Abstract
Objective:
To investigate the relationship between hypothalamic D3 dopamine receptor availability and severity of sleep problems in Parkinson disease (PD).
Methods:
Twelve patients were assessed with PET and the high-affinity dopamine D3 receptor radioligand [11C]-propyl-hexahydro-naphtho-oxazin ([11C]-PHNO). Severity of sleep problems was rated with appropriate subitems of the Unified Parkinson's Disease Rating Scale part I (patient questionnaire) and the Epworth Sleepiness Scale.
Results:
We found that lower dopamine D3 receptor availability measured with [11C]-PHNO PET was associated with greater severity of excessive daytime sleepiness but not with problems of falling asleep or insomnia.
Conclusion:
In our cohort of patients with PD, the occurrence of excessive daytime sleepiness was linked to reductions in hypothalamic dopamine D3 receptor availability. If these preliminary findings are confirmed in larger cohorts of patients with polysomnographic characterization, selective pharmacologic modulation of the dopaminergic D3 system could be used to increase daytime alertness in patients with PD.
Reduced presynaptic monoaminergic terminal function1,2 and postsynaptic D2 dopaminergic receptor availability3 have been reported in the hypothalamus of patients with Parkinson disease (PD). Sleep-dominant subtypes in PD have also been reported.4,5 These studies have suggested that altered dopaminergic transmission in the hypothalamus may contribute to the development of sleep disorders in PD. However, a direct relationship between hypothalamic dopaminergic dysfunction and the presence of sleep problems in patients with PD has not yet been demonstrated.
[11C]-propyl-hexahydro-naphtho-oxazin ([11C]-PHNO) is a dopamine D3 receptor–selective PET radioligand that shows high binding in the substantia nigra, hypothalamus, and ventral pallidum/substantia innominata.6 In this study, we have used [11C]-PHNO PET to determine in vivo dopamine D3 receptor availability in the hypothalamus of patients with PD and to assess its relationship with severity of sleep problems measured with the Epworth Sleepiness Scale (ESS) and the specific subitems of the Movement Disorder Society Unified Parkinson's Disease Rating Scale (MDS-UPDRS).
METHODS
Participants.
Twelve patients with a clinical diagnosis of idiopathic PD according to the UK Parkinson's Disease Society Brain Bank diagnostic criteria were recruited for this study.7 Patients with significant comorbidities that could cause nocturnal insomnia or excessive daytime sleepiness (EDS), a history of other neurologic conditions (e.g., stroke, head injury, epilepsy), dementia (Mini-Mental State Examination score <248) or depression (Hamilton Rating Scale for Depression >79) were excluded.
Standard protocol approvals, registrations, and patient consents.
All participants gave informed written consent in accordance with the Declaration of Helsinki, and the study received approval from the local Ethics Committee. Permission to administer [11C]-PHNO was obtained from the UK Administration of Radioactive Substances Advisory Committee.
Clinical assessment.
PD severity was rated with the modified Hoehn and Yahr (H&Y) scale and the part III motor subscale of the MDS-UPDRS.10 Sleep problems in patients with PD were scored with subitem 1.7 (sleep problems) and subitem 1.8 (daytime sleepiness) of the MDS-UPDRS part I, and the ESS, a widely used self-reported instrument,11 was used to assess the occurrence of EDS. Subitem 1.7 of the MDS-UPDRS part I (patient questionnaire) asks the question, “Over the past week, have you had trouble going to sleep at night or staying asleep through the night? Consider how rested you felt after waking up in the morning.”11 The answer score ranges from 0 (no problems) to 4 (I usually do not sleep for most of the night). Subitem 1.8 of the MDS-UPDRS part I (patient questionnaire) asks the question, “Over the past week, have you had trouble staying awake during the daytime?”11 The answer score ranges from 0 (no daytime sleepiness) to 4 (I often fall asleep when I should not, e.g., while eating or talking with other people). The ESS consists of 8 questions, each rated on a scale of 0 to 3, with a maximum score of 24; a higher score indicates a higher degree of EDS. The scale has previously been validated in PD.12 Other nonmotor symptoms were assessed with the Non-Motor Symptoms Scale.13
Daily levodopa equivalent dose (DLED) was calculated as follows: total levodopa equivalent dose = regular levodopa dose × 1 + levodopa continuous-release dose × 0.75 + pramipexole dose × 100 + ropinirole dose × 20 + rotigotine dose × 30 + selegilne oral dose × 10 + selegilne sublingual dose × 80 + rasagiline dose × 100 + amantadine dose × 1 + apomorphine dose × 10 + tolcapone × 0.5 or entacapone × 0.33.14
PET procedure.
All participants underwent [11C]-PHNO PET scan. Patients were scanned in an “off” state after overnight withdrawal of their antiparkinsonian medication. Patients receiving controlled-release dopaminergic supplementation withdrew this medication 72 hours before scanning. PET scans were acquired with a Siemens Biograph HiRez XVI PET scanner (Siemens Healthcare, Erlangen, Germany). A low-dose CT scan was performed to enable attenuation correction. [11C]-PHNO was administered intravenously with a mean tracer dose of 481.4 ± 731.8 MBq, a mean volume of 4.7 ± 2.3 mL, and a target PHNO mass of 3.18 ± 1.1 μg (0.77 ± 0.28 μg/kg). After the administration of [11C]-PHNO (as a slow bolus), dynamic emission data were collected over 90 minutes. All patients had a volumetric T1 MRI for coregistration purposes, obtained with a 3T MRI (Magneton Trio Syngo MR B13 Siemens 3T; Siemens AG, Munich, Germany) on the same day as the PET scan. All imaging procedures were performed at Imanova Ltd, Hammersmith Hospital Campus, Imperial College London (London, UK).
Image analysis.
Using in-house software (c-wave) implemented in Matlab 8.2, we generated parametric images of [11C]-PHNO nondisplaceable binding potential (BPND) at a voxel level for the whole brain via a basis function implementation of the simplified reference tissue model.15 The cerebellum was used as the reference tissue for nonspecific binding.15 For the spatial normalization of the parametric images, we also created summated images of the time series of [11C]-PHNO uptake scans collected 0 to 90 minutes after tracer administration, which reflect both tracer delivery and specific binding. Both summed dynamic images and parametric images of [11C]-PHNO uptake were spatially normalized into Montreal Neurologic Institute stereotaxic space with SPM8 software. The following procedure was followed: we spatially normalized the individual participant's MRI to the T1 MRI template available in SPM8. The transformation parameters were subsequently applied to the coregistered [11C]-PHNO images. A region-of-interest template for the hypothalamus was defined as previously described6 on an MRI scan transformed into standard Montreal Neurologic Institute space and used to sample individual normalized parametric images of [11C]-PHNO BPND and normalized MRI images with Analyze software (Mayo Clinic, Rochester, MN). Visual inspection of the normalized PET and MRI images was performed carefully to ensure correct placement of the object region over the hypothalamus. Any misalignment between [11C]-PHNO BPND images and the object map was manually corrected to ensure that the template was correctly placed over the hypothalamus in the parametric images. Finally, [11C]-PHNO BPND values were quantified with Analyze software.
Statistical analysis.
Statistical analyses were performed with Statistical Package for the Social Sciences (SPSS Inc, Chicago, IL), version 22. The Spearman ρ nonparametric statistic was used to assess clinical correlations between hypothalamic [11C]-PHNO BPND values and clinical scores. Statistical significance was set at p < 0.05 with Bonferroni correction for multiple comparisons.
RESULTS
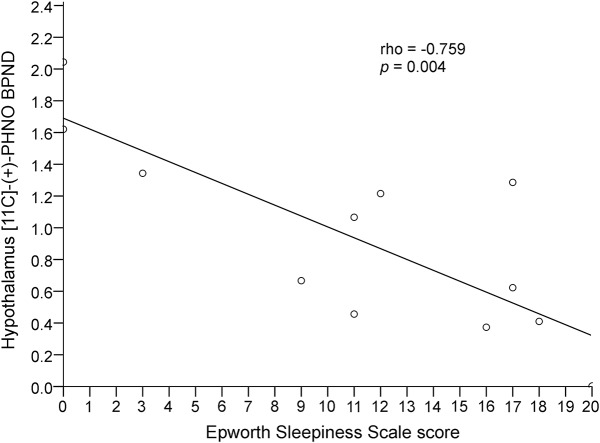
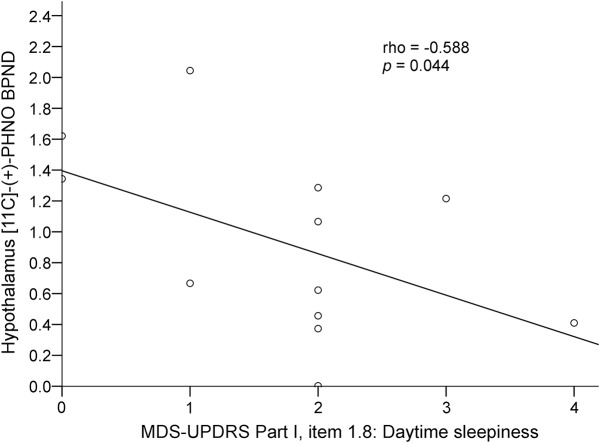
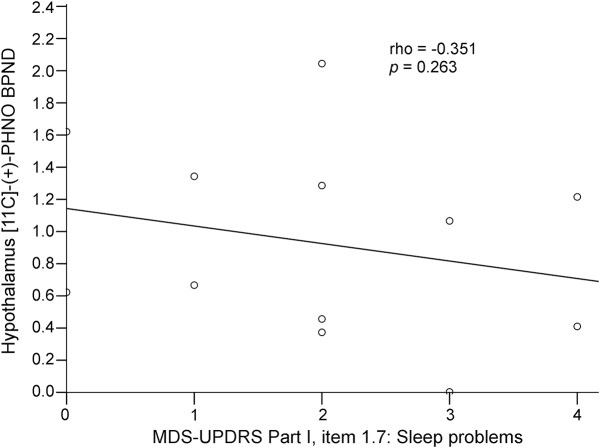
The 12 patients (7 male) included in the study had a mean ± SD age of 64.25 ± 7.3 years, disease duration of 7.5 ± 3.8 years, H&Y staging of 2.3 ± 1.4, UPDRS part III “off” score of 46 ± 18.7, UPDRS part III “on” score of 21.2 ± 13.4, and Non-Motor Symptoms Scale score of 54.7 ± 45.3. Nine patients were treated with levodopa and dopamine agonists, and 3 patients were treated with levodopa alone. Mean DLED was 670.7 ± 423.8. A correlation was found between regional mean hypothalamus [11C]-PHNO BPND values, ESS scores (ρ = −0.759, p = 0.004, figure 1), and MDS-UPDRS part I item 1.8 (daytime sleepiness) scores (ρ = −0.588, p = 0.04, figure 2) in patients with PD, indicating that lower [11C]-PHNO BPND values correlated with more severe daytime sleepiness. Hypothalamus [11C]-PHNO BPND values did not correlate with MDS-UPDRS part I item 1.7 (sleep problems) scores (ρ = −0.351, p = 0.263, figure 3) or with other nonmotor symptoms, age, disease duration, H&Y staging, UPDRS scores, and DLED. ESS scores, in addition to correlating with hypothalamus [11C]-PHNO BPND values, correlated with MDS-UPDRS part I item 1.8 (daytime sleepiness) scores (ρ = −0.766, p = 0.003) but not with age, disease duration, H&Y staging, UPDRS scores, and DLED.
Figure 1. Correlation between hypothalamus [11C]-propyl-hexahydro-naphtho-oxazin ([11C]-PHNO) nondisplaceable binding potential (BPND) and Epworth Sleepiness Scale.
Lower regional mean hypothalamus [11C]-PHNO BPND values correlated with higher Epworth Sleepiness Scale scores in patients with Parkinson disease.
Figure 2. Correlation between hypothalamus [11C]-propyl-hexahydro-naphtho-oxazin ([11C]-PHNO) nondisplaceable binding potential (BPND) and Movement Disorder Society Unified Parkinson's Disease Rating Scale (MDS-UPDRS) part I item 1.8 (daytime sleepiness).
Lower regional mean hypothalamus [11C]-PHNO BPND values correlated with higher scores on the MDS-UPDRS part I item 1.8 (daytime sleepiness) in patients with Parkinson disease.
Figure 3. Correlation between hypothalamus [11C]-propyl-hexahydro-naphtho-oxazin ([11C]-PHNO) nondisplaceable binding potential (BPND) and Movement Disorder Society Unified Parkinson's Disease Rating Scale (MDS-UPDRS) part I item 1.7 (sleep problems).
Lack of correlation between regional mean hypothalamus [11C]-PHNO BPND values and MDS-UPDRS part I item 1.7 (sleep problems) scores in patients with Parkinson disease.
DISCUSSION
Findings from our study suggest that, while EDS is associated with reduced postsynaptic dopaminergic receptor availability measured with [11C]-PHNO PET, this is not the case for sleep-onset and sleep-maintenance insomnia.
[11C]-PHNO binds primarily to the D3 receptor subtype, so the PET BPND values observed in this study most likely reflect dopamine D3 receptor availability. In nonhuman primates, [11C]-PHNO has shown a 10- to 20-fold higher affinity for the D3 over the D2 receptor in vivo, so a small percentage of the PET signal may also reflect D2 binding.16,17 When a selective D3 antagonist was administered in humans before [11C]-PHNO administration, a 100% BPND reduction was observed in the hypothalamus, suggesting that the accumulation of [11C]-PHNO in this structure represented D3 receptor binding.6
EDS is one of the most common sleep problems observed in PD, with a prevalence ranging from 15% to 50%.18–20 The mechanisms underlying EDS in patients with PD remain unclear. Possible risk factors for EDS in patients with PD include male sex, long disease duration, disease severity, and dopaminergic therapy.18,19,21 With regard to the influence of dopaminergic medication, several studies have now shown that increased daytime sleepiness or a condition mimicking narcolepsy22 develops after the introduction of dopaminergic treatment, particularly D3 agonists, in patients with PD, while there is no difference in prevalence of EDS in untreated patients with PD compared with healthy controls.23–25 These studies indicate that dopaminergic therapy plays a strong role in the emergence of EDS in PD. EDS in patients with PD who have never used dopamine agonists was found to be associated with H&Y stage23 and mood and autonomic dysfunction,25 suggesting that disease-related changes in sleep-wake regulation also contribute to EDS. This view is supported by a number of studies that have reported dysfunction of the dopaminergic, cholinergic, serotoninergic, and noradrenergic pathways26–29 and the orexin system30 within the brain arousal systems in patients with PD that could contribute to the occurrence of EDS. In line with these studies, we have recently reported that patients with PD with EDS showed significant decreases in [18F]-dopa, a marker of monoaminergic terminal function, and [11C]-DASB binding, a marker of serotonin transport availability, in sleep regulatory centers compared to age-matched healthy volunteers without sleep disorders.31
While our study suggests that EDS could be associated with changes in D3 dopamine receptor availability, it does not provide a primary cause for this reduction. It is possible that reductions of monoamine transmission within the key nuclei involved in sleep control are responsible for adaptive changes in dopamine receptor availability. Direct neuronal loss of dopamine receptor–expressing neurons within the hypothalamic dopaminergic networks is a possible alternative explanation. Finally, we cannot rule out a possible effect of chronic dopamine replacement on the expression of dopamine receptors in the hypothalamus. However, it should be noted that neither hypothalamic [11C]-PHNO PET BPND values nor ESS scores correlated with DLED, suggesting that, at least in this cohort of patients, EDS and hypothalamic dopamine D3 receptor availability were not related to medical treatment. On the other hand, it is possible that all these factors occur is a synergistic manner. Patients with underlying disease process in the brain arousal systems could be more susceptible to the side effect induced by dopamine replacement drugs, and this would explain why not all the patients on dopamine drugs develop EDS. Future studies should aim to explore the interactions between dopaminergic drugs and the neurodegenerative process in the wake-sleep regulatory system.
The mechanisms by which reduced hypothalamic dopamine D3 receptor availability could lead to EDS remain to be elucidated. In this study, we did not measure levels of orexin in the CSF of our patients. However, because dopamine appears to regulate the function of orexin cells,32,33 it is possible that changes in hypothalamic dopamine receptor availability might contribute to the dysfunction of the orexinergic system reported in patients with PD.30
Sleep-onset insomnia and sleep-maintenance insomnia are more common in patients with PD than in healthy controls.34–36 It is well recognized that in many cases these problems are secondary to poorly controlled motor symptoms at night (akinesia, tremor, rigidity, and wearing off) and nonmotor symptoms (psychosis, depression, hallucination, and pain). However, it is possible that sleep-onset insomnia and sleep-maintenance insomnia arise from midbrain pathology in PD. In our study, scores on the subitems of the MDS-UPDRS, which assess problems falling asleep and sleep-maintenance insomnia, did not correlate with hypothalamic [11C]-PHNO PET BPND values, suggesting that these symptoms in patients with PD are not related to changes in dopamine D3 receptors density. Changes in other brain neurotransmitters need to be evaluated in those patients with PD whose sleep-onset insomnia and sleep-maintenance insomnia are not caused by nocturnal motor/nonmotor complications or other age-related comorbidities.
Some aspects of this study need further consideration and must to be taken into account in the interpretation of the findings. We have not used polysomnography to characterize different types of sleep problems at night such as periodic limb movement during sleep and REM behavior disorder, which affect initiation and maintaining sleep at night. Therefore, the role of dopamine D3 receptor availability in patients with PD with these specific sleep-related disorders remains to be investigated in future studies. Similarly, it would be interesting to assess dopamine D3 receptor availability in patients with narcolepsy, a condition that has been suggested to share pathophysiologic mechanisms with EDS in patients with PD. We did not include a control group in this study because we had previously reported reduced dopamine D2/D3 receptor availability in patients with PD compared to healthy controls and suggested that this reduction could be responsible for sleep problems in PD.3 The main aim of this study was indeed to test that hypothesis in a cohort of patients with PD.
Finally, we believe that [11C]-PHNO binding in the hypothalamus is almost entirely attributable to the D3 receptor density. However, we acknowledge that changes in other dopamine receptor subtypes can also occur in this region in patients with PD and could contribute to the development of EDS.
This study provides evidence that lower dopamine D3 receptor availability in the hypothalamus may be associated with more severe EDS in patients with PD. However, because of the relatively small number of patients investigated and the lack of polysomnography, our results should still be considered preliminary, and further studies on this topic need to be performed to clarify this issue. Strategies to modulate hypothalamic dopamine D3 expression could be useful in the management of EDS in PD. This study also provides support for the emerging concept of noncognitive nonmotor subtypes of PD and, in particular, imaging surrogates to support the concept of sleep-dominant subtypes.5
GLOSSARY
- BPND
nondisplaceable binding potential
- [11C]-PHNO
[11C]-propyl-hexahydro-naphtho-oxazin
- DLED
daily levodopa equivalent dose
- EDS
excessive daytime sleepiness
- ESS
Epworth Sleepiness Scale
- H&Y
Hoehn and Yahr
- MDS-UPDRS
Movement Disorder Society Unified Parkinson's Disease Rating Scale
- PD
Parkinson disease
AUTHOR CONTRIBUTIONS
Dr. Pagano: study concept and design, acquisition of data, analysis and interpretation, statistical analysis and interpretation of data, and drafting of the manuscript. Dr. Molloy and Dr. Bain: critical revision of the manuscript for important intellectual content. Dr. Rabiner: acquisition of data, analysis and interpretation, and critical revision of the manuscript for important intellectual content. Prof. Chaudhuri and Prof. Brooks: analysis and interpretation and critical revision of the manuscript for important intellectual content. Dr. Pavese: study concept and design, acquisition of data, analysis and interpretation, study supervision, drafting of the manuscript, and final approval of the manuscript.
STUDY FUNDING
No targeted funding reported.
DISCLOSURE
G. Pagano, S. Molloy, P. Bain, E. Rabiner, K. Chaudhuri, and D. Brooks report no disclosures relevant to the manuscript. N. Pavese reports no conflict of interest. The Medical Research Council UK pays for part of the salary for Dr. Pavese's research activity. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Pavese N, Rivero-Bosch M, Lewis SJ, Whone AL, Brooks DJ. Progression of monoaminergic dysfunction in Parkinson's disease: a longitudinal 18F-dopa PET study. Neuroimage 2011;56:1463–1468. [DOI] [PubMed] [Google Scholar]
- 2.Moore RY, Whone AL, Brooks DJ. Extrastriatal monoamine neuron function in Parkinson's disease: an 18F-dopa PET study. Neurobiol Dis 2008;29:381–390. [DOI] [PubMed] [Google Scholar]
- 3.Politis M, Piccini P, Pavese N, Koh SB, Brooks DJ. Evidence of dopamine dysfunction in the hypothalamus of patients with Parkinson's disease: an in vivo 11C-raclopride PET study. Exp Neurol 2008;214:112–116. [DOI] [PubMed] [Google Scholar]
- 4.Sauerbier A, Jenner P, Todorova A, Chaudhuri KR. Non motor subtypes and Parkinson's disease. Parkinsonism Relat Disord 2016;22(suppl 1):S41–S46. [DOI] [PubMed] [Google Scholar]
- 5.Marras C, Chaudhuri KR. Nonmotor features of Parkinson's disease subtypes. Mov Disord 2016;31:1095–1102. [DOI] [PubMed] [Google Scholar]
- 6.Tziortzi AC, Searle GE, Tzimopoulou S, et al. Imaging dopamine receptors in humans with [11C]-(+)-PHNO: dissection of D3 signal and anatomy. Neuroimage 2011;54:264–277. [DOI] [PubMed] [Google Scholar]
- 7.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992;55:181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Folstein MF, Folstein SE, McHugh PR. Mini-Mental State: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 9.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry 1960;23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goetz CG, Tilley BC, Shaftman SR, et al. ; Movement Disorder Society UPDRS Revision Task Force. Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord 2008;23:2129–2170. [DOI] [PubMed] [Google Scholar]
- 11.Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep 1991;14:540–545. [DOI] [PubMed] [Google Scholar]
- 12.Högl B, Arnulf I, Comella C, et al. Scales to assess sleep impairment in Parkinson's disease: critique and recommendations. Mov Disord 2010;25:2704–2716. [DOI] [PubMed] [Google Scholar]
- 13.Chaudhuri KR, Martinez-Martin P, Brown RG, et al. The metric properties of a novel non-motor symptoms scale for Parkinson's disease: results from an international pilot study. Mov Disord 2007;22:1901–1911. [DOI] [PubMed] [Google Scholar]
- 14.Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord 2010;25:2649–2653. [DOI] [PubMed] [Google Scholar]
- 15.Gunn RN, Lammertsma AA, Hume SP, Cunningham VJ. Parametric imaging of ligand-receptor binding in PET using a simplified reference region model. Neuroimage 1997;6:279–287. [DOI] [PubMed] [Google Scholar]
- 16.Rabiner EA, Slifstein M, Gunn RN, et al. Contribution of D3 receptors to the in vivo binding of [11C]PHNO in the primate brain. Neuroimage 2008;41(suppl 2):T39. [Google Scholar]
- 17.Rabiner EA, Slifstein M, Nobrega J, et al. In vivo quantification of regional dopamine-D3 receptor binding potential of (+)-PHNO: studies in non-human primates and transgenic mice. Synapse 2009;63:782–793. [DOI] [PubMed] [Google Scholar]
- 18.Ondo WG, Dat Vuong K, Khan H, Atassi F, Kwak C, Jankovic J. Daytime sleepiness and other sleep disorders in Parkinson's disease. Neurology 2001;57:1392–1396. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki K, Miyamoto T, Miyamoto M, et al. Excessive daytime sleepiness and sleep episodes in Japanese patients with Parkinson's disease. J Neurol Sci 2008;271:47–52. [DOI] [PubMed] [Google Scholar]
- 20.Tandberg E, Larsen JP, Karlsen K. Excessive daytime sleepiness and sleep benefit in Parkinson's disease: a community-based study. Mov Disord 1999;14:922–927. [DOI] [PubMed] [Google Scholar]
- 21.Tan EK, Lum SY, Fook-Chong SM, et al. Evaluation of somnolence in Parkinson's disease: comparison with age- and sex-matched controls. Neurology 2002;58:465–468. [DOI] [PubMed] [Google Scholar]
- 22.Ylikoski A, Martikainen K, Sarkanen T, Partinen M. Parkinson's disease and narcolepsy-like symptoms. Sleep Med 2015;16:540–544. [DOI] [PubMed] [Google Scholar]
- 23.Kaynak D, Kiziltan G, Kaynak H, Benbir G, Uysal O. Sleep and sleepiness in patients with Parkinson's disease before and after dopaminergic treatment. Eur J Neurol 2005;12:199–207. [DOI] [PubMed] [Google Scholar]
- 24.Bušková J, Klempíř J, Majerová V, et al. Sleep disturbances in untreated Parkinson's disease. J Neurol 2011;258:2254–2259. [DOI] [PubMed] [Google Scholar]
- 25.Simuni T, Caspell-Garcia C, Coffey C, et al. ; PPMI Sleep Working Group on behalf of the PPMI Investigators. Correlates of excessive daytime sleepiness in de novo Parkinson's disease: a case control study. Mov Disord 2015;30:1371–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Happe S, Baier PC, Helmschmied K, Meller J, Tatsch K, Paulus W. Association of daytime sleepiness with nigrostriatal dopaminergic degeneration in early Parkinson's disease. J Neurol 2007;254:1037–1043. [DOI] [PubMed] [Google Scholar]
- 27.Hilker R, Razai N, Ghaemi M, et al. [18F]fluorodopa uptake in the upper brainstem measured with positron emission tomography correlates with decreased REM sleep duration in early Parkinson's disease. Clin Neurol Neurosurg 2003;105:262–269. [DOI] [PubMed] [Google Scholar]
- 28.Kotagal V, Albin RL, Müller ML, et al. Symptoms of rapid eye movement sleep behavior disorder are associated with cholinergic denervation in Parkinson disease. Ann Neurol 2012;71:560–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsui H, Nishinaka K, Oda M, et al. Excessive daytime sleepiness in Parkinson disease: a SPECT study. Sleep 2006;29:917–920. [DOI] [PubMed] [Google Scholar]
- 30.Fronczek R, Overeem S, Lee SY, et al. Hypocretin (orexin) loss in Parkinson's disease. Brain 2007;130:1577–1585. [DOI] [PubMed] [Google Scholar]
- 31.Pavese N, Metta V, Simpson BS, et al. Sleep regulatory centres dysfunction in Parkinson's disease patients with excessive daytime sleepiness: an in vivo PET study. Parkinsonism Relat Disord 2012;18(suppl 2):S24–S25.22166445 [Google Scholar]
- 32.Li Y, van den Pol AN. Direct and indirect inhibition by catecholamines of hypocretin/orexin neurons. J Neurosci 2005;25:173–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baldo BA, Daniel RA, Berridge CW, Kelley AE. Overlapping distributions of orexin/hypocretin- and dopamine-beta-hydroxylase immunoreactive fibers in rat brain regions mediating arousal, motivation, and stress. J Comp Neurol 2003;464:220–237. [DOI] [PubMed] [Google Scholar]
- 34.Chaudhuri KR, Pal S, DiMarco A, et al. The Parkinson's disease sleep scale: a new instrument for assessing sleep and nocturnal disability in Parkinson's disease. J Neurol Neurosurg Psychiatry 2002;73:629–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suzuki K, Okuma Y, Hattori N, et al. Characteristics of sleep disturbances in Japanese patients with Parkinson's disease: a study using Parkinson's disease sleep scale. Mov Disord 2007;22:1245–1251. [DOI] [PubMed] [Google Scholar]
- 36.Suzuki K, Miyamoto M, Miyamoto T, Hirata K. Parkinson's disease and sleep/wake disturbances. Curr Neurol Neurosci Rep 2015;15:8. [DOI] [PubMed] [Google Scholar]