Abstract
Background
While it is well established that the cell-mediated immune response plays an important role in cancer progression and spread, the role of the humoral immune response in this regard has been less studied. According to the existing literature, dense infiltration of B cells or plasma cells appears to correlate mainly with an improved prognosis in several types of cancer, but their prognostic impact in oesophageal and gastric cancer has not yet been described.
Methods
Immunohistochemistry was applied on tissue microarrays (TMA) to assess the stromal density of B cells (CD20+) and plasma cells [CD138+ or immunoglobulin kappa C (IGKC+)] in chemo-/radiotherapy-naive tumours from a consecutive cohort of 174 patients with resected oesophageal or gastric adenocarcinoma. Cox proportional hazard’s modelling was applied to examine the impact of the investigated markers on overall survival (OS) and time to recurrence (TTR).
Results
In curatively treated patients with oesophageal adenocarcinoma, high expression of IGKC was an independent predictor of a prolonged OS [hazard ratio (HR) 0.10; 95% confidence interval (CI), 0.02–0.57], and TTR (HR 0.15; 95% CI, 0.03–0.71). In curatively treated patients with gastric adenocarcinoma, high expression of IGKC independently predicted a prolonged OS (HR 0.46; 95% CI, 0.24–0.87) and TTR (HR 0.46; 95% CI, 0.21–0.98). Expression of CD20 was not prognostic, and CD138 expression was only prognostic in unadjusted analysis of TTR in gastric cancer.
Conclusions
These results demonstrate, for the first time, that abundant infiltration of IGKC+ plasma cells independently predicts a prolonged survival in both oesophageal and gastric cancer.
Keywords: Plasma cells, prognosis, gastric, oesophageal, adenocarcinoma
Introduction
Oesophageal cancer is now the eighth most common type of cancer and sixth most common cause of cancer related deaths, with an estimated number of 400,000 deaths worldwide annually (1). In the westernized world there has been a steady increase in adenocarcinoma of the esophagus, now surpassing squamous cell carcinoma (1). Gastric cancer, while overall declining in the west, is still the fifth most prevalent cancer worldwide, and the third when it comes to cancer related deaths, with an estimated number of 723,000 deaths annually (1).
Tumour-infiltrating immune cells have been shown to influence the prognosis and response to treatment in several types of cancer (2). Immune system evasion has been labelled an emerging hallmark of cancer (3), but cancer cells may also acquire help from the immune system to enable enhanced growth and metastasis (4). Hence, depending on the context, the many effector cells of the immune system may inhibit or promote tumour progression (5-7).
Hitherto, the role of the innate and cell-mediated immune response in cancer has been extensively studied. Tumour associated macrophages promote angiogenesis, epithelial-mesenchymal transition (EMT) and metastasis and have, consequently, been associated with poor prognosis in different types of cancer (8,9). Abundant T lymphocyte infiltration, in particular cytotoxic CD8+ and memory T cells, has been associated with a favourable clinical outcome in many tumour types (2,10,11). The improved understanding of cancer and immune system interactions is now paving the way for novel immunotherapy treatments, e.g., checkpoint inhibitors.
Thus far, less focus has been rewarded to the involvement of the humoral immune system in tumour development and progression. In solid cancers of the breast, cervix, colorectum and non-small cell lung cancer (NSCLC), tumour-infiltrating B cells (CD20+) have been associated with improved outcomes (12-15).
Ambiguous prognostic data exist for the plasma cell marker CD138 (syndecan-1), which can also be expressed in epithelial tumour cells and other stromal cells (e.g., fibroblasts). Tumour infiltrating CD138+ plasma cells have been associated with an improved prognosis in NSCLC and colorectal cancer (15,16), but linked to poor prognosis in breast cancer (17), and in epithelial ovarian cancer (EOC) (18). In gastric cancer, high stromal CD138 expression (unspecified cell type) has been demonstrated to have a negative impact on clinical outcome (19). In EOC, high CD138 expression in stromal fibroblasts has also been associated with an impaired prognosis (20,21).
Immunoglobulin kappa C (IGKC), exclusively expressed in plasma cells, has been associated with an improved prognosis in colorectal cancer, both at the protein expression and gene expression levels (15,22), NSCLC (16) and breast cancer (14), including being predictive for chemotherapy response in the latter (gene expression) (22). To the best of our knowledge, the prognostic significance of IGKC in oesophageal and gastric cancer has not yet been reported. The aim of this study was therefore to investigate the expression and prognostic impact of B cells (CD20+) and plasma cells (CD138+ or IGKC+) in tumours from a consecutive cohort of 174 patients with resected oesophageal and gastric adenocarcinoma.
Methods
Study design and participants
The cohort is a consecutive series of 174 patients with chemo-/radiotherapy-naive oesophageal and gastric adenocarcinoma subjected to surgical resection at the University Hospitals of Lund and Malmö between 1 January, 2006 and 31 December, 2010. The cohort has been described previously (23-26), and a new follow-up has been performed until 31 December, 2014, with additional re-examination of some of the clinicopathological data (27). Tumour stage was classified according to TNM 7. Residual tumour status was classified as: R0 = no residual tumour, R1 = microscopic residual tumour (within 1mm of the resection margin), or R2 = macroscopic residual tumour. Three patients included in the cohort had metastatic disease and were resected in order to palliate symptoms from the primary tumour. Only 7.5% of the patients received adjuvant treatment (chemoradiotherapy). Clinical data and information on recurrence and cause of death was obtained from medical charts.
Tissue microarray (TMA) construction
All cases were histopathologically re-evaluated on haematoxylin and eosin stained sections. TMAs were constructed using a semi-automated arraying device (TMArrayer, Pathology Devices, Westminster, MD, USA). Duplicate tissue cores (1 mm) were obtained from non-necrotic areas in the primary tumours, whereby each core, whenever possible, was obtained from two different donor blocks, as previously described (23,25,28).
Immunohistochemistry and staining evaluation
For immunohistochemical (IHC) analysis of CD20 and CD138, 4 µm TMA sections were pre-treated using ULTRA Cell Condition Solution 1, pH 8.5 (Ventana Medical Systems Inc., Tucson, AZ, USA), for heat induced epitope retrieval, and stained with the ready-to-use monoclonal antibodies CD20cy Clone L6 and CD138 clone MI15 in a Ventana BenchMark stainer (Ventana Medical Systems Inc.). The antibody-antigen complex was visualized with ultraView Universal DAB Detection kit (Ventana Medical Systems, Inc.).
For analysis of IGKC, the TMA slides were manually deparaffinised in xylene, rehydrated in graded alcohol and blocked for endogenous peroxidase in 0.3% hydrogen peroxide. For antigen retrieval, the slides were immersed in citrate buffer pH 6.7 and microwaved for 15 min. Automated IHC staining was performed using the Autostainer Link 48 (Dako; Glostrup, Copenhagen, Denmark) and a polyclonal rabbit anti-human kappa light chain antibody (Dako, A019; 1:40,000). The slides were incubated with the secondary antibody (EnVision™ FLEX, Rabbit/Mouse, K8000, Dako) for 30 min at RT and developed using diaminobenzidine (DAB). All TMA slides were counterstained with Mayer’s haematoxylin (Sigma-Aldrich).
CD20, CD138 and IGKC were exclusively expressed in the cytoplasm and cell membrane of the lymphocytes. The staining was assessed by two independent observers, RF and KJ. Discrepant scores were discussed in order to reach consensus. The estimated fraction of stained cells was denoted as 0.0–1.0 (1=100%) and the staining intensity was denoted as 0= negative, 1= weak, 2= moderate and 3= strong intensity. A multiplier of the fraction (0.0–1.0) and intensity (0–3) was calculated for each core and a mean value of the annotated cores was used in the analyses.
The presence of CD20+ lymphoid islets (15) was also annotated as either absent or, when present, the total number of islets.
Statistical analysis
Non-parametric Wilcoxon signed-rank, Mann-Whitney U and Kruskal-Wallis tests were applied for analyses of differences in the distribution of CD20, CD138 and IGKC expression in relation to clinicopathological characteristics. Time to recurrence (TTR) was defined as the date of surgery to the date of loco-regional or distant recurrence. The median value was used for prognostic cut-off. Kaplan Meier analysis and the log rank test were applied to estimate differences in 5-year overall survival (OS) and TTR in strata according to high and low density of CD20+, CD138+ and IGKC+ cells, respectively. Hazard ratios (HR) and confidence intervals (CI) at the 95% level for death and recurrence within 5 years were calculated by Cox proportional hazard’s regression in both univariable and multivariable analysis, adjusted for age, sex, tumour (T-) stage, nodal (N-) stage, tumour grade and resection margins. All tests were two sided. P values <0.05 were considered significant. All statistical analyses were performed using IBM SPSS Statistics version 23.0 (SPSS Inc., Chicago, IL, USA).
Results
Interrelationship of IGKC, CD20 and CD138 expression and associations with clinicopathological parameters
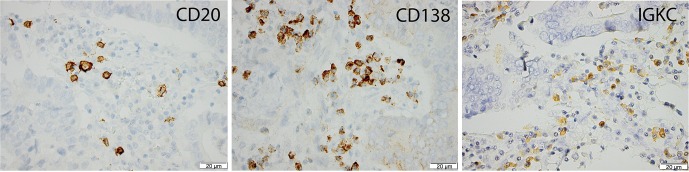
Sample IHC images are shown in Figure 1. CD20 expression could be assessed in 170 (97.7%) cases and CD138 in 172 (98.8%) cases. IGKC expression could be determined in a total of 173 (99.4%) cases and correlations between the investigated immune markers were found to be moderately strong, as shown in Table 1. In oesophageal adenocarcinoma, CD20 expression was significantly associated with IGKC expression (R=0.431, P<0.001), but not with CD138 expression. There was a significant correlation between expression of CD138 and IGKC (R=0.459, P≤0.001). In gastric adenocarcinoma, there was a significant correlation between expression of CD20 and IGKC (R=0.431, P<0.001), but not between CD20 and CD138. The correlation coefficient for CD138 and IGKC was also moderately strong (R=0.425, P≤0.001).
Figure 1.
Sample immunohistochemical images of the investigated markers in a T3 N0 M0 oesophageal adenocarcinoma. Total immunoscores for CD20, CD138 and IGKC were 39, 60 and 31.5, respectively. IGKC, immunoglobulin kappa C.
Table 1. Interrelationship between immune cell-specific CD20, CD138, and IGKC expression.
| Marker | Oesophageal adenocarcinoma | Gastric adenocarcinoma | |||||
|---|---|---|---|---|---|---|---|
| CD20 | CD138 | IGKC | CD20 | CD138 | IGKC | ||
| CD20 | |||||||
| R | 0.143 | 0.431** | 0.137 | 0.460** | |||
| P | 0.236 | <0.001 | 0.177 | <0.001 | |||
| n | 70 | 70 | 99 | 101 | |||
| CD138 | |||||||
| R | 0.143 | 0.459** | 0.137 | 0.425** | |||
| P | 0.236 | <0.001 | 0.177 | <0.001 | |||
| n | 70 | 71 | 99 | 101 | |||
| IGKC | |||||||
| R | 0.431** | 0.459** | 0.460** | 0.425** | |||
| P | <0.001 | <0.001 | <0.001 | <0.001 | |||
| n | 70 | 71 | 101 | 101 | |||
*, significance at the 5% level; **, significance at the 1% level. R, Spearman’s correlation coefficient; P, P value; n, number of cases available for analysis; IGKC, immunoglobulin kappa c.
Associations of the investigated immune cell markers and patient characteristics in oesophageal and gastric adenocarcinoma are shown in Tables 2 and 3, respectively. In oesophageal adenocarcinoma, there were significant associations between high density of CD138+ cells and less advanced N-stage (P=0.048) and M-stage (P=0.047). In gastric adenocarcinoma, high density of CD20+ cells was significantly associated with less advanced N-stage (P=0.018) and low tumour grade (P=0.007), while high CD138 as well as IGKC expression was significantly associated with less advanced T-stage (P=0.037 and P=0.008, respectively).
Table 2. Associations between IGKC, CD20, CD138 expression and clinicopathological parameters in oesophageal adenocarcinoma.
| Factor | Total CD20+ | Total CD138+ | Total IGKC+ | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Median (range) | P value | n | Median (range) | P value | n | Median (range) | P value | |||
| Age | 0.561 | 0.909 | 0.770 | ||||||||
| ≤70 | 39 | 5.00 (0.00–165.00) | 40 | 2.00 (0.00–150.00) | 40 | 2.75 (0.00–41.00) | |||||
| >70 | 31 | 6.25 (0.00–120.00) | 31 | 1.50 (0.00–50.00) | 31 | 2.50 (0.00–65.00) | |||||
| Gender | 0.510 | 0.709 | 0.305 | ||||||||
| Female | 6 | 27.25 (2.00–165.00) | 6 | 5.25 (0.00–45.00) | 6 | 10 (0.00–35.00) | |||||
| Male | 64 | 5.00 (0.00–120.00) | 65 | 1.50 (0.00–150.00) | 65 | 2.50 (0.00–65.00) | |||||
| T-stage | 0.507 | 0.579 | 0.221 | ||||||||
| 1 | 8 | 5.12 (0.00–120.00) | 9 | 29.00 (0.00–35.00) | 9 | 12.00 (0.00–41.00) | |||||
| 2 | 11 | 5.25 (0.00–165.00) | 11 | 1.50 (0.00–16.50) | 11 | 1.50 (0.00–18.00) | |||||
| 3 | 44 | 5.62 (0.00–112.50) | 44 | 1.25 (0.00–150.00) | 44 | 2.37 (0.00–65.00) | |||||
| 4 | 6 | 2.50 (0.00–21.25) | 6 | 2.50 (0.00–28.75) | 6 | 0.87 (0.00–23.00) | |||||
| N-stage | 0.060 | 0.048 | 0.069 | ||||||||
| 0 | 16 | 24.00 (0.00–165.00) | 6 | 2.50 (0.00–28.75) | 17 | 11.00 (0.00–41.00) | |||||
| 1 | 15 | 4.00 (0.00–63.00) | 15 | 1.00 (0.00–50.00) | 15 | 2.25 (0.00–31.00) | |||||
| 2 | 17 | 3.50 (0.00–82.50) | 17 | 0.00 (0.00–37.50) | 17 | 0.00 (0.00–52.50) | |||||
| M-stage | 0.404 | 0.047 | 0.298 | ||||||||
| 0 | 60 | 5.25 (0.00–165.00) | 17 | 0.00 (0.00–37.50) | 61 | 3.00 (0.00–65.00) | |||||
| 1 | 10 | 2.25 (0.00–46.25) | 10 | 0.00 (0.00–12.50) | 10 | 1.00 (0.00 –30.00) | |||||
| Tumour grade | 0.535 | 0.814 | 0.108 | ||||||||
| Low | 28 | 1.75 (0.00–63.00) | 31 | 4.00 (0.00–62.50) | 31 | 4.00 (0.00–52.50) | |||||
| High | 72 | 16.25 (0.00–150.00) | 40 | 1.50 (0.00–150.00) | 40 | 1.50 (0.00–65.00) | |||||
| Resection margin | 0.591 | 0.847 | 0.838 | ||||||||
| R0 | 44 | 5.25 (0.00–165.00) | 45 | 1.50 (0.00–150.00) | 45 | 3.00 (0.00–52.50) | |||||
| R1 & 2 | 26 | 4.00 (0.00–108.00) | 26 | 2.50 (0.00–50.00) | 26 | 2.00 (0.00–65.00) | |||||
IGKC, immunoglobulin kappa C.
Table 3. Associations between CD20, CD138, IGKC expression and clinicopathological parameters in gastric adenocarcinoma.
| Factor | Total CD20+ | Total CD138+ | Total IGKC+ | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Median (range) | P value | n | Median (range) | P value | n | Median (range) | P value | |||
| Age | 0.511 | 0.478 | 0.734 | ||||||||
| ≤70 | 45 | 15.00 (0.00–120.00) | 45 | 5.00 (0.00–150.00) | 46 | 4.50 (0.00–55.00) | |||||
| >70 | 55 | 6.00 (0.00–150.00) | 56 | 3.50 (0.00–97.50) | 56 | 3.50 (0.00–95.00) | |||||
| Gender | 0.624 | 0.497 | 0.379 | ||||||||
| Female | 33 | 4 (0.00–120.00) | 34 | 4.00 (0.00–150.00) | 34 | 3.75 (0.00–70.00) | |||||
| Male | 67 | 7.50 (0.00–150.00) | 67 | 4.00 (0.00–97.50) | 68 | 4.25 (0.00–95.00) | |||||
| T-stage | 0.139 | 0.037 | 0.008 | ||||||||
| 1 | 9 | 27.00 (0.00–106.25) | 9 | 22.50 (0.00–150.00) | 9 | 12.00 (4.50–95.00) | |||||
| 2 | 19 | 8.00 (0.00–51.00) | 20 | 12.00 (0.00–90.00) | 21 | 5.00 (0.00–70.00) | |||||
| 3 | 49 | 6.00 (0.00–68.75) | 49 | 2.50 (0.00–105.00) | 49 | 0.50 (0.00–55.00) | |||||
| 4 | 21 | 4.00 (0.00–150.00) | 21 | 1.50 (0.00–90.00) | 21 | 4.00 (0.00–24.00) | |||||
| N-stage | 0.018 | 0.627 | 0.207 | ||||||||
| 0 | 40 | 20.00 (0.00–106.25) | 40 | 4.00 (0.00–150.00) | 41 | 4.50 (0.00–95.00) | |||||
| 1 | 14 | 2.00 (0.00–150.00) | 15 | 0.00 (0.00–35.00) | 15 | 0.75 (0.00–35.00) | |||||
| 2 | 24 | 2.00 (0.00–46.50) | 24 | 5.00 (0.00–97.50) | 24 | 0.50 (0.00–43.50) | |||||
| M-stage | 0.261 | 0.842 | 0.087 | ||||||||
| 0 | 88 | 7.75 (0.00–150.00) | 89 | 4.00 (0.00–150.00) | 90 | 4.50 (0.00–95.00) | |||||
| 1 | 12 | 5.00 (0.00–52.50) | 12 | 2.75 (0.00–90.00) | 12 | 0.37 (0.00–10.00) | |||||
| Tumour grade | 0.007 | 0.407 | 0.325 | ||||||||
| Low | 28 | 1.75 (0.00–63.00) | 29 | 2.00 (0.00–97.50) | 29 | 1.50 (0.00–95.00) | |||||
| High | 72 | 16.25 (0.00–150.00) | 72 | 5.00 (0.00–150.00) | 73 | 4.50 (0.00–70.00) | |||||
| Resection margin | 0.172 | 0.920 | 0.834 | ||||||||
| R0 | 74 | 6.00 (0.00–106.25) | 75 | 4.00 (0.00–150.00) | 76 | 4.25 (0.00–95.00) | |||||
| R1 & 2 | 26 | 15.00 (0.00–150.00) | 26 | 2.50 (0.00–90.00) | 26 | 3.75 (0.00–55.00) | |||||
IGKC, immunoglobulin kappa C.
Prognostic significance of CD20, CD138 and IGKC expression in oesophageal and gastric adenocarcinoma
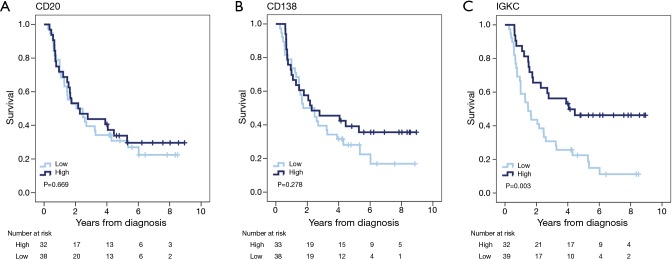
Kaplan-Meier curves for OS according to the investigated markers in all patients with oesophageal adenocarcinoma are shown in Figure 2. Whereas there were no significant associations between OS and high CD20 or CD138 expression, there was a significantly prolonged OS for patients with tumours displaying high IGKC expression (P=0.003). As shown in Table 4, in patients with M0/R0 oesophageal cancer, the association of high IGKC expression with a reduced risk of death was significant in both univariable and multivariable Cox regression analysis (HR 0.21; 95% CI, 0.08–0.60 and HR 0.10; 95% CI, 0.02–0.57, respectively). With regards to TTR, KM analysis revealed that a high IGKC expression was significantly associated with a prolonged TTR in all patients with oesophageal adenocarcinoma (P=0.003, data not shown). In patients with M0/R0 oesophageal cancer, the association of high IGKC expression with a reduced risk of recurrence was significant in both univariable and multivariable Cox regression analysis (HR 0.20; 95% CI, 0.06–0.65, and HR 0.15; 95% CI, 0.03–0.71, respectively) (Table 4).
Figure 2.
Kaplan-Meier estimates of overall survival according to expression of B cell and plasma cell markers in patients with oesophageal adenocarcinoma. Overall survival according to high and low expression of (A) CD20, (B) CD138 and (C) IGKC in all patients with oesophageal adenocarcinoma. IGKC, immunoglobulin kappa C.
Table 4. Cox proportional hazards analysis of the impact of CD20, CD138 and IGKC expression on time to recurrence and overall survival in oesophageal and gastric adenocarcinoma, respectively.
| Group | TTR | OS | |||||
|---|---|---|---|---|---|---|---|
| n [events] | HR (95% CI) | P | n [events] | HR (95% CI) | P | ||
| Oesophageal | |||||||
| CD20 | |||||||
| Univariable | |||||||
| Low | 17 [7] | 1.00 | 20 [10] | 1.00 | |||
| High | 15 [7] | 0.88 (0.31–2.73) | 0.878 | 18 [18] | 1.17 (0.49–2.81) | 0.726 | |
| Multivariable | |||||||
| Low | 16 [7] | 1.00 | 19 [10] | 1.00 | |||
| High | 15 [6] | 1.17 (0.33–4.12) | 0.805 | 18 [10] | 1.23 (0.41–3.71) | 0.716 | |
| CD138 | |||||||
| Univariable | |||||||
| Low | 15 [8] | 1.00 | 20 [13] | 1.00 | |||
| High | 18 [5] | 0.40 (0.13–1.25) | 0.116 | 19 [7] | 0.39 (0.16–1.00) | 0.050 | |
| Multivariable | |||||||
| Low | 15 [8] | 1.00 | 20 [13] | 1.00 | |||
| High | 17 [5] | 1.62 (0.49–5.31) | 0.426 | 18 [7] | 0.73 (0.24–2.21) | 0.575 | |
| IGKC | |||||||
| Univariable | |||||||
| Low | 14 [9] | 1.00 | 20 [15] | 1.00 | |||
| High | 19 [4] | 0.20 (0.06–0.65) | 0.008 | 19 [5] | 0.21 (0.08–0.60) | 0.003 | |
| Multivariable | |||||||
| Low | 13 [9] | 1.00 | 19 [15] | 1.00 | |||
| High | 19 [4] | 0.15 (0.03–0.71) | 0.017 | 19 [5] | 0.10 (0.02–0.57) | 0.009 | |
| Gastric | |||||||
| CD20 | |||||||
| Univariable | |||||||
| Low | 30 [16] | 1.00 | 32 [23] | 1.00 | |||
| High | 32 [13] | 0.55 (0.26–1.14) | 0.110 | 38 [24] | 0.73 (0.41–1.29) | 0.278 | |
| Multivariable | |||||||
| Low | 30 [16] | 1.00 | 32 [23] | 1.00 | |||
| High | 32 [13] | 0.92 (0.42–2.03) | 0.834 | 38 [24] | 0.98 (0.51–1.87) | 0.952 | |
| CD138 | |||||||
| Univariable | |||||||
| Low | 29 [14] | 1.00 | 31 [24] | 1.00 | |||
| High | 34 [15] | 0.77 (0.37–1.60) | 0.481 | 40 [23] | 0.50 (0.27–0.90) | 0.021 | |
| Multivariable | |||||||
| Low | 29 [14] | 1.00 | 31 [24] | 1.00 | |||
| High | 34 [15] | 1.17 (0.51–2.65) | 0.711 | 40 [23] | 0.0.55 (0.30–1.00) | 0.052 | |
| IGKC | |||||||
| Univariable | |||||||
| Low | 30 [16] | 1.00 | 34 [25] | 1.00 | |||
| High | 34 [13] | 0.53 (0.25–1.10) | 0.090 | 38 [22] | 0.55 (0.31–0.99) | 0.047 | |
| Multivariable | |||||||
| Low | 30 [16] | 1.00 | 34 [25] | 1.00 | |||
| High | 34 [13] | 0.45 (0.21–0.98) | 0.043 | 38 [22] | 0.46 (0.24–0.87) | 0.018 | |
IGKC, immunoglobulin kappa C; TTR, time to recurrence; OS, overall survival; HR, hazard ratio; CI, confidence interval.
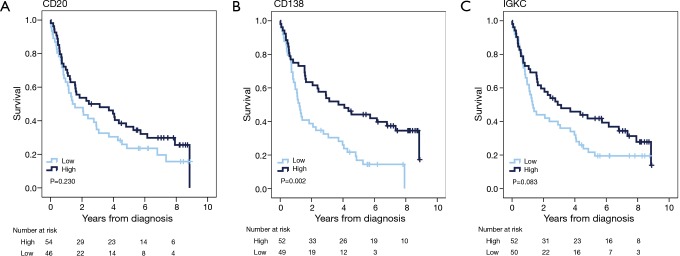
Kaplan-Meier curves for OS according to the investigated markers in all patients with gastric adenocarcinoma are shown in Figure 3. There were no significant associations between high CD20 expression and OS; however, there was a significantly prolonged OS for patients with tumours displaying high CD138 expression (P=0.002), and a non-significant trend towards an improved OS for patients with tumours displaying high IGKC expression with (P=0.083). As shown in Table 4, in patients with M0/R0 gastric cancer, the association of high CD138 expression with a reduced risk of death was significant only in univariable and borderline significant in multivariable Cox regression analysis (HR 0.50; 95% CI, 0.27–0.90 and HR 0.55; 95% CI, 0.30–1.00, respectively). The association of high IGKC expression with a reduced risk of death was however significant in both univariable and multivariable Cox regression analysis (HR 0.55; 95% CI, 0.31–0.99 and HR 0.46; 95% CI, 0.26–0.87, respectively). With regards to TTR in patients with M0/R0 oesophageal cancer, neither CD20 nor CD138 expression was prognostic, but high IGKC expression was significantly associated with a reduced risk of recurrence in both univariable and multivariable Cox regression analysis (HR 0.20; 95% CI, 0.06–0.65 and HR 0.15; 95% CI, 0.03–0.71, respectively) (Table 4). In patients with M0/R0 gastric cancer, neither CD20 nor CD138 expression was prognostic in relation to TTR, and high IGKC expression was only prognostic in multivariable analysis (HR 0.45; 95% CI, 0.21–0.98).
Figure 3.
Kaplan-Meier estimates of overall survival according to expression of B cell and plasma cell markers in patients with gastric adenocarcinoma. Overall survival according to high and low expression of (A) CD20, (B) CD138 and (C) IGKC in all patients with gastric adenocarcinoma. IGKC, immunoglobulin kappa C.
CD20+ B cell islets were present in 11 (6.3%) of the tumours, and the median was 1 islet per tumour (range, 0–3). In the Kaplan-Meier analysis there was a trend, however non-significant, towards an improved survival for patients with tumours displaying B cell islets as compared with tumours having none.
Discussion
The numerous studies investigating the prognostic effect of the cellular immune system in different forms of cancer have recently been supplemented by research exploring the role of the humoral immune system. To the best of our knowledge, this is the first report to describe the prognostic impact of IGKC in oesophageal and gastric adenocarcinoma, with the results demonstrating that abundant IGKC+ plasma cell tumour infiltration is an independent predictor of an improved OS and TTR in both oesophageal and gastric adenocarcinoma.
B cells are known to be antibody producers but they are also, alongside macrophages and dendritic cells, antigen-presenting cells with the ability to supply co-stimulatory signals for T-cells (29-31). The existence of T cells and B cells in conjunction is necessary for an effective anti-tumour immunity, as evidenced by the improved prognosis in tumours with a high number of CD8+, CD4+ and CD20+ lymphocytes (32-34). The recent report by Kroeger et al. in ovarian cancer (34), demonstrating that CD8+ T cells do not carry any prognostic value without the presence of dense infiltration of plasma cells (IgG+) in ovarian cancer further corroborates the importance of an intact immune system and more specifically the significance of the humoral immune response.
The auspicious impact of tumour infiltrating plasma cells has previously been reported in NSCLC, colorectal and breast cancer (22). Our data on esophageal and gastric adenocarcinoma, demonstrating dense plasma cell infiltration to improve OS, is in line with the vast majority of previous research.
The antibody dependent activation of CD8+ T cells and natural killer cells, and the following cellular cytotoxicity is a plausible explanation for the positive impact on survival seen with an increased number of tumour infiltrating plasma cells. Furthermore, plasma cells have been shown to be able to activate the complement system (35) and to competitively inhibit myeloid derived stem cells (36), thereby promoting tumour cell destruction and creating a favourable inflammatory microenvironment, respectively. On the other hand, Mohammed et al. (17) reported that breast cancer patients with tumours displaying a high proportion of tumour infiltrating plasma cells (CD138+) had a significantly decreased OS, in line with recent findings in EOC (18). One explanation for this may be the presence of regulatory plasma cells with the ability to inhibit the T cell response through cytokines IL-10 (37) and IL-35 (38). Moreover, CD138 has also been proposed as a novel target for immunotherapy in metastatic breast cancer (39). Notably, these are two of very few studies to demonstrate an adverse relationship between high plasma cell infiltration and survival, although plasma cell infiltration may vary in different cancer types, and its effect on survival accordingly.
Our findings are in concordance with studies on NSCLC (16,22), colorectal cancer (22,34), ovarian cancer (33) and breast cancer (22), all reporting ameliorated OS rates with high levels of plasma cells. Hitherto, only the tumour cell-specific expression of CD138 has been shown to be associated with more advanced TNM stage and poor clinical outcome in gastric cancer (19), similarly to findings in colorectal cancer (40).
There were significant intercorrelations between all three herein investigated markers, which are in consistency with previous findings in NSCLC (16). Of note, CD138 is a less specific plasma cell marker than IGKC (22), i.e., it may also be expressed in normal and malignant epithelial cells as well as in stromal fibroblasts.
There are somewhat conflicting data on the prognostic impact of CD20+ tumour infiltrating lymphocytes (TIL) in the existing literature. In a report by Shah et al. (41), mice completely lacking CD20+ TIL showed an improved anti-tumour cell response, suggesting that lifting CD20+ TIL inhibition enabled CD8+ TIL and Th1 (CD4+) cytokine anti-tumour responses. Similar data have been reported by Julien et al. (42). However, in a report on serous EOC, CD20+ TIL, when coupled with CD8+ TIL, were found to be significantly associated with decreased tumour progression, resulting in an improved OS (43). In the current study, however, there was no significant correlation between CD20+ cell density and survival.
A potential limitation to the present study is the use of TMA. More than fifteen years after its introduction (44), the TMA technique can be considered a well- established platform for tissue biomarker studies, providing similar or even better prognostic information than whole tissue section based analyses (45). However, suboptimal sampling may occur, e.g., of heterogeneously expressed markers. Therefore, whenever possible, tissue cores were obtained from different donor blocks of the primary tumours. Moreover, the use of two 1.0 mm cores can be considered a comparatively generous sampling size and, of note, heterogeneity issues may well arise even with the use of whole tissue sections. Use of the TMA technique may also not be optimal for detection of B cell islets. In the study by Berntsson et al., which was also based on TMA analysis and wherein a high density of CD20+ B-cells was found to be an independent predictor of a prolonged survival in colorectal cancer, CD20+ B cell islets were found in 8% of cases (15). The corresponding proportion in this study was 11%, and in neither of the studies, a significant association with survival could be found. The small proportion may well be due to use of the TMA-technique, and may also explain the lack of association with prognosis. It is likely that analysis of whole tissue sections slides would improve the detection rate for B cell islets, and hence also demonstrate their prognostic impact in oesophageal and gastric cancer.
Although the study cohort can be considered as medium-sized, the number of cases is more limited when stratifying for tumour location. Therefore, additional validatory studies on independent, and, if possible, larger cohorts are warranted. Another potential caveat is that several tests have been made, which increases the risk for type I errors, i.e., detecting a difference that is coincidental.
Conclusions
This study provides a first description of the prognostic significance of tumour-infiltrating B cells and plasma cells in oesophageal and gastric adenocarcinoma. The strongest association with prognosis was observed for IGKC+ plasma cells, abundant infiltration of which was found to be an independent predictor of a prolonged survival in both types of cancer.
Acknowledgements
This study was supported by grants from the Swedish Research Council, the Swedish Cancer Society, the Swedish Government Grant for Clinical Research, Lund University Faculty of Medicine, and the Lund University Hospital Research Grants. Parts of the study were supported by the Lions Cancerfond and the Erik, Karin and Gösta Selanders Foundation.
Ethical Statement: The study was approved by the regional ethics committee at Lund University (No. 445/07), whereby the committee waived the need for consent other than by the option to opt out.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893-917. 10.1002/ijc.25516 [DOI] [PubMed] [Google Scholar]
- 2.Fridman WH, Pagès F, Sautès-Fridman C, et al. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 2012;12:298-306. 10.1038/nrc3245 [DOI] [PubMed] [Google Scholar]
- 3.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74. 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 4.Ostrand-Rosenberg S. Immune surveillance: a balance between protumor and antitumor immunity. Curr Opin Genet Dev 2008;18:11-8. 10.1016/j.gde.2007.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010;140:883-99. 10.1016/j.cell.2010.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin WW, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest 2007;117:1175-83. 10.1172/JCI31537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klemm F, Joyce JA. Microenvironmental regulation of therapeutic response in cancer. Trends Cell Biol 2015;25:198-213. 10.1016/j.tcb.2014.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell 2006;124:263-6. 10.1016/j.cell.2006.01.007 [DOI] [PubMed] [Google Scholar]
- 9.Murdoch C, Muthana M, Coffelt SB, et al. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer 2008;8:618-31. 10.1038/nrc2444 [DOI] [PubMed] [Google Scholar]
- 10.Naito Y, Saito K, Shiiba K, et al. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res 1998;58:3491-4. [PubMed] [Google Scholar]
- 11.Schumacher K, Haensch W, Röefzaad C, et al. Prognostic significance of activated CD8(+) T cell infiltrations within esophageal carcinomas. Cancer Res 2001;61:3932-6. [PubMed] [Google Scholar]
- 12.Al-Shibli KI, Donnem T, Al-Saad S, et al. Prognostic effect of epithelial and stromal lymphocyte infiltration in non-small cell lung cancer. Clin Cancer Res 2008;14:5220-7. 10.1158/1078-0432.CCR-08-0133 [DOI] [PubMed] [Google Scholar]
- 13.Nedergaard BS, Ladekarl M, Nyengaard JR, et al. A comparative study of the cellular immune response in patients with stage IB cervical squamous cell carcinoma. Low numbers of several immune cell subtypes are strongly associated with relapse of disease within 5 years. Gynecol Oncol 2008;108:106-11. 10.1016/j.ygyno.2007.08.089 [DOI] [PubMed] [Google Scholar]
- 14.Schmidt M, Böhm D, von Törne C, et al. The humoral immune system has a key prognostic impact in node-negative breast cancer. Cancer Res 2008;68:5405-13. 10.1158/0008-5472.CAN-07-5206 [DOI] [PubMed] [Google Scholar]
- 15.Berntsson J, Nodin B, Eberhard J, et al. Prognostic impact of tumour-infiltrating B cells and plasma cells in colorectal cancer. Int J Cancer 2016;139:1129-39. 10.1002/ijc.30138 [DOI] [PubMed] [Google Scholar]
- 16.Lohr M, Edlund K, Botling J, et al. The prognostic relevance of tumour-infiltrating plasma cells and immunoglobulin kappa C indicates an important role of the humoral immune response in non-small cell lung cancer. Cancer Lett 2013;333:222-8. 10.1016/j.canlet.2013.01.036 [DOI] [PubMed] [Google Scholar]
- 17.Mohammed ZM, Going JJ, Edwards J, et al. The relationship between lymphocyte subsets and clinico-pathological determinants of survival in patients with primary operable invasive ductal breast cancer. Br J Cancer 2013;109:1676-84. 10.1038/bjc.2013.493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lundgren S, Berntsson J, Nodin B, et al. Prognostic impact of tumour-associated B cells and plasma cells in epithelial ovarian cancer. J Ovarian Res 2016;9:21. 10.1186/s13048-016-0232-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wiksten JP, Lundin J, Nordling S, et al. Epithelial and stromal syndecan-1 expression as predictor of outcome in patients with gastric cancer. Int J Cancer 2001;95:1-6. [DOI] [PubMed] [Google Scholar]
- 20.Kusumoto T, Kodama J, Seki N, et al. Clinical significance of syndecan-1 and versican expression in human epithelial ovarian cancer. Oncol Rep 2010;23:917-25. [DOI] [PubMed] [Google Scholar]
- 21.Davies EJ, Blackhall FH, Shanks JH, et al. Distribution and clinical significance of heparan sulfate proteoglycans in ovarian cancer. Clin Cancer Res 2004;10:5178-86. 10.1158/1078-0432.CCR-03-0103 [DOI] [PubMed] [Google Scholar]
- 22.Schmidt M, Hellwig B, Hammad S, et al. A comprehensive analysis of human gene expression profiles identifies stromal immunoglobulin κ C as a compatible prognostic marker in human solid tumors. Clin Cancer Res 2012;18:2695-703. 10.1158/1078-0432.CCR-11-2210 [DOI] [PubMed] [Google Scholar]
- 23.Fristedt R, Gaber A, Hedner C, et al. Expression and prognostic significance of the polymeric immunoglobulin receptor in esophageal and gastric adenocarcinoma. J Transl Med 2014;12:83. 10.1186/1479-5876-12-83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hedner C, Tran L, Borg D, et al. Discordant human epidermal growth factor receptor 2 overexpression in primary and metastatic upper gastrointestinal adenocarcinoma signifies poor prognosis. Histopathology 2016;68:230-40. 10.1111/his.12744 [DOI] [PubMed] [Google Scholar]
- 25.Jonsson L, Hedner C, Gaber A, et al. High expression of RNA-binding motif protein 3 in esophageal and gastric adenocarcinoma correlates with intestinal metaplasia-associated tumours and independently predicts a reduced risk of recurrence and death. Biomark Res 2014;2:11. 10.1186/2050-7771-2-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Borg D, Hedner C, Gaber A, et al. Expression of IFITM1 as a prognostic biomarker in resected gastric and esophageal adenocarcinoma. Biomark Res 2016;4:10. 10.1186/s40364-016-0064-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hedner C, Borg D, Nodin B, et al. Expression and Prognostic Significance of Human Epidermal Growth Factor Receptors 1 and 3 in Gastric and Esophageal Adenocarcinoma. PLoS One 2016;11:e0148101. 10.1371/journal.pone.0148101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hedner C, Gaber A, Korkocic D, et al. SATB1 is an independent prognostic factor in radically resected upper gastrointestinal tract adenocarcinoma. Virchows Arch 2014;465:649-59. 10.1007/s00428-014-1667-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crawford A, Macleod M, Schumacher T, et al. Primary T cell expansion and differentiation in vivo requires antigen presentation by B cells. J Immunol 2006;176:3498-506. 10.4049/jimmunol.176.6.3498 [DOI] [PubMed] [Google Scholar]
- 30.DiLillo DJ, Yanaba K, Tedder TF. B cells are required for optimal CD4+ and CD8+ T cell tumor immunity: therapeutic B cell depletion enhances B16 melanoma growth in mice. J Immunol 2010;184:4006-16. 10.4049/jimmunol.0903009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stockinger B, Zal T, Zal A, et al. B cells solicit their own help from T cells. J Exp Med 1996;183:891-9. 10.1084/jem.183.3.891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iglesia MD, Vincent BG, Parker JS, et al. Prognostic B-cell signatures using mRNA-seq in patients with subtype-specific breast and ovarian cancer. Clin Cancer Res 2014;20:3818-29. 10.1158/1078-0432.CCR-13-3368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kroeger DR, Milne K, Nelson BH. Tumor-Infiltrating Plasma Cells Are Associated with Tertiary Lymphoid Structures, Cytolytic T-Cell Responses, and Superior Prognosis in Ovarian Cancer. Clin Cancer Res 2016;22:3005-15. 10.1158/1078-0432.CCR-15-2762 [DOI] [PubMed] [Google Scholar]
- 34.Richards CH, Flegg KM, Roxburgh CS, et al. The relationships between cellular components of the peritumoural inflammatory response, clinicopathological characteristics and survival in patients with primary operable colorectal cancer. Br J Cancer 2012;106:2010-5. 10.1038/bjc.2012.211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vidarsson G, Dekkers G, Rispens T. IgG subclasses and allotypes: from structure to effector functions. Front Immunol 2014;5:520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Joyce JA, Fearon DT. T cell exclusion, immune privilege, and the tumor microenvironment. Science 2015;348:74-80. 10.1126/science.aaa6204 [DOI] [PubMed] [Google Scholar]
- 37.Neves P, Lampropoulou V, Calderon-Gomez E, et al. Signaling via the MyD88 adaptor protein in B cells suppresses protective immunity during Salmonella typhimurium infection. Immunity 2010;33:777-90. 10.1016/j.immuni.2010.10.016 [DOI] [PubMed] [Google Scholar]
- 38.Shen P, Roch T, Lampropoulou V, et al. IL-35-producing B cells are critical regulators of immunity during autoimmune and infectious diseases. Nature 2014;507:366-70. 10.1038/nature12979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rousseau C, Ruellan AL, Bernardeau K, et al. Syndecan-1 antigen, a promising new target for triple-negative breast cancer immuno-PET and radioimmunotherapy. A preclinical study on MDA-MB-468 xenograft tumors. EJNMMI Res 2011;1:20. 10.1186/2191-219X-1-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fujiya M, Watari J, Ashida T, et al. Reduced expression of syndecan-1 affects metastatic potential and clinical outcome in patients with colorectal cancer. Jpn J Cancer Res 2001;92:1074-81. 10.1111/j.1349-7006.2001.tb01062.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shah S, Divekar AA, Hilchey SP, et al. Increased rejection of primary tumors in mice lacking B cells: inhibition of anti-tumor CTL and TH1 cytokine responses by B cells. Int J Cancer 2005;117:574-86. 10.1002/ijc.21177 [DOI] [PubMed] [Google Scholar]
- 42.Julien S, Picco G, Sewell R, et al. Sialyl-Tn vaccine induces antibody-mediated tumour protection in a relevant murine model. Br J Cancer 2009;100:1746-54. 10.1038/sj.bjc.6605083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nielsen JS, Sahota RA, Milne K, et al. CD20+ tumor-infiltrating lymphocytes have an atypical CD27- memory phenotype and together with CD8+ T cells promote favorable prognosis in ovarian cancer. Clin Cancer Res 2012;18:3281-92. 10.1158/1078-0432.CCR-12-0234 [DOI] [PubMed] [Google Scholar]
- 44.Kononen J, Bubendorf L, Kallioniemi A, et al. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med 1998;4:844-7. 10.1038/nm0798-844 [DOI] [PubMed] [Google Scholar]
- 45.Torhorst J, Bucher C, Kononen J, et al. Tissue microarrays for rapid linking of molecular changes to clinical endpoints. Am J Pathol 2001;159:2249-56. 10.1016/S0002-9440(10)63075-1 [DOI] [PMC free article] [PubMed] [Google Scholar]