Abstract
Nanoscale engineering is revolutionizing the way we prevent, detect, and treat diseases. Viruses have played a special role in these developments because they can function as prefabricated nanoscaffolds that have unique properties and are easily modified. The interiors of virus particles can encapsulate and protect sensitive compounds, while the exteriors can be altered to display large and small molecules in precisely defined arrays. These properties of viruses, along with their innate biocompatibility, have led to their development as actively targeted drug delivery systems that expand on and improve current pharmaceutical options. Viruses are naturally immunogenic, and antigens displayed on their surface have been used to create vaccines against pathogens and to break self-tolerance to initiate an immune response to dysfunctional proteins. Densely and specifically aligned imaging agents on viruses have allowed for high-resolution and noninvasive visualization tools to detect and treat diseases earlier than previously possible. These and future applications of viruses have created an exciting new field within the disciplines of both nanotechnology and medicine.
Keywords: viruses, nanotechnology, drug delivery, vaccines, imaging
INTRODUCTION: VIRUSES AS BIOMATERIALS
Advances in synthetic biology and chemistry have influenced many areas of research, industry, and medicine by allowing for the fabrication of nanoscale devices with increasingly controllable structures. Even so, the large-scale manufacturing of such materials remains challenging, and it is difficult to prepare structurally homogeneous populations of particles (1, 2). In contrast, bionanomaterials based on viruses allow for the templated assembly of millions of identical nanoparticles and their production in living cells. Viruses are ubiquitous in the environment, and those that infect bacteria, mammals, or plants have all been used to manufacture virus-based nanoparticles (VNPs). Viruses are an ideal starting point because they have evolved naturally to deliver nucleic acids and can therefore be subverted for the delivery of other molecules, such as drugs and imaging reagents. Finally, viruses replicate prodigiously, allowing the inexpensive manufacture of VNPs on an industrial scale.
VNPs comprise regular arrays of virus coat proteins and have highly defined three-dimensional structure, providing an engineering scaffold that is superior to synthetic particles (Figure 1). The structure of VNPs can be altered by modifying the nucleic acid template that codes for viral proteins prior to synthesis, and by chemically decorating the particles by adding conjugates to specific amino acid side chains. VNPs are composed primarily of protein and are therefore known for their biocompatibility, biodegradability, ability to cross biological barriers, and efficient delivery of cargo to target cells. Viruses have evolved to interact with specific cellular proteins, deliver their nucleic acid cargo, and hijack the intracellular machinery to produce the components of progeny viruses. These properties have led to the development of VNPs based on mammalian viruses for use in gene therapy, but it is difficult to rule out pathogenic effects resulting from natural virus-host interactions (3–5). In contrast, VNPs based on bacteriophages and plant viruses are regarded as safe because even the fully functional viruses cannot infect humans. The majority of this article therefore focuses on the medical applications of VNPs based on bacteriophages and plant viruses.
Figure 1.
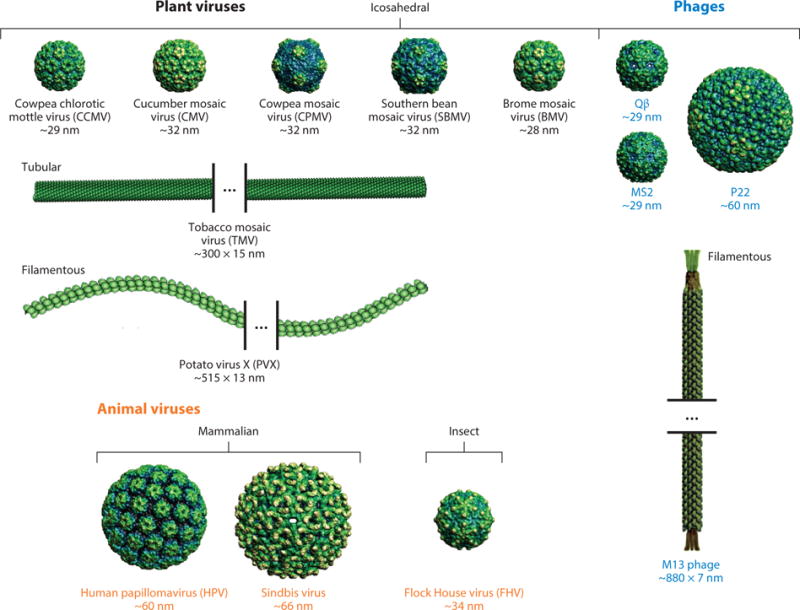
Structures of a selection of plant, bacteriophage, and animal viruses that have been used as virus-based nanoparticles. Structural data for icosahedral viruses were obtained from http://viperdb.scripps.edu/, and structural data for TMV were obtained from http://www.rcsb.org; images were produced using Chimera software (6). For PVX and M13, schematic representations of the virus particles are shown.
Bacteriophages and plant viruses are nucleoprotein assemblies in which the nucleic acids are tightly enclosed in a capsid comprising multiple copies of identical coat proteins. The capsids are generally icosahedral (roughly spherical in appearance), stiff tubes, or flexible filaments, the latter groups characterized by a high aspect ratio (Figure 1). Unlike many mammalian viruses, plant viruses and bacteriophages usually are not enveloped by a fragile lipid membrane because they must withstand more harsh environmental conditions in order to successfully infect their hosts.
The natural function of the virus capsid is to protect the viral genome from nucleases and physical hazards. Virus coat proteins are therefore stable and resistant to chemical and physical degradation, which is an advantage for the development of VNPs because it means they have a long shelf life and can withstand the chemical treatments necessary for conjugation with targeting ligands or loading with payloads such as drugs, fluorophores, or contrast agents (7, 8).
MODIFICATION STRATEGIES: FROM SCAFFOLDS TO FUNCTIONAL ENTITIES
A battery of techniques can be used to tailor and modify virus-based materials, including genetic engineering, encapsulation, biomineralization, infusion, and bioconjugation (Figure 2). Genetic engineering allows the basic structure of the coat protein to be changed by inserting, removing, or substituting particular amino acid residues (9–12). Such changes include terminal extensions (adding sequences to the N terminus or C terminus of each coat protein), the insertion of sequences that form surface loops, or the insertion or exchange of individual amino acids to introduce side chains that allow functionalization (13–15) or to alter the overall physicochemical properties of the VNP (16). Prominent examples of such modifications include the introduction of purification/immunodetection tags, the introduction of epitope sequences so that the VNP functions as a vaccine (17), and the introduction of targeting sequences that allow the VNP to target specific receptors (18). The incorporation of unnatural amino acids as unique handles for subsequent chemical reactions is also possible using similar recombinant expression strategies (19).
Figure 2.
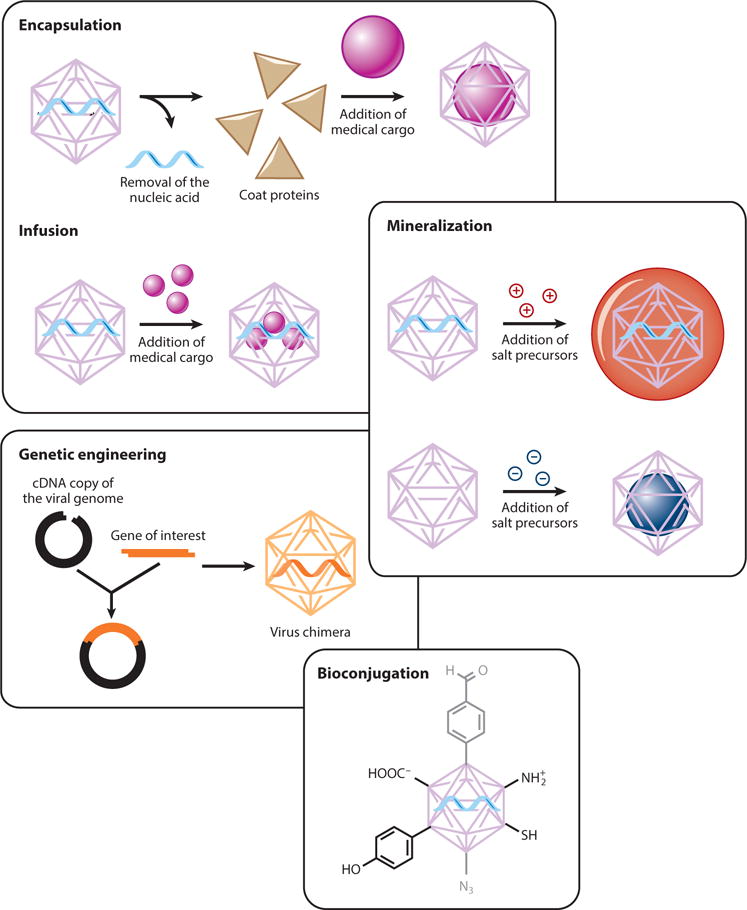
Generalized modification strategies to develop functionalized virus-based nanoparticles.
Virus coat proteins have evolved to self-assemble around nucleic acids under physiological conditions, and this property (which is shared by VNPs) can be exploited to disassemble VNPs and reassemble them into more desirable structures around other cargo molecules. Two basic principles can be used to trigger cargo encapsulation (20–23): (a) surface charge and electrostatic interactions or (b) unique binding interactions that occur during self-assembly. For example, bacteriophage MS2 contains a translational repression (TR) operator protein that binds to a TR RNA stem loop. TR operator proteins can be chemically engineered to carry small drug molecules. When intact MS2 particles are mixed with the modified TR operators, the latter diffuse inside the VNPs and bind stably to the capsid. Therapeutic molecules such as the ricin A chain and 5-fluorouridine have been successfully incorporated into MS2 particles using these design principles. In vitro cell studies using this approach have confirmed cargo delivery and the successful destruction of target cells (24, 25).
Biomineralization is the accumulation of minerals in and around the cells and tissues of living organisms, but in the context of VNPs it refers to the ability of virus coat proteins to assemble around a mineral core or nucleate mineralization. There are many applications of VNP biomineralization in the field of energy research (26), but there are also examples in medicine, particularly where mineral cargos are used as contrast agents. For example, an electrostatically engineered cowpea chlorotic mottle virus (CCMV) was used to facilitate the nucleation and oxidation of an Fe(II) cargo, leading to the formation of spatially constrained iron oxide nanocrystals suitable for magnetic resonance imaging (MRI) or hyperthermia treatment applications (16).
Whereas some materials must be encapsulated by encouraging the formation of capsids around a cargo, others can diffuse through the capsid and into the interior cavity, where they can be persuaded to remain inside by noncovalent interactions with nucleic acids or internally projecting amino acid side chains or can be permanently linked to handles by bioconjugation (27). Fluorescent dyes for optical imaging, Gd3+ ions for MRI, and small drug molecules have all been loaded using this approach (28, 29).
One of the most powerful approaches for the modification of VNPs is the use of classical chemistry to functionalize particular amino acid side chains, such as the carboxylate groups on glutamic and aspartic acid residues, reactive amines on lysine residues, sulfhydryl groups on cysteine residues, and phenol groups on tyrosine residues (Figure 3). These groups can be directly conjugated to particular molecules or modified to display functional groups necessary for more sophisticated conjugation strategies. For example, carbodiimide coupling agents can be used to link any molecule containing a primary amine to the carboxylate groups of glutamic and aspartic acid, Michael addition can be used to link maleimides to the sulfhydryl groups of cysteine residues, and N-hydroxysuccinimide (NHS)-activated esters can be added to lysine side chains to link any molecule compatible with NHS chemistry (8, 30). The solvent-exposed phenol ring of tyrosine side chains can be modified by reacting it with the diazonium salts of a particular conjugate. Alkyne-based diazonium salts are more versatile because they provide a handle for further functionalization using click chemistry (31, 32). A popular click chemistry strategy used with VNPs is copper-catalyzed azide-alkyne cycloaddition (CuAAC) between azides and alkynes in the presence of Cu(I), irreversibly yielding the biocompatible 1,4-substituted triazole derivative (19, 28, 33–37). In addition, azo-coupling can be used to introduce payloads into VNPs (32, 38–40). Typically, an aldehyde is first introduced onto the VNP surface by azo-coupling to a bifunctional linker. The aldehyde can then be used in oxime or hydrazone condensation reactions (38, 39, 41). Another method is to selectively oxidize the primary N-terminal amine using pyridoxal 5′-phosphate. The reaction is specific to the N-terminal amines because it has a lower pKa than the equivalent reaction with lysine side chains, and the resulting ketone (or aldehyde in the case of oxidizing terminal glycine residues) condenses with hydroxylamine-modified payloads to form oxime linkages. This approach has been used to conjugate imaging agents to the surface of bacteriophage M13 (42).
Figure 3.
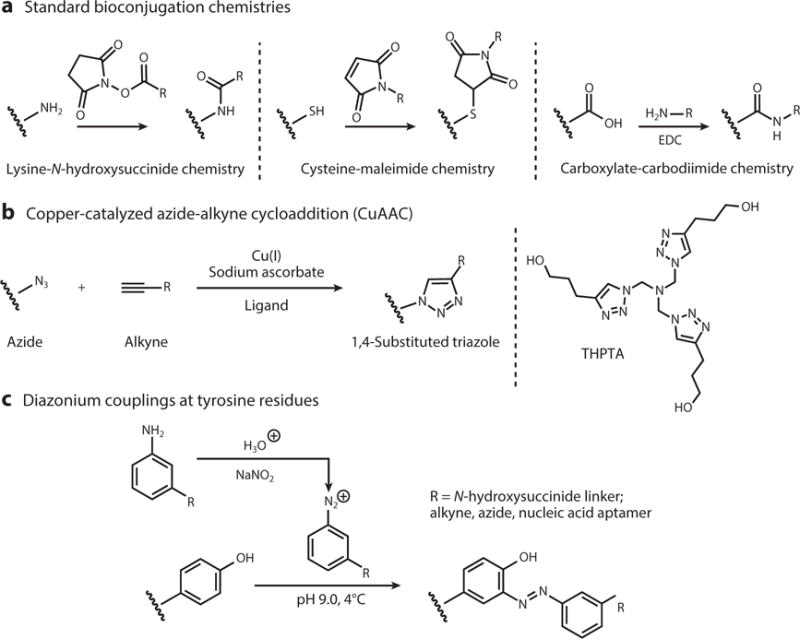
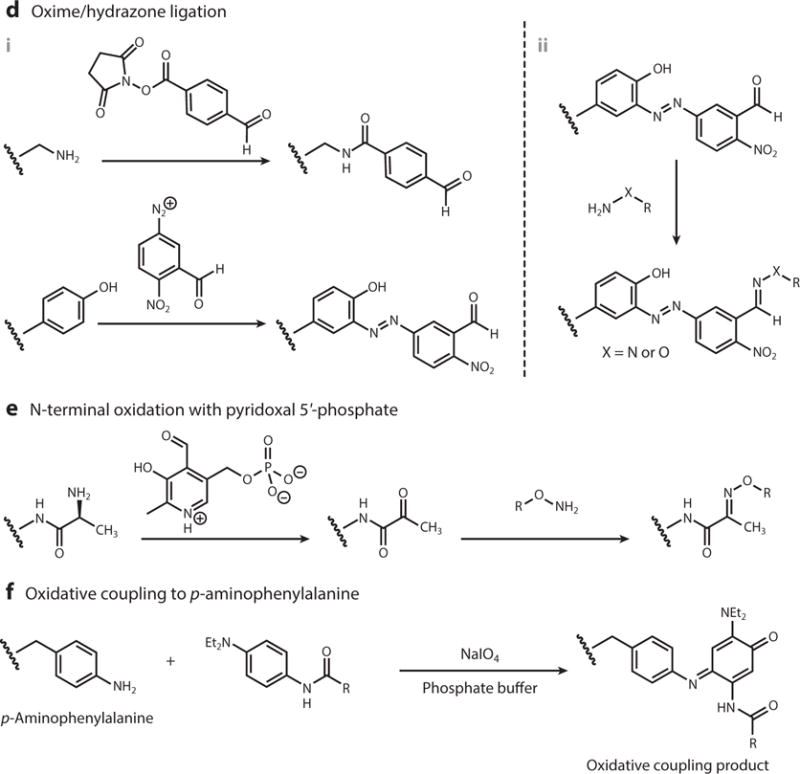
Frequently used virus-based nanoparticle amino acid bioconjugation schemes. Abbreviations: EDC, 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide; THPTA, tris(3-hydroxypropyltriazolylmethyl)amine.
VIRUS-BASED NANOPARTICLES IN THERAPEUTIC INTERVENTIONS
The ability of bacteriophages and plant viruses to enter mammalian cells without further replication makes them suitable as tools for therapeutic interventions. Virus-based nanomaterials can be tailored to target particular cells, including cancer cells and specific cells of the immune system. They can present antigens to the immune system, meaning they can also be used as vaccines. VNP interactions with the immune system are advantageous for immunotherapy and immuno/chemo combination therapies, but often not for imaging applications or drug delivery. Therefore, several strategies have been developed to shield VNPs from the immune system while directing them to specific target cells. The clearance of VNPs by the mononuclear phagocyte system (43–45) can be overcome by tailoring the surface chemistry or shape of the particles. For example, surface PEGylation can minimize nonspecific interactions between VNPs and macrophages, thus prolonging their circulation time (46–48). Targeting can be achieved by the genetic or chemical addition of ligands that bind to receptors overexpressed on particular cell types, such as cancer cells. The ligands are either displayed on the capsid surface or added to the ends of the PEG chains; for example, VNPs have been targeted using folic acid (49), transferrin (50, 51), epidermal growth factor (EGF) (52, 53), and RGD peptides (54) (Figure 4).
Figure 4.
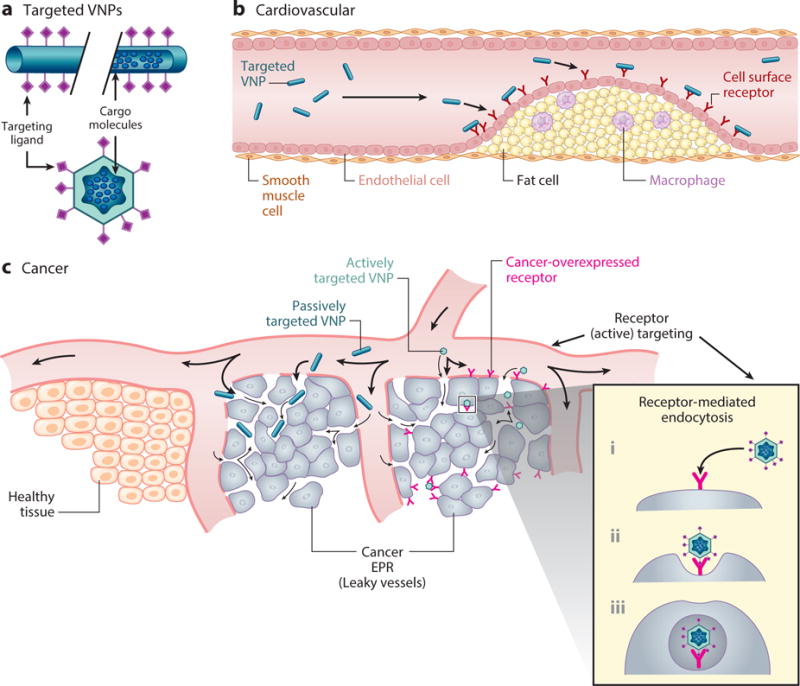
Fundamentals of tissue- or disease-targeted virus-based nanoparticles (VNPs). Targeting ligands are presented on the exterior of the VNP to facilitate active cell-specific surface binding and/or receptor-mediated endocytosis. Additional unique factors, such as the enhanced permeability and retention (EPR) effect in tumor masses or VNP shape, can further aid in passive localization.
Tissue specificity can also be influenced by the shape, size, and aspect ratio of the VNP, so these are other properties that can be considered at the design stage. Notably, tubular or filamentous VNPs can have in vivo properties preferable to those of spherical VNPs—that is, enhanced flow and margination toward the vessel wall and reduced clearance by the mononuclear phagocytic system, thus leading to increased tumor homing (55) and thrombus targeting (56). Because VNP structures are monodisperse and can be tailored with precise and reproducible spatial control, they can be used to investigate the impact of VNP size and shape on the efficiency of drug delivery and imaging. For example, a bottom-up assembly strategy based on nucleic acids of different lengths was used to control the aspect ratio of VNPs based on tobacco mosaic virus (TMV). This allowed the comparison of particles that were identical in all properties except the aspect ratio, and showed that the aspect ratio had a significant impact on VNP biodistribution, longevity, and tumor penetration (57).
DRUG DELIVERY WITH VIRUS-BASED NANOPARTICLES
The development of VNPs that target specific cell types has allowed for the addition of toxic payloads by conjugation, infusion, and/or encapsulation so that the target cells are killed, thus allowing the selective elimination of cancer cells, or other diseased cells, without off-target effects. As discussed briefly above, conjugation involves the selective covalent addition of payload molecules to particular amino acid residues of the coat protein. Infusion is achieved by incubating the intact VNP in a solution containing the cargo (29, 58), and encapsulation requires the carrier to be assembled around the payload (24) (Figure 2). Many different types of therapeutic cargo have been delivered, including genes and short interfering RNAs (50, 59), conventional small-molecule drugs, photoactive molecules that support photodynamic therapy, and even heterologous viral genomes for gene therapy, such as an alphavirus genome encapsulated in a VNP based on CCMV (60).
Toxic cargos can be preferentially loaded into the VNP cavity, rather than coated onto the external surface, to protect them from enzymatic and chemical degradation in vivo and avoid interactions with nontarget cells. The capacity of VNPs and the efficiency of loading are generally improved by removing the native viral genome, which can be achieved by expressing the coat proteins from a plasmid (for the production of bacteriophage VNPs) or from a transgene (for the production of plant VNPs) so that the viral nucleic acid is never present; the resulting empty particle is described as a virus-like particle (VLP). Alternatively, the viral genome can be removed by selective chemical or enzymatic degradation.
The covalent attachment of toxic cargo molecules to internally exposed side chains ensures that there is no early release, but noncovalent methods generally allow for higher loading efficiency because there is space within the VNP for more cargo if the entire cavity is used rather than only the internal surface. For example, up to 300 doxorubicin molecules can be conjugated to the capsid surface of cowpea mosaic virus (CPMV) (61), but up to ∼950 doxorubicin molecules could be loaded into the internal cavity of hibiscus chlorotic ringspot virus (HCRSV) by encapsulation (62). Both viruses have icosahedral capsids 30 nm in diameter. Polymerization can achieve the best of both worlds by providing a branching network of functionalized groups for payload attachment that extends from the external surface of the VNP (63) or pervades the interior (64). Although most studies thus far have focused on the design aspects of VNPs and their in vitro toxicity, the preclinical testing of a VNP-based drug delivery vehicle has demonstrated in vivo efficacy and reduced cardiotoxicity of a doxorubicin-loaded VNP, specifically cucumber mosaic virus (CMV) modified with folic acid to target ovarian cancer (49).
As well as standard chemotherapy, VNPs have also been loaded with photosensitizers for photodynamic therapy applications. For example, a VLP based on bacteriophage Qβ was loaded with a metalloporphyrin derivative for photodynamic therapy and a glycan ligand targeting cells bearing the CD22 receptor (65). Furthermore, a multifunctional MRI contrast and photodynamic therapy agent (chelated Gd3+ and Zn2+ phthalocyanine) was successfully encapsulated in CCMV as a first demonstration of theranostic VNPs (66). Hybrid VNP-based materials carrying metal nanoparticles for photothermal therapy have also been investigated (67).
IMMUNIZATION AND IMMUNOTHERAPY USING VIRUS-BASED MATERIALS
The development of vaccines to prevent infectious diseases is one of the most significant medical advances in the past 300 years (68, 69) and has had an immense socioeconomic impact worldwide (70–72). Nevertheless, several key vaccines remain elusive, including those for HIV, respiratory syncytial virus, hepatitis C virus, and the hemorrhagic fever viruses. A current public health crisis, for example, is the Ebola outbreak affecting West African countries and visiting health care workers. Without effective antivirals and vaccines, detection and monitoring are currently the best options to prevent the spread of this life-threatening disease. The rapid development of a vaccine using a safe and effective platform is urgently needed.
Vaccination and other forms of immunotherapy also hold great promise as prophylactic and therapeutic interventions for the treatment of cancer and chronic diseases (73–76). Several monoclonal antibodies, antibody-based fusion proteins, and antibody-drug conjugates are already used for clinical immunotherapy, and many others are undergoing clinical and preclinical development (77–80).
Virus-based materials have repetitive, protein-based structures and therefore elicit immune responses that make them useful for the development of vaccines and immunomodulators. Particle-based vaccines fall into several classes, including (a) chemically inactivated virus vaccines, (b) attenuated virus vaccines with minimal virulence, (c) genome-free and noninfectious VLPs, and (d) chimeric and nanoparticle vaccines, in which pathogen-derived epitopes are displayed using a noninfectious carrier such as a plant virus, bacteriophage, or synthetic platform (81–83). Particulate vaccines, including VLPs and other nanoparticle vaccines, provide several distinct advantages over DNA vaccines (84–87) and subunit vaccines (88–90). The virus-based carrier confers antigen stability, carries multiple copies of the antigen (multivalent presentation), and has the potential to present two or more different antigens. The formulation promotes passive or active uptake by antigen-presenting cells (91, 92) followed by their activation and the subsequent priming of the appropriate T and B cell responses (93–95) (Figure 5). The different categories of virus-based materials that have been developed as vaccines have been comprehensively reviewed (17, 96). Here we focus on important examples of VLPs and chimeric VNPs based on bacteriophages and plant viruses.
Figure 5.
Immune response activation by virus-based nanoparticles. Homogeneous or heterogeneous epitopes displayed on the surface of virus-based nanoparticles are taken up by antigen-presenting cell types and initiate activation of the humoral immune response, the cellular immune response, or both.
Vaccines for Infectious Diseases
VLP vaccines have enjoyed great success against viral diseases, particularly using strategies in which the structure of the noninfectious vaccine formulation closely mimics that of the natural virus [these have been described as native VLPs (96)]. The first successful example was the vaccine against hepatitis B virus (HBV), which has dramatically reduced HBV infections in immunized populations (97). Vaccines against human papillomavirus (HPV) elicit immunity against the virus that in turn protects against HPV-induced cervical carcinoma, and potentially other HPV-induced cancers (98).
Chimeric VLPs display heterologous antigens and can generate antipathogen and neutralizing antibodies such that immunization may also result in protection against pathogen challenge (99, 100). Many studies have been carried out with chimeric VLPs based on plant viruses (101–107), bacteriophages (108, 109), insect viruses (110), and animal polyomaviruses and papillomaviruses (97, 98). Chimeras have also been prepared from native vaccine platforms (e.g., HBV and HPV) and build on these platforms by displaying additional heterologous epitopes (111–113). These native-chimeric VLPs have the advantage of using an established and FDA-approved vaccine backbone.
Chimeric VLPs displaying complex antigen structures have been developed based on Flock House virus (FHV), which infects insects. This multivalent display system has been modified to include portions of the anthrax toxin receptor (ANTXR2), which in turn acts as a scaffold to display the Bacillus anthracis protective antigen. The virus-antigen complex induced protective immune responses after a single dose in the absence of adjuvant (110). Additional mechanisms for chemical attachment of multivalent antigens achieve a similarly efficient induction of immune responses (41). The FHV system has the advantage of accepting protein and peptide insertions in a variety of locations on the capsid surface and benefits from the availability of detailed structural and genetic information that allows the precise placement and arrangement of antigenic domains. For example, the influenza hemagglutinin (HA) protein is a major antigen for all strains of influenza, but it is difficult to develop broadly neutralizing immune responses due to antigenic variation. There are small regions of the protein that are highly conserved, but they are difficult to display in a structural context, which would allow the induction of specific and neutralizing antibody responses. Displaying the conserved regions of HA in a trimeric arrangement on FHV allows for the induction of these antibodies (114). The utility and scope of native and chimeric VLPs for vaccine applications continue to grow. For example, the combination of bioengineering VLP vaccines and administering them into the lungs was recently demonstrated as a powerful strategy for future vaccine development and immunotherapy (115).
Vaccines for Cancer
Cancer vaccines are based on our increasing knowledge of the molecular identities of tumor-associated antigens (116–118), combined with the ability to elicit efficient immune responses against these self-antigens despite active immunosuppression by the tumor. Anti-tumor vaccination has several advantages over chemotherapy, including a simplified outpatient procedure, fewer side effects, the avoidance of drug resistance, training the immune system to eliminate residual drug-resistant cells, and the induction of long-term immunological memory to protect against metastases and relapse.
Because tumor antigens are derived from the host, it is necessary to break immunological tolerance to induce immune responses against nonimmunogenic or weakly immunogenic targets. Several VNP-based cancer vaccine approaches that involve the patterned display of tumor-associated carbohydrate or peptide antigens have been evaluated. For example, CPMV particles were used as scaffolds to chemically display the Tn antigen, a glycoprotein that is overexpressed on numerous cancer cells including breast, colon, and prostate cancer cells. Conjugation to the virus-based scaffold and multivalent display resulted in production of high titers of Tn-specific antibodies (75). Similarly, Tn antigen conjugated to TMV can elicit antigen-specific IgG and IgM responses (76). The presentation of cancer epitopes on virus-based scaffolds enables the presentation of these self-epitopes in a nonnative molecular environment, which is a promising strategy to overcome self-tolerance, as shown for human epidermal growth factor receptor 2 (HER2)-positive breast cancer patients (119).
Vaccines for Neurological Diseases and Addiction
VLPs have been used as scaffolds to display the amyloid beta (Aβ) protein implicated in the progression of Alzheimer’s disease. Papillomavirus and Qβ VLPs displaying Aβ antigens elicited anti-Aβ antibodies in the absence of adjuvant, and with limited T cell responses. The antibody subclasses varied according to the use of the whole antigen or peptide antigens (73, 120). An HBV core antigen (HBcAg) carrying N-terminal epitopes of Aβ has also been developed as a vaccine and demonstrated efficacy in a transgenic mouse model of Alzheimer’s disease (121, 122).
A potential vaccine against nicotine addiction has been recently developed using the 30-nm icosahedral capsid of bacteriophage Qβ chemically modified to display nicotine in a multivalent fashion. The multivalent and particulate nature of the Qβ-based vaccine boosts the production of antinicotine neutralizing antibodies, thereby reducing blood nicotine levels and limiting transport across the blood-brain barrier. The nicotine vaccine is currently under investigation in clinical trials with the goal to reduce smoking addiction (123, 124).
VIRUS-BASED MATERIALS AS IMAGING PROBES
Imaging agents with an optimal signal-to-noise ratio, tailored circulation time, reduced toxicity, and targeted delivery can achieve higher-resolution imaging and earlier disease detection to facilitate better patient outcomes. VNPs are particularly suitable for this application, as demonstrated by recent advances in oncology and cardiovascular medicine (see Figure 4). As described above for drugs, imaging molecules can be added to the external surface or internal cavity of VNPs by genetic modification (bioluminescent proteins), infusion, encapsulation, and/or bioconjugation. The precise spacing and controlled loading of imaging molecules can be used to tweak the signal-to-noise ratio depending on the dye or contrast agent used.
The attachment of fluorescent dyes to VNPs can facilitate flow cytometry, confocal microscopy, and in vivo imaging experiments because a large number of dye molecules can be incorporated per particle and the sensitivity of detection can be modulated by controlling the spacing of the molecules to optimize individual particle detection (125). Dye molecules are usually attached by conjugation to the surface of the VNP (126, 127), but some investigators have used genetic engineering to incorporate polypeptides such as green fluorescent protein (GFP) into the coat protein, thus creating stable virus chimeras that are fluorescent. In order to complete these genetic manipulations, viral genomes often must be refactored to eliminate overlaps in the protein-coding sequences that are to be modified (128). The genetic addition of GFP or the red fluorescent protein mCherry to the potato virus X (PVX) coat protein allowed for in vivo imaging of human tumor xenographs in mice (129). Similarly, bacteriophage λ has been coated with fluorescent molecules via bioconjugation to addressable lysine side chains (130) and also by the genetic engineering of the coat protein gpD to decorate the particle with GFP (131). Most VNPs for imaging are based on nonenveloped viruses, but the E2 spike protein of Sindbis virus (an enveloped alphavirus) has been used to decorate enveloped particles with the fluorescent proteins mApple and Venus, while maintaining gross particle morphology (132). Sindbis virus capsid proteins can also be manipulated to self-assemble around different imaging cargos (133). Filamentous bacteriophage M13 has been developed that incorporates 2,700 copies of an HPQ biotin-like sequence in the capsid, which was subsequently used as a handle to attach streptavidin-modified fluorophores (134). Infusion with imaging agents can be achieved by soaking VNPs in a solution of imaging agents that bind nucleic acids or proteins. For example, CPMV was shown to deliver noncovalently retained DAPI, propidium iodide, and acridine orange to mammalian cancer cells (29). In addition, these VNPs were stable for several weeks at 4°C without significant dye leakage (29). Such highly fluorescently modified VNPs can be used with new high-resolution technologies, such as stochastic optical reconstruction microscopy (135), to promote our understanding of viral delivery systems and their clinical deployment.
Imaging in vivo often requires light to penetrate deep into a tissue. Near-infrared (NIR) imaging can accomplish this goal because proteins and water absorb few photons in the NIR range (650–950 nm), allowing access to deeper layers of living tissue. There is also little tissue autofluorescence in the NIR window. Bacteriophage MS2 has therefore been loaded internally with NIR dyes and decorated on the outside with fibrin-targeting ligands, allowing for the detection of blood clots (136). Similarly, VLPs based on brome mosaic virus (BMV) were used to encapsulate the FDA-approved NIR imaging agent indocyanine green (137). Further studies showed that the presence of serum proteins enhanced the absorption and fluorescence emission properties of this VNP formulation (138). Second near-infrared light (NIR2) imaging (950–1,400 nm) has an even greater penetration depth than NIR. Single-wall carbon nanotubes (SWNT) can fluoresce in the NIR2 window and have previously been used alone for in vivo imaging (139). The coat protein of filamentous bacteriophage M13 was genetically modified to align a hydrophobic SWNT along the long axis of the VNP, and the resulting M13/SWNT complexes were also modified with targeting ligands on the distal end, allowing for the successful NIR2 imaging of deep tumors in vivo (140). A similar M13 formulation was successfully used for surgical guidance during tumor resection (141).
MRI is widely used for the in vivo noninvasive characterization of both healthy and diseased tissues. Tailored MRI contrast agents can further increase signal differences between types of tissues and help resolve anatomical differences. VNPs are advantageous in this field because they can concentrate contrast probes to increase the sensitivity of detection, they reduce tumbling rates and increase relaxivity to optimize the signal, and by encapsulating the contrast reagents they reduce their toxicity. Paramagnetic gadolinium (Gd3+) is often used as a positive MRI contrast agent, but it must be presented in a chelated form to reduce toxicity, usually with tetraazacyclododecane tetraacetic acid (Gd-DOTA) or diethylenetriamine pentaacetic acid (Gd-DTPA). In order to make a better MRI contrast agent, the internal cavity of spherical bacteriophage P22 capsids were decorated with multiple branched oligomers that each held several Gd-DTPA complexes (142). This formulation allowed researchers to achieve high contrast agent density with high relaxivity (142). Additional studies using bacteriophage P22 capsids showed that the internal or external attachment of chelated Gd3+ did not significantly change the relaxivity (143), but that larger particles caused slower tumbling and faster T1 relaxation (143). Less toxic Mn3+ chelators have also been attached successfully to bacteriophage P22 (144). As stated earlier, a multifunctional MRI contrast and photodynamic therapy agent was created by the coencapsulation of chelated Gd3+ and Zn2+ phthalocyanine in a VNP based on CCMV particles (66). The amphipathic nature of Zn2+ phthalocyanine allowed the chelated Gd3+ to be loaded at a higher density, thus achieving a greater overall contrast (66).
The potential of virus-based MRI probes was validated in a recent study that achieved sensitive delineation of atherosclerotic plaques using a Gd-DOTA-loaded TMV nanoparticle followed by detection and imaging at submicromolar doses (400× lower than the typical clinical dose) (145, 146). The high contrast-to-noise ratio, and resulting sensitivity of the agent, was attributed to slower tumbling and enhanced relaxivity, multivalency, recognition chemistry (the probes were targeted to VCAM-1 receptors), and carrier shape. The elongated shape of the VNP enhanced its flow properties and promoted margination toward the vessel wall.
Virus scaffolds have also been developed for T2 imaging. Whereas T1 shortening agents create a bright signal, T2 shortening agents create a dark signal. Iron oxide–based nanoparticles are used as T2 contrast agents and have been combined with VNPs. For example, iron oxide nanoparticles have been aligned on bacteriophage M13 (147) or have been encapsulated into VLPs derived from BMV (148) to achieve both biomedical and agricultural imaging.
Newer approaches include the use of virus-based materials in chemical exchange saturation transfer (CEST) and hyperCEST imaging. These are MRI techniques in which exogenous nuclei are selectively saturated using radio frequency signals after the transfer of this saturation to the surrounding water protons, and are detected indirectly through the water signal with enhanced sensitivity. Hyperpolarizable and chemically inert xenon-based MRI sensors, which can increase the signal sensitivity 10,000-fold, have therefore been added to both icosahedral bacteriophage MS2 (149) and filamentous bacteriophage fd (150).
VNPs have also been investigated as potential tools for radioimaging. DOTA groups covalently attached to the interior surface of PEGylated VNPs based on bacteriophage MS2 have been used to chelate 64Cu radioisotopes for positron emission tomography (PET). This allowed PET imaging for up to several hours in mice, and small concentrations of these 64Cu-carrying VNPs stayed in the bloodstream 24 h postinjection, much longer than similar-sized VNPs (151).
OPPORTUNITIES AND IMPLICATIONS
VNPs are high-precision materials that self-assemble into symmetrical and polyvalent structures that can be tailored at the atomic level. Virus-based materials come in a variety of shapes and sizes, but most are monodisperse with geometries that can be custom modified. For example, gold-core BMV-shell hybrid structures can be self-assembled in vitro from coat proteins into hierarchical structures, in which the overall size of the assembly is governed by the size of the core nanoparticle (23). Not only can different-sized particles be generated, but the geometries can also be switched. For example, isolated coat proteins from icosahedral CCMV particles form nanotubes when assembled in the presence of DNA templates (152). Going in the other direction, TMV rods can be converted into spherical nanoparticles by thermally controlled shape-switching (153).
Synthetic biology and orthogonal chemistry allow the addition of reproducible functionalities to VNPs. In contrast to synthetic nanoparticles (in which modifications follow a statistically randomized distribution), the proteinaceous scaffolds of VNPs allow the deterministic display of drugs, imaging reagents, targeting peptides, or epitopes, thus enabling structure-based engineering. Not only can small chemical modifiers such as contrast agents, drugs, and peptides be displayed, but there is also an opportunity to present entire proteins as genetic fusions. Examples include the display of full-length fluorescent proteins for optical imaging (129); the genetic fusion of functional enzymes to the internal (154) or external (155) surfaces of VNPs, allowing them to be used as nanoreactors; and the presentation of complex protein structures such as the I-domain of the anthrax toxin cellular receptor in a complex with the anthrax protective antigen as a vaccination platform (110). Another interesting avenue is the use of subviral structures such as the tail, which functions as a molecular motor (156).
As well as providing a three-dimensional scaffold to facilitate VNP design, plant viruses and bacteriophages can also be manufactured on a large scale by molecular farming in plants or fermentation in bacteria or yeast, providing a realistic avenue for commercialization (157). The commercialization of VNPs must also take into account any potential risks to health and the environment. As stated above, mammalian viruses pose a risk of infection, so bacteriophages and plant viruses are preferred for medical use, but even these pose a risk to the environment and in particular to agriculture. Procedures have therefore been established to render them noninfectious, including the heterologous expression of genome-free VLPs (157) and in vitro disassembly and reassembly to yield VLPs from self-assembled purified coat proteins (11, 158). Alternatively, nucleic acids can be removed from intact particles by alkaline hydrolysis (159, 160) either during extraction or following the recovery of pure virus particles (161). Shortwave (254 nm) UV irradiation can be used to cross-link and inactivate the genomes of intact particles (162).
As is the case for any platform technology, preclinical safety and efficacy must be established before any promising strategies can be considered for clinical development and translational medicine. Bacteriophages and plant viruses have been shown to be well tolerated by mammalian cells in vitro and by animal models. For example, no evidence of toxicity was observed in mice injected with up to 1016 CPMV particles per kilogram of body weight (100 mg/kg) (44). Following intravenous administration, bacteriophage- and plant virus–based materials are cleared from the circulation by the mononuclear phagocytic system and deposited in liver and spleen, followed by hepatobiliary extraction (44, 55, 163, 164). High-aspect-ratio, anisotropic, and filamentous plant viruses, much like nanotubes (165, 166), are also cleared by renal filtration (167). Overall, the clearance of VNPs from the circulation follows the same rules observed with synthetic materials, but there are significant differences when it comes to the clearance of VNPs from tissues. Whereas some synthetic nanoparticles persist in the body for weeks or even longer (168–171), virus-based materials are subject to proteolytic degradation and thus are removed safely from the body within days (172, 173).
Like many other exogenous materials, protein carriers derived from bacteria and plants are immunogenic. Such immunostimulatory properties can be exploited in the development of VNP-based vaccines, for example, in which the capsid carrier can be exploited to overcome self-tolerance or boost the immunogenicity of otherwise unstable or weakly immunogenic molecules such as peptides and carbohydrates (17). The elicitation of an immune response is also desirable in certain therapeutic approaches (e.g., immuno/chemo combination therapies) but not for the straightforward delivery of drugs or imaging reagents, particularly when repeat administration is required and an immune response (particularly carrier-specific antibodies) would dampen the efficacy of the VNP by accelerating its clearance. Immunogenicity is not a challenge unique to VNPs, because antibodies are also elicited against many inorganic and synthetic carriers (164, 174–178). Several strategies have been developed to address this challenge. For example, polymers such as PEG grafted to and grafted from the capsid surface can prevent antibody recognition (179–181). Another elegant strategy to reduce interactions with the immune system (which ultimately lead to the production of antibodies and potential inflammatory responses) is to camouflage the particles as endogenous proteins. This approach was recently demonstrated using synthetic nanoparticles tagged with peptides derived from the active domain of CD47, which prevented clearance by the mononuclear phagocyte system (182).
A detailed understanding of the biological fate of VNPs will provide opportunities to control their fate in vivo and increase their efficacy. Natural molecular recognition between VNPs and target cells can be harnessed; for example, the discovery of CPMV-vimentin interactions has been exploited for molecular imaging and drug targeting applications in cancer, neurological disease, and atherosclerosis (183–189). Molecular functionality can also be incorporated by shape engineering and molecular recognition chemistry. The internal and external surfaces, coat protein interfaces, and interior cavity are all amenable to functionalization with medically relevant cargos. Mammalian viruses have been investigated in detail for gene delivery applications (3), with numerous VLP and VNP systems undergoing clinical development and several already on the market, including the HPV vaccine Gardasil (17). Novel approaches in the development pipeline include VNPs used for theranostics and tissue engineering (190). Within the nanomedicine sector, at least one start-up company has been established focusing on the development of plant virus–based chemotherapy (Nanovector Inc.). Bacteriophages and plant viruses have therefore emerged as versatile research tools and as platforms for nanomedical research, and there is now a clear path for their adoption in the clinic.
Acknowledgments
This work was supported by National Science Foundation grants CMMI NM 1333651 (VNP nanomanufacturing), CHEM MSN 1306447 (VNP polymer hybrids), and CMMI NM 1509232 (VNP diagnostics); National Institutes of Health grant NHLBI R21 HL121130 (VNP-based materials in cardiovascular research); Susan G. Komen grant CCR14298962 (VNP research targeting breast cancer); and Mt. Sinai Foundation and Case Western Reserve University start-up funds to N.F.S. Additional support was provided by Point Loma Nazarene University start-up funds to K.J.K. M.M. was supported by grants from the National Institutes of Health (NIAID, NCI) and the American Heart Association, as well as the Skaggs Foundation at the University of California, San Diego. M.M. is presently affiliated with Translational Technologies and Bioinformatics, Pharmaceutical Sciences, Pharma Research and Early Development (pRED), Roche Pharmaceuticals, Basel, Switzerland.
Footnotes
The Annual Review of Virology is online at virology.annualreviews.org
DISCLOSURE STATEMENT
M.M. is a full-time employee of F. Hoffmann–La Roche, Basel, Switzerland. The authors are not aware of any other affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Desai N. Challenges in development of nanoparticle-based therapeutics. AAPS J. 2012;14:282–95. doi: 10.1208/s12248-012-9339-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Franca R, Zhang XF, Veres T, Yahia L, Sacher E. Core-shell nanoparticles as prodrugs: possible cytotoxicological and biomedical impacts of batch-to-batch inconsistencies. J Colloid Interface Sci. 2013;389:292–97. doi: 10.1016/j.jcis.2012.08.065. [DOI] [PubMed] [Google Scholar]
- 3.Guenther CM, Kuypers BE, Lam MT, Robinson TM, Zhao J, Suh J. Synthetic virology: engineering viruses for gene delivery. WIRES Nanomed Nanobiotechnol. 2014;6:548–58. doi: 10.1002/wnan.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wirth T, Parker N, Ylä-Herttuala S. History of gene therapy. Gene. 2013;525:162–69. doi: 10.1016/j.gene.2013.03.137. [DOI] [PubMed] [Google Scholar]
- 5.Ylä-Herttuala S. Endgame: Glybera finally recommended for approval as the first gene therapy drug in the European Union. Mol Ther. 2012;20:1831–32. doi: 10.1038/mt.2012.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, et al. UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–12. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 7.Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2:751–60. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 8.Steinmetz NF. Viral nanoparticles as platforms for next-generation therapeutics and imaging devices. Nanomedicine. 2010;6:634–41. doi: 10.1016/j.nano.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Q, Lin T, Johnson JE, Finn MG. Natural supramolecular building blocks: cysteine-added mutants of Cowpea mosaic virus. Chem Biol. 2002;9:813–19. doi: 10.1016/s1074-5521(02)00166-7. [DOI] [PubMed] [Google Scholar]
- 10.Peabody DS. A viral platform for chemical modification and multivalent display. J Nanobiotechnol. 2003;1:5. doi: 10.1186/1477-3155-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller RA, Presley AD, Francis MB. Self-assembling light-harvesting systems from synthetically modified tobacco mosaic virus coat proteins. J Am Chem Soc. 2007;129:3104–9. doi: 10.1021/ja063887t. [DOI] [PubMed] [Google Scholar]
- 12.Klem MT, Willits D, Young M, Douglas T. 2-D array formation of genetically engineered viral cages on Au surfaces and imaging by atomic force microscopy. J Am Chem Soc. 2003;125:10806–7. doi: 10.1021/ja0363718. [DOI] [PubMed] [Google Scholar]
- 13.Udit AK, Brown S, Baksh MM, Finn MG. Immobilization of bacteriophage Qβ on metal-derivatized surfaces via polyvalent display of hexahistidine tags. J Inorg Biochem. 2008;102:2142–46. doi: 10.1016/j.jinorgbio.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 14.Medintz IL, Sapsford KE, Konnert JH, Chatterji A, Lin T, et al. Decoration of discretely immobilized cowpea mosaic virus with luminescent quantum dots. Langmuir. 2005;21:5501–10. doi: 10.1021/la0468287. [DOI] [PubMed] [Google Scholar]
- 15.Chatterji A, Ochoa WF, Ueno T, Lin T, Johnson JE. A virus-based nanoblock with tunable electrostatic properties. Nano Lett. 2005;5:597–602. doi: 10.1021/nl048007s. [DOI] [PubMed] [Google Scholar]
- 16.Douglas T, Strable E, Willits D. Protein engineering of a viral cage for constrained material synthesis. Adv Mater. 2002;14:415–18. [Google Scholar]
- 17.Plummer EM, Manchester M. Viral nanoparticles and virus-like particles: platforms for contemporary vaccine design. WIRES Nanomed Nanobiotechnol. 2010;3:174–96. doi: 10.1002/wnan.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yildiz I, Shukla S, Steinmetz NF. Applications of viral nanoparticles in medicine. Curr Opin Biotechnol. 2011;22:901–8. doi: 10.1016/j.copbio.2011.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strable E, Prasuhn DE, Jr, Udit AK, Brown S, Link AJ, et al. Unnatural amino acid incorporation into virus-like particles. Bioconjug Chem. 2008;19:866–75. doi: 10.1021/bc700390r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daniel MC, Tsvetkova IB, Quinkert ZT, Murali A, De M, et al. Role of surface charge density in nanoparticle-templated assembly of bromovirus protein cages. ACS Nano. 2010;4:3853–60. doi: 10.1021/nn1005073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dixit SK, Goicochea NL, Daniel MC, Murali A, Bronstein L, et al. Quantum dot encapsulation in viral capsids. Nano Lett. 2006;6:1993–99. doi: 10.1021/nl061165u. [DOI] [PubMed] [Google Scholar]
- 22.Huang X, Bronstein LM, Retrum J, Dufort C, Tsvetkova I, et al. Self-assembled virus-like particles with magnetic cores. Nano Lett. 2007;7:2407–16. doi: 10.1021/nl071083l. [DOI] [PubMed] [Google Scholar]
- 23.Sun J, DuFort C, Daniel MC, Murali A, Chen C, et al. Core-controlled polymorphism in virus-like particles. PNAS. 2007;104:1354–59. doi: 10.1073/pnas.0610542104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown WL, Mastico RA, Wu M, Heal KG, Adams CJ, et al. RNA bacteriophage capsid-mediated drug delivery and epitope presentation. Intervirology. 2002;45:371–80. doi: 10.1159/000067930. [DOI] [PubMed] [Google Scholar]
- 25.Wu M, Brown WL, Stockley PG. Cell-specific delivery of bacteriophage-encapsidated ricin A chain. Bioconjug Chem. 1995;6:587–95. doi: 10.1021/bc00035a013. [DOI] [PubMed] [Google Scholar]
- 26.Pokorski JK, Steinmetz NF. The art of engineering viral nanoparticles. Mol Pharm. 2011;8:29–43. doi: 10.1021/mp100225y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wen AM, Shukla S, Saxena P, Aljabali AA, Yildiz I, et al. Interior engineering of a viral nanoparticle and its tumor homing properties. Biomacromolecules. 2012;13:3990–4001. doi: 10.1021/bm301278f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prasuhn DE, Jr, Yeh RM, Obenaus A, Manchester M, Finn MG. Viral MRI contrast agents: coordination of Gd by native virions and attachment of Gd complexes by azide-alkyne cycloaddition. Chem Commun. 2007;2007:1269–71. doi: 10.1039/b615084e. [DOI] [PubMed] [Google Scholar]
- 29.Yildiz I, Lee KL, Chen K, Shukla S, Steinmetz NF. Infusion of imaging and therapeutic molecules into the plant virus-based carrier cowpea mosaic virus: cargo-loading and delivery. J Control Release. 2013;172:568–78. doi: 10.1016/j.jconrel.2013.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Young M, Willits D, Uchida M, Douglas T. Plant viruses as biotemplates for materials and their use in nanotechnology. Annu Rev Phytopathol. 2008;46:361–84. doi: 10.1146/annurev.phyto.032508.131939. [DOI] [PubMed] [Google Scholar]
- 31.Kovacs EW, Hooker JM, Romanini DW, Holder PG, Berry KE, Francis MB. Dual-surface-modified bacteriophage MS2 as an ideal scaffold for a viral capsid-based drug delivery system. Bioconjug Chem. 2007;18:1140–47. doi: 10.1021/bc070006e. [DOI] [PubMed] [Google Scholar]
- 32.Schlick TL, Ding Z, Kovacs EW, Francis MB. Dual-surface modification of the tobacco mosaic virus. J Am Chem Soc. 2005;127:3718–23. doi: 10.1021/ja046239n. [DOI] [PubMed] [Google Scholar]
- 33.Bruckman MA, Kaur G, Lee LA, Xie F, Sepulveda J, et al. Surface modification of tobacco mosaic virus with “click” chemistry. ChemBioChem. 2008;9:519–23. doi: 10.1002/cbic.200700559. [DOI] [PubMed] [Google Scholar]
- 34.Hong V, Presolski SI, Ma C, Finn MG. Analysis and optimization of copper-catalyzed azide-alkyne cycloaddition for bioconjugation. Angew Chem Int Ed. 2009;48:9879–83. doi: 10.1002/anie.200905087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steinmetz NF, Hong V, Spoerke ED, Lu P, Breitenkamp K, et al. Buckyballs meet viral nanoparticles: candidates for biomedicine. J Am Chem Soc. 2009;131:17093–95. doi: 10.1021/ja902293w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steinmetz NF, Mertens ME, Taurog RE, Johnson JE, Commandeur U, et al. Potato virus X as a novel platform for potential biomedical applications. Nano Lett. 2010;10:305–12. doi: 10.1021/nl9035753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uchida M, Morris DS, Kang S, Jolley CC, Lucon J, et al. Site-directed coordination chemistry with P22 virus-like particles. Langmuir. 2012;28:1998–2006. doi: 10.1021/la203866c. [DOI] [PubMed] [Google Scholar]
- 38.Datta A, Hooker JM, Botta M, Francis MB, Aime S, Raymond KN. High relaxivity gadolinium hydroxypyridonate-viral capsid conjugates: nanosized MRI contrast agents. J Am Chem Soc. 2008;130:2546–52. doi: 10.1021/ja0765363. [DOI] [PubMed] [Google Scholar]
- 39.Hooker JM, Datta A, Botta M, Raymond KN, Francis MB. Magnetic resonance contrast agents from viral capsid shells: a comparison of exterior and interior cargo strategies. Nano Lett. 2007;7:2207–10. doi: 10.1021/nl070512c. [DOI] [PubMed] [Google Scholar]
- 40.Hooker J, O’Neil J, Romanini D, Taylor S, Francis M. Genome-free viral capsids as carriers for positron emission tomography radiolabels. Mol Imaging Biol. 2008;10:182–91. doi: 10.1007/s11307-008-0136-5. [DOI] [PubMed] [Google Scholar]
- 41.Venter PA, Dirksen A, Thomas D, Manchester M, Dawson PE, Schneemann A. Multivalent display of proteins on viral nanoparticles using molecular recognition and chemical ligation strategies. Biomacromolecules. 2011;12:2293–301. doi: 10.1021/bm200369e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carrico ZM, Farkas ME, Zhou Y, Hsiao SC, Marks JD, et al. N-Terminal labeling of filamentous phage to create cancer marker imaging agents. ACS Nano. 2012;6:6675–80. doi: 10.1021/nn301134z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gatto D, Ruedl C, Odermatt B, Bachmann MF. Rapid response of marginal zone B cells to viral particles. J Immunol. 2004;173:4308–16. doi: 10.4049/jimmunol.173.7.4308. [DOI] [PubMed] [Google Scholar]
- 44.Singh P, Prasuhn D, Yeh RM, Destito G, Rae CS, et al. Bio-distribution, toxicity and pathology of cowpea mosaic virus nanoparticles in vivo. J Control Release. 2007;120:41–50. doi: 10.1016/j.jconrel.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Srivastava AS, Kaido T, Carrier E. Immunological factors that affect the in vivo fate of T7 phage in the mouse. J Virol Methods. 2004;115:99–104. doi: 10.1016/j.jviromet.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 46.Kwon OJ, Kang E, Choi JW, Kim SW, Yun CO. Therapeutic targeting of chitosan-PEG-folate-complexed oncolytic adenovirus for active and systemic cancer gene therapy. J Control Release. 2013;169:257–65. doi: 10.1016/j.jconrel.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 47.Pasut G, Veronese FM. PEG conjugates in clinical development or use as anticancer agents: an overview. Adv Drug Deliv Rev. 2009;61:1177–88. doi: 10.1016/j.addr.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 48.Tesfay MZ, Kirk AC, Hadac EM, Griesmann GE, Federspiel MJ, et al. PEGylation of vesicular stomatitis virus extends virus persistence in blood circulation of passively immunized mice. J Virol. 2013;87:3752–59. doi: 10.1128/JVI.02832-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zeng Q, Wen H, Wen Q, Chen X, Wang Y, et al. Cucumber mosaic virus as drug delivery vehicle for doxorubicin. Biomaterials. 2013;34:4632–42. doi: 10.1016/j.biomaterials.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 50.Galaway FA, Stockley PG. MS2 viruslike particles: a robust, semisynthetic targeted drug delivery platform. Mol Pharm. 2013;10:59–68. doi: 10.1021/mp3003368. [DOI] [PubMed] [Google Scholar]
- 51.Huang RK, Steinmetz NF, Fu CY, Manchester M, Johnson JE. Transferrin-mediated targeting of bacteriophage HK97 nanoparticles into tumor cells. Nanomedicine. 2011;6:55–68. doi: 10.2217/nnm.10.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chariou PL, Lee KL, Wen AM, Gulati NM, Stewart PL, Steinmetz NF. Detection and imaging of aggressive cancer cells using an epidermal growth factor receptor (EGFR)-targeted filamentous plant virus-based nanoparticle. Bioconjug Chem. 2015;26:262–69. doi: 10.1021/bc500545z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pokorski JK, Hovlid ML, Finn MG. Cell targeting with hybrid Qβ virus-like particles displaying epidermal growth factor. ChemBioChem. 2011;12:2441–47. doi: 10.1002/cbic.201100469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hovlid ML, Steinmetz NF, Laufer B, Lau JL, Kuzelka J, et al. Guiding plant virus particles to integrin-displaying cells. Nanoscale. 2012;4:3698–705. doi: 10.1039/c2nr30571b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shukla S, Ablack AL, Wen AM, Lee KL, Lewis JD, Steinmetz NF. Increased tumor homing and tissue penetration of the filamentous plant viral nanoparticle Potato virus X. Mol Pharm. 2013;10:33–42. doi: 10.1021/mp300240m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wen AM, Wang Y, Jiang K, Hsu GC, Gao H, et al. Shaping bio-inspired nanotechnologies to target thrombosis for dual optical-magnetic resonance imaging. J Mater Chem B. 2015;3:6037–45. doi: 10.1039/C5TB00879D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shukla S, Eber FJ, Nagarajan AS, DiFranco NA, Schmidt N, et al. The impact of aspect ratio on the biodistribution and tumor homing of rigid soft-matter nanorods. Adv Healthc Mater. 2015;4:874–82. doi: 10.1002/adhm.201400641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cao J, Guenther RH, Sit TL, Opperman CH, Lommel SA, Willoughby JA. Loading and release mechanism of Red clover necrotic mosaic virus derived plant viral nanoparticles for drug delivery of doxorubicin. Small. 2014;10:5126–36. doi: 10.1002/smll.201400558. [DOI] [PubMed] [Google Scholar]
- 59.Choi KM, Kim K, Kwon IC, Kim IS, Ahn HJ. Systemic delivery of siRNA by chimeric capsid protein: tumor targeting and RNAi activity in vivo. Mol Pharm. 2012;10:18–25. doi: 10.1021/mp300211a. [DOI] [PubMed] [Google Scholar]
- 60.Azizgolshani O, Garmann RF, Cadena-Nava R, Knobler CM, Gelbart WM. Reconstituted plant viral capsids can release genes to mammalian cells. Virology. 2013;441:12–17. doi: 10.1016/j.virol.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 61.Aljabali AA, Shukla S, Lomonossoff GP, Steinmetz NF, Evans DJ. CPMV-DOX delivers. Mol Pharm. 2013;10:3–10. doi: 10.1021/mp3002057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ren Y, Wong SM, Lim LY. Folic acid-conjugated protein cages of a plant virus: a novel delivery platform for doxorubicin. Bioconjug Chem. 2007;18:836–43. doi: 10.1021/bc060361p. [DOI] [PubMed] [Google Scholar]
- 63.Pokorski JK, Breitenkamp K, Liepold LO, Qazi S, Finn MG. Functional virus-based polymer-protein nanoparticles by atom transfer radical polymerization. J Am Chem Soc. 2011;133:9242–45. doi: 10.1021/ja203286n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hovlid ML, Lau JL, Breitenkamp K, Higginson CJ, Laufer B, et al. Encapsidated atom-transfer radical polymerization in Qβ virus-like nanoparticles. ACS Nano. 2014;8:8003–14. doi: 10.1021/nn502043d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rhee JK, Baksh M, Nycholat C, Paulson JC, Kitagishi H, Finn MG. Glycan-targeted virus-like nanoparticles for photodynamic therapy. Biomacromolecules. 2012;13:2333–38. doi: 10.1021/bm300578p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Millán JG, Brasch M, Anaya-Plaza E, de la Escosura A, Velders AH, et al. Self-assembly triggered by self-assembly: optically active, paramagnetic micelles encapsulated in protein cage nanoparticles. J Inorg Biochem. 2014;136:140–46. doi: 10.1016/j.jinorgbio.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 67.Everts M, Saini V, Leddon JL, Kok RJ, Stoff-Khalili M, et al. Covalently linked Au nanoparticles to a viral vector: potential for combined photothermal and gene cancer therapy. Nano Lett. 2006;6:587–91. doi: 10.1021/nl0500555. [DOI] [PubMed] [Google Scholar]
- 68.Greenwood B, Salisbury D, Hill AV. Vaccines and global health. Philos Trans R Soc B. 2011;366:2733–42. doi: 10.1098/rstb.2011.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Levine MM, Robins-Browne R. Vaccines, global health and social equity. Immunol Cell Biol. 2009;87:274–78. doi: 10.1038/icb.2009.15. [DOI] [PubMed] [Google Scholar]
- 70.McElrath MJ, Walker BD. Is an HIV vaccine possible? JAIDS. 2012;60(Suppl. 2):S41–43. doi: 10.1097/QAI.0b013e31825b7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ottenhoff TH, Kaufmann SH. Vaccines against tuberculosis: Where are we and where do we need to go? PLOS Pathog. 2012;8:e1002607. doi: 10.1371/journal.ppat.1002607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thera MA, Plowe CV. Vaccines for malaria: How close are we? Annu Rev Med. 2012;63:345–57. doi: 10.1146/annurev-med-022411-192402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chackerian B, Rangel M, Hunter Z, Peabody DS. Virus and virus-like particle-based immunogens for Alzheimer’s disease induce antibody responses against amyloid-β without concomitant T cell responses. Vaccine. 2006;24:6321–31. doi: 10.1016/j.vaccine.2006.05.059. [DOI] [PubMed] [Google Scholar]
- 74.Kushnir N, Streatfield SJ, Yusibov V. Virus-like particles as a highly efficient vaccine platform: diversity of targets and production systems and advances in clinical development. Vaccine. 2012;31:58–83. doi: 10.1016/j.vaccine.2012.10.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Miermont A, Barnhill H, Strable E, Lu X, Wall KA, et al. Cowpea mosaic virus capsid: a promising carrier for the development of carbohydrate based antitumor vaccines. Chemistry. 2008;14:4939–47. doi: 10.1002/chem.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yin Z, Nguyen HG, Chowdhury S, Bentley P, Bruckman MA, et al. Tobacco mosaic virus as a new carrier for tumor associated carbohydrate antigens. Bioconjug Chem. 2012;23:1694–703. doi: 10.1021/bc300244a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Parmiani G, Russo V, Maccalli C, Parolini D, Rizzo N, Maio M. Peptide-based vaccines for cancer therapy. Hum Vaccines Immunother. 2014;10:3175–78. doi: 10.4161/hv.29418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Geynisman D, Chien CR, Smieliauskas F, Shen C, Tina Shih YC. Economic evaluation of therapeutic cancer vaccines and immunotherapy: a systematic review. Hum Vaccines Immunother. 2014;10:3415–24. doi: 10.4161/hv.29407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tagliamonte M, Petrizzo A, Tornesello ML, Buonaguro FM, Buonaguro L. Antigen-specific vaccines for cancer treatment. Hum Vaccines Immunother. 2014;10:3332–46. doi: 10.4161/21645515.2014.973317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Winter H, Fox BA, Ruttinger D. Future of cancer vaccines. Methods Mol Biol. 2014;1139:555–64. doi: 10.1007/978-1-4939-0345-0_40. [DOI] [PubMed] [Google Scholar]
- 81.Garcea RL, Gissmann L. Virus-like particles as vaccines and vessels for the delivery of small molecules. Curr Opin Biotechnol. 2004;15:513–17. doi: 10.1016/j.copbio.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 82.Grgacic EV, Anderson DA. Virus-like particles: passport to immune recognition. Methods. 2006;40:60–65. doi: 10.1016/j.ymeth.2006.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ludwig C, Wagner R. Virus-like particles—universal molecular toolboxes. Curr Opin Biotechnol. 2007;18:537–45. doi: 10.1016/j.copbio.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Folb PI, Bernatowska E, Chen R, Clemens J, Dodoo AN, et al. A global perspective on vaccine safety and public health: the Global Advisory Committee on Vaccine Safety. Am J Public Health. 2004;94:1926–31. doi: 10.2105/ajph.94.11.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kelso JM. Safety of influenza vaccines. Curr Opin Allergy Clin Immunol. 2012;12:383–88. doi: 10.1097/ACI.0b013e328354395d. [DOI] [PubMed] [Google Scholar]
- 86.Tozzi AE, Asturias EJ, Balakrishnan MR, Halsey NA, Law B, Zuber PL. Assessment of causality of individual adverse events following immunization (AEFI): a WHO tool for global use. Vaccine. 2013;44:5041–46. doi: 10.1016/j.vaccine.2013.08.087. [DOI] [PubMed] [Google Scholar]
- 87.Klinman DM, Takeno M, Ichino M, Gu M, Yamshchikov G, et al. DNA vaccines: safety and efficacy issues. Springer Semin Immunopathol. 1997;19:245–56. doi: 10.1007/BF00870272. [DOI] [PubMed] [Google Scholar]
- 88.Aguilar JC, Rodriguez EG. Vaccine adjuvants revisited. Vaccine. 2007;25:3752–62. doi: 10.1016/j.vaccine.2007.01.111. [DOI] [PubMed] [Google Scholar]
- 89.Awate S, Babiuk LA, Mutwiri G. Mechanisms of action of adjuvants. Front Immunol. 2013;4:114. doi: 10.3389/fimmu.2013.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bramwell VW, Perrie Y. The rational design of vaccines. Drug Discov Today. 2005;10:1527–34. doi: 10.1016/S1359-6446(05)03600-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Leleux J, Roy K. Micro and nanoparticle-based delivery systems for vaccine immunotherapy: an immunological and materials perspective. Adv Healthc Mater. 2013;2:72–94. doi: 10.1002/adhm.201200268. [DOI] [PubMed] [Google Scholar]
- 92.De Temmerman ML, Rejman J, Demeester J, Irvine DJ, Gander B, De Smedt SC. Particulate vaccines: on the quest for optimal delivery and immune response. Drug Discov Today. 2011;16:569–82. doi: 10.1016/j.drudis.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 93.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 94.Neefjes J, Jongsma ML, Paul P, Bakke O. Towards a systems understanding of MHC class I and MHC class II antigen presentation. Nat Rev Immunol. 2011;11:823–36. doi: 10.1038/nri3084. [DOI] [PubMed] [Google Scholar]
- 95.Vyas JM, Van der Veen AG, Ploegh HL. The known unknowns of antigen processing and presentation. Nat Rev Immunol. 2008;8:607–18. doi: 10.1038/nri2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pushko P, Pumpens P, Grens E. Development of virus-like particle technology from small highly symmetric to large complex virus-like particle structures. Intervirology. 2013;56:141–65. doi: 10.1159/000346773. [DOI] [PubMed] [Google Scholar]
- 97.Roldao A, Mellado MC, Castilho LR, Carrondo MJ, Alves PM. Virus-like particles in vaccine development. Expert Rev Vaccines. 2010;9:1149–76. doi: 10.1586/erv.10.115. [DOI] [PubMed] [Google Scholar]
- 98.Chan JK, Berek JS. Impact of the human papilloma vaccine on cervical cancer. J Clin Oncol. 2007;25:2975–82. doi: 10.1200/JCO.2007.10.8662. [DOI] [PubMed] [Google Scholar]
- 99.Sominskaya I, Skrastina D, Dislers A, Vasiljev D, Mihailova M, et al. Construction and immunological evaluation of multivalent hepatitis B virus (HBV) core virus-like particles carrying HBV and HCV epitopes. Clin Vaccine Immunol. 2010;17:1027–33. doi: 10.1128/CVI.00468-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cheong WS, Reiseger J, Turner SJ, Boyd R, Netter HJ. Chimeric virus-like particles for the delivery of an inserted conserved influenza A-specific CTL epitope. Antiviral Res. 2009;81:113–22. doi: 10.1016/j.antiviral.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 101.Brennan FR, Gilleland LB, Staczek J, Bendig MM, Hamilton WD, Gilleland HE., Jr A chimaeric plant virus vaccine protects mice against a bacterial infection. Microbiology. 1999;145:2061–67. doi: 10.1099/13500872-145-8-2061. [DOI] [PubMed] [Google Scholar]
- 102.Brennan K, McSherry EA, Hudson L, Kay EW, Hill AD, et al. Junctional adhesion molecule-A is co-expressed with HER2 in breast tumors and acts as a novel regulator of HER2 protein degradation and signaling. Oncogene. 2013;32:2799–804. doi: 10.1038/onc.2012.276. [DOI] [PubMed] [Google Scholar]
- 103.Koo M, Bendahmane M, Lettieri GA, Paoletti AD, Lane TE, et al. Protective immunity against murine hepatitis virus (MHV) induced by intranasal or subcutaneous administration of hybrids of tobacco mosaic virus that carries an MHV epitope. PNAS. 1999;96:7774–79. doi: 10.1073/pnas.96.14.7774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nuzzaci M, Piazzolla G, Vitti A, Lapelosa M, Tortorella C, et al. Cucumber mosaic virus as a presentation system for a double hepatitis C virus-derived epitope. Arch Virol. 2007;152:915–28. doi: 10.1007/s00705-006-0916-7. [DOI] [PubMed] [Google Scholar]
- 105.Rennermalm A, Li YH, Bohaufs L, Jarstrand C, Brauner A, et al. Antibodies against a truncated Staphylococcus aureus fibronectin-binding protein protect against dissemination of infection in the rat. Vaccine. 2001;19:3376–83. doi: 10.1016/s0264-410x(01)00080-9. [DOI] [PubMed] [Google Scholar]
- 106.Wu L, Jiang L, Zhou Z, Fan J, Zhang Q, et al. Expression of foot-and-mouth disease virus epitopes in tobacco by a tobacco mosaic virus-based vector. Vaccine. 2003;21:4390–98. doi: 10.1016/s0264-410x(03)00428-6. [DOI] [PubMed] [Google Scholar]
- 107.Yusibov V, Mett V, Mett V, Davidson C, Musiychuk K, et al. Peptide-based candidate vaccine against respiratory syncytial virus. Vaccine. 2005;23:2261–65. doi: 10.1016/j.vaccine.2005.01.039. [DOI] [PubMed] [Google Scholar]
- 108.Caldeira JdC, Medford A, Kines RC, Lino CA, Schiller JT, et al. Immunogenic display of diverse peptides, including a broadly cross-type neutralizing human papillomavirus L2 epitope, on virus-like particles of the RNA bacteriophage PP7. Vaccine. 2010;28:4384–93. doi: 10.1016/j.vaccine.2010.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Richert LE, Rynda-Apple A, Harmsen AL, Han S, Wiley JA, et al. CD11c+ cells primed with unrelated antigens facilitate an accelerated immune response to influenza virus in mice. Eur J Immunol. 2014;44:397–408. doi: 10.1002/eji.201343587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Manayani DJ, Thomas D, Dryden KA, Reddy V, Siladi ME, et al. A viral nanoparticle with dual function as an anthrax antitoxin and vaccine. PLOS Pathog. 2007;3:1422–31. doi: 10.1371/journal.ppat.0030142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bhatia BD, Chug S, Narang P, Singh MN. Bacterial flora of newborns at birth and 72 hours of age. Indian Pediatr. 1988;25:1058–65. [PubMed] [Google Scholar]
- 112.Schiller JT, Lowy DR. Papillomavirus-like particle vaccines. J Natl Cancer Inst Monogr. 2001;2000:50–54. doi: 10.1093/oxfordjournals.jncimonographs.a024258. [DOI] [PubMed] [Google Scholar]
- 113.Skrastina D, Petrovskis I, Petraityte R, Sominskaya I, Ose V, et al. Chimeric derivatives of hepatitis B virus core particles carrying major epitopes of the rubella virus E1 glycoprotein. Clin Vaccine Immunol. 2013;20:1719–28. doi: 10.1128/CVI.00533-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schneemann A, Speir JA, Tan GS, Khayat R, Ekiert DC, et al. A virus-like particle that elicits cross-reactive antibodies to the conserved stem of influenza virus hemagglutinin. J Virol. 2012;86:11686–97. doi: 10.1128/JVI.01694-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rynda-Apple A, Patterson DP, Douglas T. Virus-like particles as antigenic nanomaterials for inducing protective immune responses in the lung. Nanomedicine. 2014;9:1857–68. doi: 10.2217/nnm.14.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yaddanapudi K, Mitchell RA, Eaton JW. Cancer vaccines: looking to the future. Oncoimmunology. 2013;2:e23403. doi: 10.4161/onci.23403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ryan SO, Turner MS, Gariepy J, Finn OJ. Tumor antigen epitopes interpreted by the immune system as self or abnormal-self differentially affect cancer vaccine responses. Cancer Res. 2010;70:5788–96. doi: 10.1158/0008-5472.CAN-09-4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rosenberg SA. The identification of cancer antigens: impact on the development of cancer vaccines. Cancer J. 2000;6(Suppl. 2):S142–49. [PubMed] [Google Scholar]
- 119.Shukla S, Wen AM, Commandeur U, Steinmetz NF. Presentation of HER2 epitopes using a filamentous plant virus-based vaccination platform. J Mater Chem B. 2014;2:6249–58. doi: 10.1039/c4tb00749b. [DOI] [PubMed] [Google Scholar]
- 120.Chackerian B. Virus-like particle based vaccines for Alzheimer disease. Hum Vaccines. 2010;6:926–30. doi: 10.4161/hv.7.1.12655. [DOI] [PubMed] [Google Scholar]
- 121.Jin H, Wang W, Zhao S, Yang W, Qian Y, et al. Aβ-HBc virus-like particles immunization without additional adjuvant ameliorates the learning and memory and reduces Aβ deposit in PDAPP mice. Vaccine. 2014;32:4450–56. doi: 10.1016/j.vaccine.2014.06.051. [DOI] [PubMed] [Google Scholar]
- 122.Feng G, Wang W, Qian Y, Jin H. Anti-Aβ antibodies induced by Aβ-HBc virus-like particles prevent Aβ aggregation and protect PC12 cells against toxicity of Aβ1–40. J Neurosci Methods. 2013;218:48–54. doi: 10.1016/j.jneumeth.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 123.Maurer P, Jennings GT, Willers J, Rohner F, Lindman Y, et al. A therapeutic vaccine for nicotine dependence: preclinical efficacy, and phase I safety and immunogenicity. Eur J Immunol. 2005;35:2031–40. doi: 10.1002/eji.200526285. [DOI] [PubMed] [Google Scholar]
- 124.Cornuz J, Zwahlen S, Jungi WF, Osterwalder J, Klingler K, et al. A vaccine against nicotine for smoking cessation: a randomized controlled trial. PLOS ONE. 2008;3:e2547. doi: 10.1371/journal.pone.0002547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Robertson KL, Liu JL. Engineered viral nanoparticles for flow cytometry and fluorescence microscopy applications. WIRES Nanomed Nanobiotechnol. 2012;4:511–24. doi: 10.1002/wnan.1177. [DOI] [PubMed] [Google Scholar]
- 126.Koudelka KJ, Manchester M. Chemically modified viruses: principles and applications. Curr Opin Chem Biol. 2010;14:810–17. doi: 10.1016/j.cbpa.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 127.Schoonen L, van Hest JC. Functionalization of protein-based nanocages for drug delivery applications. Nanoscale. 2014;6:7124–41. doi: 10.1039/c4nr00915k. [DOI] [PubMed] [Google Scholar]
- 128.Ghosh D, Kohli AG, Moser F, Endy D, Belcher AM. Refactored M13 bacteriophage as a platform for tumor cell imaging and drug delivery. ACS Synth Biol. 2012;1:576–82. doi: 10.1021/sb300052u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shukla S, Dickmeis C, Nagarajan AS, Fischer R, Commandeur U, Steinmetz NF. Molecular farming of fluorescent virus-based nanoparticles for optical imaging in plants, human cells and mouse models. Biomater Sci. 2014;2:784–97. doi: 10.1039/c3bm60277j. [DOI] [PubMed] [Google Scholar]
- 130.Koudelka KJ, Ippoliti S, Medina E, Shriver LP, Trauger SA, et al. Lysine addressability and mammalian cell interactions of bacteriophage λ procapsids. Biomacromolecules. 2013;14:4169–76. doi: 10.1021/bm401577f. [DOI] [PubMed] [Google Scholar]
- 131.Chang JR, Song EH, Nakatani-Webster E, Monkkonen L, Ratner DM, Catalano CE. Phage lambda capsids as tunable display nanoparticles. Biomacromolecules. 2014;15:4410–19. doi: 10.1021/bm5011646. [DOI] [PubMed] [Google Scholar]
- 132.Tsvetkova IB, Cheng F, Ma Z, Moore AW, Howard B, et al. Fusion of mApple and Venus fluorescent proteins to the Sindbis virus E2 protein leads to different cell-binding properties. Virus Res. 2013;177:138–46. doi: 10.1016/j.virusres.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cheng F, Tsvetkova IB, Khuong YL, Moore AW, Arnold RJ, et al. The packaging of different cargo into enveloped viral nanoparticles. Mol Pharm. 2012;10:51–58. doi: 10.1021/mp3002667. [DOI] [PubMed] [Google Scholar]
- 134.Jin HE, Farr R, Lee SW. Collagen mimetic peptide engineered M13 bacteriophage for collagen targeting and imaging in cancer. Biomaterials. 2014;33:9236–45. doi: 10.1016/j.biomaterials.2014.07.044. [DOI] [PubMed] [Google Scholar]
- 135.Grove J. Super-resolution microscopy: a virus’ eye view of the cell. Viruses. 2014;6:1365–78. doi: 10.3390/v6031365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Obermeyer AC, Capehart SL, Jarman JB, Francis MB. Multivalent viral capsids with internal cargo for fibrin imaging. PLOS ONE. 2014;9:e100678. doi: 10.1371/journal.pone.0100678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jung B, Rao AL, Anvari B. Optical nano-constructs composed of genome-depleted brome mosaic virus doped with a near infrared chromophore for potential biomedical applications. ACS Nano. 2011;5:1243–52. doi: 10.1021/nn1028696. [DOI] [PubMed] [Google Scholar]
- 138.Jung B, Anvari B. Virus-mimicking optical nanomaterials: near infrared absorption and fluorescence characteristics and physical stability in biological environments. ACS Appl Mater Interfaces. 2013;5:7492–500. doi: 10.1021/am401800w. [DOI] [PubMed] [Google Scholar]
- 139.Leeuw TK, Reith RM, Simonette RA, Harden ME, Cherukuri P, et al. Single-walled carbon nanotubes in the intact organism: near-IR imaging and biocompatibility studies in Drosophila. Nano Lett. 2007;7:2650–54. doi: 10.1021/nl0710452. [DOI] [PubMed] [Google Scholar]
- 140.Yi H, Ghosh D, Ham MH, Qi J, Barone PW, et al. M13 phage-functionalized single-walled carbon nanotubes as nanoprobes for second near-infrared window fluorescence imaging of targeted tumors. Nano Lett. 2012;12:1176–83. doi: 10.1021/nl2031663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ghosh D, Bagley AF, Na YJ, Birrer MJ, Bhatia SN, Belcher AM. Deep, noninvasive imaging and surgical guidance of submillimeter tumors using targeted M13-stabilized single-walled carbon nanotubes. PNAS. 2014;111:13948–53. doi: 10.1073/pnas.1400821111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Qazi S, Liepold LO, Abedin MJ, Johnson B, Prevelige P, et al. P22 viral capsids as nanocomposite high-relaxivity MRI contrast agents. Mol Pharm. 2013;10:11–17. doi: 10.1021/mp300208g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Min J, Jung H, Shin HH, Cho G, Cho H, Kang S. Implementation of P22 viral capsids as intravascular magnetic resonance T1 contrast conjugates via site-selective attachment of Gd(III)-chelating agents. Biomacromolecules. 2013;14:2332–39. doi: 10.1021/bm400461j. [DOI] [PubMed] [Google Scholar]
- 144.Qazi S, Uchida M, Usselman R, Shearer R, Edwards E, Douglas T. Manganese(III) porphyrins complexed with P22 virus-like particles as T1-enhanced contrast agents for magnetic resonance imaging. J Biol Inorg Chem. 2014;19:237–46. doi: 10.1007/s00775-013-1075-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bruckman MA, Hern S, Jiang K, Flask CA, Yu X, Steinmetz NF. Tobacco mosaic virus rods and spheres as supramolecular high-relaxivity MRI contrast agents. J Mater Chem B. 2013;1:1482–90. doi: 10.1039/C3TB00461A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bruckman MA, Jiang K, Simpson EJ, Randolph LN, Luyt LG, et al. Dual-modal magnetic resonance and fluorescence imaging of atherosclerotic plaques in vivo using VCAM-1 targeted tobacco mosaic virus. Nano Lett. 2014;14:1551–58. doi: 10.1021/nl404816m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ghosh D, Lee Y, Thomas S, Kohli AG, Yun DS, et al. M13-templated magnetic nanoparticles for targeted in vivo imaging of prostate cancer. Nat Nanotechnol. 2012;7:677–82. doi: 10.1038/nnano.2012.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Huang X, Stein BD, Cheng H, Malyutin A, Tsvetkova IB, et al. Magnetic virus-like nanoparticles in N. benthamiana plants: a new paradigm for environmental and agronomic biotechnological research. ACS Nano. 2011;5:4037–45. doi: 10.1021/nn200629g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Meldrum T, Seim KL, Bajaj VS, Palaniappan KK, Wu W, et al. A xenon-based molecular sensor assembled on an MS2 viral capsid scaffold. J Am Chem Soc. 2010;132:5936–37. doi: 10.1021/ja100319f. [DOI] [PubMed] [Google Scholar]
- 150.Palaniappan KK, Ramirez RM, Bajaj VS, Wemmer DE, Pines A, Francis MB. Molecular imaging of cancer cells using a bacteriophage-based 129Xe NMR biosensor. Angew Chem Int Ed. 2013;52:4849–53. doi: 10.1002/anie.201300170. [DOI] [PubMed] [Google Scholar]
- 151.Farkas ME, Aanei IL, Behrens CR, Tong GJ, Murphy ST, et al. PET imaging and biodistribution of chemically modified bacteriophage MS2. Mol Pharm. 2013;10:69–76. doi: 10.1021/mp3003754. [DOI] [PubMed] [Google Scholar]
- 152.Mukherjee S, Pfeifer CM, Johnson JM, Liu J, Zlotnick A. Redirecting the coat protein of a spherical virus to assemble into tubular nanostructures. J Am Chem Soc. 2006;128:2538–39. doi: 10.1021/ja056656f. [DOI] [PubMed] [Google Scholar]
- 153.Bruckman MA, VanMeter A, Steinmetz NF. Nanomanufacturing of tobacco mosaic virus-based spherical biomaterials using a continuous flow method. ACS Biomater Sci Eng. 2015;1:13–18. doi: 10.1021/ab500059s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Patterson DP, Schwarz B, El-Boubbou K, van der Oost J, Prevelige PE, Douglas T. Virus-like particle nanoreactors: programmed encapsulation of the thermostable CelB glycosidase inside the P22 capsid. Soft Matter. 2012;8:10158–66. [Google Scholar]
- 155.Carette N, Engelkamp H, Akpa E, Pierre SJ, Cameron NR, et al. A virus-based biocatalyst. Nat Nanotechnol. 2007;2:226–29. doi: 10.1038/nnano.2007.76. [DOI] [PubMed] [Google Scholar]
- 156.Guo P, Lee TJ. Viral nanomotors for packaging of dsDNA and dsRNA. Mol Microbiol. 2007;64:886–903. doi: 10.1111/j.1365-2958.2007.05706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Schneemann A, Young MJ. Viral assembly using heterologous expression systems and cell extracts. Adv Protein Chem. 2003;64:1–36. doi: 10.1016/s0065-3233(03)01001-5. [DOI] [PubMed] [Google Scholar]
- 158.Gillitzer E, Suci P, Young M, Douglas T. Controlled ligand display on a symmetrical protein-cage architecture through mixed assembly. Small. 2006;2:962–66. doi: 10.1002/smll.200500433. [DOI] [PubMed] [Google Scholar]
- 159.Douglas T, Young M. Host-guest encapsulation of materials by assembled virus protein cages. Nature. 1998;393:152–55. [Google Scholar]
- 160.Ren Y, Wong SM, Lim LY. In vitro-reassembled plant virus-like particles for loading of polyacids. J Gen Virol. 2006;87:2749–54. doi: 10.1099/vir.0.81944-0. [DOI] [PubMed] [Google Scholar]
- 161.Phelps JP, Dang N, Rasochova L. Inactivation and purification of cowpea mosaic virus-like particles displaying peptide antigens from Bacillus anthracis. J Virol Methods. 2007;141:146–53. doi: 10.1016/j.jviromet.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lytle CD, Sagripanti JL. Predicted inactivation of viruses of relevance to biodefense by solar radiation. J Virol. 2005;79:14244–52. doi: 10.1128/JVI.79.22.14244-14252.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bruckman MA, Randolph LN, Vanmeter A, Hern S, Shoffstall AJ, et al. Biodistribution, pharmacokinetics, and blood compatibility of native and PEGylated tobacco mosaic virus nano-rods and-spheres in mice. Virology. 2014;449:163–73. doi: 10.1016/j.virol.2013.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kaiser CR, Flenniken ML, Gillitzer E, Harmsen AL, Harmsen AG, et al. Biodistribution studies of protein cage nanoparticles demonstrate broad tissue distribution and rapid clearance in vivo. Int J Nanomed. 2007;2:715–33. [PMC free article] [PubMed] [Google Scholar]
- 165.Ruggiero A, Villa CH, Bander E, Rey DA, Bergkvist M, et al. Paradoxical glomerular filtration of carbon nanotubes. PNAS. 2010;107:12369–74. doi: 10.1073/pnas.0913667107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Singh R, Pantarotto D, Lacerda L, Pastorin G, Klumpp C, et al. Tissue biodistribution and blood clearance rates of intravenously administered carbon nanotube radiotracers. PNAS. 2006;103:3357–62. doi: 10.1073/pnas.0509009103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lee KL, Shukla S, Wu M, Ayat NR, El Sanadi CE, et al. Stealth filaments: Polymer chain length and conformation affect the in vivo fate of PEGylated potato virus X. Acta Biomater. 2015;19:166–79. doi: 10.1016/j.actbio.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Semmler-Behnke M, Kreyling WG, Lipka J, Fertsch S, Wenk A, et al. Biodistribution of 1.4- and 18-nm gold particles in rats. Small. 2008;4:2108–11. doi: 10.1002/smll.200800922. [DOI] [PubMed] [Google Scholar]
- 169.Tarn D, Ashley CE, Xue M, Carnes EC, Zink JI, Brinker CJ. Mesoporous silica nanoparticle nanocarriers: biofunctionality and biocompatibility. Acc Chem Res. 2013;46:792–801. doi: 10.1021/ar3000986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Jaganathan H, Godin B. Biocompatibility assessment of Si-based nano- and micro-particles. Adv Drug Deliv Rev. 2012;64:1800–19. doi: 10.1016/j.addr.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Liu Y, Zhao Y, Sun B, Chen C. Understanding the toxicity of carbon nanotubes. Acc Chem Res. 2013;46:702–13. doi: 10.1021/ar300028m. [DOI] [PubMed] [Google Scholar]
- 172.Shukla S, Wen AM, Ayat NR, Commandeur U, Gopalkrishnan R, et al. Biodistribution and clearance of a filamentous plant virus in healthy and tumor-bearing mice. Nanomedicine. 2014;9:221–35. doi: 10.2217/nnm.13.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Rae CS, Khor IW, Wang Q, Destito G, Gonzalez MJ, et al. Systemic trafficking of plant virus nanoparticles in mice via the oral route. Virology. 2005;343:224–35. doi: 10.1016/j.virol.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 174.Baiu DC, Brazel CS, Bao Y, Otto M. Interactions of iron oxide nanoparticles with the immune system: challenges and opportunities for their use in nano-oncology. Curr Pharm Des. 2013;19:6606–21. doi: 10.2174/13816128113199990409. [DOI] [PubMed] [Google Scholar]
- 175.Meng J, Yang M, Jia F, Xu Z, Kong H, Xu H. Immune responses of BALB/c mice to subcutaneously injected multi-walled carbon nanotubes. Nanotoxicology. 2011;5:583–91. doi: 10.3109/17435390.2010.523483. [DOI] [PubMed] [Google Scholar]
- 176.Raja KS, Wang Q, Gonzalez MJ, Manchester M, Johnson JE, Finn MG. Hybrid virus-polymer materials. 1. Synthesis and properties of PEG-decorated cowpea mosaic virus. Biomacromolecules. 2003;4:472–76. doi: 10.1021/bm025740+. [DOI] [PubMed] [Google Scholar]
- 177.Semple SC, Harasym TO, Clow KA, Ansell SM, Klimuk SK, Hope MJ. Immunogenicity and rapid blood clearance of liposomes containing polyethylene glycol-lipid conjugates and nucleic acid. J Pharmacol Exp Ther. 2005;312:1020–26. doi: 10.1124/jpet.104.078113. [DOI] [PubMed] [Google Scholar]
- 178.Shimizu T, Ishida T, Kiwada H. Transport of PEGylated liposomes from the splenic marginal zone to the follicle in the induction phase of the accelerated blood clearance phenomenon. Immunobiology. 2013;218:725–32. doi: 10.1016/j.imbio.2012.08.274. [DOI] [PubMed] [Google Scholar]
- 179.Harris JM, Chess RB. Effect of pegylation on pharmaceuticals. Nat Rev Drug Discov. 2003;2:214–21. doi: 10.1038/nrd1033. [DOI] [PubMed] [Google Scholar]
- 180.Roberts MJ, Bentley MD, Harris JM. Chemistry for peptide and protein PEGylation. Adv Drug Deliv Rev. 2002;54:459–76. doi: 10.1016/s0169-409x(02)00022-4. [DOI] [PubMed] [Google Scholar]
- 181.Wattendorf U, Merkle HP. PEGylation as a tool for the biomedical engineering of surface modified microparticles. J Pharm Sci. 2008;97:4655–69. doi: 10.1002/jps.21350. [DOI] [PubMed] [Google Scholar]
- 182.Rodriguez PL, Harada T, Christian DA, Pantano DA, Tsai RK, Discher DE. Minimal “self” peptides that inhibit phagocytic clearance and enhance delivery of nanoparticles. Science. 2013;339:971–75. doi: 10.1126/science.1229568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Agrawal A, Manchester M. Differential uptake of chemically modified cowpea mosaic virus nanoparticles in macrophage subpopulations present in inflammatory and tumor microenvironments. Biomacromolecules. 2012;13:3320–26. doi: 10.1021/bm3010885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Plummer EM, Manchester M. Endocytic uptake pathways utilized by CPMV nanoparticles. Mol Pharm. 2013;10:26–32. doi: 10.1021/mp300238w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Plummer EM, Thomas D, Destito G, Shriver LP, Manchester M. Interaction of cowpea mosaic virus nanoparticles with surface vimentin and inflammatory cells in atherosclerotic lesions. Nanomedicine. 2012;7:877–88. doi: 10.2217/nnm.11.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Shriver LP, Koudelka KJ, Manchester M. Viral nanoparticles associate with regions of inflammation and blood brain barrier disruption during CNS infection. J Neuroimmunol. 2009;211:66–72. doi: 10.1016/j.jneuroim.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Steinmetz NF, Cho CF, Ablack A, Lewis JD, Manchester M. Cowpea mosaic virus nanoparticles target surface vimentin on cancer cells. Nanomedicine. 2011;6:351–64. doi: 10.2217/nnm.10.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Steinmetz NF, Maurer J, Sheng H, Bensussan A, Maricic I, et al. Two domains of vimentin are expressed on the surface of lymph node, bone and brain metastatic prostate cancer lines along with the putative stem cell marker proteins CD44 and CD133. Cancers. 2011;3:2870–85. doi: 10.3390/cancers3032870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Koudelka KJ, Destito G, Plummer EM, Trauger SA, Siuzdak G, Manchester M. Endothelial targeting of cowpea mosaic virus (CPMV) via surface vimentin. PLOS Pathog. 2009;5:e1000417. doi: 10.1371/journal.ppat.1000417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Desai MS, Lee SW. Protein-based functional nanomaterial design for bioengineering applications. WIRES Nanomed Nanobiotechnol. 2014;7:69–97. doi: 10.1002/wnan.1303. [DOI] [PubMed] [Google Scholar]


