Abstract
In plant cells, an increase in cellular oxidants can have multiple effects, including the promotion of mixed disulfide bonds between glutathione and some proteins (S-glutathionylation). The present study focuses on the cytosolic isoform of the glycolytic enzyme triosephosphate isomerase (cTPI) from Arabidopsis thaliana and its reversible modification by glutathione. We used purified recombinant cTPI to demonstrate the enzyme sensitivity to inhibition by N-ethylmaleimide, hydrogen peroxide and diamide. Treatment of cTPI with diamide in the presence of reduced glutathione (GSH) led to a virtually complete inhibition of its enzymatic activity by S-glutathionylation. Recombinant cTPI was also sensitive to the oxidized form of glutathione (GSSG) in the micromolar range. Activity of cTPI was restored after reversion of S-glutathionylation by two purified recombinant A. thaliana cytosolic glutaredoxins (GRXs). GRXs-mediated deglutathionylation of cTPI was dependent on a GSH-regenerating system. Analysis of cTPI by mass spectrometry after S-glutathionylation by GSSG revealed that two Cys residues (Cys127 and Cys218) were modified by glutathione. The role of these two residues was assessed using site-directed mutagenesis. Mutation of Cys127 and Cys218 to Ser separately or together caused different levels of decrease in enzyme activity, loss of stability, as well as alteration of intrinsic fluorescence, underlining the importance of these Cys residues in protein conformation. Comparison of wild-type and mutant proteins modified with biotinyl glutathione ethyl ester (BioGEE) showed partial binding with single mutants and total loss of binding with the double mutant, demonstrating that both Cys residues were significantly S-glutathionylated. cTPI modification with BioGEE was reversed using DTT. Our study provides the first identification of the amino acid residues involved in cTPI S-glutathionylation and supports the hypothesis that this reversible modification could be part of an oxidative stress response pathway.
Keywords: cytosolic triosephosphate isomerase, Arabidopsis thaliana, S-glutathionylation, deglutathionylation, glutaredoxin, redox post-translational modification, cysteine
Introduction
In plant cells, ROS such as superoxide anion (), hydrogen peroxide (H2O2) and hydroxyl radical (OH•) are normal by-products of many cellular processes like photosynthesis (Karpinski et al., 2003; Apel and Hirt, 2004) and aerobic metabolism (Miwa and Brand, 2003). In addition, many biotic and abiotic stresses can lead to abnormal accumulation of ROS (Inzé and Montagu, 1995; Mittler, 2002) which can potentially damage cell structures and impair metabolic activities through oxidation of biomolecules such as nucleic acids, fatty acids and proteins (Gill and Tuteja, 2010). Due to their nature, ROS also play important roles in plant redox signaling under basal conditions and during stress response by triggering defense mechanisms against oxidative stress (Foyer and Noctor, 2009).
Cysteine thiol groups are sensitive to oxidation by ROS due to their electron-rich sulfur center, making Cys residues potential targets of various redox PTMs (Davies, 2005). In cells, the reactivity of Cys thiol groups increases through stabilization of the thiolate anion in the Cys microenvironment. This can occur for example because of the proximity of basic amino acids. Thiolate anions are much stronger nucleophiles than protonated thiol groups. They are more prone to undergo ROS-mediated oxidation such as the formation of sulfenic acid thiol and other redox PTMs such as S-nitrosylation and S-glutathionylation (Bykova and Rampitsch, 2013). Protein S-glutathionylation results from the reaction of sulfenic acid thiol with GSH or by thiol disulfide exchange between a reduced thiol group and GSSG leading to the formation of a mixed disulfide bond between glutathione and the targeted protein (Dalle-Donne et al., 2009; Zaffagnini et al., 2012d). Protein S-glutathionylation was first described as a mechanism to protect Cys residues from irreversible oxidation (Klatt and Lamas, 2000; Bedhomme et al., 2012). Recently, S-glutathionylation has also emerged as part of different redox signaling pathways in many organisms including plants (Gallogly and Mieyal, 2007; Pastore and Piemonte, 2012). Indeed, PTM of Cys by glutathione can alter the three-dimensional structure of targeted proteins and modify enzymes activity, thus affecting specific biochemical pathways (Zaffagnini et al., 2012d). S-glutathionylation occurs spontaneously in vitro but was also reported to be catalyzed by glutathione S-transferases (GSTs) of the π class (GSTπ) in animals (Townsend et al., 2009) and by a GRX in Arabidopsis thaliana (Bender et al., 2015). Despite in vitro evidence for catalysis of protein S-glutathionylation, the main mechanism leading to this modification in vivo remains unclear. Nevertheless, treatments with GSSG, GSH + H2O2, and GSH + diamide have been used to study protein S-glutathionylation in vitro (Barrett et al., 1999; Ito et al., 2003; Holtgrefe et al., 2008; van der Linde et al., 2011; Bedhomme et al., 2012; Butturini et al., 2014; Zaffagnini et al., 2014). S-glutathionylation is reversible and protein deglutathionylation is mainly catalyzed by GRXs. GRXs are small thiol-disulfide oxidoreductase belonging to the thioredoxin superfamily. Class I (CPYC-like active site) and class II (CGFS active site) GRXs are present in almost all living organism (Rouhier et al., 2008; Zaffagnini et al., 2012b). A third class, CC-type GRXs, is only present in higher plants and their biochemical properties are still largely unknown. Class I GRXs are the most recognized for their protein deglutathionylation functions in plants (Zaffagnini et al., 2008; Gao et al., 2010; Bedhomme et al., 2012; Zaffagnini et al., 2012c). A. thaliana possesses two cytosolic class I GRXs: GRXC1 and GRXC2 and both can reduce peroxiredoxin IIB (Riondet et al., 2012). The poplar (Populus trichocarpa) ortholog of A. thaliana GRXC1 has been shown to perform in vitro deglutathionylation of cytosolic glyceraldehyde 3-phosphate dehydrogenase (GAPC1) (Bedhomme et al., 2012). Hence, GRXC1 and GRXC2 might play major roles in deglutathionylation of plant cytosolic enzymes in vivo.
In photosynthetic organisms, more than 200 S-glutathionylation targets have been identified using different types of approaches (Dixon et al., 2005; Michelet et al., 2008; Zaffagnini et al., 2012a). Proteins identified in these surveys are part of many metabolic pathways including glycolysis and the Calvin–Benson cycle. Triosephosphate isomerase (TPI; EC 5.3.1.1) is a housekeeping enzyme that plays essential role in glycolysis by catalyzing the interconversion between GAP and DHAP. Photosynthetic organisms have a chloroplastic isoform (pTPI) that also participates in photosynthetic carbon metabolism. The S-glutathionylation of A. thaliana cTPI was shown to inhibit enzyme activity (Ito et al., 2003). More recently, pTPI from Chlamydomonas reinhardtii was also shown to be S-glutathionylated in vitro, with a negative impact on its activity (Zaffagnini et al., 2014). It is important to note that these results were obtained with relatively high concentrations (2–2.5 mM) of GSSG. Other evidence for redox modification of the cytosolic isoform is the interaction between cTPI and TRXs in A. thaliana roots and leaves (Marchand et al., 2004, 2010). Analysis of the TPI-TRX complex from Plasmodium falciparum suggested that TRX could perform deglutathionylation on PfTPI Cys217 (Shahul and Sarma, 2011).
In human, TPI deficiency (OMIM: 615512) is a rare genetic disorder leading to premature death and is the object of intensive research (Orosz et al., 2009). Interestingly, low TPI activity has been associated with higher resistance to the thiol-oxidizing reagent diamide in yeast (Ralser et al., 2006) and Caenorhabditis elegans (Ralser et al., 2007). It has been demonstrated that cells with reduced TPI activity have a higher NADPH/NADP+ ratio due to an increase in carbon flux through the PPP, thus providing more reducing power within the cytosol (Ralser et al., 2007). In plants, transgenic potato roots with reduced cTPI activity also showed a greater carbon flux through the PPP compared to controls (Dorion et al., 2012). Metabolic modeling simulations also suggested a rerouting of carbon flux through PPP in plant cells with low cTPI activity (Valancin et al., 2013). These studies strongly suggest that cTPI as well as other glycolytic enzymes can partake in a cellular switch to influence the cytosolic redox status by redirecting the carbon flux through the PPP.
In the present study, we show that activity of A. thaliana recombinant cTPI is sensitive to inhibition by oxidation and S-glutathionylation. We also demonstrate that S-glutathionylation of cTPI is reversible by A. thaliana GRXC1 and GRXC2. We use nanoLC-MS/MS analysis to identify two Cys residues targeted by modification with glutathione in cTPI. Analysis of purified site directed mutant proteins for these Cys residues suggests differences in enzyme activity, structure and stability. This indicates that S-glutathionylation targets residues that are important for cTPI structure and function.
Materials and Methods
Chemicals
BioGEE was purchased from Molecular Probes (Eugene, OR, USA) or synthesized from 25 mM EZ-Link-Sulfo-NHS-Biotin (Thermo Fisher Scientific, Nepean, ON, Canada) and 25 mM glutathione ethyl ester (Sigma Chemical Co., St Louis, MO, USA) in 50 mM phosphate buffered saline at pH 8. After 1 h incubation, NH4HCO3 was added to a final concentration of 138 mM to quench the remaining EZ-Link-Sulfo-NHS-Biotin. The concentration of the newly synthesized BioGEE was determined spectrophotometrically with 5,5′-dithiobis-(2-nitrobenzoic acid) using GSH as standard. Except when indicated otherwise, buffers, chemicals, and reagents were of analytical grade from Sigma Chemical Co. or Thermo Fisher Scientific.
Plasmid Constructions and Site Directed Mutagenesis
Standard techniques were used for recombinant DNA manipulations (Sambrook et al., 1989). A. thaliana sequence for cTPI (At3g55440) and GRXC1 (At5g63030) in pUNI51 vector, and GRXC2 (At5g40370) in PENTR/SD-DTOPO vector, were obtained from ABRC (respective stock numbers U12445, U16036, and U21122) and amplified by PCR using the following primers: cTPI forward 5′ATGGCCAGAAAGTTCTTCGTC3′ and reverse 5′TGTTAGCAGCCGGATCTTCTA3′; GRXC1 forward 5′ATGGGTTCAATGTTCAGTGGA3′ and reverse 5′ATTATAGCGGCCGCTACAAGAAAGCTGGGTCGG3′; GRXC2 forward, 5′ATGGCGATGCAGAAAGCTAAG3′ and reverse 5′TGGCTGGCAACTAGAAGGCAC3′.
The resulting amplicons were purified using a QIAquick gel extraction kit (Qiagen, Chatsworth, CA, USA) and digested by NotI. All digested inserts were cloned into the expression vector pProEx HTb (Invitrogen Canada Inc., Burlington, ON, Canada) previously digested with EheI and NotI. Restriction digestions were used to confirm the presence of the insert. The ligated plasmids were used to transform competent Escherichia coli cells (HB101 strain for cTPI and GRXC2 or RosettaTM strain for GRXC1). cTPI Cys residues detected to be involved in mixed disulfide modification were modified to Ser to form three mutants (C127S, C218S, and C127/218S). Mutagenesis was performed by NorClone (London, ON, Canada) on pProEx HTb containing the cTPI insert. All the constructions used in this study were verified by full sequencing. WT designates the original cTPI protein.
Production and Purification of Recombinant Enzymes
Recombinant proteins were expressed in E. coli as previously described (Dorion et al., 2005) except that cells were grown overnight at room temperature for cTPI or 4 h at 37°C after the addition of 0.6 mM isopropyl β-D-thiogalactoside to the medium for GRXC1 and GRXC2. The purification of recombinant enzymes was performed under native conditions according to the manufacturer’s instructions (Invitrogen Canada Inc, Burlington, ON, Canada). Purified fractions were pooled and dialyzed against a buffer containing 25 mM Tris-Cl pH 7.5, 1 mM MgCl2 and 10 mM DTT. To remove DTT, samples were dialyzed twice against the same buffer containing no DTT. The purified and dialyzed enzymes were stored at -20°C in 50% (v/v) glycerol and were stable for over 3 months without any significant loss of activity. Different aliquots of the stored enzymes were used as material for independent experiments.
cTPI Activity Assays, Kinetic Analysis, and Protein Concentration
cTPI activity was measured by following the isomerization of GAP to DHAP at 30°C using a coupled enzyme assay on a VERSAmax microplate reader (Molecular Devices, San Diego, CA, USA) (Dorion et al., 2005). GAP used as a substrate was prepared from DL-GAP diethyl acetal barium salt (G5376, Sigma Chemical Co., St Louis, MO, USA) according to the procedure provided by the supplier. cTPI assays mixture contained 100 mM Tris-Cl pH 7.8, 1 mM GAP, 0.5 mM EDTA, 2.5 U/mL α-glycerophosphate dehydrogenase and 0.2 mM NADH. For stability assays, WT and mutant proteins were incubated at room temperature (21–23°C) with 100 mM Tris-Cl pH 7.8, 0.005% (w/v) BSA with or without 5 mM DTT and activity was measured after different incubation times. After 145 min, the samples without DTT were treated with 10 mM DTT for 30 min and TPI activity was measured again. To determine Km and kcat values, activity assays were performed with varying concentrations of GAP (0–4 mM) in the presence of 5 mM DTT. Km for GAP was determined using a non-linear regression analysis software (SigmaPlot 8.0, SPSS Inc., Chicago, IL, USA). Protein concentration was measured by the method of Bradford using BSA as a standard (Bradford, 1976).
Electrophoresis, Immunoblotting, Immunodetection, and Activity Staining
SDS-PAGE analysis was performed according to Laemmli (1970) on 15% (w/v) polyacrylamide gels. For immunoblotting, gels were transferred onto a nitrocellulose membrane for 60 min at 70 V. Immunodetection of cTPI was carried out with rabbit anti-cTPI polyclonal immune serum (1/500 dilution) (Dorion et al., 2005). The detection was done using a goat anti-rabbit IgG conjugated to alkaline phosphatase secondary antibody (1/10,000 dilution) (Promega, Nepean, ON, Canada). Native PAGE was performed on 10% (w/v) acrylamide gels according to Laemmli (1970) except that gels and electrode buffers were exempt of SDS. cTPI activity staining was performed essentially according to a previously described method (Dorion et al., 2005). Control gels incubated without GAP did not show any activity band. Detection of BioGEE was performed using streptavidin conjugated with alkaline phosphatase (Molecular Probes, Eugene, OR, USA) using a 1/40,000 dilution for reducing conditions and 1/20,000 for native conditions.
GRXs GSH-Disulfide Transhydrogenase Activity Assay
The protocol to assay GRX activity using HED as substrate was adapted from Zaffagnini et al. (2008). Briefly, various amounts of GRXs (0.05 to 0.4 μg/ml final concentrations) were added to a reaction mixture containing 100 mM Tris-Cl pH 8.0, 2 mM EDTA, 0.01% (w/v) BSA, 1 mM GSH, 0.2 mM NADPH, 6 μg/ml yeast glutathione reductase and 0.7 mM HED in a final volume of 200 μl. Reactions were carried out in 96-well microplates at 30°C and NADPH disappearance over time was measured at 340 nm on a microplate reader. Background activity measured in the absence of GRX was subtracted from activity obtained in the presence of GRX. GRX activity was expressed as μmoles of NADPH oxidized/min and was proportional to the quantity of GRX added.
Sensitivity of cTPI to H2O2, Diamide, NEM, and GSSG
Purified recombinant cTPI (0.2 μg/ml) was incubated at room temperature with 100 mM Tris-Cl pH 7.8, 0.005% (w/v) BSA with or without addition of 1 mM GSH and then treated with 0.5 mM H2O2, 1 mM diamide or 0.5 mM NEM. TPI activity was determined at different incubation times. After 120 min, DTT was added to all the samples at a final concentration of 10 mM and activity was measured 30 min later. To test the immediate effect of diamide on cTPI, the purified enzyme was first incubated with 100 mM Tris-Cl pH 7.8, 0.005% (w/v) BSA with or without addition of 1 mM GSH or 1 mM GSSG. The activity was determined before and within 5 min of addition of diamide at a final concentration of 1 mM. DTT was then immediately added to a final concentration of 10 mM for 90 min. For inhibition of cTPI by GSSG, purified cTPI (0.2 μg/ml) was incubated in 100 mM Tris-Cl pH 7.8, 0.005% (w/v) BSA with different concentrations of GSSG (0–2.5 mM). TPI activity was assayed after various times of incubation.
Reactivation of cTPI Activity by GRX-Mediated Deglutathionylation
For BioGEE detection on nitrocellulose membranes, recombinant cTPI (75 μg/ml) was incubated at room temperature with 100 mM Tris-Cl pH 7.8, 0.5 mM BioGEE and 0.5 mM diamide to induce spontaneous S-glutathionylation. After 5 min incubation, the cTPI sample was desalted using a PD10 column to remove excess of diamide and BioGEE. The desalted cTPI sample was then divided into different incubation mixtures containing 100 mM Tris-HCl pH 7.8, 0.01% (w/v) BSA (CTL), or with the addition of a GRS (2 mM EDTA, 1 mM GSH, 0.2 mM NADPH, 0.6 μg/ml glutathione reductase) or with 10 μM of recombinant GRXs (GRXC1 or GRXC2 samples), or with the addition of both GRS and GRXs (GRS + GRXC1/C2 samples) or with 10 mM DTT. Samples were incubated at room temperature for 30 min. An aliquot of the different treatments was used for Native-PAGE analysis and another aliquot was denatured for 5 min at 94°C for SDS-PAGE analysis. Electrophoresis and immunoblotting were performed as described above. For cTPI reactivation assays, recombinant cTPI (5.3 μg/ml) was incubated with 0.005% (w/v) BSA, 100 mM Tris-HCl pH 7.8, 1 mM GSH and 1 mM diamide for 5 min and desalted as described above. A non-glutathionylated control was also prepared identically but without addition of diamide. Aliquots of the desalted cTPI sample were used in the same treatments as described for the BioGEE detection except that 0.2 μM of GRXs was used. An aliquot of the different samples was taken at different time points to measure cTPI activity.
Fluorescence Emission Spectra
Fluorescence was determined using a SpectraMax M5 Multi-Mode Microplate Reader (Molecular Devices, San Diego, CA, USA). Recombinant enzymes were diluted (0.1 mg/ml) in 100 mM Tris-Cl pH 7.8 and fluorescence emission spectra were recorded using an excitation wavelength of 274 nm.
Effect of GSSG on WT and Mutant cTPI Electrophoretic Mobility
The recombinant proteins (5 μg/ml) were incubated for 120 min with 100 mM Tris-Cl pH 7.8, 1 mM GSSG or with 100 mM Tris-Cl pH 7.8, 15 mM DTT. Samples were then denatured for 5 min at 94°C. Immunoblot analyses against cTPI were performed as described above.
BioGEE Labeling of Recombinant Proteins
Recombinant proteins (5 μg/ml for SDS-PAGE and 20 μg/ml for Native-PAGE) were incubated for 120 min with 100 mM Tris-Cl pH 7.8, 0.5 mM BioGEE, 0.1 mM H2O2 for native gels and with the addition of 0.005% (w/v) BSA to the incubation mix for denaturing gels. As a control, 50 mM DTT was added to the loading buffer. Samples for SDS-PAGE were denatured for 5 min at 94°C and analyzed by immunoblotting.
Trypsin Digestion and NanoLC-MS/MS Analysis
For determination of modified Cys residues, cTPI (100 pmol) was incubated with 2.5 mM GSSG or left untreated as a control and dialyzed at room temperature overnight against 100 mM NH4HCO3. In-solution protein digestion was carried out overnight at 37°C in 100 mM NH4HCO3 buffer supplemented with 10% (v/v) acetonitrile, 2.5 mM CaCl2 and trypsin (modified, sequencing grade, Promega, Madison, WI, USA) in a trypsin-to-protein ratio 1:50, followed by drying down in a speed vac. In control experiments GSSG-treated and untreated cTPI proteins were also reduced with 30 mM DTT at 56°C for 45 min and alkylated at room temperature in the dark with 70 mM iodoacetamide prior to dialysis and trypsin digestion. All samples were re-dissolved in loading buffer containing 1% (v/v) formic acid and 0.5% (v/v) acetic acid. Automated nano-flow LC-MS/MS analysis of peptide digests was performed using a linear ion trap Finnigan LTQ XL (Thermo Finnigan, San Jose, CA, USA) mass spectrometer connected on-line with nano-HPLC (Dionex UltiMateTM 3000). The chromatographic separation was accomplished with a 10 cm reversed-phase nano-column (75 μm OD, 360 μm OD; packed in-house with Vydac C18, 5 μm bead and 300 Å pore size resin). The flow rate was set at 250 nl/min and peptide elution was performed using a linear gradient of 4–40% (v/v) ACN for 44 min, followed by a gradient of 40–80% (v/v) ACN for 3 min, and 80% (v/v) acetonitrile for 2 min in 1% (v/v) formic acid, 0.5% (v/v) acetic acid. The mass spectrometer was operated in the positive ion mode with the source temperature set at 200°C. It was tuned in nano-spray mode using 10 μM (Glu)-Fibrinopeptide B singly charged ion at m/z 1552.67. Data-dependent analysis was employed with one single MS full survey scan set at m/z range 450–2000 followed by CID MS/MS product ion scans of the five most abundant ions in each cycle with 20 s dynamic exclusion. The results were reproduced, and two independent runs were performed for each sample with 10 and 36 pmol total protein loaded per LC-MS/MS analysis.
Protein Identification and Assignment of Modification Sites
Peptide CID fragmentation spectra were searched using Mascot v. 2.4 search engine (Matrix Science, London, UK) against the NCBInr protein database (A. thaliana) first using a general ID search followed by the Error Tolerant search to allow for protein modification screening. The raw data created by XCalibur (Thermo Scientific) version 2.2 on LTQ-XL instrument were converted into Mascot Generic Format with Mascot Distiller 2.4 (Matrix Science, London, UK) software and used for peptide/protein identification and modification screening. The Mascot MS/MS Ion Search parameters were as follows: (1) tryptic digest with maximum one missed cleavage; (2) monoisotopic peptide masses were used; (3) the peptide mass tolerance was kept at 2 Da; and the fragment ions mass tolerance was set at 0.8 Da; (4) variable modifications Glutathione (C), Carbamidomethyl (C), Pyro-carbamidomethyl (N-term C), Oxidation (M), and Deamidation (NQ) were used; (5) peptide charge state +1, +2, and +3 for LC-MS/MS spectra. A single peptide probability of identification MOWSE score greater than 42 for cTPI experiments indicated identity or extensive homology (p < 0.05). Spectra with modifications were verified manually using the GPMAW 9.2 (Lighthouse Data, Odense, Denmark) software.
Replication and Statistical Analysis
Data were analyzed using the unpaired Student t-test tool of SigmaPlot 8.0, with P < 0.05 considered as a significant difference. The number of independent experiments (n) is indicated in each table or figure. Otherwise, figures show representative data of at least three experiments. Each independent experiment was the mean of at least three technical replicates. Several protein purifications were used during this work with low variations between purification batches (<10% variation in specific activity).
Results
Purification of Recombinant His-Tagged cTPI from A. thaliana
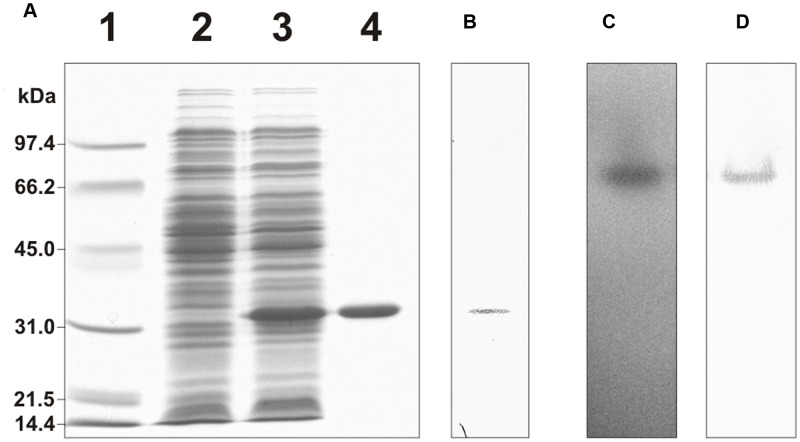
His-tagged cTPI was expressed in E. coli and affinity-purified. In the presence of DTT, the recombinant protein migrated as a single band after SDS-PAGE (Figure 1A) and was recognized by a rabbit anti-cTPI polyclonal immune serum on a Western blot (Figure 1B). Recombinant cTPI was also visualized after native gel electrophoresis and activity staining (Figure 1C). The native gel showed a single band of activity which was recognized by the anti-cTPI immune serum (Figure 1D).
FIGURE 1.
Purification of His-tagged recombinant cTPI. (A) SDS-PAGE analysis of the purification of recombinant cTPI. Lane 1, molecular weight standards; lane 2, E. coli protein extract without induction; lane 3, E. coli protein extract after isopropyl β-D-thiogalactoside induction; lane 4, affinity-purified recombinant cTPI. (B) Western blot analysis of the purified recombinant cTPI (25 ng) using an isoform-specific antibody against cTPI. (C) Native gel electrophoresis of the purified recombinant cTPI (50 ng) followed by TPI activity staining. (D) Western blot analysis of (C) using anti-cTPI immune serum.
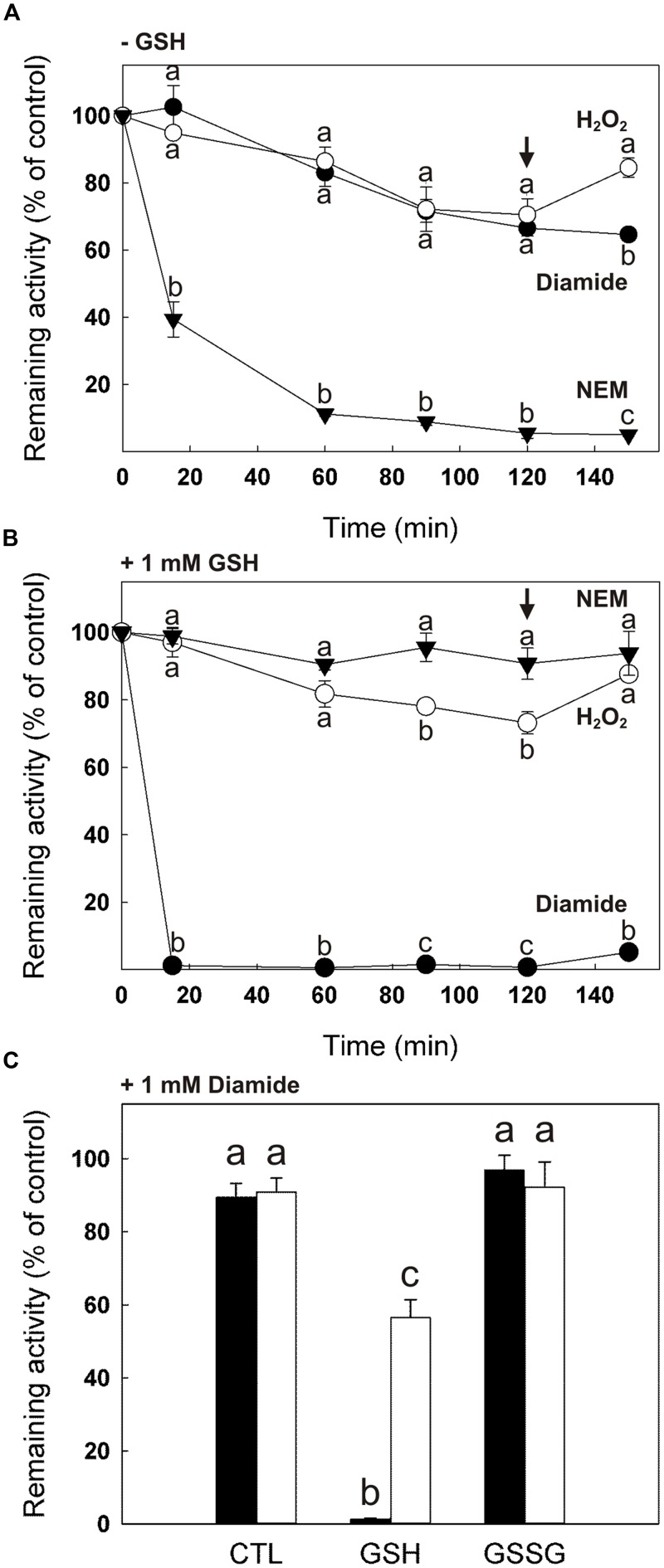
Inhibition of cTPI Activity by Oxidants and S-Glutathionylation
We found that, upon dilution in the absence of DTT, cTPI activity was unstable with incubation time (Supplemental Figure S1). The effect of H2O2, diamide and NEM on cTPI activity was therefore expressed as percentage of remaining activity of the control to visualize the loss of activity due to the treatment. Figures 2A,B show the evolution of cTPI activity over time in the presence of 0.5 mM H2O2, 1 mM diamide or 0.5 mM NEM with or without addition of 1 mM GSH. Alkylation of cTPI Cys residues by NEM led to a loss of more than 90% in cTPI activity after 60 min of incubation (Figure 2A). As expected, addition of 10 mM DTT after 120 min of incubation with NEM did not restore cTPI activity due to irreversible alkylation of its Cys residues. However, in the presence of 1 mM GSH, the addition of 0.5 mM NEM did not affect significantly cTPI activity (Figure 2B). This is probably due to the fact that GSH is subject to alkylation and that its concentration in the assay is two times higher than NEM, thus protecting cTPI from being targeted.
FIGURE 2.
Effect of GSH and GSSG on the sensitivity of cTPI activity to NEM, H2O2 and diamide. Recombinant cTPI was incubated without (A) or with (B) 1 mM GSH prior to treatment with NEM (black triangles), H2O2 (white circles) or diamide (black circles). Activity (mean ± SE, n = 3) was measured at different time points during the treatment and DTT (10 mM) was added to all samples after 120 min (black arrow). In (C), cTPI was incubated with 1 mM GSH (GSH), 1 mM GSSG (GSSG) or no addition (CTL). Samples were treated with 1 mM diamide and cTPI activity (mean ± SE, n = 3) was measured within 5 min (black bars). After, DTT was added at a final concentration of 10 mM and cTPI activity was measured again after 90 min of incubation (white bars). Data with different letters are significantly different (Student’s t-test, P < 0.05). Activity is expressed as percentage of the control (untreated) sample remaining activity in order to take into account the loss of cTPI activity upon dilution without reductant.
Oxidation of recombinant cTPI treated with 0.5 mM H2O2 alone or in the presence of 1 mM GSH caused about 30% loss of cTPI activity after 120 min compared to the untreated enzyme (Figures 2A,B). Addition of DTT after 120 min of incubation partially restored cTPI activity for both samples. Unlike NEM treated samples, presence of 1 mM GSH did not protect cTPI from inactivation by H2O2, suggesting that cTPI contains Cys residues more sensitive to oxidation.
When incubated with the thiol-specific oxidant diamide (1 mM), cTPI activity decreases similarly to the H2O2 treatment (Figure 2A). However, no restoration of cTPI activity was observed when DTT was added to diamide treated samples. Irreversible protein inactivation by diamide has been suggested to be caused by the rapid oxidation of Cys residues into sulfinic and sulfonic acid groups (Bender et al., 2015). cTPI inhibition by diamide was much more pronounced in the presence of 1 mM GSH in the samples (Figure 2B). Figure 2C shows the effect of treatment of cTPI within 5 min of incubation by diamide alone (CTL), in the presence of 1 mM GSH or 1 mM GSSG. As expected from results in Figure 2B, addition of diamide in the presence of GSH caused almost total inhibition of cTPI activity. Incubation for 90 min following the addition of 10 mM DTT partially restored cTPI activity showing that the inhibition is reversible. Interestingly, incubation with diamide + GSSG did not affect cTPI activity within 5 min of incubation. It is to be noted that incubation of GSSG affects negatively cTPI activity, but at longer incubation times (Figure 3).
FIGURE 3.
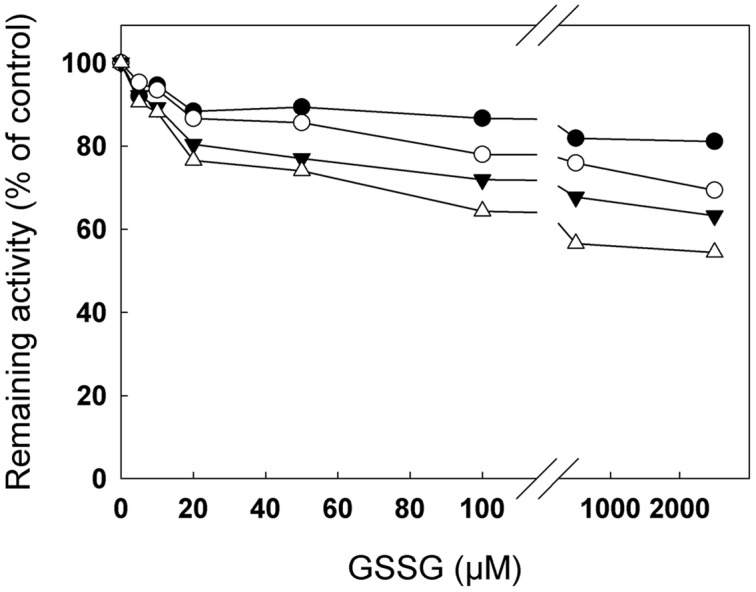
Inhibition of cTPI activity by GSSG. Recombinant cTPI was incubated with different GSSG concentrations. TPI activity is represented as a function of GSSG concentration for different incubation times: 75 min (black circles); 130 min (white circles); 180 min (black triangles) and 240 min (white triangles). The data are representative of three independent experiments. Activity is expressed as percentage of the control (untreated) sample remaining activity in order to take into account the loss of cTPI activity upon dilution without reductant.
Figure 3 shows remaining cTPI activity as a function of GSSG concentration for different incubation times. At concentrations as low as 5–10 μM, GSSG produced an observable decrease in recombinant cTPI activity. At much higher concentrations (500–2500 μM) inhibition reached approximately 40% after 240 min of incubation, but the effect of varying GSSG was less substantial on the enzyme activity. Hence, small variations of GSSG in the low concentrations range caused the greatest changes in cTPI activity. The same data plotted as a function of time show that cTPI inactivation by GSSG progresses relatively linearly with time (Supplemental Figure S2). Equal amounts of cTPI (Supplemental Figure S3, right) were subjected to native gel electrophoresis with and without prior to GSSG treatment (Supplemental Figure S3, left). Upon modification of cTPI with GSSG a less intense activity staining was observed. However, it did not change significantly the electrophoretic mobility of the enzyme. These results supported the previous data (Figure 3) showing that the GSSG treated cTPI was less active than the control.
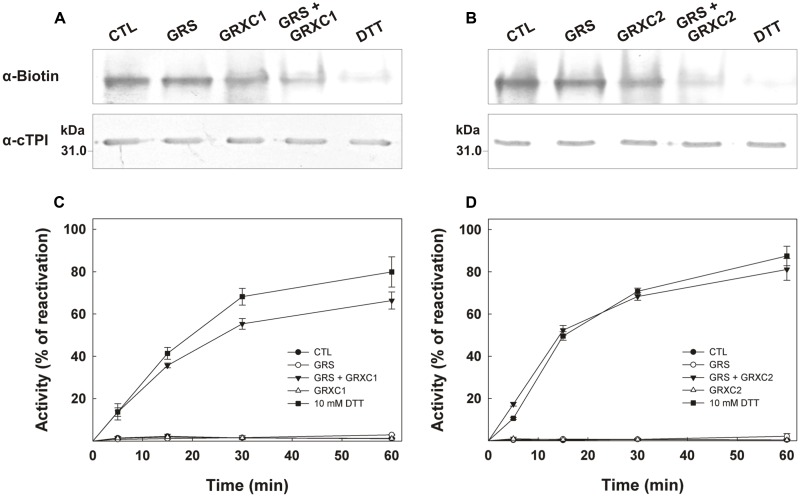
Deglutathionylation of cTPI by Recombinant GRXC1 and GRXC2
In order to study the possible deglutathionylation of cTPI by GRXs, His-tagged GRXC1 and GRXC2 from A. thaliana were expressed in E. coli and affinity-purified (Supplemental Figure S4). The specific activities of recombinant GRXs were measured using the artificial substrate HED as described in material and methods. Calculated specific activities for GRXC1 and GRXC2 were, respectively, 28.8 ± 3.4 μmol min-1 mg-1 (kcat = 8.0 ± 0.9 s-1) and 57.7 ± 4.1 μmol min-1 mg-1 (kcat = 14.2 ± 1.0 s-1). Both recombinant GRXs were able to catalyze deglutathionylation of cTPI in vitro (Figure 4). In Figures 4A,B, we used BioGEE, a biotinylated glutathione analog and diamide to induce S-glutathionylation of recombinant cTPI and observed the impact of different treatments on the enzyme by native gel electrophoresis and BioGEE detection using streptavidin conjugated with alkaline phosphatase on nitrocellulose membranes. The presence of GRS did not affect the intensity of band corresponding to the S-glutathionylated cTPI compared to the control. However, addition of GRS + GRXC1 (Figure 4A) or GRXC2 (Figure 4B) decreased the BioGEE signal compared to the control, GRS alone and GRXs alone, meaning that turnover of GRXs was required to obtain deglutathionylation activity. Incubation with 10 mM DTT was also used as a control to show removal of BioGEE on the native enzyme. Deglutathionylation of cTPI by GRXs was also tested by reactivation of its enzymatic activity after virtually complete inhibition by diamide + GSH treatment (Figures 4C,D). After cTPI S-glutathionylation and desalting, addition of GRS or 0.2 μM GRXs alone did not rescue cTPI activity in both experiments. Addition of both GRS and 0.2 μM GRXC1 (Figure 4C) or 0.2 μM GRXC2 (Figure 4D) was able to reverse cTPI inhibition in a time dependent manner. The reactivation rates were comparable to those obtained with the addition of 10 mM DTT. Based on the experimental observations shown in Figure 4, GRXC2 was slightly more effective than GRXC1 in catalyzing in vitro deglutathionylation of cTPI (which is consistent with specific activities measured by HED assays).
FIGURE 4.
Deglutathionylation of cTPI mediated by recombinant GRXC1 and GRXC2. BioGEE detection by streptavidin conjugated with alkaline phosphatase after native PAGE analysis of 350 ng recombinant cTPI (A,B, upper). Recombinant cTPI was S-glutathionylated in the presence of BioGEE followed by desalting as described in Section “Materials and Methods.” The enzyme was then treated for 30 min with GSH-regeneration system (GRS), 10 μM (A) GRXC1, (B) GRXC2 or both GRS + GRXC1 (A) or GRXC2 (B). A control sample without treatment (CTL) and another control deglutathionylated in 10 mM DTT (DTT) were also prepared. Aliquots of the samples were denatured and analyzed by Western blot (80 ng proteins) after SDS-PAGE using an anti-cTPI antibody to show equal protein concentrations in samples (A,B, lower). For (C,D), S-glutathionylated cTPI (0.2 μg/ml) was incubated with 0.01% BSA (CTL, black circles) and GRS (open circles) or 10 mM DTT (black squares) or 0.2 μM (C) GRXC1 (D) GRXC2 (open triangles) or both GRS + GRXs (black triangles). Aliquots of the different treatment were withdrawn at different time points to measure cTPI activity (mean ± SE, n = 3). Activity is expressed as percentage of the control (non-glutathionylated) sample.
Identification of S-Glutathionylated Residues by Mass Spectrometry Analysis
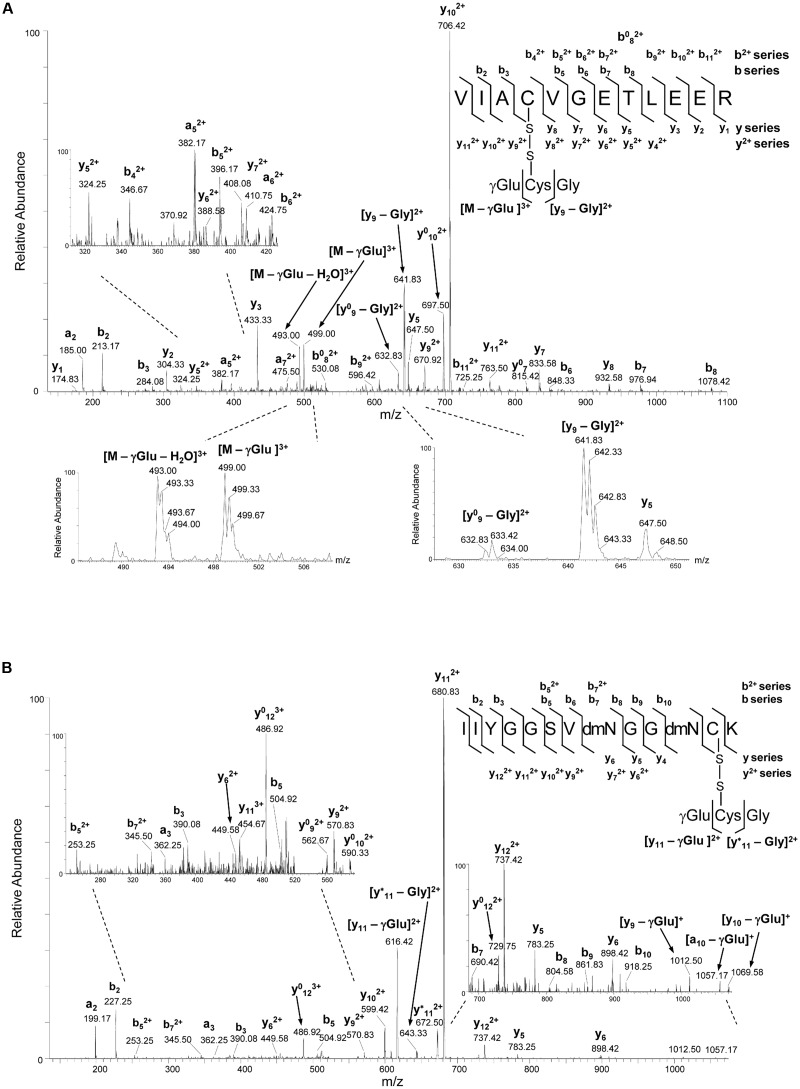
S-Glutathionylation of cTPI from A. thaliana was further confirmed by mass spectrometry analysis. Purified S-glutathionylated recombinant cTPI was digested with trypsin and analyzed by nanoLC-MS/MS. A triply charged ion at m/z value corresponding to the predicted S-glutathionylated cTPI tryptic peptide 124VIACVGETLEER135 (at m/z of 542.173+) was detected in the recombinant cTPI sample previously treated with GSSG (Supplemental Figure S5). This peptide ion was not found in control samples or S-glutathionylated samples that were previously reduced with DTT and alkylated with iodoacetamide (data not shown). This peptide was also found in two other oxidized forms with Cys-SO2H sulfinic acid (at m/z of 676.232+) and Cys-SO3H sulfonic acid (at m/z of 683.992+) indicating high reactivity of Cys127 site possibly caused by the local environment (Supplemental Figure S5). Similarly, a triply charged peptide ion corresponding to the sequence 207IIYGGSVNGGNCK219 with calculated m/z value of the S-glutathionylated form of 529.923+ was only found in GSSG-treated samples. Since each of these peptides contains only one Cys residue, the data indicate that Cys127 and Cys218 are subject to S-glutathionylation by GSSG in vitro.
The CID MS/MS fragmentation spectra of precursor ions corresponding to S-glutathionylated forms of peptides with sequence 124VIACVGETLEER135 (Figure 5A) and 207IIYGGSVNGGNCK219 (Figure 5B) provided further specific information for the amino acid composition and identity of the two S-glutathionylated peptides in cTPI as well as for the site-specific assignment of this modification. The signature neutral loss of 129 Da was observed from the precursor [M – γGlu]3+ ion at m/z 542.173+ characteristic to the loss of γ-glutamyl moiety from glutathione adduct (Figure 5A). A number of additional high intensity internal fragment ions were present in the product ion spectra of both modified peptides. They were the results of glutathione components γGlu- and Gly-residue loss from y-type ions upon collision in the mass spectrometer (Figures 5A,B). These ions provided further structural information about the nature of the adduct, thus, confirming the presence of glutathione in these peptides.
FIGURE 5.
NanoLC-MS/MS analysis of cTPI tryptic peptides modified by GSSG. (A) CID MS/MS fragmentation spectrum of the precursor ion at m/z of 542.573+ corresponding to S-glutathionylated peptide with the sequence 124VIACVGETLEER135. The detection of ion at m/z 499.00 indicate the loss of γGlu (-129 Da) from the triply charged precursor resulting in the ion [M – γGlu]3+, while the concomitant loss of water (-18 Da) resulted in the ion at m/z 493.00 corresponding to [M – γGlu – H2O]3+. Sequence specific y- and b-type fragment ion signals of different charge states (+1 and +2) identifying the peptide, and metastable decomposition product ions resulting from further internal fragmentation of glutathione adduct by elimination of γGlu or Gly residues are indicated. The peaks denoted y0 and b0 are the result of water (-18 Da) loss from corresponding ion. Spectral portions containing signature ions resulting from the loss of γGlu or Gly residues in glutathione with partly isotopically resolved peaks indicating ions charge state are shown. (B) CID MS/MS fragmentation spectrum of the precursor ion at m/z of 530.213+ corresponding to S-glutathionylated peptide with the sequence 207IIYGGSVNGGNCK219. The expanded regions of the product ion spectra showing detailed fragmentation pathways of S-glutathionylated peptides are given on the left side of panel (A) and on both left and right sides of panel (B). Product maps indicating the cleavage sites in CID MS/MS are given on the right side of panels (A,B).
We performed cTPI sequence comparisons between different photosynthetic species using Clustal W (Larkin et al., 2007) (Supplemental Figure S6). Four Cys residues were found to be strictly or semi-conserved in cTPI sequences. A. thaliana Cys13 and Cys127 were strictly conserved while Cys67 and Cys218 were semi-conserved between different photosynthetic organisms. Interestingly, the Cys residue corresponding to Cys127 is present in the pTPI sequence of A. thaliana, but Cys218 is absent from the chloroplastic enzyme.
Analysis of Cys127 and Cys218 by Site-Directed Mutagenesis
Site directed mutagenesis was performed to obtain three recombinant cTPI mutants (C127S, C218S, C127/218S) in which selected Cys residues were replaced by a Ser residue. Recombinant cTPI mutants were purified as described for the WT protein to obtain a homogeneous purified protein of each mutant (Supplemental Figure S7). Mutation of the targeted Cys residues altered cTPI activity distinctively. A loss of more than 97% of specific activity was observed for C127S and C127/218S mutants (Table 1). In the C218S mutant, loss of specific activity was less striking (around 21%). Interestingly, the Km value for GAP was lower in the C127S mutant (Table 1).
Table 1.
Kinetic analysis of recombinant WT and mutant cTPI proteins.
| Protein | Specific activity (μmol min-1 mg-1) | kcat (s-1) | Km (mM) | kcat/Km (×103 M-1s-1) |
|---|---|---|---|---|
| WT | 6580 ± 520 | 3406 ± 269 | 1.32 ± 0.06 | 2580 ± 321 |
| C127S | 136 ± 14 | 70.4 ± 7.4 | 0.48 ± 0.04 | 147 ± 28 |
| C218S | 5197 ± 140 | 2690 ± 73 | 1.68 ± 0.03 | 1601 ± 72 |
| C127/218S | 28.6 ± 1.6 | 14.8 ± 0.8 | 0.50 ± 0.06 | 29.6 ± 5.2 |
Activities were measured using a coupled enzymatic assay in the presence of 5 mM DTT.
In order to investigate a possible difference in protein conformation in the mutants compared to the WT, fluorescence emission spectra were studied. Recombinant proteins were excited at 274 nm and intrinsic fluorescence emission was recorded between 300 nm and 450 nm. The spectra of WT and the C218S mutant were relatively similar and differed from those of C127S and C127/218S, in particular with respect to the intensity of the peak between 320 and 340 nm (Figure 6).
FIGURE 6.
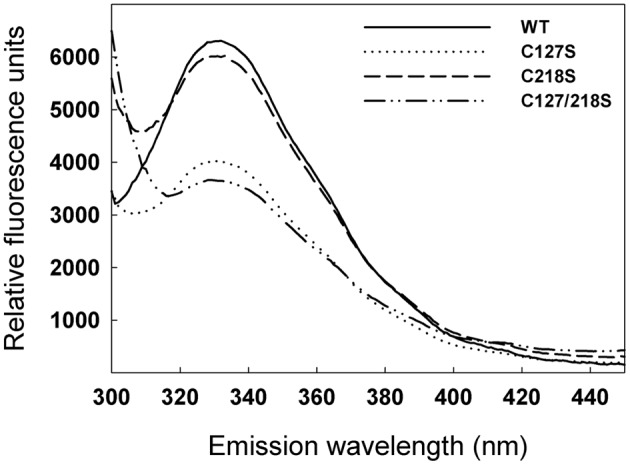
Effect of C127S and C218S mutations on the fluorescence emission spectra of the cTPI recombinant protein. Fluorescence emission spectra of recombinant WT and mutant cTPI proteins were recorded using an excitation wavelength of 274 nm.
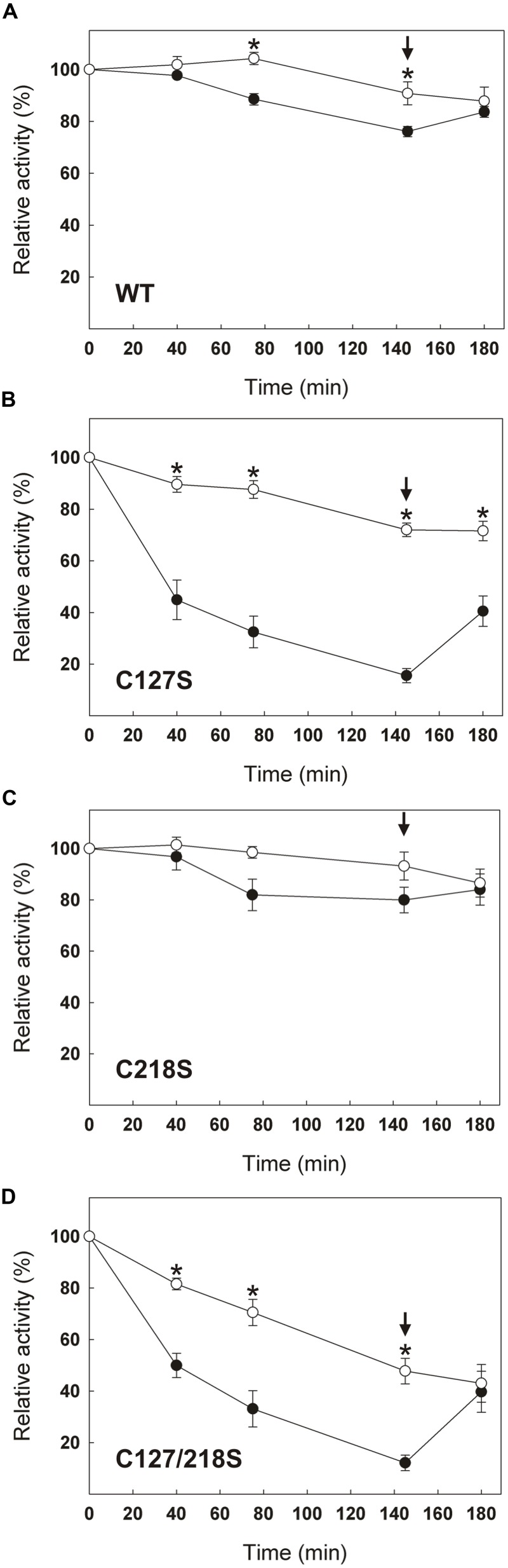
In addition to changes in their specific activity and fluorescence spectrum, C127S and C127/218S mutants were much less redox stable over time than the WT and the C218S mutant. Monitoring the time-dependent evolution of cTPI activity in the presence or absence of 5 mM DTT showed that the WT (Figure 7A) and the C218S (Figure 7C) mutant were more stable when incubated with DTT indicating that cTPI was sensitive to spontaneous oxidation. The two proteins kept more than 75% of their initial activity after 145 min when incubated without DTT. In contrast, within the same time frame, the C127S (Figure 7B) and C127/218S (Figure 7D) mutants lost 80% of their initial activity in absence of DTT. The activity of C127S and C127/218S mutants did not recover completely even after the addition of DTT. Due to the presence of DTT in these samples, the decrease in activity is probably not related to a spontaneous oxidation and could perhaps be due to an intrinsically less stable protein structure.
FIGURE 7.
Effect of C127S and C218S mutations on the stability of cTPI activity in presence or absence of DTT. Recombinant (A) WT, (B) C127S, (C) C218S, or (D) C127/218S cTPI enzymes were incubated with (white circles) or without (black circles) 5 mM DTT. Activity was measured at different time points. A star indicates a significant difference between treatments (Student’s t-test, P < 0.05). After 145 min (black arrow), the samples incubated in absence of reductant received DTT (10 mM final concentration) and TPI activity was measured at 180 min. Data points are mean ± SE (n ≥ 3).
Effect of Glutathione on cTPI Mutants
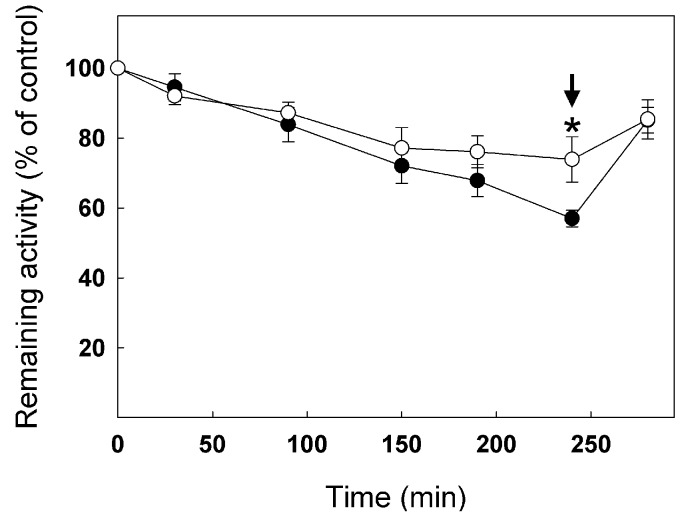
Because the C127S mutation leads to very low stability of enzyme activity and high inhibition due to spontaneous oxidation over time, it was not possible to test the effect of GSSG on the activity of the C127S and C127/218S mutants. Figure 8 shows inhibition of WT cTPI and C218S mutant by 100 μM GSSG over time. The C218S cTPI mutant behaved similarly to the WT, except that it significantly retained more activity than the WT after 240 min of incubation. Activity was partially restored by 10 mM DTT for both WT and C218S samples. These results show that C218S is sensitive to inhibition by GSSG. Thus, in A. thaliana, we observed that mutation of Cys218 does not confer resistance of cTPI activity to inhibition by GSSG.
FIGURE 8.
Inhibition of TPI activity by GSSG in WT and C218S mutant. Recombinant WT (black circles) and C218S mutant (white circle) cTPI proteins were incubated with 100 μM GSSG. TPI activity was measured at different incubation times. A star indicates a significant difference between treatments (Student’s t-test, P < 0.05). After 240 min (black arrow), both samples received 10 mM DTT before TPI activity was measured. Activity (mean ± SE, n = 3) is expressed as percentage of the control (untreated) sample remaining activity in order to take into account the loss of cTPI activity upon dilution without reductant.
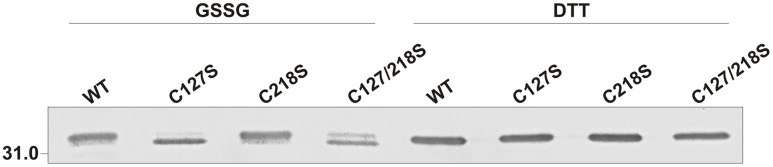
We performed non-reducing SDS-PAGE followed by anti-cTPI Western blot with the different cTPI mutants to visualize the effect of GSSG on the oxidation state of cTPI (Figure 9). Upon incubation with GSSG, two bands were observed indicating differences in electrophoretic mobility. Addition of DTT to the samples led to resolution of the proteins as a single band. The band with higher mobility suggests the formation of intramolecular disulfide bond in cTPI structure. After GSSG treatment, the WT protein and the C218S mutant showed a barely visible band with higher mobility, while most of the signal was associated with the upper band. On the contrary, the C127S and C127/218S mutants displayed a much higher signal corresponding to the high mobility band when incubated with GSSG. In this case, it remains unclear whether GSSG promotes formation of intramolecular disulfide bond in cTPI structure and if it is related to S-glutathionylation.
FIGURE 9.
Effect of GSSG on recombinant WT and mutant cTPI proteins mobility in non-reducing SDS-PAGE. Enzymes were incubated with 1 mM GSSG or 15 mM DTT. The figure shows immunoblot analysis after non-reducing SDS-PAGE of recombinant cTPI (50 ng). The identity of the protein is indicated on top of each lane.
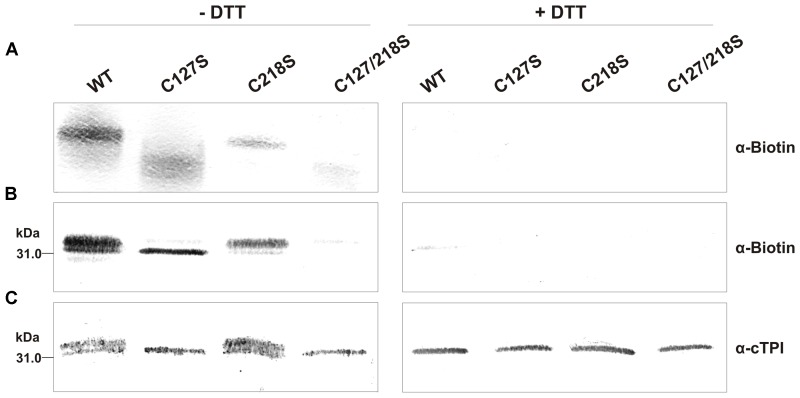
We next incubated the WT and mutant proteins with 0.5 mM of BioGEE and 0.1 mM of H2O2 followed by Western blot analyses using streptavidin detection (Figures 10A,B) and anti-cTPI antibody (Figure 10C). These experiments were performed with or without DTT to observe the reversibility of the modification. Streptavidin detection showed the presence of bands with different intensity for WT, C127S and C218S, but no significant signal for the C127/218S mutant. Most of the BioGEE signal was removed following addition of DTT. These results suggest that Cys127 and Cys218 are the major targets of S-glutathionylation in cTPI. The anti-cTPI detection (Figure 10C) showed a pattern similar to that previously observed in Figure 9, indicating differences in cTPI mobility due to the treatment. In these conditions, WT and C218S form two bands while only the bottom band is visible for C127S and C127/218S.
FIGURE 10.
BioGEE/Streptavidin detection and immunoblot analysis of recombinant WT and mutant cTPI proteins after native and SDS-PAGE. BioGEE detection by streptavidin conjugated with alkaline phosphatase after (A) Native PAGE analysis of recombinant proteins (300 ng) and Western blotting or (B) SDS-PAGE analysis of recombinant proteins (40 ng) and Western blotting. (C) Immunoblot analysis after SDS-PAGE of recombinant proteins (80 ng) using an isoform-specific cTPI antibody. Enzymes were incubated in absence (left panels) or presence (right panels) of 50 mM DTT in a buffer containing: (A) 100 mM Tris-Cl pH 7.8, 0.5 mM BioGEE, 0.1 mM H2O2 and (for B,C only) 0.005% (w/v) BSA.
Discussion
S-Glutathionylation has recently emerged as a PTM that takes part in redox signaling in photosynthetic organisms. Enzymes from carbon metabolism constitute major targets for redox PTMs including S-glutathionylation. In the plant cytosolic compartment, many glycolytic enzymes have been reported to be inhibited by this modification. Among these targets, enzymatic activities of cTPI, aldolase and GAPDH (GAPC1 and GAPC2) were reported to be inhibited by S-glutathionylation (Ito et al., 2003; Holtgrefe et al., 2008; van der Linde et al., 2011; Bedhomme et al., 2012). Low cTPI activity in transgenic Solanum tuberosum roots carrying cTPI antisense constructs has been associated with increased carbon flux through the PPP (Dorion et al., 2012; Valancin et al., 2013). The effect of TPI inhibition in cell response to oxidant treatment has also been well documented in non-plant systems. Reduced TPI activity has been linked to resistance against diamide, rerouting of metabolic flux through PPP and accumulation of PPP metabolites in Saccharomyces cerevisiae and C. elegans (Ralser et al., 2007; Grüning et al., 2014). Interestingly, reduction of GAPDH activity, a well-known redox regulated enzyme, led to similar observations (Ralser et al., 2007).
Recombinant cTPI Is Sensitive to Oxidant and S-Glutathionylation Treatments
In this work, we incubated cTPI with NEM, H2O2 and diamide with or without addition of GSH to study the effect of derivation of Cys residues on cTPI activity (Figure 2). Diamide is a chemical thiol-specific oxidant whereas H2O2 is a physiological oxidant. NEM is an alkylating reagent that blocks thiol groups irreversibly. Here, we showed that alkylation of cTPI Cys residues by NEM led to inhibition of the enzyme. Alkylation can be buffered by GSH when its concentration is higher than NEM thus protecting cTPI from inactivation. In contrast, we observed that addition of GSH did not protect cTPI from oxidation by H2O2. This suggests the presence of nucleophilic Cys residues in cTPI sequence. It is known that H2O2 oxidizes preferentially nucleophilic thiol groups. GSH has a high pKa in solution and does not constitute a major target for spontaneous oxidation by ROS (Zaffagnini et al., 2012d). However, the pKa of protein Cys residues can be lowered by their microenvironment thus stabilizing the thiolate anion which is more prone to react with ROS (Zaffagnini et al., 2012d). Advanced oxidized forms of Cys127 (sulfinic and sulfonic acid) were detected by mass spectrometry (Supplemental Figure S5) showing high reactivity of this residue.
Diamide is a thiol-specific oxidant that has been used in mammalian (Fratelli et al., 2002; Lock et al., 2011) and photosynthetic (Michelet et al., 2008) cells to promote S-glutathionylation in vivo. Diamide is known to react rapidly with cellular GSH to form GSSG (Kosower and Kosower, 1995). In rat erythrocytes, diamide was shown to induce S-glutathionylation by first reacting with hemoglobin and then promoting S-glutathionylation with GSH (Di Simplicio et al., 1998; Ghezzi and Di Simplicio, 2007). Coupled with GSH, diamide has also been used in vitro to show the inhibition of a mammalian protein tyrosine phosphatase by S-glutathionylation (Barrett et al., 1999). Here, we show that incubation of cTPI with GSH + diamide lead to rapid and almost total inhibition of cTPI activity while diamide alone as limited effects on cTPI activity especially in a short period of time (Figure 2). We suggest that, in the conditions used in our experiments, diamide could react rapidly with GSH to form a reactive diamide-GS conjugate as described in Kosower and Kosower (1995). This intermediate could then spontaneously react with reduced thiols present in the samples thus inducing protein S-glutathionylation and inhibition of cTPI activity. Being already oxidized, GSSG cannot form a reactive diamide-GS conjugate which can explain why incubation of cTPI with diamide + GSSG does not affect activity within 5 min. Although it does not seem to induce diamide-mediated S-glutathionylation, GSSG is known to induce S-glutathionylation by thiol disulfide exchange (Ghezzi, 2005). However, in our experiments inhibition of cTPI activity with GSSG was slower than that observed by treatment with diamide + GSH.
Activity of recombinant A. thaliana cTPI was previously reported to be inhibited by 2.5 mM GSSG (Ito et al., 2003). However, this inhibition has never been studied under more physiological concentrations of GSSG. GSSG concentrations in plant cells cytosol are very low under basal conditions. In situ experiments using a ratiometric redox-sensitive green fluorescent protein suggested that cytosolic GSSG concentrations in normal conditions are found in the low nanomolar range in A. thaliana (Meyer et al., 2007). Such reducing conditions could be explained by the reduction of GSSG by glutathione reductase activity but also by the sequestration of GSSG, particularly in the vacuole (Meyer et al., 2007; Noctor et al., 2013). By using a GSSG-accumulating catalase-deficient (cat2) A. thaliana mutant as a model for oxidative stressed cells, it was estimated that GSSG concentrations could reach the micromolar range in stressed plant cytosol by assuming that 99.9% of the GSSG is sequestrated inside the vacuole (Noctor et al., 2013). These data suggest that cytosolic GSSG concentrations remain low in plant cells even under oxidative stress. In this work, we showed that cTPI activity can be affected by micromolar concentrations of GSSG in vitro (Figure 3). These data support the view that cTPI activity could be regulated by conditions similar to those prevailing under oxidative stress. As previously mentioned, treatment of proteins with GSSG can lead to S-glutathionylation by thiol-disulfide exchange (Ghezzi, 2005), but GSSG has also been reported to promote formation of other types of disulfide bonds (Cumming et al., 2004). Moreover, catalysis of thiol disulfide exchange could also accelerate cTPI S-glutathionylation and inhibition. Indeed, a recent study has shown that S-glutathionylation of BAK1 by GSSG can be catalyzed by GRXC2 in A. thaliana (Bender et al., 2015). The authors showed that lower concentrations of GSSG were required for inhibition of the BAK1 kinase activity in the presence of GRXC2. However, catalysis of S-glutathionylation is still poorly understood in plants and needs further investigation.
S-Glutathionylation of Recombinant cTPI Is Reversed by GRXs
Since deglutathionylation is known to be catalyzed by GRXs (Rouhier et al., 2008; Bedhomme et al., 2012) we expressed and purified recombinant GRXC1 and GRXC2 from A. thaliana (Supplemental Figure S4). Determination of kcat values showed that they were similar to those obtained for poplar homologs PtGRXC1 and PtGRXC2 (Couturier et al., 2013). Our results were also in the range of activities measured for C. reinhardtii GRX1 and GRX2, two class I GRXs predicted to be cytosolic (Zaffagnini et al., 2008). However, kcat value reported here for A. thaliana GRXC1 (kcat = 8.0 ± 0.9 s-1) was lower than that reported by Riondet et al. (2012) (38.91 ± 0.43 s-1). Both A. thaliana GRXC1 and GRXC2 are class I GRXs (CPYC-like motif) well known for their reductase activity and localized in the cytosol (Riondet et al., 2012; Couturier et al., 2013). Despite the well-known ability of GRXC1 but not GRXC2 to bind [2Fe-2S] clusters (Rouhier et al., 2007, 2010; Riondet et al., 2012; Couturier et al., 2015), both GRXC1 and GRXC2 are suggested to have redundant functions in plant cells (Riondet et al., 2012). In our experiments, both GRXC1 and GRXC2 were able to restore cTPI activity with a similar efficiency. Both GRXs were dependent on GRS for deglutathionylation of cTPI (Figure 4). Similar reactivation experiments have previously shown GRXs-mediated deglutathionylation. Cytosolic enzyme GAPC1 from A. thaliana was shown to be inactivated by S-glutathionylation and rescued by cytosolic poplar PtGRXC1 through a monothiol mechanism (Bedhomme et al., 2012). Other experiments showed reactivation of S-glutathionylated isocitrate lyase from C. reinhardtii by CrGRX1, CrGRX2 (Gao et al., 2010) and poplar chloroplastic class I GRXS12 (Zaffagnini et al., 2012c). Overall, our results suggest that cTPI activity can be inhibited by S-glutathionylation and rescued by cytosolic GRXs such as GRXC1 and GRXC2.
cTPI Cys127 and Cys218 Residues Are Targets of Glutathione and Other Redox PTMs
In animals, the Cys residue from Gallus gallus TPI corresponding to Cys127 was described to be sensitive to oxidation leading to inhibition of the enzyme (Tang et al., 1990). Subsequently, a proteomic survey of human T lymphocytes identified TPI as a target of S-glutathionylation, but the modified Cys were not identified (Fratelli et al., 2002). Recently, the Cys127 residue of photosynthetic cyanobacterium Synechocystis sp. PCC6803 TPI was identified as a target of S-glutathionylation (Chardonnet et al., 2014). In higher plants, cTPI is known to be S-glutathionylationed (Ito et al., 2003), however, the modified amino acids were not identified. Here we used nanoLC-MS/MS analysis to show that recombinant cTPI from A. thaliana was modified by GSSG on residues Cys127 and Cys218 (Figure 5). Interestingly, Cys218 is located next to a Lys residue (Lys219) (Supplemental Figure S5) which could contribute to the stabilization of the thiolate anion. Plant cTPI was also found to be modified by S-nitrosylation (Romero-Puertas et al., 2008; Fares et al., 2011). Moreover, Cys127 and Cys218 residues were shown to be S-nitrosylated in A. thaliana cell culture (Fares et al., 2011). Cys residues S-nitrosylation is a spontaneous redox PTM induced by reactive nitrogen species that shows some similarities with sulfenic acid formation. S-nitrosylated Cys thiols are considered to be reactive and can thus be modified by GSH leading to S-glutathionylation (Martínez-Ruiz and Lamas, 2007). Considering our results, plant cTPI Cys127 and Cys218 residues appear to be major targets of redox modification including S-glutathionylation.
Mutation Cys127 and Cys218 Affects cTPI Activity, Structure, Stability, and Interaction with Glutathione
Arabidopsis thaliana cTPI contains four Cys residues (Supplemental Figure S5), none of which is known to be directly involved in the TPI catalytic site (Komives et al., 1991; Zaffagnini et al., 2014). In the structure of C. reinhardtii pTPI and in G. gallus TPI, Cys residues corresponding to Cys127 are located near the catalytic site, while Cys residues corresponding to Cys218 are exposed to the solvent (Banner et al., 1975; Zaffagnini et al., 2014). Being close to the active site, modification of Cys127 is more likely to affect cTPI activity. Our data demonstrate that mutation of Cys127 to a Ser residue lead to strong perturbation of A. thaliana cTPI kinetics parameters (Table 1). Nonetheless, mutation of Cys126 (corresponding to Cys127) to a Ser residue in S. cerevisiae TPI was reported to have a lower impact on enzyme kinetics (González-Mondragón et al., 2004). This mutation caused a four-fold decrease in yeast TPI kcat while we observed a 48-fold decrease for the same mutation in A. thaliana cTPI. Yeast TPI C126S mutant was also reported to be less stable and more subject to irreversible denaturation than the WT. Similarly, mutation of Cys126 to a Ser in TPI from P. falciparum caused a five-fold decrease of activity. It was also proposed that mutation of this Cys residue decreased the dimer stability at elevated temperatures (Samanta et al., 2011). Later, it was suggested that the low stability of yeast C126S was due to penetration of water molecules in the region near the residue (Hernández-Santoyo et al., 2012). It should be noted that the amino acids sequence of S. cerevisiae TPI has no Cys corresponding to Cys218 in A. thaliana cTPI.
While it was impossible to test the effect of GSSG on C127S and C127/218S mutant due to their low stability, we showed that incubation of C218S with GSSG mutant caused a decrease in activity similar to that observed for the WT (Figure 8). These results differ from data obtained with Giardia lamblia TPI. In this latter case, inactivation was mostly caused by derivatization of Cys222 (corresponding to Cys218 in A. thaliana cTPI). Indeed, the activity of the C222A mutant was completely resistant to inactivation by sulfhydryl reagents such as 2-carboxyethyl methanethiosulfonate and 5,50-dithio-bis (2-nitrobenzoic acid) while the WT and all other mutants for Cys residues were strongly inhibited by these reagents (Enríquez-Flores et al., 2011).
Difference in electrophoretic mobility of oxidized and reduced TPI has already been observed in chicken where the oxidized form was suggested to have lower catalytic activity (Tang et al., 1990). Here, we found that exposure of cTPI to GSSG or H2O2 and BioGEE led to the formation of two bands with different mobility only in the absence of DTT (Figures 9 and 10). Surprisingly, mutation of both targets of S-glutathionylation, Cys127 and Cys218, did not confer oxidant resistance. On the contrary, C127S and C127/218S mutants were less stable (Figure 7) and more likely to form a higher mobility band corresponding to an oxidized form of cTPI. However, the C127/218S mutant does not seem to be significantly modified by BioGEE since both S-glutathionylation sites are mutated. It still remains unclear whether Cys13 and Cys67 residues are redox sensitive in cTPI. Formation of a higher mobility band for the oxidized C127/218S mutant and spontaneous loss of activity could be attributed to the formation of an intramolecular disulfide bond between the two remaining Cys residues. Moreover, rapid formation of disulfide bond between Cys13 and Cys67 could also prevent interaction of the C127/218S mutant with BioGEE as observed here. This redox sensitivity could be linked to perturbations in cTPI structure due to the mutation of the strictly conserved Cys127 residue. Differences in fluorescence emission spectra provide an indication of alterations in protein structure. A mutation of the redox sensitive Cys127 seemed to significantly affect protein fluorescence and kinetics parameters. Given our results, we hypothesize that the C127S substitution had more impact on protein structure than the C218S mutation. This is in agreement with results of specific activity showing a drastic decrease in cTPI activity in the C127S mutant protein.
Conclusion
In the present study, we showed that recombinant cTPI from A. thaliana is sensitive to inhibition by different treatments promoting Cys oxidation or S-glutathionylation. Inhibition of cTPI by S-glutathionylation was reversed by the activity of recombinant GRXC1 and GRXC2 from A. thaliana in the presence of a GRS. We used nanoLC-MS/MS to identify Cys127 and Cys218 as the two residues targeted by GSSG. Mutation of targeted Cys appeared to affect cTPI structure, activity and redox stability. When incubated with BioGEE, the cTPI mutant that lacks both targeted Cys residues did not show a significant signal on nitrocellulose membrane after streptavidin detection.
We propose that S-glutathionylation of cTPI in A. thaliana could be involved in the response to oxidizing conditions in the cytoplasmic compartment. In this hypothesis, a stress-induced S-glutathionylation would lead to a decrease in the activity of cTPI and other glycolytic enzymes such as cytosolic GAPDH and aldolase. This mechanism could lead to an increase in carbon flux through the PPP and enhanced production of NADPH used for restoration of GSH.
Author Contributions
SDu, NB, GP, and SDo performed the experimental work; SDu, NB, and JR planned the experiments, interpreted the data and wrote the paper; all the authors discussed the data and revised the manuscript prior to submission.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
SDu is grateful for a graduate scholarship from the Fond de Recherche du Québec Nature et Technologie. GP was a recipient of an Undergraduate Student Research Award from the National Science and Engineering Research Council of Canada.
Abbreviations
- ABRC
Arabidopsis Biological Resource Center
- BAK1
Brassinosteroid insensitive 1-Associated receptor-like Kinase 1
- BioGEE
biotinylated glutathione ethyl ester
- BioGSSG
biotinylated oxidized glutathione
- BSA
bovine serum albumin
- CID
collision-induced dissociation
- cTPI
cytosolic triosephosphate isomerase
- DHAP
dihydroxyacetone phosphate
- GAP
glyceraldehyde-3-phosphate
- GAPDH
GAP dehydrogenase
- GRXC1
glutaredoxin C1
- GRXC2
glutaredoxin C2
- GRS
GSH-regeneration system
- GSH
reduced glutathione
- GSNO
S-nitrosoglutathione
- GSSG
oxidized glutathione
- HED
2-hydroxyethyl disulfide
- H2O2
hydrogen peroxide
- LC-MS/MS
liquid chromatography-tandem mass spectrometry
- NEM
N-ethylmaleimide
- PPP
pentose phosphate pathway
- PTM
post-translational modification
- pTPI
plastidic TPI
- ROS
reactive oxygen species
- TRX
thioredoxin
- WT
wild-type
Footnotes
Funding. This work was supported by a Discovery Grant from the National Science and Engineering Research Council of Canada to JR (grant number RGPIN 227271), and by Agriculture and Agri-Food Canada Growing Forward funding to NB (AAFC AgriInnovation project 1203).
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fpls.2016.01942/full#supplementary-material
References
- Apel K., Hirt H. (2004). Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 55 373–399. 10.1146/annurev.arplant.55.031903.141701 [DOI] [PubMed] [Google Scholar]
- Banner D., Bloomer A., Petsko G., Phillips D., Pogson C., Wilson I., et al. (1975). Structure of chicken muscle triose phosphate isomerase determined crystallographically at 2.5 Å resolution: using amino acid sequence data. Nature 255 609–614. 10.1038/255609a0 [DOI] [PubMed] [Google Scholar]
- Barrett W. C., Degnore J. P., König S., Fales H. M., Keng Y.-F., Zhang Z.-Y., et al. (1999). Regulation of PTP1B via glutathionylation of the active site cysteine 215. Biochemistry 38 6699–6705. 10.1021/bi990240v [DOI] [PubMed] [Google Scholar]
- Bedhomme M., Adamo M. H., Marchand C., Couturier J., Rouhier N., Lemaire D. S., et al. (2012). Glutathionylation of cytosolic glyceraldehyde-3-phosphate dehydrogenase from the model plant Arabidopsis thaliana is reversed by both glutaredoxins and thioredoxins in vitro. Biochem. J. 445 337–347. 10.1042/BJ20120505 [DOI] [PubMed] [Google Scholar]
- Bender K. W., Wang X. J., Cheng G. B., Kim H. S., Zielinski R. E., Huber S. C. (2015). Glutaredoxin AtGRXC2 catalyses inhibitory glutathionylation of Arabidopsis BRI1-associated receptor-like kinase 1 (BAK1) in vitro. Biochem. J. 467 399–413. 10.1042/BJ20141403 [DOI] [PubMed] [Google Scholar]
- Bradford M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72 248–254. 10.1016/0003-2697(76)90527-3 [DOI] [PubMed] [Google Scholar]
- Butturini E., Darra E., Chiavegato G., Cellini B., Cozzolino F., Monti M., et al. (2014). S-glutathionylation at Cys328 and Cys542 impairs STAT3 phosphorylation. ACS Chem. Biol. 9 1885–1893. 10.1021/cb500407d [DOI] [PubMed] [Google Scholar]
- Bykova N. V., Rampitsch C. (2013). Modulating protein function through reversible oxidation: redox-mediated processes in plants revealed through proteomics. Proteomics 13 579–596. 10.1002/pmic.201200270 [DOI] [PubMed] [Google Scholar]
- Chardonnet S., Sakr S., Cassier-Chauvat C., Le MareìChal P., Chauvat F., Lemaire S. P. D., et al. (2014). First proteomic study of S-glutathionylation in cyanobacteria. J. Proteome Res. 14 59–71. 10.1021/pr500625a [DOI] [PubMed] [Google Scholar]
- Couturier J., Jacquot J. P., Rouhier N. (2013). Toward a refined classification of class I dithiol glutaredoxins from poplar: biochemical basis for the definition of two subclasses. Front. Plant Sci. 4:518 10.3389/fpls.2013.00518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couturier J., Przybyla-Toscano J., Roret T., Didierjean C., Rouhier N. (2015). The roles of glutaredoxins ligating Fe–S clusters: sensing, transfer or repair functions? Biochim. Biophys. Acta 1853 1513–1527. 10.1016/j.bbamcr.2014.09.018 [DOI] [PubMed] [Google Scholar]
- Cumming R. C., Andon N. L., Haynes P. A., Park M., Fischer W. H., Schubert D. (2004). Protein disulfide bond formation in the cytoplasm during oxidative stress. J. Biol. Chem. 279 21749–21758. 10.1074/jbc.M312267200 [DOI] [PubMed] [Google Scholar]
- Dalle-Donne I., Rossi R., Colombo G., Giustarini D., Milzani A. (2009). Protein S-glutathionylation: a regulatory device from bacteria to humans. Trends Biochem. Sci. 34 85–96. 10.1016/j.tibs.2008.11.002 [DOI] [PubMed] [Google Scholar]
- Davies M. J. (2005). The oxidative environment and protein damage. Biochim. Biophys. Acta 1703 93–109. 10.1016/j.bbapap.2004.08.007 [DOI] [PubMed] [Google Scholar]
- Di Simplicio P., Cacace M. G., Lusini L., Giannerini F., Giustarini D., Rossi R. (1998). Role of protein -SH groups in redox homeostasis - The erythrocyte as a model system. Arch. Biochem. Biophys. 355 145–152. 10.1006/abbi.1998.0694 [DOI] [PubMed] [Google Scholar]
- Dixon D. P., Skipsey M., Grundy N. M., Edwards R. (2005). Stress-induced protein S-glutathionylation in Arabidopsis. Plant Physiol. 138 2233–2244. 10.1104/pp.104.058917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorion S., Clendenning A., Jeukens J., Salas J. J., Parveen N., Haner A. A., et al. (2012). A large decrease of cytosolic triosephosphate isomerase in transgenic potato roots affects the distribution of carbon in primary metabolism. Planta 236 1177–1190. 10.1007/s00425-012-1675-1 [DOI] [PubMed] [Google Scholar]
- Dorion S., Jeukens J., Matton D. P., Rivoal J. (2005). Cloning and characterization of a cytosolic isoform of triosephosphate isomerase developmentally regulated in potato leaves. Plant Sci. 168 183–194. 10.1016/j.plantsci.2004.07.029 [DOI] [Google Scholar]
- Enríquez-Flores S., Rodríguez-Romero A., Hernández-Alcántara G., Oria-Hernández J., Gutiérrez-Castrellón P., Pérez-Hernández G., et al. (2011). Determining the molecular mechanism of inactivation by chemical modification of triosephosphate isomerase from the human parasite Giardia lamblia: a study for antiparasitic drug design. Proteins 79 2711–2724. 10.1002/prot.23100 [DOI] [PubMed] [Google Scholar]
- Fares A., Rossignol M., Peltier J.-B. (2011). Proteomics investigation of endogenous S-nitrosylation in Arabidopsis. Biochem. Biophys. Res. Commun. 416 331–336. 10.1016/j.bbrc.2011.11.036 [DOI] [PubMed] [Google Scholar]
- Foyer C. H., Noctor G. (2009). Redox regulation in photosynthetic organisms: signaling, acclimation, and practical implications. Antioxid. Redox Signal. 11 861–905. 10.1089/ars.2008.2177 [DOI] [PubMed] [Google Scholar]
- Fratelli M., Demol H., Puype M., Casagrande S., Eberini I., Salmona M., et al. (2002). Identification by redox proteomics of glutathionylated proteins in oxidatively stressed human T lymphocytes. Proc. Natl. Acad. Sci. U.S.A. 99 3505–3510. 10.1073/pnas.052592699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallogly M. M., Mieyal J. J. (2007). Mechanisms of reversible protein glutathionylation in redox signaling and oxidative stress. Curr. Opin. Pharmacol. 7 381–391. 10.1016/j.coph.2007.06.003 [DOI] [PubMed] [Google Scholar]
- Gao X.-H., Zaffagnini M., Bedhomme M., Michelet L., Cassier-Chauvat C., Decottignies P., et al. (2010). Biochemical characterization of glutaredoxins from Chlamydomonas reinhardtii: kinetics and specificity in deglutathionylation reactions. FEBS Lett. 584 2242–2248. 10.1016/j.febslet.2010.04.034 [DOI] [PubMed] [Google Scholar]
- Ghezzi P. (2005). Review regulation of protein function by glutathionylation. Free Radic. Res. 39 573–580. 10.1080/10715760500072172 [DOI] [PubMed] [Google Scholar]
- Ghezzi P., Di Simplicio P. (2007). Glutathionylation pathways in drug response. Curr. Opin. Pharmacol. 7 398–403. 10.1016/j.coph.2007.04.006 [DOI] [PubMed] [Google Scholar]
- Gill S. S., Tuteja N. (2010). Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 48 909–930. 10.1016/j.plaphy.2010.08.016 [DOI] [PubMed] [Google Scholar]
- González-Mondragón E., Zubillaga R. A., Saavedra E., Chánez-Cárdenas M. E., Pérez-Montfort R., Hernández-Arana A. (2004). Conserved cysteine 126 in triosephosphate isomerase is required not for enzymatic activity but for proper folding and stability. Biochemistry 43 3255–3263. 10.1021/bi036077s [DOI] [PubMed] [Google Scholar]
- Grüning N.-M., Du D., Keller M. A., Luisi B. F., Ralser M. (2014). Inhibition of triosephosphate isomerase by phosphoenolpyruvate in the feedback-regulation of glycolysis. Open Biol. 4 130232 10.1098/rsob.130232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Santoyo A., Domínguez-Ramírez L., Reyes-López C. A., González-Mondragón E., Hernández-Arana A., Rodríguez-Romero A. (2012). Effects of a buried cysteine-to-serine mutation on yeast triosephosphate isomerase structure and stability. Int. J. Mol. Sci. 13 10010–10021. 10.3390/ijms130810010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtgrefe S., Gohlke J., Starmann J., Druce S., Klocke S., Altmann B., et al. (2008). Regulation of plant cytosolic glyceraldehyde 3-phosphate dehydrogenase isoforms by thiol modifications. Physiol. Plant. 133 211–228. 10.1111/j.1399-3054.2008.01066.x [DOI] [PubMed] [Google Scholar]
- Inzé D., Montagu M. V. (1995). Oxidative stress in plants. Curr. Opin. Biotechnol. 6 153–158. 10.1016/0958-1669(95)80024-7 [DOI] [Google Scholar]
- Ito H., Iwabuchi M., Ogawa K. (2003). The sugar-metabolic enzymes aldolase and triose-phosphate isomerase are targets of glutathionylation in Arabidopsis thaliana: detection using biotinylated glutathione. Plant Cell Physiol. 44 655–660. 10.1093/pcp/pcg098 [DOI] [PubMed] [Google Scholar]
- Karpinski S., Gabrys H., Mateo A., Karpinska B., Mullineaux P. M. (2003). Light perception in plant disease defence signalling. Curr. Opin. Plant Biol. 6 390–396. 10.1016/S1369-5266(03)00061-X [DOI] [PubMed] [Google Scholar]
- Klatt P., Lamas S. (2000). Regulation of protein function by S-glutathiolation in response to oxidative and nitrosative stress. Eur. J. Biochem. 267 4928–4944. 10.1046/j.1432-1327.2000.01601.x [DOI] [PubMed] [Google Scholar]
- Komives E. A., Chang L. C., Lolis E., Tilton R. F., Petsko G. A., Knowles J. R. (1991). Electrophilic catalysis in triosephosphate isomerase: the role of histidine-95. Biochemistry 30 3011–3019. 10.1021/bi00226a005 [DOI] [PubMed] [Google Scholar]
- Kosower N. S., Kosower E. M. (1995). Diamide: an oxidant probe for thiols. Methods Enzymol. 251 123–133. 10.1016/0076-6879(95)51116-4 [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227 680–685. 10.1038/227680a0 [DOI] [PubMed] [Google Scholar]
- Larkin M. A., Blackshields G., Brown N. P., Chenna R., Mcgettigan P. A., Mcwilliam H., et al. (2007). Clustal W and Clustal X version 2.0. Bioinformatics 23 2947–2948. 10.1093/bioinformatics/btm404 [DOI] [PubMed] [Google Scholar]
- Lock J. T., Sinkins W. G., Schilling W. P. (2011). Effect of protein S-glutathionylation on Ca2+ homeostasis in cultured aortic endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 300 H493–H506. 10.1152/ajpheart.01073.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand C., Le Maréchal P., Meyer Y., Miginiac-Maslow M., Issakidis-Bourguet E., Decottignies P. (2004). New targets of Arabidopsis thioredoxins revealed by proteomic analysis. Proteomics 4 2696–2706. 10.1002/pmic.200400805 [DOI] [PubMed] [Google Scholar]
- Marchand C. H., Vanacker H., Collin V., Issakidis-Bourguet E., Maréchal P. L., Decottignies P. (2010). Thioredoxin targets in Arabidopsis roots. Proteomics 10 2418–2428. 10.1002/pmic.200900835 [DOI] [PubMed] [Google Scholar]
- Martínez-Ruiz A., Lamas S. (2007). Signalling by NO-induced protein S-nitrosylation and S-glutathionylation: convergences and divergences. Cardiovasc. Res. 75 220–228. 10.1016/j.cardiores.2007.03.016 [DOI] [PubMed] [Google Scholar]
- Meyer A. J., Brach T., Marty L., Kreye S., Rouhier N., Jacquot J. P., et al. (2007). Redox-sensitive GFP in Arabidopsis thaliana is a quantitative biosensor for the redox potential of the cellular glutathione redox buffer. Plant J. 52 973–986. 10.1111/j.1365-313X.2007.03280.x [DOI] [PubMed] [Google Scholar]
- Michelet L., Zaffagnini M., Vanacker H., Le Marechal P., Marchand C., Schroda M., et al. (2008). In vivo targets of S-thiolation in Chlamydomonas reinhardtii. J. Biol. Chem. 283 21571–21578. 10.1074/jbc.M802331200 [DOI] [PubMed] [Google Scholar]
- Mittler R. (2002). Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 7 405–410. 10.1016/S1360-1385(02)02312-9 [DOI] [PubMed] [Google Scholar]
- Miwa S., Brand M. (2003). Mitochondrial matrix reactive oxygen species production is very sensitive to mild uncoupling. Biochem. Soc. Trans. 31 1300–1301. 10.1042/bst0311300 [DOI] [PubMed] [Google Scholar]
- Noctor G., Mhamdi A., Queval G., Foyer C. H. (2013). Regulating the redox gatekeeper: vacuolar sequestration puts glutathione disulfide in its place. Plant Physiol. 163 665–671. 10.1104/pp.113.223545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orosz F., Oláh J., Ovádi J. (2009). Triosephosphate isomerase deficiency: new insights into an enigmatic disease. Biochim. Biophys. Acta 1792 1168–1174. 10.1016/j.bbadis.2009.09.012 [DOI] [PubMed] [Google Scholar]
- Pastore A., Piemonte F. (2012). S-Glutathionylation signaling in cell biology: progress and prospects. Eur. J. Pharm. Sci. 46 279–292. 10.1016/j.ejps.2012.03.010 [DOI] [PubMed] [Google Scholar]
- Ralser M., Heeren G., Breitenbach M., Lehrach H., Krobitsch S. (2006). Triose phosphate isomerase deficiency is caused by altered dimerization–not catalytic inactivity–of the mutant enzymes. PLoS ONE 1:e30 10.1371/journal.pone.0000030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralser M., Wamelink M. M., Kowald A., Gerisch B., Heeren G., Struys E. A., et al. (2007). Dynamic rerouting of the carbohydrate flux is key to counteracting oxidative stress. J. Biol. 6 301–312. 10.1186/jbiol61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riondet C., Desouris J. P., Montoya J. G., Chartier Y., Meyer Y., Reichheld J. P. (2012). A dicotyledon-specific glutaredoxin GRXC1 family with dimer-dependent redox regulation is functionally redundant with GRXC2. Plant Cell Environ. 35 360–373. 10.1111/j.1365-3040.2011.02355.x [DOI] [PubMed] [Google Scholar]
- Romero-Puertas M. C., Campostrini N., Mattè A., Righetti P. G., Perazzolli M., Zolla L., et al. (2008). Proteomic analysis of S-nitrosylated proteins in Arabidopsis thaliana undergoing hypersensitive response. Proteomics 8 1459–1469. 10.1002/pmic.200700536 [DOI] [PubMed] [Google Scholar]
- Rouhier N., Couturier J., Johnson M. K., Jacquot J.-P. (2010). Glutaredoxins: roles in iron homeostasis. Trends Biochem. Sci. 35 43–52. 10.1016/j.tibs.2009.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouhier N., Lemaire S. D., Jacquot J.-P. (2008). The role of glutathione in photosynthetic organisms: emerging functions for glutaredoxins and glutathionylation. Annu. Rev. Plant Biol. 59 143–166. 10.1146/annurev.arplant.59.032607.092811 [DOI] [PubMed] [Google Scholar]
- Rouhier N., Unno H., Bandyopadhyay S., Masip L., Kim S.-K., Hirasawa M., et al. (2007). Functional, structural, and spectroscopic characterization of a glutathione-ligated [2Fe–2S] cluster in poplar glutaredoxin C1. Proc. Natl. Acad. Sci. U.S.A. 104 7379–7384. 10.1073/pnas.0702268104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanta M., Banerjee M., Murthy M., Balaram H., Balaram P. (2011). Probing the role of the fully conserved Cys126 in triosephosphate isomerase by site-specific mutagenesis–distal effects on dimer stability. FEBS J. 278 1932–1943. 10.1111/j.1742-4658.2011.08110.x [DOI] [PubMed] [Google Scholar]
- Sambrook J., Fritsch E. F., Maniatis T. (1989). Molecular Cloning. New York, NY: Cold spring harbor laboratory press. [Google Scholar]
- Shahul M. S., Sarma S. P. (2011). The structure of the thioredoxin–triosephosphate isomerase complex provides insights into the reversible glutathione-mediated regulation of triosephosphate isomerase. Biochemistry 51 533–544. [DOI] [PubMed] [Google Scholar]
- Tang C.-Y., Yüksel K. Ü., Jacobson T. M., Gracy R. W. (1990). Isoforms of chicken triosephosphate isomerase are due to specific oxidation of cysteine126. Arch. Biochem. Biophys. 283 12–19. 10.1016/0003-9861(90)90605-X [DOI] [PubMed] [Google Scholar]
- Townsend D. M., Manevich Y., He L., Hutchens S., Pazoles C. J., Tew K. D. (2009). Novel role for glutathione S-transferase π regulator of protein S-glutathionylation following oxidative and nitrosative stress. J. Biol. Chem. 284 436–445. 10.1074/jbc.M805586200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valancin A., Srinivasan B., Rivoal J., Jolicoeur M. (2013). Analyzing the effect of decreasing cytosolic triosephosphate isomerase on Solanum tuberosum hairy root cells using a kineticmetabolic model. Biotechnol. Bioeng. 110 924–935. 10.1002/bit.24747 [DOI] [PubMed] [Google Scholar]
- van der Linde K., Gutsche N., Letters H.-M., Lindermayr C., Mueller B., Holtgrefe S., et al. (2011). Regulation of plant cytosolic aldolase functions by redox-modifications. Plant Physiol. Biochem. 49 946–957. 10.1016/j.plaphy.2011.06.009 [DOI] [PubMed] [Google Scholar]
- Zaffagnini M., Bedhomme M., Groni H., Marchand C. H., Puppo C., Gontero B., et al. (2012a). Glutathionylation in the photosynthetic model organism Chlamydomonas reinhardtii: a proteomic survey. Mol. Cell. Proteom. 11 M111.014142 10.1074/mcp.M111.014142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaffagnini M., Bedhomme M., Lemaire S. D., Trost P. (2012b). The emerging roles of protein glutathionylation in chloroplasts. Plant Sci. 185 86–96. 10.1016/j.plantsci.2012.01.005 [DOI] [PubMed] [Google Scholar]
- Zaffagnini M., Bedhomme M., Marchand C. H., Couturier J., Gao X.-H., Rouhier N., et al. (2012c). Glutaredoxin s12: unique properties for redox signaling. Antioxid. Redox Signal. 16 17–32. 10.1089/ars.2011.3933 [DOI] [PubMed] [Google Scholar]
- Zaffagnini M., Bedhomme M., Marchand C. H., Morisse S., Trost P., Lemaire S. D. (2012d). Redox regulation in photosynthetic organisms: focus on glutathionylation. Antioxid. Redox. Signal. 16 567–586. 10.1089/ars.2011.4255 [DOI] [PubMed] [Google Scholar]
- Zaffagnini M., Michelet L., Massot V., Trost P., Lemaire S. D. (2008). Biochemical characterization of glutaredoxins from Chlamydomonas reinhardtii reveals the unique properties of a chloroplastic CGFS-type glutaredoxin. J. Biol. Chem. 283 8868–8876. 10.1074/jbc.M709567200 [DOI] [PubMed] [Google Scholar]
- Zaffagnini M., Michelet L., Sciabolini C., Di Giacinto N., Morisse S., Marchand C. H., et al. (2014). High-resolution crystal structure and redox properties of chloroplastic triosephosphate isomerase from Chlamydomonas reinhardtii. Mol. Plant 7 101–120. 10.1093/mp/sst139 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.