Abstract
Medication-related osteonecrosis of the jaw (MRONJ) has been associated with medications that include bisphosphonates (BPs), denosumab, bevacizumab and sunitinib. Ziv-aflibercept is a recombinant human vascular endothelial growth factor (VEGF) receptor which has been used to treat patients with various advanced solid tumors. We report three patients without a history of the use of medications known to cause MRONJ presenting with jaw osteonecrosis typical for MRONJ following therapy with ziv-aflibercept. All patients had metastatic gastrointestinal cancer treated with ziv-aflibercept and were evaluated for MRONJ because of exposed bone in the oral cavity. None of the patients had received antiresorptive therapies or any other medication known to cause MRONJ, and none had received radiation therapy to the jaws. Patients were aged 43, 51, 63 and all were males. Patients received 7, 16 and 23 cycles of ziv-aflibercept treatment and developed necrotic bone. All three patients presented with mandibular involvement, with two reporting pain. Patients were managed with anti-microbial mouth rinse, antibiotics and non-surgical sequestrectomy and followed up for 1.5, 2, and 2 months; two patients became asymptomatic while one patient continued to have pain. These three reported patients with a history of ziv-aflibercept therapy and no reported use of other medications known to cause MRONJ developed exposed necrotic bone of the jaw. We believe that ziv-aflibercept is another medication that can potentially cause MRONJ probably through its anti-VEGF activity, similar to bevacizumab and sunitinib.
Keywords: Osteonecrosis, jaw, ziv-aflibercept
Introduction
Medication-related osteonecrosis of the jaw (MRONJ) is a condition of jaw necrosis that is caused by a number of medications via different mechanisms (1). Initial reports in the mid 2000s revealed that use of bisphosphonates (BPs) in patients with metastatic cancers to the bone and in patients with osteoporosis led to necrosis of the jaw bones (2-4). However, other medications have also been shown to cause this condition and these include denosumab, an antibody targeting receptor activator of nuclear factor κ-B (RANK) ligand; bevacizumab, an antibody targeting vascular endothelial growth factor (VEGF); and sunitinib, a multi-targeted receptor tyrosine kinase inhibitor with activity against VEGF (5-8). As such, the name of this condition was changed from BP-related osteonecrosis of the jaws to MRONJ (1).
Ziv-aflibercept is a recombinant fusion protein consisting of human VEGF receptor extracellular domains fused to the Fc portion of human immunoglobulin G1 (IgG1) (9). The binding of ziv-aflibercept to its ligands can block tumor angiogenesis and vascular permeability (10). To date, ziv-aflibercept has been approved for refractory metastatic colorectal cancer in combination with 5-fluorouracil, leucovorin and irinotecan (FOLFIRI) (9,11,12). Reported toxicities associated with ziv-aflibercept include delayed wound healing, reduction in female reproductive function and increase in blood urea nitrogen (12-14). To date, there has been a single reported case of MRONJ that resulted from taking this medication (15).
We report three patients with a history of ziv-aflibercept use who developed MRONJ.
Case presentation
Three patients were seen at the Division of Oral Medicine and Dentistry, Brigham and Women Hospital, Boston, MA, at the request of the patients’ oncologists because of exposed bone in the oral cavity. All patients were males, with metastatic cancer of gastrointestinal origin (Table 1). One patient with metastatic colorectal cancer received FOLFIRI (5-fluorouracil, leucovorin and irinotecan) and ziv-aflibercept. One patient with metastatic carcinoid tumor received ziv-aflibercept monotherapy as part of a phase II clinical trial, and one patient was enrolled in a randomized, double-blinded study of mFOLFOX6 (oxaliplatin, leucovorin and 5-fluorouracil) plus either ziv-aflibercept or placebo. Ziv-aflibercept was given to patients at dose of 4 mg/kg/cycle every 2 weeks. On unblinding at the end of the study, the patient was on ziv-aflibercept. None of the patients had had BP therapy, or any other medication known to cause MRONJ, and none had received radiation to the jaws. All patients were consented for publication of this case series and any accompanying images. A summary of clinical data is presented in Table 1. Extraoral examination was unremarkable in all patients.
Table 1. Patient characteristics.
| Patient No./description | Age/diagnosis | No. of cycles of treatment at initial visit/at follow up | Site and size of MRONJ | MRONJ treatment | Stage of MRONJ at initial consultation/final visit status of patient |
|---|---|---|---|---|---|
| Patient 1 | 43/metastatic colorectal carcinoma | 16/20 | Left lingual mandible area of second molar (0.1 cm × 0.1 cm) | Bone sequestration; amoxicillin 500 mg/TID for 14 days, CHX rinses BID | 1/0, alive |
| Patient 2 | 63/metastatic carcinoid | 23/23 | Left lingual mandible area of second molar (0.1 cm × 0.5 cm) | Amoxicillin with clavulanic acid 875–125 mg/BID for 14 days, CHX rinses BID | 1/0, deceased |
| Patient 3 | 51/metastatic esophageal carcinoma | 7/12 | Right lingual mandible area of second molar (0.2 cm × 0.4 cm) | Amoxicillin 500 mg/TID for 14 days, CHX rinses BID | 0/1, deceased |
All patients received ziv-aflibercept as a 4 mg/kg infusion once every 2 weeks. MRONJ, medication-related osteonecrosis of the jaw; TID, three times a day; BID, two times a day.
Case 1
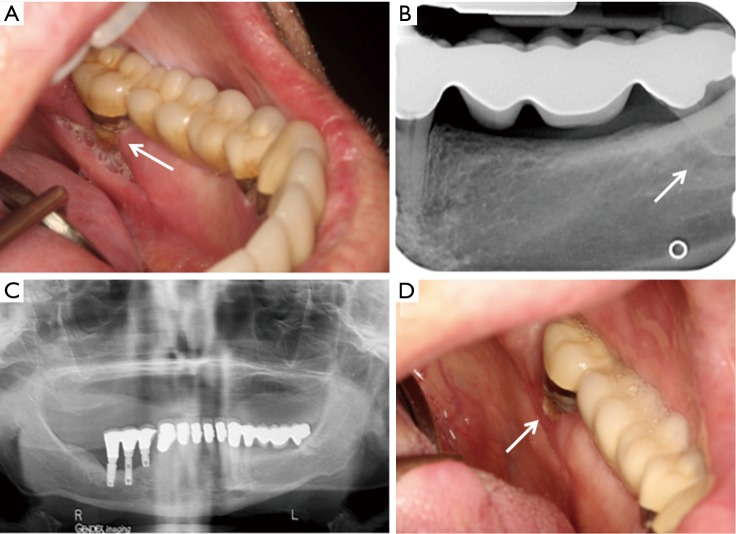
The patient reported an asymptomatic, rough area corresponding to an area of exposed bone on the left mandibular lingual mucosa present for 6 months. On examination, there were two 0.1 cm × 0.1 cm areas of exposed necrotic bone on the lingual gingiva of the left mandibular second molar (stage 1 MRONJ) (Figure 1A). The surrounding gingiva was erythematous with slight bleeding on palpation. The panoramic radiograph showed no signs of periapical radiolucency, osteosclerosis or bony sequestrum (Figure 1B). The exposed bone was removed by non-surgical sequestrectomy and this was submitted for histopathologic examination. The patient was prescribed amoxicillin because of concern that the area may become infected and complicate his course of therapy; he was also prescribed chlorhexidine rinses. Ziv-aflibercept was discontinued per study protocol. At 1.5-month follow up, the patient had received one additional cycle of FOLFOX without ziv-aflibercept. At this visit, the patient was asymptomatic without exposed bone (stage 0 MRONJ) but with a sinus tract located on the lingual gingiva of right mandibular second molar with surrounding erythema.
Figure 1.
Case 1. (A) Exposed bone on lingual mandible in the area of the left second molar (arrow); (B) panoramic radiograph without significant pathological findings.
Case 2
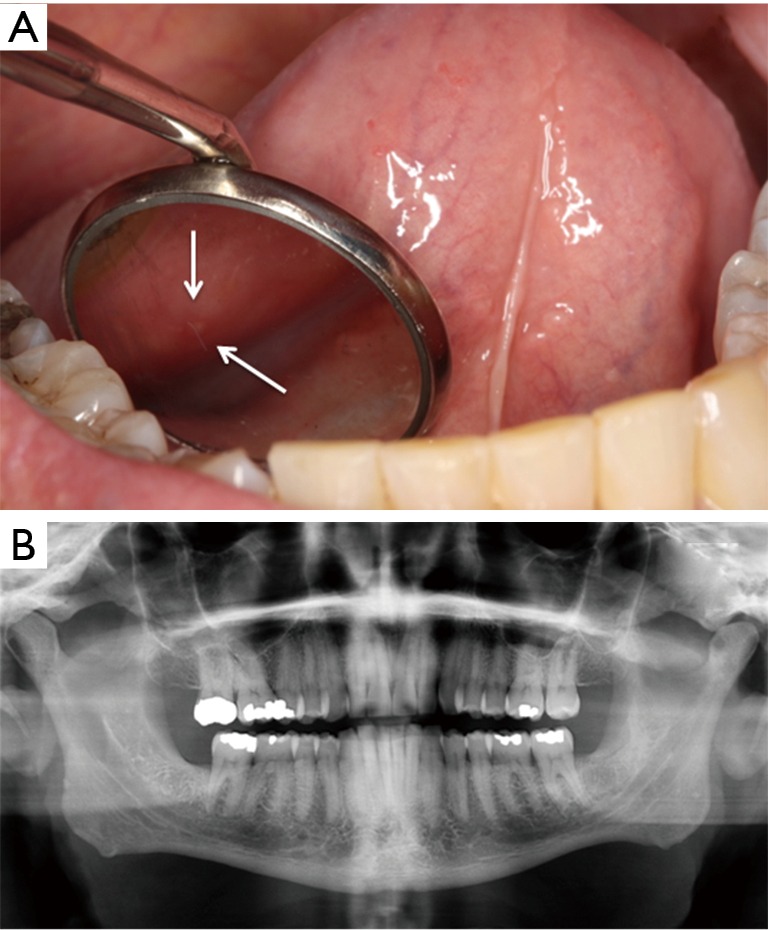
The patient reported that his dentist had noticed a painless area of exposed bone around the root of the left mandibular second molar two weeks prior to his consultation. On examination, more than 70% of the left mandibular second molar roots were exposed with root caries and the roots were surrounded by a rim of necrotic exposed bone measuring 0.5 cm × 0.1 cm (stage 1 MRONJ) (Figure 2A). The surrounding gingiva was normal without erythema or suppuration. Periapical and panoramic radiographs showed a discrete, 0.6 cm × 0.8 cm circumscribed periapical radiolucency around the roots of the left mandibular second molar (Figure 2B,C). The patient was treated with amoxicillin with clavulanic acid and chlorhexidine digluconate rinse. At two-week follow up, the tooth root was partially covered by mucosa with exposed bone still present (Figure 2D). He was asymptomatic although he had not filled his prescription. At two-month follow up, the area continued to have exposed necrotic bone and he died of progressive cancer shortly thereafter.
Figure 2.
Case 2. (A) Exposed necrotic bone on lingual mandible in the area of the left second molar (arrow); (B) periapical radiograph shows radiolucency around the root of the mandibular left second molar (arrow); (C) panoramic radiograph without other significant pathological changes; (D) two-week follow up showed a thin rim of exposed bone around the tooth (arrow).
Case 3
The patient reported a rough area on the right mandibular mucosa at extraction sites of the right mandibular second and third molars. These teeth had been extracted two weeks prior to initial consultation because of mobility and the presence of an abscess. On examination, both extraction sites were healing but not fully epithelialized and were slightly tender with a 0.2 cm × 0.4 cm area of exposed necrotic bone. Despite treatment with amoxicillin and chlorhexidine, and non-surgical sequestrectomy at interim visits, asymptomatic necrotic bone was still present at a 2-month follow-up fulfilling the criteria for stage 1 MRONJ (Figure 3A). Periapical and panoramic radiographs demonstrated a 0.5 cm × 0.3 cm radiolucency consistent with a healing extraction socket (Figure 3B,C). A non-surgical sequestrectomy of the exposed bone was performed and the patient died of progressive cancer shortly thereafter.
Figure 3.
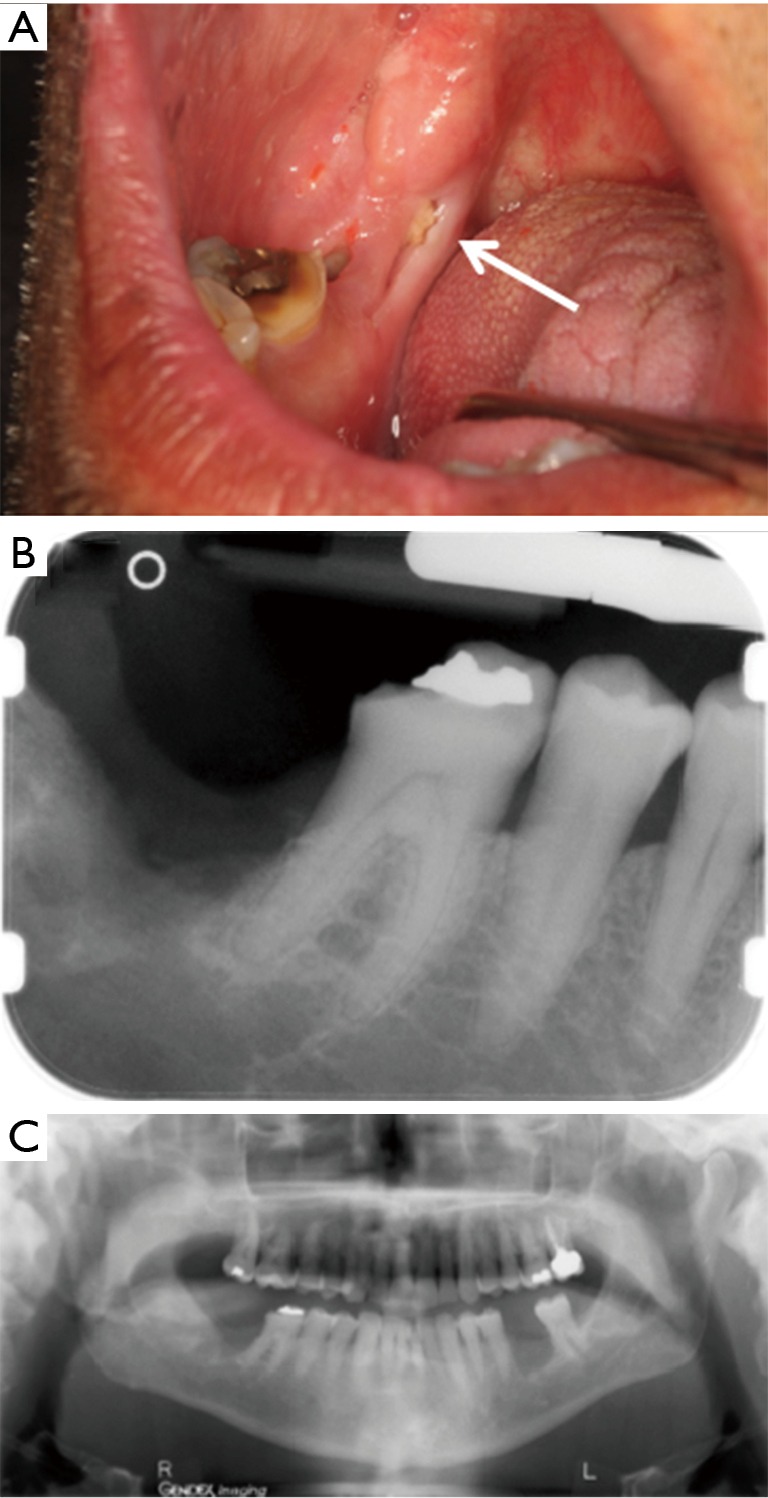
Case 3. (A) Exposed necrotic bone close to extraction site of right mandibular second and third molars (arrow); (B) periapical radiograph shows ill-defined area of radiolucency distal to mandibular right first molar; (C) panoramic radiograph shows a similar radiolucency but no other lesions.
Histopathologic examination
Necrotic bone from case 1 showed nonvital bone sequestra with empty lacunae and ragged borders consistent with osteonecrosis of the jaws (Figure 4).
Figure 4.
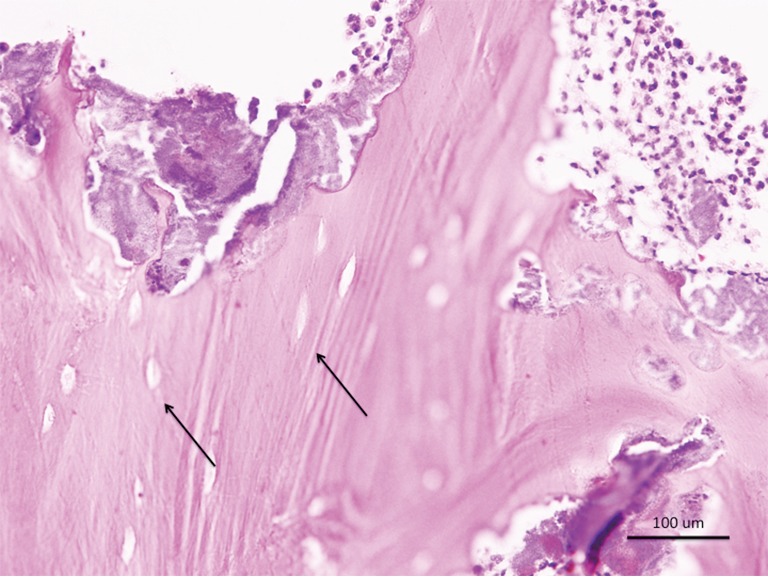
From case 1: histopathological examination shows nonvital bone sequestra with empty lacunae (arrows) and ragged borders consistent with medication-related osteonecrosis of the jaws (scale bar measures 100 µm).
Discussion
MRONJ is defined as the presence of exposed bone, or bone that can be probed through an intraoral or extraoral fistula in the maxillofacial region, present for more than 8 weeks and without a history of radiation therapy to the jaws (1). The prevalence of MRONJ associated with antiresorptive therapy (whether BPs or denosumab) has been well characterized in the oncology population and has a prevalence ranging from 0.019% to 6.7% (5,16-18). Risk factors are high cumulative dose, local odontogenic infection and trauma such as extractions (19). MRONJ associated with anti-angiogenic agents such as bevacizumab has also been reported, with a prevalence of ranging between 0.3% to 3.1% while there are only sporadic reports of those caused by sunitinib (1,6,18). These are the first reported cases of MRONJ associated with use of ziv-aflibercept, another anti-angiogenic agent.
Ziv-aflibercept is a recombinant fusion protein consisting of human VEGF receptor extracellular domain fused with the Fc receptor of human IgG1 (9,20). The toxicity profile of ziv-aflibercept includes oral mucositis, delayed wound healing, degeneration of respiratory epithelial lining and atrophy or loss of the nasal septum (14,21). Ziv-aflibercept was approved by the US Food and Drug Administration in August 2012 for treatment of refractory metastatic colorectal cancer. It inhibits vascular growth in tumors and distant metastasis. This action is similar to that of bevacizumab and sunitinib, both of which exhibit activity against VEGF and which have been associated with sporadic cases of MRONJ (7,8,22). Because of its activity against vascular growth, it is not surprising that ziv-aflibercept use may result in jaw osteonecrosis.
There is a single case report describing ONJ in a 64-year-old woman following administration of 11 cycles of ziv-aflibercept for adenocarcinoma of the transverse colon. However this case report did not document exposed necrotic bone and the diagnosis was based on CT imaging findings only (15). It is difficult to be certain if this is a bona fide case because although MRONJ may occur without any evidence of exposed bone, clinical and imaging criteria for the diagnosis must be stringent.
One additional patient with bone necrosis was treated in our clinic but was not included in this series because the necrotic bone had not been present for 8 weeks, which is one of the criteria for the diagnosis of MRONJ. He had a 0.1 cm × 0.1 cm piece of asymptomatic exposed bone on the lingual gingiva of the left mandibular third molar which was removed and confirmed by histopathology to be necrotic bone. He reported that other pieces of necrotic bone had worked their way out of the gingiva over the following weeks. The mucosa was normal at 2-month follow-up after treatment with amoxicillin and chlorhexidine.
Many new targeted therapeutic agents on the market have activity against VEGF either directly or indirectly and these may potentially cause MRONJ. However, cases should not have a history of other medications known to cause MRONJ. One case purportedly caused by ipilimumab, a monoclonal antibody against CTLA-4 showed an area of necrotic bone on the lingual mandible, a site typical for MRONJ but it was unclear it if had been present for 8 weeks (23). Two cases purportedly caused by mammalian target of rapamycin (mTOR) inhibitor which may result in reduced secretion of VEGF, were noted in patients who had received monthly zoledronic acid (24). These latter cases cannot be considered at this time to be bona fide cases of mTOR-inhibitor-associated osteonecrosis but rather BP-associated disease (25,26). Whether the mTOR inhibitor aggravated the osteonecrosis is unclear but there have been no other cases of mTOR inhibitor associated osteonecrosis in many years of use of this medication. MRONJ apparently linked to use of sorafenib and and mTOR inhibitors have been reported to the United States Food and Drug Administration via the Adverse Event Reporting System. However, none of these cases have been published and it is unclear if they have bee adjudicated (27).
We report 3 patients with a history of ziv-aflibercept use who developed ONJ. All had typical exposed necrotic bone and one had a persistent extraction socket with necrotic bone and radiographic evidence of an ill-defined radiolucency. Patients were followed up for 1.5 to 2 months and two showed complete mucosal healing at follow-up with one patient still exhibited exposed bone but without pain (stage 1). Although 2 of the patients were also on standard chemotherapy (FOLFIRI and mFOLFOX6), these medications have not been associated with development of MRONJ. It should also be noted that in the mFOLFOX6 and ziv-aflibercept versus placebo trial, none of the placebo group developed MRONJ (unpublished data). In addition, none of the patients had received BPs or radiation to the head and neck area. Patients developed MRONJ after receiving a mean of 7 months of ziv-aflibercept treatment (range, 3.5–11.5 months) compared to 2 to 15 months for bevacizumab (6,28).
Other conditions that may lead to jaw osteonecrosis besides medications and radiation include physiologic trauma, chronic odontogenic infection leading to osteomyelitis, and varicella zoster infection (29). Trauma from physiologic activity sometimes results in injury of the lingual mandibular mucosa and focal damage to the periosteum and leads to a condition known as benign spontaneous sequestration of the lingual plate which is not uncommon in healthy adults (30). In this condition, 0.1 to 0.3 cm of necrotic bone exfoliates and the mucosa heals spontaneously. The important features are the small size of the bone fragments and its self-limited presentation. Although the first case could have represented this benign condition because of the small size of the lesions, the degree of bone exposure in cases 2 and 3 are consistent with MRONJ which lends support to the diagnosis of MRONJ for case 1.
Conclusions
We report three patients with metastatic cancer of gastrointestinal origin treated with ziv-aflibercept therapy who developed MRONJ. This is likely mediated via its effects on VEGF similar to MRONJ reported in association with bevacizumab and sunitinib. As ziv-aflibercept appears to be a new medication that is associated with MRONJ, the authors recommend that all patients taking this medication be monitored for this potential adverse event.
Acknowledgements
None.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Ruggiero SL, Dodson TB, Fantasia J, et al. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw--2014 update. J Oral Maxillofac Surg 2014;72:1938-56. 10.1016/j.joms.2014.04.031 [DOI] [PubMed] [Google Scholar]
- 2.Ruggiero SL, Mehrotra B, Rosenberg TJ, et al. Osteonecrosis of the jaws associated with the use of bisphosphonates: a review of 63 cases. J Oral Maxillofac Surg 2004;62:527-34. 10.1016/j.joms.2004.02.004 [DOI] [PubMed] [Google Scholar]
- 3.Ruggiero SL, Mehrotra B. Ten years of alendronate treatment for osteoporosis in postmenopausal women. N Engl J Med 2004;351:190-2. 10.1056/NEJM200407083510218 [DOI] [PubMed] [Google Scholar]
- 4.Marx RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg 2003;61:1115-7. 10.1016/S0278-2391(03)00720-1 [DOI] [PubMed] [Google Scholar]
- 5.Qi WX, Tang LN, He AN, et al. Risk of osteonecrosis of the jaw in cancer patients receiving denosumab: a meta-analysis of seven randomized controlled trials. Int J Clin Oncol 2014;19:403-10. 10.1007/s10147-013-0561-6 [DOI] [PubMed] [Google Scholar]
- 6.Guarneri V, Miles D, Robert N, et al. Bevacizumab and osteonecrosis of the jaw: incidence and association with bisphosphonate therapy in three large prospective trials in advanced breast cancer. Breast Cancer Res Treat 2010;122:181-8. 10.1007/s10549-010-0866-3 [DOI] [PubMed] [Google Scholar]
- 7.Fleissig Y, Regev E, Lehman H. Sunitinib related osteonecrosis of jaw: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol 2012;113:e1-3. 10.1016/j.tripleo.2011.06.023 [DOI] [PubMed] [Google Scholar]
- 8.Koch FP, Walter C, Hansen T, et al. Osteonecrosis of the jaw related to sunitinib. Oral Maxillofac Surg 2011;15:63-6. 10.1007/s10006-010-0224-y [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez M. Ziv-Aflibercept Use in Metastatic Colorectal Cancer. J Adv Pract Oncol 2013;4:348-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clarke JM, Hurwitz HI. Ziv-aflibercept: binding to more than VEGF-A--does more matter? Nat Rev Clin Oncol 2013;10:10-1. 10.1038/nrclinonc.2012.197 [DOI] [PubMed] [Google Scholar]
- 11.Mansour AM, Al-Ghadban SI, Yunis MH, et al. Ziv-aflibercept in macular disease. Br J Ophthalmol 2015;99:1055-9. 10.1136/bjophthalmol-2014-306319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allen JW, Moon J, Redman M, et al. Southwest Oncology Group S0802: a randomized, phase II trial of weekly topotecan with and without ziv-aflibercept in patients with platinum-treated small-cell lung cancer. J Clin Oncol 2014;32:2463-70. 10.1200/JCO.2013.51.4109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malik D, Tarek M, Caceres del Carpio J, et al. Safety profiles of anti-VEGF drugs: bevacizumab, ranibizumab, aflibercept and ziv-aflibercept on human retinal pigment epithelium cells in culture. Br J Ophthalmol 2014;98 Suppl 1:i11-16. 10.1136/bjophthalmol-2014-305302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saif MW, Relias V, Syrigos K, et al. Incidence and management of ZIv-aflibercept related toxicities in colorectal cancer. World J Clin Oncol 2014;5:1028-35. 10.5306/wjco.v5.i5.1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ponzetti A, Pinta F, Spadi R, et al. Jaw osteonecrosis associated with aflibercept, irinotecan and fluorouracil: attention to oral district. Tumori 2016;102. [DOI] [PubMed] [Google Scholar]
- 16.Coleman R, Woodward E, Brown J, et al. Safety of zoledronic acid and incidence of osteonecrosis of the jaw (ONJ) during adjuvant therapy in a randomised phase III trial (AZURE: BIG 01-04) for women with stage II/III breast cancer. Breast Cancer Res Treat 2011;127:429-38. 10.1007/s10549-011-1429-y [DOI] [PubMed] [Google Scholar]
- 17.Mauri D, Valachis A, Polyzos IP, et al. Osteonecrosis of the jaw and use of bisphosphonates in adjuvant breast cancer treatment: a meta-analysis. Breast Cancer Res Treat 2009;116:433-9. 10.1007/s10549-009-0432-z [DOI] [PubMed] [Google Scholar]
- 18.Vahtsevanos K, Kyrgidis A, Verrou E, et al. Longitudinal cohort study of risk factors in cancer patients of bisphosphonate-related osteonecrosis of the jaw. J Clin Oncol 2009;27:5356-62. 10.1200/JCO.2009.21.9584 [DOI] [PubMed] [Google Scholar]
- 19.Campisi G, Fedele S, Fusco V, et al. Epidemiology, clinical manifestations, risk reduction and treatment strategies of jaw osteonecrosis in cancer patients exposed to antiresorptive agents. Future Oncol 2014;10:257-75. 10.2217/fon.13.211 [DOI] [PubMed] [Google Scholar]
- 20.Freund KB, Mrejen S, Gallego-Pinazo R. An update on the pharmacotherapy of neovascular age-related macular degeneration. Expert Opin Pharmacother 2013;14:1017-28. 10.1517/14656566.2013.787410 [DOI] [PubMed] [Google Scholar]
- 21.Chung C, Pherwani N. Ziv-aflibercept: a novel angiogenesis inhibitor for the treatment of metastatic colorectal cancer. Am J Health Syst Pharm 2013;70:1887-96. 10.2146/ajhp130143 [DOI] [PubMed] [Google Scholar]
- 22.Hoefert S, Eufinger H. Sunitinib may raise the risk of bisphosphonate-related osteonecrosis of the jaw: presentation of three cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2010;110:463-9. 10.1016/j.tripleo.2010.04.049 [DOI] [PubMed] [Google Scholar]
- 23.Owosho AA, Scordo M, Yom SK, et al. Osteonecrosis of the jaw a new complication related to Ipilimumab. Oral Oncol 2015;51:e100-1. 10.1016/j.oraloncology.2015.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Villaume K, Blanc M, Gouysse G, et al. VEGF secretion by neuroendocrine tumor cells is inhibited by octreotide and by inhibitors of the PI3K/AKT/mTOR pathway. Neuroendocrinology 2010;91:268-78. 10.1159/000289569 [DOI] [PubMed] [Google Scholar]
- 25.Giancola F, Campisi G, Lo Russo L, et al. Osteonecrosis of the jaw related to everolimus and bisphosphonate: a unique case report? Ann Stomatol (Roma) 2013;4:20-1. [PMC free article] [PubMed] [Google Scholar]
- 26.Kim DW, Jung YS, Park HS, et al. Osteonecrosis of the jaw related to everolimus: a case report. Br J Oral Maxillofac Surg 2013;51:e302-4. 10.1016/j.bjoms.2013.09.008 [DOI] [PubMed] [Google Scholar]
- 27.Zhang X, Hamadeh IS, Song S, et al. Osteonecrosis of the Jaw in the United States Food and Drug Administration's Adverse Event Reporting System (FAERS). J Bone Miner Res 2016;31:336-40. 10.1002/jbmr.2693 [DOI] [PubMed] [Google Scholar]
- 28.Troeltzsch M, Woodlock T, Kriegelstein S, et al. Physiology and pharmacology of nonbisphosphonate drugs implicated in osteonecrosis of the jaw. J Can Dent Assoc 2012;78:c85. [PubMed] [Google Scholar]
- 29.Almazrooa SA, Woo SB. Bisphosphonate and nonbisphosphonate-associated osteonecrosis of the jaw: a review. J Am Dent Assoc 2009;140:864-75. 10.14219/jada.archive.2009.0280 [DOI] [PubMed] [Google Scholar]
- 30.Danesh-Meyer MJ. Spontaneous alveolar bone sequestration. Case reports. J N Z Soc Periodontol 1999;18-20. [PubMed] [Google Scholar]