Abstract
Background
Role of chemotherapy in patients who continue to have unresectable disease after pre-operative chemo-radiotherapy (CRT) remains largely unaddressed.
Methods
Patients with LA rectal cancer from January 2013 to June 2015 were evaluated. Post-CRT, patients, who were deemed unresectable, were considered for further interim chemotherapy (i-CT).
Results
Seventy six patients (15%) with median age of 38.5 years received i-CT after CRT. About 61.8% patients receiving i-CT managed to undergo a definitive surgery and the extent of surgery was reduced in 48.7% patients. With the median follow up of 19 months, the estimated 2-year event free survival (EFS) of 48% and OS was 56%. The estimated 2-year OS was 81% in mucinous tumors whereas it was 44.4% in signet ring pathology (P=0.045). The 2-year OS of 86% for whom surgery was done vs. 38% (2-year OS) in whom surgery was not done (P=0.011). Survival was better in conservative surgery group vs. total pelvic exenteration (TPE) vs. no surgery (2-year OS: 84% vs. 59.1% vs. 38%; P=0.033). In the CAPE-OX group, 71.4% (14/23) underwent surgery whereas 75.9% (29/47) in the 5-FU plus irinotecan plus oxaliplatin (FOLFIRINOX) group with EFS (P=0.570) and OS (P=0.120). In conservative surgery group, OS was better in FOLFIRINOX (2-year OS: 95.7%) vs. capecitabine plus oxaliplatin (CAPOX) (2-year OS: 70%) (P=0.012).
Conclusions
i-CT can lead to improved resection rates, improved survivals and downstaging with acceptable toxicity. FOLFIRINOX appears to better over CAPOX, specifically in whom conservative surgery is feasible.
Keywords: Locally advanced, rectal adenocarcinoma, unresectable, interim chemotherapy (i-CT), 5-FU plus irinotecan plus oxaliplatin (FOLFIRINOX), downstaging
Introduction
Colorectal cancer ranks as the fourth most common malignancy in the world (1). Rectal carcinomas present a unique problem in local management due to their anatomic peculiarity. The addition of chemotherapy to pre-operative radiotherapy (RT) has improved local control and DFS rates but has not significantly affected the distant metastatic rates and OS. Methods to reduce distant failure include the use of neo-adjuvant chemotherapy (NACT) pre or post chemo-radiotherapy (CRT) prior to surgery and early studies support these strategies in terms of increased pathological complete response (pCR) rates and R0 resection rates (2,3).
The principle of sharp dissection in the TME plane as advocated by Bill Heald and implementation of national training programmes have improved outcomes of rectal cancer surgery (4-6), primarily due to improved local control rates and disease free survival (DFS). The current standard in locally advanced non-metastatic rectal cancers is neoadjuvant long course radiotherapy (LCRT) with concurrent chemotherapy. This is based on the German Rectal Cancer Study Group trial showing the benefit of LCRT over surgery alone and the EORTC 22921 which concluded that the addition of fluorouracil and leucovorin to the preoperative radiotherapy reduced locoregional recurrence (7-10). However, both trials showed improved in local control rates without DFS or OS benefit. The addition of oxaliplatin or biological therapy to CRT regimen has not been consistently associated with higher response rates and definitely not without increasing toxicity (11-15).
There is some evidence supporting that additional NACT after pre-operative CRT and delaying surgery can lead to improvement in pathological complete response rates (2,3). Limited experience with NACT prior to CRT in MRI defined high-risk disease has shown good response rates and improvement in R0 resection rates suggesting that NACT is a feasible option (16). However, the role of NACT in local down staging of patients who continue to have unresectable disease after pre-operative CRT remains largely unaddressed. Such patients, if operated, may have high CRM involvement and may require TPE/extended resection for achieving negative margins. This would reflect on further decreased compliance to treatment, and decremental quality of life after surgery.
Our hypothesis is that administering chemotherapy after a gap of 6 weeks post CTRT in rectal cancer patients not feasible for conservative surgeries may downstage the disease, allowing less extensive and aggressive resections. The aim of our study was to retrospectively assess the feasibility of administering chemotherapy after CRT [interim chemotherapy (i-CT)] in unresectable rectal cancers, as a modality to improve chances of surgical resectability, i.e., R0 resection as well as potentially less invasive treatment strategy.
Methods
Patient population and treatment
Patients with locally advanced rectal cancer (treated in the Medical Oncology GI unit between January 2013 to June 2015 were evaluated and those who received i-CT post CRT (were identified from a prospective database and included in analysis. Baseline MRI (MRI 1) was available in all patients. All patients had received CRT with 45 Gray in 25 fractions with boost of 3.2 Gray with concurrent capecitabine 825 mg/m2 twice daily throughout the course of radiation. Response evaluation with MRI (MRI-2), 6 weeks post completion of CRT was performed. Post CRT, patients, who were deemed unresectable, but with ECOG PS 0–2, were discussed in the multidisciplinary joint clinic (MDJC) and then considered for further i-CT if any of the following criteria were fulfilled (I) mesorectal invasion; or (II) threatened circumferential margin (CRM), defined as disease within 2 mm of CRM on MRI; or (III) morbid surgery required for R0 resection. Chemotherapeutic regimens administered were as per physician’s recommendation and included: (I) capecitabine plus oxaliplatin (CAPE-OX); (II) 5-FU plus irinotecan plus oxaliplatin (FOLFIRINOX); (III) 5-FU plus oxaliplatin (FOLFOX); (IV) capecitabine plus oxaliplatin (CAPOX) + bevacizumab; (V) 5-FU plus irinotecan (FOLFIRI).
Patients receiving all regimens were included for analysis regarding efficacy and benefit of i-CT, but only those receiving CAPOX and FOLFIRINOX were compared for prognosis and outcomes. Standard doses as per previous published schedules were used.
Grade 3/4 toxicities were recorded using CTCAE version 4.03 (17). Response assessments with pelvic MRI (MRI-3) were performed post two to four cycles of chemotherapy. Computed Tomography (CT) scan of chest and abdomen was also done post i-CT to look for distant metastasis. Further management including the extent of surgery was discussed in MDJC. Patients undergoing surgery received adjuvant chemotherapy to complete a total 6 months of chemotherapy including pre-operative CRT phase and i-CT. Those patients who were considered unresectable or refused surgery were continued on chemotherapy with palliative intent.
Clinical data collection
For the purposes of this study demographic data and baseline clinical and tumor characteristics, chemotherapy regimens, toxicity, surgical procedures, and outcomes were collected from a prospectively maintained GI database and electronic medical record system.
Statistical analysis
The data was analysed using SPSS software version 20. Descriptive statistics including median, frequency and percentage for categorical variables were used to describe age, sex distribution, primary T staging, neoadjuvant chemotherapy regimen type and toxicity profile, definitive surgery were used for categorical variables. Pearson’s Chi-square test and Fisher’s exact test were performed to compare the baseline demography, the T stage, N stage and CRM based on MRI 2, baseline histopathology and qualitative parameters (response and toxicity) between the CAPOX and FOLFIRINOX regimens. Survival analysis was done using Kaplan Meir Method and comparison between survival of subgroups was done by log rank test. OS was defined as the time from date of diagnosis to the date of last follow up or death. Event free survival (EFS) was defined as the time from the date of diagnosis to the event (non-conservative surgery, progression, decision of inoperability or unresectable disease or death). The patients were subgrouped with respect to conservative surgery, exenterative/non-conservative surgery and inoperable disease for purpose of overall survival analysis.
Results
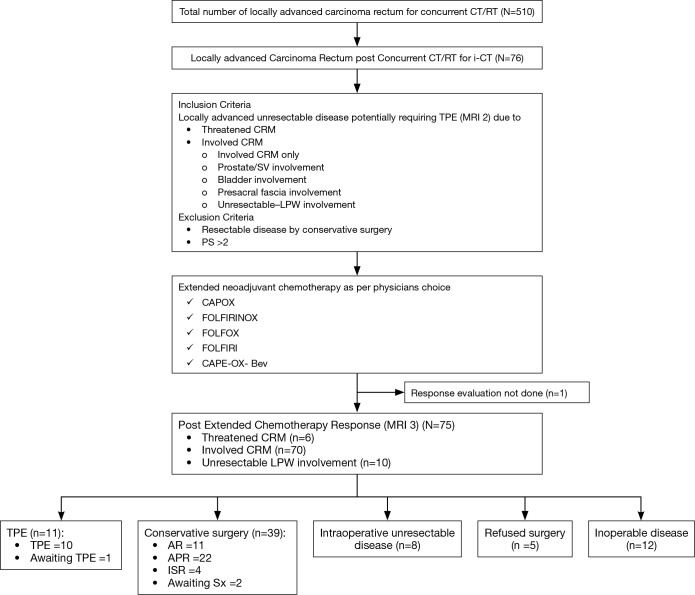
Between January 2013 and June 2015, 510 patients with locally advanced rectal cancer treated in the Medical Oncology GI unit were evaluated. Seventy six patients (15%) received i-CT after CTRT as shown in the study outline (Figure 1), and were considered for analysis.
Figure 1.
Outline of our study. i-CT, interim chemotherapy; TPE, total pelvic exenteration; CRM, circumferential resection margin; LPW, lateral pelvic wall; CAPOX, capecitabine plus oxaliplatin; FOLFIRINOX, 5-FU plus irinotecan plus oxaliplatin; FOLFOX, 5-FU plus oxaliplatin; CAPE-OX, capecitabine plus oxaliplatin; AR, anterior resection; APR, abdominal pelvic resections; ISR, intersphincteric resection.
The median age of patients in the i-CT cohort was 38.5 years (range, 15–72 years). Male to female ratio was 4:1. Mucin secreting adenocarcinoma was the predominant histology accounting for 76.3% (58/76) patients and signet ring adenocarcinoma accounting for the remaining 23.7% patients. Baseline staging ‘T’, ‘N’ pre-CT-RT and indications for NACT are provided in Table 1.
Table 1. Tumour staging (post-CRT, pre-chemo and post-surgery).
| Tumor staging | Baseline stage (%) | Post-op stage (%) |
|---|---|---|
| T staging | ||
| T0 | 0 | 11 (14.5) |
| T1 | 0 | 0 |
| T2 | 0 | 8 (10.5) |
| T3 | 59 (77.6) | 20 (26.3) |
| T4a | 10 (13.1) | 2 (2.6) |
| T4b | 7 (9.3) | 4 (5.3) |
| Nodal staging | ||
| N0 | 14 (18.4) | 26 (34.2) |
| N1 | 9 (11.8) | 9 (11.8) |
| N2 | 38 (50.0) | 8 (10.5) |
| N3 | 15 (19.7) | 2 (2.6) |
| HPR not available | – | 2 (2.6) |
| Surgery not done | – | 29 (38.1) |
CRT, chemo-radiotherapy; HPR, histopathological review.
Post-CRT and i-CT
Of the 76 patients, post-CRT assessment with pelvic MRI (MRI 2) showed mesorectal invasion in 70 patients and threatened CRM in 6 patients (Table 2), thereby placing them as candidates for non-conservative TPE. These patients were taken up for i-CT. Characteristics of i-CT administered.
Table 2. Comparison of disease extent in pre NACT (MRI-2) and post NACT MRI (MRI-3).
| Disease extent | Pre-chemo assessment | Post-chemo assessment | Response/reductions (%) |
|---|---|---|---|
| Prostate/SV involvement | 15 | 7 | 53 |
| Lateral pelvic wall involvement | 8 | 2 | 75 |
| Bladder involvement | 1 | 0 | 100 |
| Presacral fascia involvement | 2 | 1 | 50 |
| CRM involved | |||
| CRM only | 52 | 37 | 28 |
| CRM + prostate/SV involved | 15 | 7 | 47 |
| CRM + bladder involvement | 1 | 0 | 100 |
| CRM + presacral involvement | 2 | 1 | 50 |
| CRM threatened | 6 | 2 | 66 |
NACT, neo-adjuvant chemotherapy; CRM, circumferential resection margin.
CAPE-Ox was used in 23 patients, FOLFIRINOX in 47 patients, FOLFIRI in 1, FOLFOX in 4 and CAPE-Ox + bevacizumab in 1 patient. Median number of cycles of chemotherapy administered to patients who underwent surgery was four in both the CAPE-OX group and FOLFOXIRI group with a minimum of two cycles being given to all. Demographic and tumor characteristics of patients receiving CAPE-OX and FOLFIRINOX, respectively are shown in Table 3, along with the incidence of grade III/IV toxicity in each cohort (Table 4). There was no statistically significant difference between the baseline tumor characteristics in both subgroups. Incidence of grade III/IV toxicity was 17.4% in CAPE-OX group and 29.5% in FOLFIRINOX group (P value: not significant) grade III/IV toxicities in the two groups are shown in Table 4. One patient died in FOLFIRINOX group due to neutropenic sepsis.
Table 3. Basic demographic, histology and pre chemotherapy MRI 2 based T, N and CRM comparison in CAPOX vs. FOLFIRINOX group.
| Characteristics | CAPOX | FOLFIRINOX | P value |
|---|---|---|---|
| Age (years) | 0.525 | ||
| <50 | 17 | 39 | |
| ≥50 | 6 | 8 | |
| Gender | 0.87 | ||
| Males | 22 | 37 | |
| Females | 1 | 10 | |
| Histopathology | 0.554 | ||
| Mucinous | 16 | 37 | |
| Signet ring | 7 | 10 | |
| T stage on MRI 2 | 0.764 | ||
| T3 | 17 | 37 | |
| T4 | 6 | 10 | |
| N stage on MRI 2 | 0.159 | ||
| N0 | 1 | 11 | |
| N1 | 2 | 7 | |
| N2 | 15 | 21 | |
| N3 | 5 | 8 | |
| CRM on MRI 2 | 0.649 | ||
| Involved | 21 | 43 | |
| Threatened | – | – |
CRM, circumferential resection margin; CAPOX, capecitabine plus oxaliplatin; FOLFIRINOX, 5-FU plus irinotecan plus oxaliplatin.
Table 4. Chemotherapy toxicity.
| G3/4 toxicity | CAPOX (%) | FOLFIRINOX (%) | P value |
|---|---|---|---|
| CINV | 1 (4.3) | 2 (4.3) | Not significant |
| Mucositis | 0 | 0 | |
| Diarrhea | 2 (8.7) | 7 (14.9) | |
| FN | 0 | 3 (6.4) | |
| Neutropenia | 0 | 1 (2.1) | |
| Thrombocytopenia | 0 | 2 (4.3) | |
| HFS | 1 (4.3) | 2 (4.3) | |
| Death | 0 | 1 (2.1) |
CAPOX, capecitabine plus oxaliplatin; FOLFIRINOX, 5-FU plus irinotecan plus oxaliplatin; CINV, chemotherapy induced nausea and vomiting; FN, febrile neutropenia; HFS, Hand Foot Syndrome.
MRI 3 post i-CT
Response evaluation MRI pelvis done post-i-CT showed disease regression in 50/76 (65.3%) patients, stable disease in 19/76 (25%) patients and progression in 6/76 (7.9%) patients. Response assessment data was not available in 1 patient who died of sepsis and febrile neutropenia during the i-CT.
Post i-CT assessment showed mesorectal invasion in 45 patients and CRM threatened in 2 patients. There was a 35.7% reduction in the rate of mesorectal invasion and 66% reduction in the rate of threatened CRM as compared to pre i-CT MRI. Extent of disease in pre and post i-CT MRI is shown in Table 2.
Type of surgery performed
Definitive surgery was performed in 61.8% (47/76) patients—intersphincteric resection (ISR) in 4 (5.3%), low anterior resection (LAR) or anterior resection (AR) in 11 (14.5%), abdominal pelvic resections (APR) in 22 (28.9%), total pelvic exenteration (TPE) in 10 (13.2%) patients. Out of the patients undergoing TPE, four had CRM positive on MRI-3, CRM threatened in one, urinary bladder involvement in one, prostate and seminal vesical involvement in four patients. Two patients are awaiting non-exenterative surgery and one patient is awaiting exenterative Sx.
No patients developed distant metastasis on i-CT. Eight (10.5%) patients were found to be unresectable intraoperatively when considered for surgery post i-CT and 12 (15.8%) patients were considered inoperable based on MRI-3 findings. Five (6.6%) patients who were considered for surgery refused surgery and were continued on chemotherapy.
Downstaging with i-CT
Downstaging of tumour post i-CT leading to a less extensive surgery was possible in 37 (48.7%) patients with two more patients waiting for a non-exenterative/conservative surgery. This accounted for an overall downstaging rate of 51.3% (39/76). A cross comparison of baseline, and pathological tumour staging of the patients post-surgery is shown in Table 1.
Pathological response and TRG post i-CT
Nine out of 45 (20%) patients (two patients’ pathology details were not available at the time of manuscript writing) had pCR which did not show any EFS (P=0.594) or OS benefit (P=0.758) compared to those who did not achieve pCR. Mandard’s tumor regression grade (TRG) 1/5 and 2/5 were seen in 24 of 45 patients (53.3%), whereas the remaining 21 patients had TRG of 3/5 to 5/5. This was not statistically significant with respect to EFS or OS.
Outcomes and prognostic factors
The median follow up duration of the cohort of patients was 19 months. The median EFS of the patient was 19.22 months with 2-year EFS of 48%. The estimated 2-year OS was 56%. None of the factors evaluated for prognosis in terms of EFS reached statistical significance. The estimated 2-year OS was 81% in mucinous tumors whereas it was 44.4% in signet ring pathology tumors and this was statistically significant (P=0.045) (Figure 2) but this has no effect on EFS (P=0.122). Patients who underwent any surgery as compared to those who did not undergo any surgery had significantly better outcomes with a 2-year OS of 86% for patients in whom surgery was done vs. 38% (2-year OS) in whom surgery was not done (P=0.011) (Figure 3). There was also a significant difference (Figure 4) in survival between patients undergoing Conservative surgery vs. TPE vs. no surgery (median OS: 47 vs. 36 vs. 20.7 months; 2-year OS: 84% vs. 59.1% vs. 38%; P=0.033).
Figure 2.
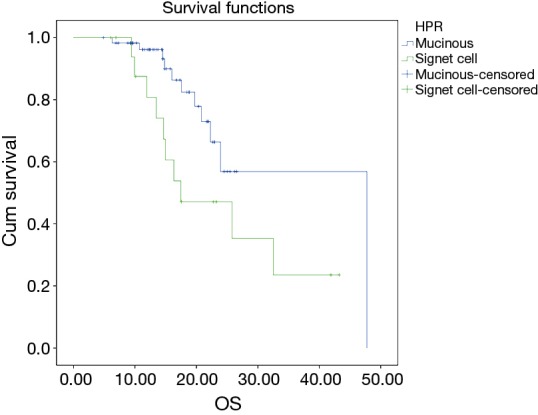
Kaplan-Meir analysis for OS (mucinous vs. signet ring histology).
Figure 3.
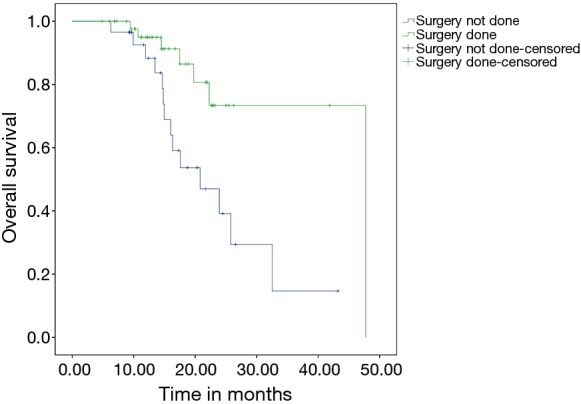
Kaplan-Meir analysis for OS (surgery vs. no Sx group).
Figure 4.
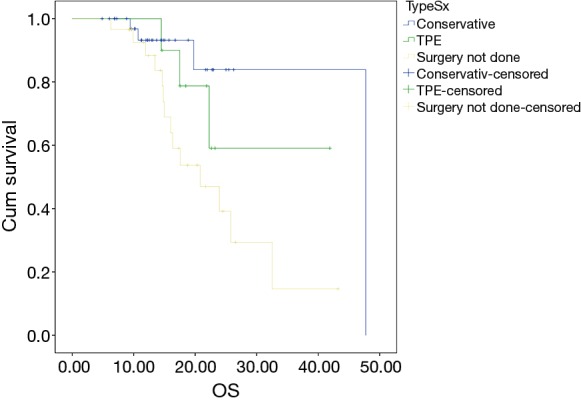
Kaplan-Meir analysis for OS for conservative surgery vs. exenteration vs. no surgery.
CAPOX vs. FOLFIRINOX (Table 5)
Table 5. Comparative analysis of CAPOX vs. FOLFIRINOX.
| Characteristic | Overall | Significance | CAPOX (n=23) | FOLFIRINOX (n=47) | Significance (CAPOX vs. FOLFIRINOX) |
|---|---|---|---|---|---|
| TRG (n=45) | NS | 5 (21.7%) | 19 (59.3%) | NS | |
| TRG (1/5 and 2/5) | 53.5% | ||||
| TRG (3/5–5/5) | 46.5% | ||||
| Pathological CR (pCR) (n=45) | NS | NS | |||
| pCR | 20 (44.4%) | 3 | 5 | ||
| No pCR | 25 (55.6%) | 10 | 24 | ||
| Surgery | NA | NS | |||
| Conservative | 37 | 10 | 23 | ||
| TPE | 10 | 4 | 6 | ||
| Awaiting conservative surgery | 2 | 0 | 2 | ||
| Awaiting TPE | 1 | 0 | 1 | ||
| Refused surgery | 5 | 3 | 2 | ||
| Inoperable/unresectable | 20 | 7 | 11 | ||
| Response evaluation not done | 1 | 0 | 1 | ||
| EFS | |||||
| Median EFS (months) | 19.2 | NA | 16.9 | 19.2 | NS |
| 2-year EFS | 48% | – | – | – | – |
| 2-year OS | |||||
| Whole cohort | 56% | NA | 43.8% | 67.1% | NS |
| Outcomes in patients undergoing surgery (n=47) | 86% | NA | – | – | – |
| Outcomes in patients undergoing conservative surgery (n=37) | 84% | NA | 70% | 95.7% | 0.012 |
CAPOX, capecitabine plus oxaliplatin; FOLFIRINOX, 5-FU plus irinotecan plus oxaliplatin; TRG, tumor regression grade; NS, not significant; TPE, total pelvic exenteration; NA, not applicable; EFS, event free survival.
There were no significant differences between pCR rates and TRG rates between the 2 regimens. In the CAPE-OX group, 71.4% (14/23) underwent surgery whereas this number was 75.9% (29/47) in the FOLFIRINOX group with no significant difference in EFS (P=0.570) and OS (P=0.120) relating to the type of i-CT used. There was no significant difference between the 2 regimens in terms of ability to undergo conservative surgery [CAPE-OX group, 43.5% (10/23) vs. 48.9% (23/47); P=0.74].
The estimated 2-year OS and EFS in CAPOX vs. FOLFIRINOX group as a whole were 43.8% vs. 67.1% (P=0.389) and 39.1% vs. 49.4% (P=0.794) respectively, with no statistically significant difference.
In patients who underwent conservative surgery, there was a significant difference between the overall survival in whom FOLFIRINOX (2-year OS, 95.7%) was considered over CAPOX (2-year OS 70%) as an i-CT (P=0.012) (Figure 5), whereas there is no significant difference in EFS (P=0.231) in these two regimes (Figure 6).
Figure 5.
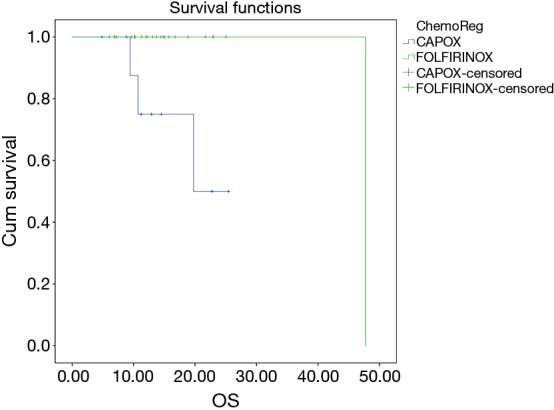
OS difference between FOLFIRINOX vs. CAPOX in conservative surgery group. FOLFIRINOX, 5-FU plus irinotecan plus oxaliplatin; CAPOX, capecitabine plus oxaliplatin.
Figure 6.
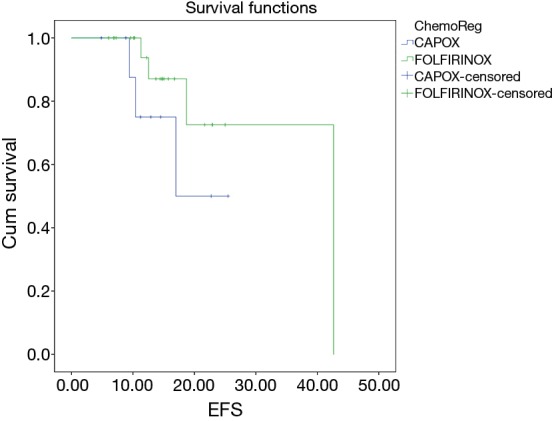
EFS difference between FOLFIRINOX vs. CAPOX in conservative surgery group. FOLFIRINOX, 5-FU plus irinotecan plus oxaliplatin; CAPOX, capecitabine plus oxaliplatin.
Discussion
Multimodality therapy has resulted in improved local control rates in rectal cancers. However the results for locally advanced tumours continue to remain far from satisfactory. Role of NACT post CRT in improving survival is one of the potential ways to improve upon existing survival rates. But this has always resulted in only local outcome without OS benefit. In their study, Habr-Gama et al. looked at the used of 5-fluorouracil/leucovorin therapy to improve complete response rates in rectal cancers (2). NACT post CRT resulted in improved CR rates with roughly two thirds of the study population having sustained CR at 12 months post therapy and were kept on close follow-up without undergoing surgery. The role of observation alone even in complete responders is not established and with the possibility of poor follow up, radical surgery is recommended. Hence adding chemotherapy in all patients with good response to CRT may not be completely appropriate. Chau et al. examined the role of NACT prior to CRT in MRI defined poor risk rectal cancers and found that capecitabine and oxaliplatin prior to CRT resulted in sustained tumour regression, rapid symptomatic response and achievement of R0 resection (16). These studies have clearly defined the feasibility of NACT in the cohort of resectable Ca-rectum post CRT.
However, to the best of our knowledge no study has examined the role of chemotherapy to improve resection rates, extent of resection, survival outcome and 2 different types of i-CT in unresectable disease after preoperative chemoradiation. pCR has been considered as a surrogate marker of improved responses and EFS in rectal adenocarcinoma (11). These studies emphasizes on the addition of chemotherapy pre or post CRT to improvise pCR rates. Our study, primarily concentrates on the downstaging achieved in terms of less extensive surgery in patients who would otherwise require extensive exenterative surgeries as well as evaluate any differences in EFS or OS with the approach of 2 different types of i-CT.
All patients in this study had CRM positive or threatened on MRI done post CRT. Out of these, 61.8% patients receiving i-CT managed to undergo a definitive surgery and the extent of surgery was reduced in 48.7% patients after the use of i-CT. Our study shows that patients achieving pCR or significant TRG (TRG1 and TRG2), did not have better outcomes in terms of EFS or OS compared to those who did not achieve these parameters, reiterating the fact that these are soft endpoints while evaluating the impact of newer therapies. The superior survival in patients who are undergoing surgery vs. who are not and improved survival in conservative surgery group vs. exenteration vs. no surgery group, validates the fact that these tumors were advanced and the option of observation (like the Habr-Gama study) (2) or going ahead with aggressive surgery would have resulted in poorer outcomes in these group of patients.
Regimens predominantly used were FOLFIRINOX and CAPE-OX in majority of our patients. FOLFIRINOX was considered based on the results of improved response rates in metastatic colorectal cancers to achieve radical curative resection (18). The patient selection and use of FOLFIRINOX vs. CAPOX was based on the discretion of treating physician, which translated into the 2 groups being almost equally matched in terms of baseline characteristics (Table 3).
The major achievement of i-CT (either CAPOX or FOLFIRINOX) seen in this study was the improvement in OS by making these patients surgically resectable with a conservative approach. While no regimen proved to be statistically superior over the other in terms of improving surgical outcomes, the patients receiving FOLFIRINOX had higher rates of tumour downstaging and grade III/IV toxicities as compared to CAPE-OX but these differences were not statistically significant. The most important finding of our study is, use of FOLFIRINOX over CAPOX as an i-CT improves OS and not EFS when patients after i-CT are downstaged and undergo conservative surgery. Thus, path CR or improved TRG may potentially be the surrogate markers of improved local control, but may not translate into improved OS. This has been proved frequently by many studies incorporating CRT for LA rectal tumors showing improving local control without any OS benefit. Effective downstaging and effective systemic chemotherapy probably are the key factors in translating the local control into OS benefit.
One of the concerns with the use of NACT was the delay in definitive surgical intervention that occurs potentially allowing tumour to progress. None of the patients developed distant metastases on chemotherapy and only 7.9% patients had local tumour progression on NACT, probably reflecting an aggressive disease biology. Similar results have been seen in the study by Garcia-Aguilar et al. (3).
About 76% patients with mucinous histology and 24% signet ring histology in our study indicates that we treated poor biology disease who usually have poorer outcome. This is one of the reasons to add aggressive chemotherapy like FOLFIRINOX, to achieve better response rates.
In our study the patients who responded and were downstaged for less extensive surgery had a 2-year OS of 84% as compared to the 2-year OS of 38% who did not undergo any surgery. Zampino et al. in their study used single agent capecitabine as extended chemotherapy post CRT in resectable rectal adenocarcinoma and had a resection rate of 100% and a 5-year DFS of 85% (19). Resection rates and survival in our study was were lower—this can be accounted for by the heavy tumour burden with significant number of our patients having mesorectal fascial invasion as well as considering the fact that these patients were not candidates for conservative surgery primarily. van DijK et al. studied the role of extended CAPE-OX–bevacizumab after CRT and had a 2-year OS of 80% which is similar to our study (20).
The major caveat of our study is its retrospective nature with no control arm for comparison. Our study has early outcomes and long term follow up is required. However, i-CT in this clinical situation surely looks promising and merits further investigation.
Conclusions
i-CT for unresectable disease after pre-operative chemoradiation can lead to improved resection rates, improved survivals and downstaging of tumours with acceptable toxicity. FOLFIRINOX appears to improve overall survival over CAPOX, specifically in patients who are amenable to conservative surgery post CRT and iCT.
Acknowledgements
None.
Ethical Statement: This study has been approved by Institutional Ethics Committee (IEC/1215/1569/001) from Tata memorial centre, Mumbai, India. Consent waiver was granted as it was a retrospective audit of prospective database.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. 10.1002/ijc.29210 [DOI] [PubMed] [Google Scholar]
- 2.Habr-Gama A, Perez RO, Sabbaga J, et al. Increasing the rates of complete response to neoadjuvant chemoradiotherapy for distal rectal cancer: results of a prospective study using additional chemotherapy during the resting period. Dis Colon Rectum 2009;52:1927-34. 10.1007/DCR.0b013e3181ba14ed [DOI] [PubMed] [Google Scholar]
- 3.Garcia-Aguilar J, Chow OS, Smith DD, et al. Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: a multicentre, phase 2 trial. Lancet Oncol 2015;16:957-66. 10.1016/S1470-2045(15)00004-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kapiteijn E, Putter H, van de Velde CJ, et al. Impact of the introduction and training of total mesorectal excision on recurrence and survival in rectal cancer in The Netherlands. Br J Surg 2002;89:1142-9. 10.1046/j.1365-2168.2002.02196.x [DOI] [PubMed] [Google Scholar]
- 5.Wibe A, Møller B, Norstein J, et al. A national strategic change in treatment policy for rectal cancer--implementation of total mesorectal excision as routine treatment in Norway. A national audit. Dis Colon Rectum 2002;45:857-66. 10.1007/s10350-004-6317-7 [DOI] [PubMed] [Google Scholar]
- 6.Martling A, Holm T, Rutqvist LE, et al. Impact of a surgical training programme on rectal cancer outcomes in Stockholm. Br J Surg 2005;92:225-9. 10.1002/bjs.4834 [DOI] [PubMed] [Google Scholar]
- 7.Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004;351:1731-40. 10.1056/NEJMoa040694 [DOI] [PubMed] [Google Scholar]
- 8.Sauer R, Liersch T, Merkel S, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol 2012;30:1926-33. 10.1200/JCO.2011.40.1836 [DOI] [PubMed] [Google Scholar]
- 9.Bosset JF, Calais G, Mineur L, et al. Preoperative radiation (Preop RT) in rectal cancer: Effect and timing of additional chemotherapy (CT) 5-year results of the EORTC 22921 trial. J Clin Oncol (Meeting Abstracts) 2005;23:3505 10.1200/JCO.2005.02.113 [DOI] [PubMed] [Google Scholar]
- 10.Bosset JF, Collette L, Calais G, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med 2006;355:1114-23. 10.1056/NEJMoa060829 [DOI] [PubMed] [Google Scholar]
- 11.Tulchinsky H, Shmueli E, Figer A, et al. An interval >7 weeks between neoadjuvant therapy and surgery improves pathologic complete response and disease-free survival in patients with locally advanced rectal cancer. Ann Surg Oncol 2008;15:2661-7. 10.1245/s10434-008-9892-3 [DOI] [PubMed] [Google Scholar]
- 12.Avallone A, Delrio P, Pecori B, et al. Oxaliplatin plus dual inhibition of thymidilate synthase during preoperative pelvic radiotherapy for locally advanced rectal carcinoma: long-term outcome. Int J Radiat Oncol Biol Phys 2011;79:670-6. 10.1016/j.ijrobp.2009.12.007 [DOI] [PubMed] [Google Scholar]
- 13.Hong YS, Kim DY, Lim SB, et al. Preoperative chemoradiation with irinotecan and capecitabine in patients with locally advanced resectable rectal cancer: long-term results of a Phase II study. Int J Radiat Oncol Biol Phys 2011;79:1171-8. 10.1016/j.ijrobp.2009.12.073 [DOI] [PubMed] [Google Scholar]
- 14.Kim SY, Hong YS, Kim DY, et al. Preoperative chemoradiation with cetuximab, irinotecan, and capecitabine in patients with locally advanced resectable rectal cancer: a multicenter Phase II study. Int J Radiat Oncol Biol Phys 2011;81:677-83. 10.1016/j.ijrobp.2010.06.035 [DOI] [PubMed] [Google Scholar]
- 15.Chua YJ, Barbachano Y, Cunningham D, et al. Neoadjuvant capecitabine and oxaliplatin before chemoradiotherapy and total mesorectal excision in MRI-defined poor-risk rectal cancer: a phase 2 trial. Lancet Oncol 2010;11:241-8. 10.1016/S1470-2045(09)70381-X [DOI] [PubMed] [Google Scholar]
- 16.Chau I, Brown G, Cunningham D, et al. Neoadjuvant capecitabine and oxaliplatin followed by synchronous chemoradiation and total mesorectal excision in magnetic resonance imaging-defined poor-risk rectal cancer. J Clin Oncol 2006;24:668-74. 10.1200/JCO.2005.04.4875 [DOI] [PubMed] [Google Scholar]
- 17.Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004;351:1731-40. 10.1056/NEJMoa040694 [DOI] [PubMed] [Google Scholar]
- 18.Falcone A, Ricci S, Brunetti I, et al. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the Gruppo Oncologico Nord Ovest. J Clin Oncol 2007;25:1670-6. 10.1200/JCO.2006.09.0928 [DOI] [PubMed] [Google Scholar]
- 19.Zampino MG, Magni E, Leonardi MC, et al. Capecitabine initially concomitant to radiotherapy then perioperatively administered in locally advanced rectal cancer. Int J Radiat Oncol Biol Phys 2009;75:421-7. 10.1016/j.ijrobp.2008.11.002 [DOI] [PubMed] [Google Scholar]
- 20.van Dijk TH, Tamas K, Beukema JC, et al. Evaluation of short-course radiotherapy followed by neoadjuvant bevacizumab, capecitabine, and oxaliplatin and subsequent radical surgical treatment in primary stage IV rectal cancer. Ann Oncol 2013;24:1762-9. 10.1093/annonc/mdt124 [DOI] [PubMed] [Google Scholar]