Abstract
Treatment goals for epilepsy are no seizures, no side effects, as soon as possible, but these goals are too often unmet. Approximately 1 million people in the United States continue to have seizures despite adequate treatment with antiseizure drugs, representing 40% of those with epilepsy, and 80% of the cost of epilepsy. Drug-resistant epilepsy (DRE) can be associated with developmental delay in infants and young children, and severe disability and morbidity in older children and adults, as well as a mortality rate 5–10 times that of the general population. While diagnosis and treatment at a full-service (levels 3 and 4) epilepsy center are demonstrated to improve seizure control, fewer than 1% of people with DRE are referred, and those who are, are referred an average of over 20 years after onset of habitual seizures. A possible reason for this is the misconception that all these epilepsy centers offer is surgery. Specialized multidisciplinary teams, consisting of neurologists, clinical neurophysiologists, neurosurgeons, neuroradiologists, psychologists, psychiatrists, social workers, and counselors, which constitute full-service epilepsy centers, can recognize and address pseudopharmacoresistance due to nonadherence, seizures that are not epilepsy, treatable underlying conditions, misdiagnosis of epilepsy syndromes, treatment with the wrong drug or wrong dosage, and lifestyle issues that are remediable. A variety of alternative treatment approaches are offered in addition to surgery, and for patients who continue to have seizures, full-service epilepsy centers have psychologists, psychiatrists, social workers, and counselors specialized in recognizing, and addressing, the psychological and social challenges experienced by people with epilepsy. Surgery for epilepsy remains, arguably, the most underutilized of all acceptable medical interventions, and the reasons for this are unclear. Often, excellent surgical candidates are not recognized as such by general neurologists, but if more patients with DRE were referred to full-service epilepsy centers, more surgical candidates would be identified by epilepsy specialists. All patients with medication-resistant epilepsy, defined as failure of 2 appropriate trials of antiseizure drugs due to inefficacy and not intolerance, who continue to be compromised by seizures deserve a timely consultation at a full-service epilepsy center. Early referral provides the best opportunity to avoid irreversible psychological and social problems, a lifetime of disability, and premature death.
The treatment goals for epilepsy are no seizures, no side effects, as soon as possible, but these goals are too often unmet. Promising basic and clinical research will continue to improve our ability to diagnose and treat the causes of drug-resistant epilepsy (DRE), and public health obstacles need to be resolved for the 80% of people with epilepsy in the developing world; however, the greatest progress in improving quality of life for those patients who live in industrialized countries could be made now, if neurologists would identify pharmacoresistance early, and refer for consultation at full-service epilepsy centers that include a team of multidisciplinary epilepsy specialists (level 3 or 4 as defined by the National Association of Epilepsy Centers [NAEC])1 (tables 1 and 2). Although I have wanted to strongly promote this simple message for some time, I have been hesitant to do so because I have a serious conflict of interest: I am the director of a level 4 epilepsy center. There is no way I can continue this discussion without seeming self-serving and critical of the neurologic community. I ask readers to consider what I have to say with an open mind and not to be too judgmental.
Table 1.
Third-level epilepsy centers
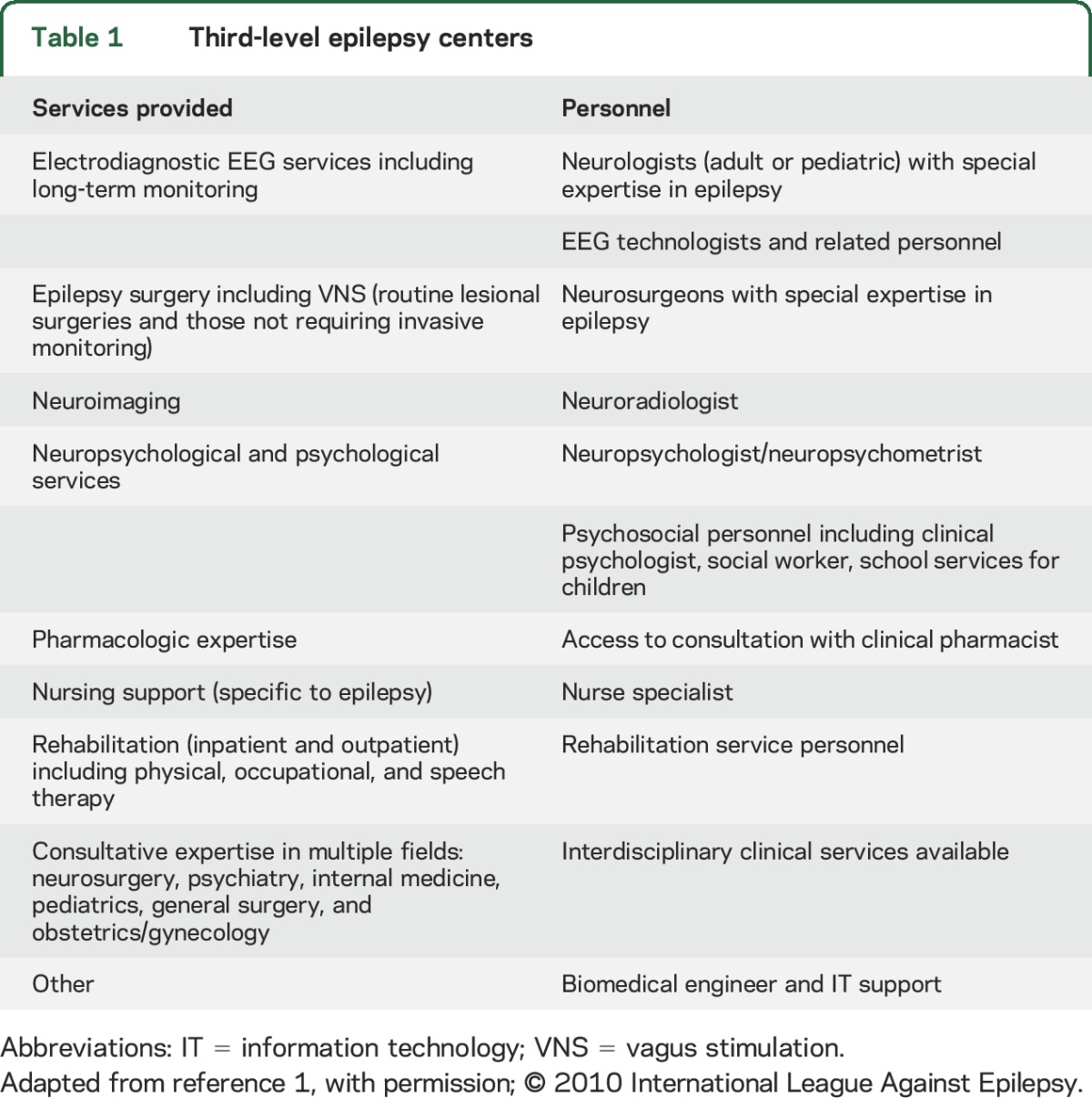
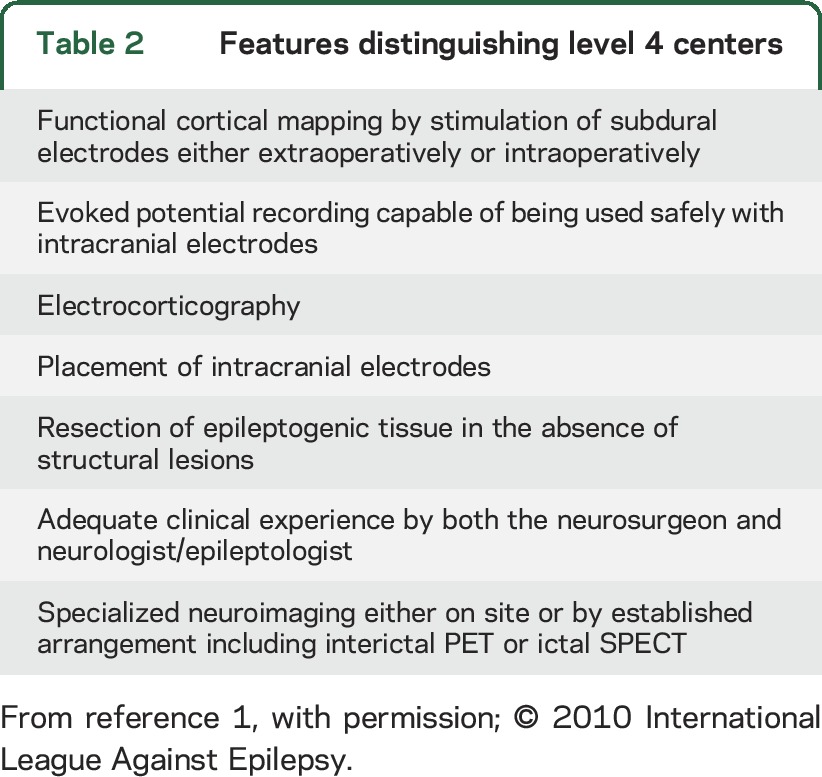
Table 2.
Features distinguishing level 4 centers
The following is a position statement and should not be taken as a comprehensive literature review. The principal issues presented are as follows: (1) DRE is a serious problem—it constitutes 40% of people with epilepsy and is associated with a mortality rate 5–10 times that of the general population; (2) fewer than 1% of people with DRE are evaluated at a full-service epilepsy center; (3) full-service epilepsy centers offer more than just surgery; and (4) surgical treatment for epilepsy remains severely underutilized. The overriding message is that all patients who continue to be compromised by seizures, after failure of 2 appropriate antiseizure medication trials, deserve a consultation at a specialized full-service epilepsy center, as recommended by the American Academy of Neurology (AAN) Epilepsy Quality Measurement Set.2 Early referral provides the best opportunity to avoid irreversible psychological and social problems, a lifetime of disability, and premature death.
DRE is a serious problem.
People who live a normal lifespan have a 5% to 10% risk of experiencing at least one seizure, and one-third of these will develop epilepsy.3 In the United States, 40% of people with epilepsy will continue to have seizures despite adequate treatment with antiseizure drugs,4 and they are responsible for 80% of the cost of epilepsy.5 According to the WHO, epilepsy accounts for 1% of the global burden of disease, more than breast cancer in women and almost as much as lung cancer in men.6
Adverse consequences of DRE in infants and small children include epileptic encephalopathies with developmental delay, often leading to institutionalization. Frequent seizures in this age group can be life-threatening. In older children, adolescents, and young adults, DRE prevents acquisition of vocational and interpersonal skills, leading to dependence on family and society and a lifetime of disability. Interictal behavioral problems such as depression, and neurologic impairment such as memory loss, are common, with increased morbidity, and a mortality rate 5–10 times that of the general population, due primarily to sudden unexpected death in epilepsy, accidents, and suicide.7
Despite the introduction of over 20 new antiseizure drugs over the past several decades, the proportion of patients with DRE has not changed appreciably. This indicates that the new drugs are treating the same population of patients as the old ones, albeit with different side effect profiles, which make them useful. Patients with DRE, however, must represent a different population, which requires new pharmacologic approaches. Current basic research is focused on identifying unique targets for pharmacotherapy of DRE. In the meantime, it is the responsibility of the clinical community to address the urgent needs of this patient population now.
Given the large number of antiseizure drugs available today, it would literally take multiple lifetimes to carry out an appropriate trial of each one in high-dose monotherapy, and in all conceivable combinations, in any given patient. Consequently, it is no longer practical to prove that a patient has epilepsy that is absolutely refractory to all antiseizure medications. As early intervention is essential to avoid the development of irreversible adverse consequences of continuing disabling seizures, the International League against Epilepsy has proposed that “drug-resistant epilepsy is defined as a failure of adequate drug trials of 2 tolerated appropriately chosen and used antiepileptic drugs (whether as monotherapy or in combination) to achieve sustained seizure freedom.”8 This definition results from prospective evidence that only 11% of patients eventually become seizure-free after failure of the first antiseizure drug trial, and only 3% after failure of the second, due to inefficacy and not intolerance.9
Studies indicating that the failure of the first 2 antiseizure medication trials is 97% reliable in identifying DRE demonstrate that there is a population of patients resistant to antiseizure drug therapy from the start. There is, however, another population of patients who respond initially to medication, but develop pharmacoresistance over time. One retrospective study of patients who underwent surgery at 7 different epilepsy surgery centers found that it took an average of 9 years to determine failure of 2 drugs.10 This observation does not mean that treating physicians were lax in beginning a second drug, but, rather, that patients were initially pharmacoresponsive and later developed pharmacoresistance, indicating that some forms of DRE represent a progressive epileptogenic disease process.
In addition to pharmacoresistance from the start, and progressive pharmacoresistance, there is a third type that I will call pseudopharmacoresistance. This refers to patients who have epileptic seizures that appear to be pharmacoresistant, but on more detailed evaluation, an effective approach to pharmacotherapy can eventually render the patient seizure-free, or in some cases the patients do not have epilepsy at all. One-third of admissions to most epilepsy monitoring units are for nonepileptic seizures. Better education of primary care clinicians about epilepsy might improve the recognition of pharmacoresistance in the community.
A simple triage of the epilepsies divides them into those that are easily treated and the more severe epilepsies, which may be remediable or nonremediable. Remediable severe epilepsies are those that require specialized diagnostic and therapeutic approaches available at full-service epilepsy centers—these are the patients with pseudopharmacoresistance, as well as those who are surgical candidates. The remainder have nonremediable epilepsy and require psychological and social supportive care. A distinction between remediable and nonremediable severe epilepsy disorders must be made quickly to avoid irreversible adverse consequences, and usually requires referral to an epilepsy center. A general rule of thumb should be that, if the first 2 antiseizure drug trials fail, and seizures are interfering with school, work, or interpersonal relationships, or there is developmental delay in infants or young children, refer to an epilepsy center. The patient may not have pharmacoresistant seizures, or may have a surgically remediable syndrome.
Fewer than 1% of people with DRE are evaluated at an epilepsy center.
Given that somewhat less than 1% of the United States population has active epilepsy, and 40% of these continue to have seizures, despite appropriate trials of 2 antiseizure drugs, approximately 1 million people in the United States have DRE. According to the NAEC, fewer than 2,000 patients undergo surgery annually,11 which may reflect about one-quarter of those patients referred to full-service epilepsy centers (including those with nonepileptic seizures). The total referred, therefore, is well under 1% of those with DRE. Whether or not this figure is taken literally, even if it is off by an order of magnitude, it indicates that the vast majority of people with DRE are not given the benefit of evaluation by a specialized multidisciplinary team of epilepsy experts. Furthermore, based on retrospective studies of patients who undergo surgical treatment, when patients are referred to epilepsy centers for DRE, they are referred an average of over 20 years after onset,10 often too late to prevent or reverse the disabling consequences of seizures, or to affect quality of life.
There have been a number of reports in recent years documenting the fact that only a very small proportion of patients who are potential surgical candidates are referred to full-service epilepsy centers, and within some of these studies there are data demonstrating that an even smaller proportion of patients with DRE who might otherwise benefit from evaluation by a multidisciplinary team of epilepsy experts at a full-service epilepsy center have this opportunity. One such study of hospital referrals in the United States for DRE between 1999 and 2008 found increasing referrals to low-volume hospitals and decreasing referrals to full-service epilepsy centers over this time period.12 Another, more recent Canadian study utilizing the Ontario provincial database between 2001 and 2010 found that 10,661 patients, with substantial comorbidities, on financial assistance, failed at least 2 trials of antiseizure drugs, and therefore had DRE. Only 124 underwent surgery within 2 years, and 12% of those who did not receive surgery died.13 It is not clear, however, how these data might apply to the general population of people with epilepsy.
One explanation offered for the recent reduction in referrals to full-service epilepsy centers is that the incidence of DRE is decreasing.14 It seems unreasonable, however, to draw any conclusions regarding the total population of patients with DRE, based on the extremely small percentage who are referred to epilepsy centers. A recent study in Finland utilizing long-term national hospital records that captured all patients with new-onset epilepsy found no change in the incidence of epilepsy between 1973 and 2013 in people younger than 65 and a nearly 5-fold increase after the age of 65.15 Given that there also is no change in the percentage of patients with epilepsy who are pharmacoresistant, if Finland is representative of industrialized countries, including the United States, this should put to rest any suggestion that a reduction in referral to epilepsy centers reflects a reduction in the DRE population.
More recently, a study of data derived from the Centers for Medicare and Medicaid Services Part B, and the American College of Surgeons, gathered between 2000 and 2013, indicated that a decrease in surgeries performed at high-volume hospitals (presumably full-service epilepsy centers) over this time was compensated by an increase in surgeries performed at low-volume hospitals (presumably not epilepsy centers), suggesting no change in utilization.16 Importantly, the increase in surgeries at low-volume hospitals was associated with an increase in adverse major and minor complications, including mortality.
A randomized controlled trial (RCT) of surgery for temporal lobe epilepsy (TLE) carried out at the University of Western Ontario was published in 2001.17 Sixty-four percent of patients who had surgery were seizure-free after 1 year, compared to only 8% in the medical arm, and there was 1 death, which occurred in the medical arm. The AAN, in association with the American Epilepsy Society and the American Association of Neurological Surgeons, subsequently issued a practice parameter,18 based on this study and 24 Class IV series of 1,952 patients who underwent surgery for TLE. Sixty-seven percent in the Class IV series were seizure-free, compared to a drug trial meta-analysis, where the best result was 54% with a greater than 50% seizure reduction and very few seizure-free.19 The conclusion was that patients with disabling limbic seizures who have failed appropriate trials of first-line antiseizure drugs should be referred to an epilepsy center (Level A rating), and that patients referred to an epilepsy center for these reasons who meet established criteria for surgery should be offered surgical treatment (Level A rating). Subsequently, 2 studies found that the delay from onset of TLE to referral for surgery, measured for a period of time before, and after, the RCT and practice parameter, did not change.20,21
The reluctance on the part of neurologists, and their patients with DRE, to consider surgery as a viable alternative treatment is an understandable longstanding concern; however, the reluctance to refer to an epilepsy center for a consultation that could lead to other beneficial therapeutic outcomes very likely results, at least in part, from the misconception that all epilepsy centers offer is surgery. In this case, it is unfortunate when neurologists reason that “My patient is not a surgical candidate or does not want surgery, and therefore there is no reason to refer him or her to an epilepsy center.” In fact, there are many other excellent reasons to request a consultation with a multidisciplinary team of experts in the diagnosis and treatment of epilepsy. Validated web-based tools designed to help general neurologists identify surgical candidates22 only serve to perpetuate the counterproductive belief that epilepsy centers only do surgery.
Epilepsy centers offer more than just surgery.
Many patients who appear to have DRE in the community improve, and actually can become seizure-free, as a result of specialized diagnostic and therapeutic approaches, other than surgery, offered by full-service epilepsy centers. At least one retrospective study has documented this fact for the general population,23 and another showed the benefit of specialized epilepsy consultations for institutionalized patients with mental retardation.24 Common causes of pseudopharmacoresistance include nonadherence, seizures that are not epileptic, failure to identify a treatable underlying cause, misdiagnosis of the epilepsy condition, treatment with the wrong drug or the wrong dosage, and lifestyle issues such as substance abuse or sleep deprivation. Epilepsy monitoring units, along with multidisciplinary teams of specialists at full-service epilepsy centers, can identify specific epilepsy syndromes; recognize nonepileptic seizures, particularly psychogenic nonepileptic seizures (PNES); diagnose underlying treatable causes that may not be apparent in the community; employ specialized pharmacologic approaches, including experimental drug trials; and consider alternative treatments. In addition to experimental drug trials, changes in the timing and combination of medications tailored to the patient's particular seizure type, and sensitivity to side effects, can greatly improve efficacy and tolerance, and render some patients with apparent DRE seizure-free. There are a number of stimulation approaches, including vagus nerve stimulation25; trigeminal nerve stimulation (TNS),26 where the device does not need to be implanted and can be used overnight and removed during the day; responsive neurostimulation,27 where a computer is imbedded in the skull that is connected to electrodes in the brain used to detect ictal onset and stimulate to abort the behavioral seizure; and deep brain stimulation (DBS).28 TNS and DBS are currently available in Europe, but not yet in the United States. Other approaches, such as the ketogenic diet or modified Atkins diet, which can be extremely effective in some patients,29 and various complementary and behavioral approaches,30 are available at most full-service epilepsy centers. Also, in addition to the standard surgical approaches, laser thermal ablation,31 a new, less invasive technique performed through a small drill hole, is particularly suited for small epileptogenic regions that are difficult to reach, such as hypothalamic hamartomas, and for patients with medical contraindications to standard open surgery.
Realistically, most patients with DRE have nonremediable epilepsy, and, for these patients, although evaluation at a full-service epilepsy center may greatly decrease the frequency and severity of their ictal events, they continue to be disabled by recurrent epileptic seizures. A major contribution of epilepsy centers for these patients, however, is access to programs that utilize the expertise of psychologists, psychiatrists, social workers, and counselors, specialized in recognizing, and potentially resolving, the psychological and social challenges experienced by people with epilepsy and PNES. People with epilepsy come to physicians not because they have seizures, but because the seizures are interfering with their lives.32 Although the first approach to address the patient's complaint is to eliminate the seizures, when this is not possible, there is much else that can be done to lessen the adverse impact of seizures on quality of life. Furthermore, even when seizure freedom is achieved, there can remain psychological and social issues that need to be addressed.
Surgery remains a severely underutilized alternative treatment.
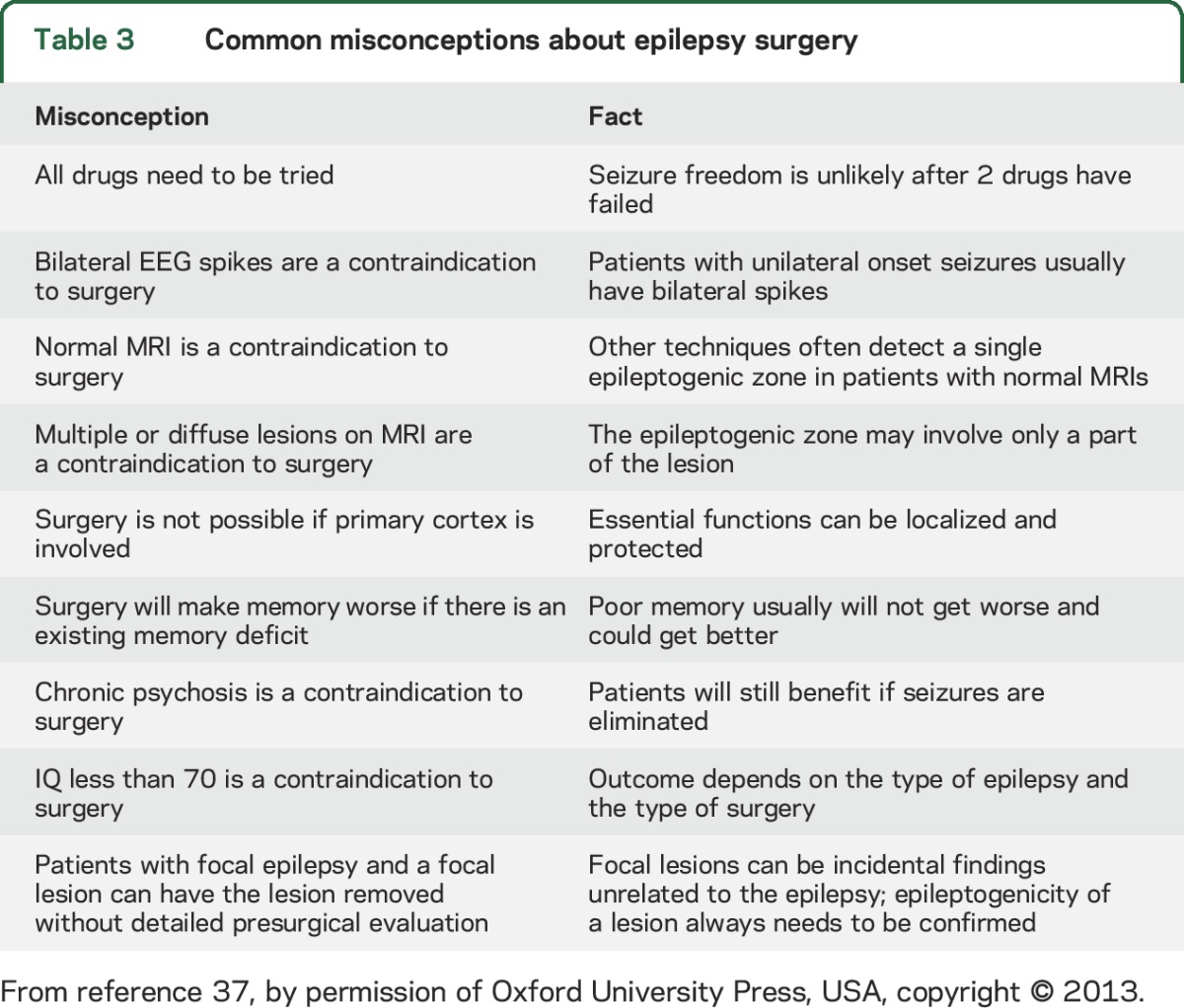
There continue to be tremendous advances in presurgical diagnosis, as well as surgical approaches, that have improved the efficacy and safety of surgical therapy,33 but to dwell on these would detract from the major point of this article, that epilepsy centers offer more than surgery. However, it is worthwhile to consider common misconceptions (table 3) responsible for failure to recognize and refer excellent surgical candidates. Some reasons offered for reluctance to consider surgery include the following: (1) fear of surgery, although the mortality rate from surgery is close to zero, while that for DRE is 5–10 times that of the general population7; (2) lack of information about improvements in safety and efficacy, although there have been hundreds of articles and over 20 books published in the last 15 years; (3) expense, although if performed early, the cost of epilepsy surgery is considerably less than the cost of a lifetime of disability34; and (4) in the past, there was concern that there had never been an RCT of epilepsy surgery, although an RCT of TLE was published in the New England Journal of Medicine in 2001,17 as discussed above, and another RCT was published in the Journal of the American Medical Association in 2012.35
Table 3.
Common misconceptions about epilepsy surgery
This latter study, the Early Randomized Surgical Epilepsy Trial, differed from the Western Ontario trial in that patients were recruited within 2 years after failure of 2 antiseizure drug trials, and underwent presurgical evaluation to confirm that they were surgical candidates prior to randomization. There were 38 participants (18 men) and, with an intention-to-treat analysis, 11 of 15 (73%) in the surgical arm were seizure-free after 2 years compared to none in the medical arm. With analysis of data limited to patients who completed the study, 11 of 13 (85%) in the surgical arm were seizure-free compared to none in the medical arm. Quality of life and socialization were significantly better in the surgical group, but the sample size was too small to assess effects on cognitive function, particularly memory. There were 3 hospitalizations for status epilepticus in the medical group, and one participant in the surgical group experienced a stroke with a transient neurologic deficit.
Memory decline continues to be a legitimate concern with temporal lobe surgery that involves removal of the hippocampus. Patients rarely, if ever, notice a memory decline with surgery of the non-language-dominant temporal lobe, even when visual-spatial problems can be demonstrated with specialized neurocognitive testing. Also, patients rarely complain of memory decline if they already have impaired memory at the time of surgery, and some actually experience improvement. Memory decline is a concern, however, in patients who undergo surgery of the language-dominant hemisphere, and who have normal verbal memory at time of surgery. In one study36 of 138 patients with 2- and 5-year follow-up, 113 (82%) were in remission and quality of life improved whether or not memory declined, while 25 (18%) were not in remission. Of the latter, 14 (10%) had no memory decline and quality of life was stable, while 11 (8%) experienced memory decline and quality of life also declined. This suggests that memory decline appears to be a fair tradeoff for seizure freedom, and is detrimental only when seizures persist after surgery. Nevertheless, careful consideration should be given to resection of the hippocampus in the language-dominant hemisphere for patients who do not already have a verbal memory deficit, and who are dependent on verbal memory for their work or lifestyle. On the other hand, it can be argued that memory decline will eventually occur without surgery in most patients, if seizures continue.
DISCUSSION
Treatment objectives for epilepsy are as follows: no seizures, no side effects, as soon as possible. Pharmacoresistant epilepsy remains a serious health burden; 40% of all people with epilepsy continue to be compromised by seizures despite adequate antiseizure medications. Fewer than 1% are referred to a full-service epilepsy center, and those who are, are referred more than 20 years after onset. Full-service epilepsy centers do more than surgery; many patients who appear to be pharmacoresistant are not, or do not have epilepsy at all; and specialized programs that address the psychological and social consequences of epilepsy can greatly improve quality of life for patients whose seizures are truly nonremediable. All people who continue to be compromised by seizures after failure of 2 appropriate trials of antiseizure drugs deserve a timely consultation with a multidisciplinary team of epilepsy experts at a full-service epilepsy center, many are not drug-resistant, some do not have epilepsy, some are surgical candidates, and the remainder benefit from the broad range of support services available at these centers. Early referral to a full-service epilepsy center provides the best opportunity to avoid irreversible psychological and social problems, a lifetime of disability, and premature death.
ACKNOWLEDGMENT
The author thanks Drs. Nathan Fountain, John Stern, and Sam Wiebe for comments on the manuscript.
GLOSSARY
- AAN
American Academy of Neurology
- DBS
deep brain stimulation
- DRE
drug-resistant epilepsy
- NAEC
National Association of Epilepsy Centers
- PNES
psychogenic nonepileptic seizures
- RCT
randomized controlled trial
- TLE
temporal lobe epilepsy
- TNS
trigeminal nerve stimulation
STUDY FUNDING
Original research reported by the author was supported in part by grants NS-02808, NS-15654, NS-33310, and NS-42372.
DISCLOSURE
J. Engel is director of a level 4 epilepsy center. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Labiner DM, Bagic AI, Herman ST, et al. Essential services, personnel, and facilities in specialized epilepsy centers: revised 2010 guidelines. Epilepsia 2010;51:2322–2333. [DOI] [PubMed] [Google Scholar]
- 2.Fountain NB, Van Ness PC, Bennett A, et al. Quality improvement in neurology: epilepsy update quality measurement set. Neurology 2015;84:1483–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hauser WA, Hesdorffer DC. Epilepsy: Frequency, Causes and Consequences. New York: Demos Press; 1990. [Google Scholar]
- 4.Kobau R, Zahran H, Thurman DJ, et al. Epilepsy surveillance among adults: 19 states. MMWR Surveill Summ 2008;57:1–20. [PubMed] [Google Scholar]
- 5.Begley CE, Famulari M, Annegers JF, et al. The cost of epilepsy in the United States: an estimate from population-based clinical and survey data. Epilepsia 2000;41:342–351. [DOI] [PubMed] [Google Scholar]
- 6.Murray CJL, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2197–2223. [DOI] [PubMed] [Google Scholar]
- 7.Sperling MR, Barshow S, Nei M, AsadiPooya AA. A reappraisal of mortality after epilepsy surgery. Neurology 2016;86:1938–1944. [DOI] [PubMed] [Google Scholar]
- 8.Kwan P, Arzimanoglou A, Berg AT, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on therapeutic strategies. Epilepsia 2010;51:1069–1077. [DOI] [PubMed] [Google Scholar]
- 9.Kwan P, Brodie MJ. Early identification of refractory epilepsy. New Engl J Med 2000;342:314–319. [DOI] [PubMed] [Google Scholar]
- 10.Berg AT, Langfitt J, Shinnar S, et al. How long does it take for partial epilepsy to become intractable? Neurology 2003;60:186–190. [DOI] [PubMed] [Google Scholar]
- 11.Kaiboriboon K, Malkhachroum AM, Zrik A, et al. Epilepsy surgery in the United States: analysis of data from the national association of epilepsy centers. Epilepsy Res 2015;116:105–109. [DOI] [PubMed] [Google Scholar]
- 12.Englot DJ, Ouyang D, Garcia PA, Barbaro NM, Chang EF. Epilepsy surgery trends in the United States, 1990–2008. Neurology 2012;78:1200–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burneo JG, Shariff SZ, Liu K, et al. Disparities in surgery among patients with intractable epilepsy in a universal health system. Neurology 2016;86:72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jehi L, Friedman D, Carlson C, et al. The evolution of epilepsy surgery between 1991 and 2011 in nine major epilepsy centers across the United States, Germany, and Australia. Epilepsia 2015;56:1526–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sillanpää M, Gissler M, Schmidt PD. Efforts in epilepsy prevention in the last 40 years: lessons from a large nationwide study. JAMA Neurol 2016;73:390–395. [DOI] [PubMed] [Google Scholar]
- 16.Rolston JD, Englot DJ, Knowlton RC, Chang EF. Rate and complications of adult epilepsy surgery in North America: analysis of multiple databases. Epilepsy Res 2016;124:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wiebe S, Blume WT, Girvin JP, Eliasziw M. A randomized, controlled trial of surgery for temporal lobe epilepsy. N Engl J Med 2001;345:311–318. [DOI] [PubMed] [Google Scholar]
- 18.Engel J Jr, Wiebe S, French J, et al. Practice parameter: temporal lobe and localized neocortical resections for epilepsy. Neurology 2003;60:538–547. [DOI] [PubMed] [Google Scholar]
- 19.Cramer JA, Fisher R, Ben-Menachem E, French JA, Mattson RH. New antiepileptic drugs: comparison of key clinical trials. Epilepsia 1999;40:590–600. [DOI] [PubMed] [Google Scholar]
- 20.Choi H, Carlino R, Heiman G, Hauser WA, Gilliam FG. Evaluation of duration of epilepsy prior to temporal lobe epilepsy surgery during the past two decades. Epilepsy Res 2009;86:224–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haneef Z, Stern J, Dewar S, Engel J Jr. Referral pattern for epilepsy surgery after evidence-based recommendations: a retrospective study. Neurology 2010;75:699–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jette N, Quan H, Tellez-Zenteno JF, Macrodimitris S, et al. Development of an online tool to determine appropriateness for an epilepsy surgery evaluation. Neurology 2012;79:1084–1093. [DOI] [PubMed] [Google Scholar]
- 23.Szaflarski JP, Rackley AY, Lindsell CJ, Szaflarski M, Yates SL. Seizure control in patients with epilepsy: the physician vs. medication factors. BMC Health Serv Res 2008;8:264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arain A, Shihabuddin B, Niaz F, et al. Epilepsy and the impact of an epileptology clinic for patients with mental retardation and associated disabilities in an institutional setting. Epilepsia 2006;47:2052–2057. [DOI] [PubMed] [Google Scholar]
- 25.Morris GL III, Gloss D, Buchhalter J, et al. Evidence-based guideline update: vagus nerve stimulation for the treatment of epilepsy. Neurology 2013;81:1453–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeGiorgio CM, Soss J, Cook IA, et al. Randomized controlled trial of trigeminal nerve stimulation for drug-resistant epilepsy. Neurology 2013;80:786–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morrell M; RNS System in Epilepsy Study Group. Responsive-cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology 2011;77:1295–1304. [DOI] [PubMed] [Google Scholar]
- 28.Fisher R, Salanova V, Witt T, et al. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia 2010;51:899–908. [DOI] [PubMed] [Google Scholar]
- 29.Kossoff EH, Haney CA. Ketogenic diets. In: Shorvon S, Perucca E, Engel J Jr, eds. The Treatment of Epilepsy, 4th ed. Oxford: Wiley; 2016:288–297. [Google Scholar]
- 30.Baxendale S. Complementary and alternative treatments for epilepsy. In: Shorvon S, Perucca E, Engel J Jr, eds. The Treatment of Epilepsy, 4th ed. Hoboken, NJ: Wiley-Blackwell; 2016:298–310. [Google Scholar]
- 31.Drane DL, Loring DW, Voets NL, et al. Better object recognition and naming outcome with MRI-guided stereotactic laser amygdalohippocampectomy for temporal lobe epilepsy. Epilepsia 2015;56:101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor D. Epilepsy as a chronic sickness: remediating its impact. In: Engel J Jr, ed. Surgical Treatment of the Epilepsies, 2d ed. Ch 2. New York: Raven Press; 1993:11–22. [Google Scholar]
- 33.Engel J Jr. Overview of surgical treatment for epilepsy. In: Shorvon S, Perucca E, Engel J Jr, eds. The Treatment of Epilepsy, 4th ed. London: Wiley-Blackwell; 2015:709–722. [Google Scholar]
- 34.Wiebe S, Gafni A, Blume WT, Girvin JP. An economic evaluation of surgery for temporal lobe epilepsy. J Epilepsy 1995;8:227–235. [Google Scholar]
- 35.Engel J Jr, McDermott MP, Wiebe S, et al. Early surgical therapy for drug-resistant temporal lobe epilepsy: a randomized trial. JAMA 2012;307:922–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Langfitt JT, Westerveld M, Hamberger MJ, et al. Worsening of quality of life after epilepsy surgery: effect of seizures and memory decline. Neurology 2007;68:1988–1994. [DOI] [PubMed] [Google Scholar]
- 37.Engel J Jr. Seizures and Epilepsy, 2nd ed. Oxford: Oxford University Press; 2013:607. [Google Scholar]


