2.74, P=0.01) were independent negative prognostic factors.
Conclusions
The two prognostic factors identified herein are useful in the identification of patients with a poor prognosis and subsequent administration of supportive care alone, which may help avoid the unnecessary adverse effects and complications of systemic chemotherapy.
Keywords: Pancreatic cancer (PC), prognostic factor, elderly patient, neutrophil-lymphocyte ratio (NLR)
Introduction
Globally, pancreatic cancer (PC) is considered to be the seventh most prevalent cause of cancer-related death (1). It was estimated that approximately 39,590 patients in the United States will die of this disease in 2014 (2). In Japan, more than 33,000 cases are diagnosed annually, with the majority of patients dying as a result of the disease, culminating in an estimated 30,600 deaths. The mortality rates closely follow incidence rates because of poor prognosis (3).
The incidence of cancer is increasing with age and the current median age for PC in the United States is 72 years (4). Unfortunately, although 69% of the patients with PC are more than 65 years old (4), this group of older patients is very much under-represented in the clinical trials on which treatment decisions are based (5). This under-representation makes it difficult to evaluate treatment options and survival benefit for this older age group. Several factors have been reported to be associated with the treatment of elderly patients without the use of chemotherapy, including advanced stage at the time of diagnosis, age, and comorbidities (6,7). Regardless of this, some studies have shown that chemotherapy treatment may be beneficial to the elderly (8,9).
Due to the moderate improvement achieved by chemotherapeutics, recent studies have evaluated whether subgroups of patients who would benefit the most from specific treatment strategies may be identified (8,10-12). This may improve the identification of patients with a poor prognosis and subsequent administration of supportive care alone, which may help avoid the unnecessary adverse effects and complications of systemic chemotherapy.
Surgical and pathological factors, clinical factors, laboratory and molecular factors have been routinely used to predict the outcome for PC patients (13). These parameters are generally useful; however, they are often insufficient in optimally predicting individual patient prognosis and they are often unavailable in everyday practice due to their special characteristics. Several markers, including the neutrophil-lymphocyte ratio (NLR) and modified Glasgow prognostic score (mGPS), have been proposed to estimate the magnitude of systemic inflammation in cancer patients. The usefulness of these prognostic factors for PC has been reported previously (14,15), but these studies contain disproportionately fewer elderly patients compared with everyday practice. The purpose of the present study was to investigate the prognostic factors for unresectable PC in elderly patients.
Methods
Study population and data collection
We conducted this retrospective study at Kofu Municipal Hospital and University of Yamanashi Hospital between January 2006 and December 2014. All elderly (age ≥75 years) patients with unresectable PC who were admitted during the study period for any kind of treatment for their cancer were eligible for participation. Patients who underwent only examination were excluded. We retrieved patient records from a computerized database at our institution and performed a retrospective systematic review of patient diagnosis, treatment, and laboratory data. Laboratory assessment at baseline included complete blood cell count, serum biochemistry, and levels of serum tumor markers, such as carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9). For comparison, 102 unresectable patients aged <75 years and treated during the same time period were also retrieved and analyzed. The institutional review board of our hospital approved this study.
Prognostic variables
To identify potential prognostic factor, we systematically searched PubMed with a search strategy based on the terms “pancreas cancer” and “prognostic factor” published within last 5 years (the last search was performed on February 1, 2016). Total 525 studies were extracted. We excluded these studies following criteria: (I) case reports or case series; (II) proceedings of meetings; (III) studies dealing with surgical resection cases; (IV) studies dealing with prognostic factor that cannot obtain within daily practice of community hospital. Finally, 25 studies were remained and age (16), distant metastasis (17), Eastern Cooperative Oncology Group (ECOG) performance status (PS) (16,18-21), CRP level (17,20,22), CEA level (23,24), CA19-9 level (19,20,25), mGPS (14,26), NLR (15,22,26-30) were extracted as potential prognostic factor. mGPS was calculated as follows: patients with both an elevated C-reactive protein (CRP) concentration (>1 mg/dL) and low albumin concentration (<3.5 g/dL) were allocated a score of 2; patients in whom only the CRP concentration was elevated (>1 mg/dL) were allocated a score of 1, and those with normal CRP concentration were allocated a score of 0, irrespective of their serum albumin concentration. Serum albumin and CRP concentration were measured before any kind of treatment. If infection and/or jaundice were present, these parameters were measured after symptoms had been relieved. The cut-off value for defining high CRP was 1.2, calculated based on the receiver operating curve (ROC). In the same way, the cut-off value for defining high NLR was 3.3.
Treatment and treatment evaluation
Standard doses and regimen schedules were adjusted at the discretion of treating physicians according to the incidence of adverse events or the general condition of the individual patient. Tumor response was assessed by computed tomography scans at intervals of at least three month according to Response Evaluation Criteria In Solid Tumors (RECIST). Evaluation procedures were performed ahead of schedule if the patient’s general condition worsened or severe toxicity occurred. Toxicity was graded according to the Common Terminology Criteria for Adverse Events (CTCAE) version 3.0.
Statistical analysis
Survival curves were estimated according to the Kaplan-Meier method, and differences were evaluated with the log-rank test. Variables that achieved statistical significance (P<0.05) in univariate analysis were used for multivariate Cox regression analysis to identify significant independent factors. We also calculated the hazard ratio (HR) and 95% confidence intervals (CIs). All P values of <0.05 obtained by the two-tailed test were considered significant. All statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). More precisely, it is a modified version of the R commander designed to add the statistical functions frequently used in biostatistics.
Results
Patient characteristics
A total of 98 unresectable PC patients were investigated. Of these patients, 31 patients were classified as suitable for best supportive care (BSC) alone because of poor PS or absence of chemotherapy. Ultimately, we analyzed 67 patients in this study. Patient demographics are shown in Table 1. Thirty-nine patients suffered from distant metastasis, and the remaining 28 patents had locally advanced PC. The mean tumor size of the primary lesion was 35 mm. First-line chemotherapy was gemcitabine alone in 31 patients, S-1 (oral fluoropyrimidine prodrug) alone in 18 patients, and gemcitabine and S-1 combination therapy in 18 patients.
Table 1. Patient characteristic.
| Category | Chemotherapy (n=67) | BSC (n=31) | P value |
|---|---|---|---|
| Gender (%) | 0.05† | ||
| Male | 36 (53.7) | 10 (32.3) | |
| Female | 31 (46.3) | 21 (67.7) | |
| Age at diagnosis (years) | <0.001* | ||
| Mean | 79.3 | 82.5 | |
| Median [range] | 79 [75–87] | 82 [75–90] | |
| ECOG performance status | <0.001† | ||
| 0–1 | 55 (82.1) | 5 (16.1) | |
| ≥2 | 12 (17.9) | 26 (83.9) | |
| Tumor size of primary lesion (mm) | 0.32* | ||
| Mean | 35 | 37 | |
| Median [range] | 32 [10–83] | 35 [15–70] | |
| Disease status | 0.13† | ||
| Locally advanced | 28 (41.8) | 8 (25.8) | |
| Distant metastasis | 39 (58.2) | 23 (74.2) | |
| Metastatic sites at diagnosis | — | ||
| Peritoneum | 7 | 7 | |
| Liver | 31 | 14 | |
| Lung | 3 | 4 | |
| First-line chemotherapy | — | ||
| Gem | 31 (46.2) | — | |
| S-1 | 18 (26.9) | — | |
| GEM + S-1 | 18 (26.9) | — | |
| CRP (mg/dL) | |||
| Mean | 1.7 | 2.9 | 0.21* |
| Median (range) | 0.5 (0–15.1) | 0.9 (0–30.8) | |
| <1.2 | 45 (67.2) | 19 (61.3) | 0.57† |
| ≥1.2 | 22 (32.8) | 12 (38.7) | |
| NLR | |||
| Median (range) | 2.8 (0.9–19.0) | 5.86 (1.5–22.6) | <0.001* |
| <3.3 | 40 (59.7) | 7 (22.6) | <0.001† |
| ≥3.3 | 27 (40.3) | 24 (77.4) | |
| mGPS | — | ||
| 0 | 43 (64.2) | 18 (58.1) | |
| 1 | 7 (10.4) | 4 (12.9) | |
| 2 | 17 (25.4) | 9 (29.0) | |
| CEA (ng/mL) | 0.28† | ||
| <5.0 | 27 (40.3) | 9 (29.0) | |
| ≥5.0 | 40 (59.7) | 22 (71.0) | |
| CA19-9 (U/mL) | 0.36† | ||
| <37.0 | 13 (19.4) | 4 (6.5) | |
| ≥37.0 | 54 (80.6) | 29 (93.5) | |
*, Mann-Whitney’s U test; †, Fisher’s exact test. ECOG, Eastern Cooperative Oncology Group; CRP, C-reactive protein; NLR, neutrophil-lymphocyte ratio; NLR, neutrophil-lymphocyte ratio; mGPS, modified Glasgow prognostic score; CEA, carcinoembryonic antigen; CA19-9, carbohydrate antigen 19-9.
Response and survival
None of our patients achieved a complete response. Partial response rate was observed in 6 patients (9.0%) and stable disease was documented in 31 patients. Consequently, this indicated that the disease control rate was 55.2% (Table 2). The overall survival was 9.2 months, which was significantly longer compared with BSC only (Figure 1A), and non-inferiority was proved compared with 102 unresectable patients aged less than 75 years (Figure 1B).
Table 2. Response rate and disease control rate.
| Best response | Number of patients |
|---|---|
| Complete response | 0 |
| Partial response | 6 |
| Stable disease | 31 |
| Progressive disease | 24 |
| Not evaluated | 6 |
| Response rate | 9% |
| Disease control rate | 55.2% |
Figure 1.
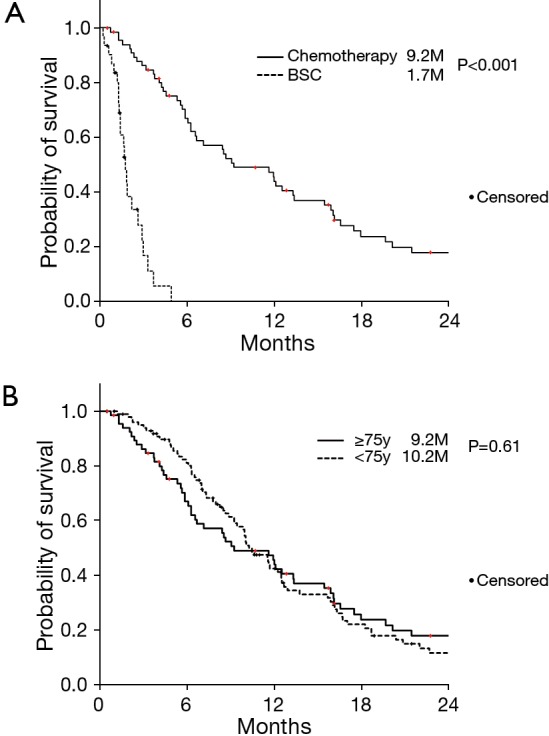
Kaplan-Meier estimates for overall survival. (A) According to treatment; (B) according to patient age.
Safety evaluation
Six patients were discontinued as a result of adverse events (interstitial pneumonia in three patients and gastrointestinal bleeding, nausea, and neutropenia, respectively, in one patient each). Although not directly related to chemotherapy, cerebral infarction was observed in 2 patients.
Multivariate analysis to detect independent prognostic factors
First, we explored prognostic factors (Table 3). ECOG PS 2 (P<0.001), distant metastasis (P=0.04), CRP ≥1.2 (P=0.001), NLR ≥3.3 (P=0.009), and mGPS 1–2 (P<0.001) were extracted from univariate analysis. Multivariate analysis was undertaken to identify pretreatment variables that correlated with prognosis. Multivariate analysis revealed that NLR ≥3.3 (HR 1.91, P=0.03) and ECOG PS 2 (HR 2.74, P=0.01) were independent negative prognostic factors.
Table 3. Univariate and multivariate analyses to detect independent prognostic factors.
| Variable | n | Survival month | Univariate, P value | Multivariate | |
|---|---|---|---|---|---|
| HR (95% CI) | P value | ||||
| Gender | 0.78 | — | — | ||
| Male | 31 | 6.9 | |||
| Female | 36 | 11.8 | |||
| ECOG PS | <0.001 | 2.74 (1.25–6.02) | 0.01 | ||
| 0–1 | 55 | 12.1 | |||
| 2 | 12 | 5.0 | |||
| Tumor size (mm) | 0.06 | — | — | ||
| <35 | 26 | 6.4 | |||
| ≥35 | 41 | 12.3 | |||
| Disease status | 0.04 | 1.27 (0.67–2.42) | 0.47 | ||
| Locally advanced | 28 | 7.8 | |||
| Distant metastasis | 39 | 12.6 | |||
| CRP (mg/dL) | 0.001 | 0.82 (0.18–3.70) | 0.79 | ||
| <1.2 | 45 | 13.3 | |||
| ≥1.2 | 22 | 5.7 | |||
| NLR | 0.009 | 1.91 (1.06–3.44) | 0.03 | ||
| <3.3 | 40 | 12.7 | |||
| ≥3.3 | 27 | 5.7 | |||
| mGPS | <0.001 | 2.57 (0.57–11.5) | 0.22 | ||
| 0 | 43 | 13.3 | |||
| 1–2 | 24 | 5.7 | |||
| CEA (ng/mL) | 0.21 | — | — | ||
| <5.0 | 27 | 11.8 | |||
| ≥5.0 | 40 | 6.6 | |||
| CA19-9 (U/mL) | 0.57 | — | — | ||
| <37.0 | 13 | 12.1 | |||
| ≥37.0 | 54 | 8.6 | |||
HR, hazard ratio; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; CRP, C-reactive protein; NLR, neutrophil-lymphocyte ratio; NLR, neutrophil-lymphocyte ratio; mGPS, modified Glasgow prognostic score; CEA, carcinoembryonic antigen; CA19-9, carbohydrate antigen 19-9.
Discussion
At present, there is a clear lack of knowledge with regard to specific prognostic factors for chemotherapy in elderly patients with unresectable PC. The geriatric population is always under-represented in clinical trials, representing only 25–30% of study participants (5), which makes it more difficult for physicians to determine if the benefit of treatment seen in younger patients can be seen in the elderly population with the same type of cancer. However, we believe that chemotherapy should be considered in all patients regardless of age, if clinically possible. Establishing definitive prognostic variables during initial diagnosis may help physicians determine which patients should be considered for supportive care alone or chemotherapy. There are some retrospective analyses and studies in elderly patients showing that elderly patients benefit from chemotherapy to a similar extent as younger patients, with manageable toxicity (8,31), but there are also analyses that show the opposite result (12). One of the biggest reasons for withholding chemotherapy in the elderly is the fear of intolerance and the possibility of greater toxicity compared with younger patients, but in the present retrospective analysis, elderly patients benefited from chemotherapy to a similar extent as younger patients, with manageable side effects. Patients of identical age may greatly differ in their functional status. PS is an independent prognostic factor and is a useful guide in making treatment decisions for elderly patients.
Studies until date have shown that higher NLR (15,27,29) is correlated with adverse survival outcomes in patients with PC. Elevated NLR is often accompanied by elevated neutrophil levels and relative lymphocytopenia. Elevated neutrophil levels can promote tumor cell progression by upregulating a variety of inflammatory cytokines and providing a suitable microenvironment for tumor growth (32,33). Furthermore, lymphocytopenia arising from numerous inhibitory immunological mediators released by tumor cells represents an immunosuppressive condition in cancer patients and contributes to a poorer outcome (34).
The major problem of NLR is the inability to provide an optimal cut-off value. The cut-off value for NLR ranges from 2 to 5 in previous reports (28). The mGPS also represents a useful systemic inflammatory prognostic factor. The cut-off value of mGPS is relatively clear, but the usefulness of mGPS is rather controversial (14,35).
This retrospective analysis demonstrated that NLR and ECOG PS were independent prognostic factors, we categorized patients according to the two prognostic factors; prognostic score 2 (NLR ≥3.3 and PS 2), score 1 (one of the two factors), and score 0 (neither elevated NLR nor poor PS). Survival was significantly shorter with increasing the score (Figure 2).
Figure 2.
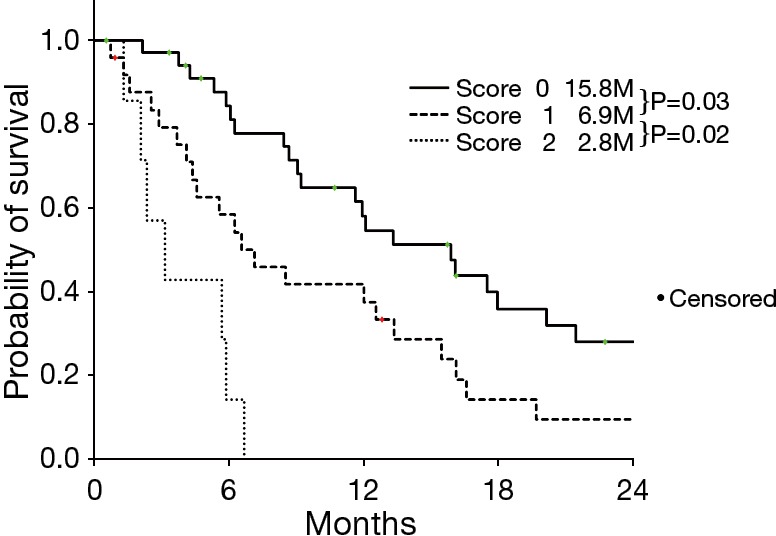
Kaplan-Meier estimates for overall survival according to risk score.
This study was limited by its retrospective design. In addition, chemotherapy regimens differed among patients; however, it is unlikely that chemotherapy regimen heterogeneity affected the current results because all patients received gemcitabine, S-1, or gemcitabine/S-1 combination therapy, and the efficacies of these three regimens were not statistically different in a previous large randomized phase III study (36). Furthermore, unreviewed potentially prognostic factors extracted PubMed search such as uric acid (37) and coagulation assays (38).
In conclusion, this retrospective analysis demonstrated that NLR and ECOG PS were independent prognostic factors in elderly patients with unresectable PC.
Acknowledgements
We are grateful to Takeshi Ishida, Naruki Shimamura, and Dai Yoshimura for their cooperation and advice. The authors would like to thank Enago (www.enago.jp) for the English language review.
Ethical Statement: The study was approved by the institutional review board of our hospital.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. 10.1002/ijc.29210 [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9-29. 10.3322/caac.21208 [DOI] [PubMed] [Google Scholar]
- 3.VItal Statistics Japan. Ministry of Health, Labour and Welfare. Available online: http://www.mhlw.go.jp/english/database/db-hw
- 4.Niederhuber JE, Brennan MF, Menck HR. The National Cancer Data Base report on pancreatic cancer. Cancer 1995;76:1671-7. [DOI] [PubMed] [Google Scholar]
- 5.Hutchins LF, Unger JM, Crowley JJ, et al. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med 1999;341:2061-7. 10.1056/NEJM199912303412706 [DOI] [PubMed] [Google Scholar]
- 6.Mor V, Masterson-Allen S, Goldberg RJ, et al. Relationship between age at diagnosis and treatments received by cancer patients. J Am Geriatr Soc 1985;33:585-9. 10.1111/j.1532-5415.1985.tb06313.x [DOI] [PubMed] [Google Scholar]
- 7.Samet J, Hunt WC, Key C, et al. Choice of cancer therapy varies with age of patient. JAMA 1986;255:3385-90. 10.1001/jama.1986.03370240055036 [DOI] [PubMed] [Google Scholar]
- 8.Hentic O, Dreyer C, Rebours V, et al. Gemcitabine in elderly patients with advanced pancreatic cancer. World J Gastroenterol 2011;17:3497-502. 10.3748/wjg.v17.i30.3497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sehgal R, Alsharedi M, Larck C, et al. Pancreatic cancer survival in elderly patients treated with chemotherapy. Pancreas 2014;43:306-10. 10.1097/MPA.0000000000000091 [DOI] [PubMed] [Google Scholar]
- 10.Aldoss IT, Tashi T, Gonsalves W, et al. Role of chemotherapy in the very elderly patients with metastatic pancreatic cancer — A Veterans Affairs Cancer Registry analysis. J Geriatr Oncol 2011;2:209-14. 10.1016/j.jgo.2011.02.003 [DOI] [Google Scholar]
- 11.Oziel-Taieb S, Faure M, Gilabert M, et al. Treatment of Pancreatic Adenocarcinoma in Elderly Patients over 75 Years of Age: A Retrospective Series of 129 Patients. J Gastrointest Cancer 2016;47:15-9. 10.1007/s12029-015-9774-4 [DOI] [PubMed] [Google Scholar]
- 12.Tas F, Sen F, Keskin S, et al. Prognostic factors in metastatic pancreatic cancer: Older patients are associated with reduced overall survival. Mol Clin Oncol 2013;1:788-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bilici A. Prognostic factors related with survival in patients with pancreatic adenocarcinoma. World J Gastroenterol 2014;20:10802-12. 10.3748/wjg.v20.i31.10802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Imaoka H, Mizuno N, Hara K, et al. Evaluation of Modified Glasgow Prognostic Score for Pancreatic Cancer: A Retrospective Cohort Study. Pancreas 2016;45:211-7. 10.1097/MPA.0000000000000446 [DOI] [PubMed] [Google Scholar]
- 15.Xue P, Kanai M, Mori Y, et al. Neutrophil-to-lymphocyte ratio for predicting palliative chemotherapy outcomes in advanced pancreatic cancer patients. Cancer Med 2014;3:406-15. 10.1002/cam4.204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tabernero J, Chiorean EG, Infante JR, et al. Prognostic factors of survival in a randomized phase III trial (MPACT) of weekly nab-paclitaxel plus gemcitabine versus gemcitabine alone in patients with metastatic pancreatic cancer. Oncologist 2015;20:143-50. 10.1634/theoncologist.2014-0394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yi JH, Lee J, Park SH, et al. A prognostic model to predict clinical outcomes with first-line gemcitabine-based chemotherapy in advanced pancreatic cancer. Oncology 2011;80:175-80. 10.1159/000328449 [DOI] [PubMed] [Google Scholar]
- 18.Erdogan B, Turkmen E, Uzunoglu S, et al. Performance status is an important prognostic factor in second line treatment of pancreaticobiliary adenocarcinoma. Hepatogastroenterology 2013;60:1479-83. [DOI] [PubMed] [Google Scholar]
- 19.Lim KH, Kim TY, Lee KH, et al. Efficacy of infusional 5-fluorouracil, doxorubicin, and mitomycin-C (iFAM) in the treatment of patients with gemcitabine-pretreated pancreatic cancer and analysis of prognostic factors in a salvage setting. Cancer Chemother Pharmacol 2011;68:1017-26. 10.1007/s00280-011-1584-1 [DOI] [PubMed] [Google Scholar]
- 20.Tanaka T, Ikeda M, Okusaka T, et al. Prognostic factors in japanese patients with advanced pancreatic cancer treated with single-agent gemcitabine as first-line therapy. Jpn J Clin Oncol 2008;38:755-61. 10.1093/jjco/hyn098 [DOI] [PubMed] [Google Scholar]
- 21.Tas F, Sen F, Odabas H, et al. Performance status of patients is the major prognostic factor at all stages of pancreatic cancer. Int J Clin Oncol 2013;18:839-46. 10.1007/s10147-012-0474-9 [DOI] [PubMed] [Google Scholar]
- 22.Szkandera J, Stotz M, Absenger G, et al. Validation of C-reactive protein levels as a prognostic indicator for survival in a large cohort of pancreatic cancer patients. Br J Cancer 2014;110:183-8. 10.1038/bjc.2013.701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inal A, Kos FT, Algin E, et al. Prognostic factors in patients with advanced pancreatic cancer treated with gemcitabine alone or gemcitabine plus cisplatin: retrospective analysis of a multicenter study. J BUON 2012;17:102-5. [PubMed] [Google Scholar]
- 24.Lee KJ, Yi SW, Chung MJ, et al. Serum CA 19-9 and CEA levels as a prognostic factor in pancreatic adenocarcinoma. Yonsei Med J 2013;54:643-9. 10.3349/ymj.2013.54.3.643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin LK, Wei L, Trolli E, et al. Elevated baseline CA19-9 levels correlate with adverse prognosis in patients with early- or advanced-stage pancreas cancer. Med Oncol 2012;29:3101-7. 10.1007/s12032-012-0278-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmad J, Grimes N, Farid S, et al. Inflammatory response related scoring systems in assessing the prognosis of patients with pancreatic ductal adenocarcinoma: a systematic review. Hepatobiliary Pancreat Dis Int 2014;13:474-81. 10.1016/S1499-3872(14)60284-8 [DOI] [PubMed] [Google Scholar]
- 27.Ben Q, An W, Wang L, et al. Validation of the pretreatment neutrophil-lymphocyte ratio as a predictor of overall survival in a cohort of patients with pancreatic ductal adenocarcinoma. Pancreas 2015;44:471-7. [DOI] [PubMed] [Google Scholar]
- 28.Cheng H, Long F, Jaiswar M, et al. Prognostic role of the neutrophil-to-lymphocyte ratio in pancreatic cancer: a meta-analysis. Sci Rep 2015;5:11026. 10.1038/srep11026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stotz M, Gerger A, Eisner F, et al. Increased neutrophil-lymphocyte ratio is a poor prognostic factor in patients with primary operable and inoperable pancreatic cancer. Br J Cancer 2013;109:416-21. 10.1038/bjc.2013.332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szkandera J, Stotz M, Eisner F, et al. External validation of the derived neutrophil to lymphocyte ratio as a prognostic marker on a large cohort of pancreatic cancer patients. PLoS One 2013;8:e78225. 10.1371/journal.pone.0078225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maréchal R, Demols A, Gay F, et al. Tolerance and efficacy of gemcitabine and gemcitabine-based regimens in elderly patients with advanced pancreatic cancer. Pancreas 2008;36:e16-21. 10.1097/MPA.0b013e31815f3920 [DOI] [PubMed] [Google Scholar]
- 32.Jablonska J, Leschner S, Westphal K, et al. Neutrophils responsive to endogenous IFN-beta regulate tumor angiogenesis and growth in a mouse tumor model. J Clin Invest 2010;120:1151-64. 10.1172/JCI37223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fridlender ZG, Sun J, Kim S, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: "N1" versus "N2" TAN. Cancer Cell 2009;16:183-94. 10.1016/j.ccr.2009.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salazar-Onfray F, López MN, Mendoza-Naranjo A. Paradoxical effects of cytokines in tumor immune surveillance and tumor immune escape. Cytokine Growth Factor Rev 2007;18:171-82. 10.1016/j.cytogfr.2007.01.015 [DOI] [PubMed] [Google Scholar]
- 35.Inoue D, Ozaka M, Matsuyama M, et al. Prognostic value of neutrophil-lymphocyte ratio and level of C-reactive protein in a large cohort of pancreatic cancer patients: a retrospective study in a single institute in Japan. Jpn J Clin Oncol 2015;45:61-6. 10.1093/jjco/hyu159 [DOI] [PubMed] [Google Scholar]
- 36.Ueno H, Ioka T, Ikeda M, et al. Randomized phase III study of gemcitabine plus S-1, S-1 alone, or gemcitabine alone in patients with locally advanced and metastatic pancreatic cancer in Japan and Taiwan: GEST study. J Clin Oncol 2013;31:1640-8. 10.1200/JCO.2012.43.3680 [DOI] [PubMed] [Google Scholar]
- 37.Stotz M, Szkandera J, Seidel J, et al. Evaluation of uric acid as a prognostic blood-based marker in a large cohort of pancreatic cancer patients. PLoS One 2014;9:e104730. 10.1371/journal.pone.0104730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tas F, Karabulut S, Bilgin E, et al. Clinical significance of coagulation assays in metastatic pancreatic adenocarcinoma. J Gastrointest Cancer 2013;44:404-9. 10.1007/s12029-013-9512-8 [DOI] [PubMed] [Google Scholar]


