Abstract
Understanding the hepatic regenerative process has clinical interest, since the effectiveness of many treatments for chronic liver diseases is conditioned by an efficient liver regeneration. Experimental evidence points to the need of a temporal coordination between cytokines, growth factors and metabolic signaling pathways to enable successful liver regeneration. One intracellular mediator that acts as a signal integration node for these processes is the serine-threonine kinase Akt/PKB (Akt). To investigate the contribution of Akt during hepatic regeneration, we performed partial hepatectomy in mice lacking Akt1, Akt2 or both isoforms. We found that absence of Akt1 or Akt2 does not influence liver regeneration after partial hepatectomy. However, hepatic-specific Akt1 and Akt2 null mice show impaired liver regeneration and increase mortality. The major abnormal cellular events observed in total Akt deficient livers were a marked reduction in cell proliferation, cell hypertrophy, glycogenesis and lipid droplets formation. Most importantly, liver-specific deletion of FoxO1, a transcription factor regulated by Akt, rescued the hepatic regenerative capability in Akt1 and Akt2 deficient mice and normalized the cellular events associated with liver regeneration. These results establish an essential role for the Akt-FoxO1 signaling pathway during liver regeneration that has not been previously described.
Keywords: Liver regeneration, Akt, FoxO1
The liver is the only human internal organ that maintains a remarkable ability to regenerate through a process of compensatory hepatocellular hypertrophy and hyperplasia (1, 2). This phenomenon has also been described in experimental models of liver regeneration, such as the well-characterized model of two-thirds partial hepatectomy (PH) in rodents. For instance, partially hepatectomyzed mice recover their total hepatic mass after 7–10 days (3). This regenerative capability of the liver protects the organisms from hepatic parenchyma loss that may be caused by chronic infection, traumatic injury or liver poisoning. Understanding the hepatic regenerative process has clinical interest, since the effectiveness of many treatments for chronic liver diseases, such as resection of tumors and donor liver transplantation, is conditioned by an efficient liver regeneration (4, 5). In the context of hepatocellular carcinoma (HCC), these malignant tumors usually develop in cirrhotic livers, which are characterized by a poor regenerative capacity. Thus, liver resection in cirrhotic patients is a risky therapeutic decision. This is due to several factors, being the diminished regenerative capability of the remnant liver one of them (6, 7). Accordingly, it is necessary to identify new therapeutic targets to stimulate liver regeneration.
Abundant experimental evidence points to multiple signaling molecules and mechanical events that start very early after PHx and enable successful liver regeneration. These include changes in the enzymatic activation of extracellular matrix remodelers (e.g. urokinase plasminogen activator, MMP-9), relative changes in blood flow volume in relation to the remaining hepatic sinusoid, the increment on the bioavailability of certain growth factors (HGF, EGFR ligands), hormones (noradrenalin, serotonin) and cytokines; the activation of transcription factors (STAT3, NF-κB, Notch-NICD, β-catenin) and changes in the transcriptome of hepatocytes. These initial changes prime quiescent hepatocytes to progress through the cell cycle, while preserving their homeostatic metabolic function. Subsequently, parenchyma and then non-parenchymal cells proliferate until the liver recovers its lost mass and, concurrently, a no well define termination phase coordinates the repression of cell proliferation (8, 9). All these findings have helped to increase our knowledge on the mechanisms accounting for liver regeneration. However, we have not reached a full understanding of this process and some questions are still poorly define, such as for example how the metabolic state of the organism is integrated with this regenerative process. One intracellular mediator that plays a major role in cell growth, cell proliferation and glucose/fat metabolism is the serine-threonine kinase Akt/PKB (Akt). In mammals, there are three Akt isoforms (Akt1, Akt2 and Akt3) encoded by three separate genes that share sequence homology and that recognize the same phosphorylation motif (10). Experiments performed in mice that were deficient in each of the Akt/PKB isoforms have shown that despite their homology, the three Akt isoforms have distinct biological functions. For instance, mice deficient in Akt1 exhibit impaired growth (11), vascular dysfunction and decreased leukocyte recruitment in inflammation model (12, 13). Mice deficient in Akt2 show defects in glucose homeostasis and exhibit insulin resistance (14) and Akt3−/− mice exhibit impaired brain development (15). Another factor that may diversify the biological role of the Akt isoforms is their differential tissular expression. In this regard, Akt1 and Akt2 are the only isoforms expressed in the liver, being Akt2 the most abundant isoform accounting for near 85% of total Akt hepatic protein(15).
Preliminary evidence suggests that Akt may play a major role in liver regeneration. For instance, 1) partial hepatectomy induces Akt phosphorylation (16–18). 2) The blockade of Akt activators, such as PI3-K or PDK1, is associated with poor liver regeneration (19, 20). 3) Persistent stimulation of PI3-K/Akt pathway in hepatocytes resulted in an enlarged liver mass (21). Despite these observations, the role of Akt and its different isoforms during liver regeneration remains poorly understood. Thus, to define the role of Akt during liver regeneration, we performed PH in mice deficient in Akt1, Akt2 or both isoforms. We show that absence of Akt1 or Akt2 does not influence liver regeneration after PH. Importantly, deletion of both isoforms results in impaired liver regeneration and increased mortality. The hepatic double mutant mice show a marked reduction in cell proliferation, cell hypertrophy, glycogenesis and neutral lipid accumulation lipid droplets after PH. Of note, genetic ablation of FoxO1 in the liver rescues hepatocyte proliferation, glucose homeostasis and survival in Akt1/Akt2 deficient mice. In agreement with these in vivo results, pharmacological inhibition of FoxO1 in mouse primary hepatocytes stimulates the expression of proliferative markers. These results demonstrate that Akt-FoxO1 signaling pathway is essential for proper liver regeneration.
EXPERIMENTAL PROCEDURES
Mice
Experiments were performed in eight-old-week male Akt1−/− and Akt2−/− (Jackson Laboratory, CA, USA), Akt1loxP/loxP (22), Akt2loxP/loxP (23) FoxO1loxP/loxP (24) mice. In addition, we generated the Akt1loxP/loxP;Akt2loxP/loxP;FoxO1loxP/loxP mice (TLKO) by crossing the Akt1loxP/loxP;Akt2loxP/loxP mice (DLKO) with the FoxO1loxP/loxP mice, as previously reported (25). These mice were on the 129–C57BL–6J/FVB mixed background. We recovered the Akt1loxP/loxP;Akt2loxP/loxP mice on the same background and used them to compare the DLKO against TLKO (Akt1loxP/loxP;Akt2loxP/loxP and FoxO1loxP/loxP). All animals were kept under constant temperature and humidity in a 12 hours controlled dark/light cycle. Mice were fed ad libitum on a standard pellet diet. The liver specific Akt1/Akt2 (DLKO) and Akt1/Akt2/FoxO1 (TLKO) deficient mice were obtained by injecting eight-old week male with adeno-associated virus encoding Cre-recombinase under the control of the thyroxine-binding globulin (Tbg) promoter (AAV8-Tgb-Cre), which led to the hepatocyte-specific deletion of both Akt1 and Akt2. The experiments were performed 2 weeks after virus injection.
Surgical Procedure
All surgeries were carried out under Isoflurane (Sigma-Aldrich, St. Louis, MO) anesthesia. Partial hepatectomy (PH) was performed according to the technique described by Higgins and Anderson (3). The abdomen was opened via a midline incision. Two thirds of the liver (median and left lobes) was removed. After PH, WT mice (n=21), Akt1−/− mice (n=12), Akt2−/− mice (n=21), DLKO (n=12) and TLKO (n=12) were sacrificed at different time points: 2, 4 and 6 days. The regenerating bottom right lobe was snap-frozen into liquid nitrogen and the upper right lobe was fixed in either: 1) 4% paraformaldehyde at 4°C, cryoprotected overnight in 30% sucrose solution, embedded in OCT medium (Tissue-Tek® O.C.T™ Compound, SAKURA) or 2) 10% buffered formaldehyde and embedded in paraffin for future processing. The percentage of liver regeneration was calculated following the formula: weight of non-removed lobes/ Total body weight of mice.
Histology
Frozen sections of 8-µm were rehydrated and lipid droplets deposition was detected by Oil Red O staining (Sigma-Aldrich, St Louis, MO, USA). Sections were rinsed with 60% isopropanol and stained for 20 minutes with filtered Oil Red-O solution (0,5% in isopropanol followed by a 60% dilution in distilled water). After two rinses with 60% isopropanol and distilled water, slides were counterstained with hematoxylin for 4 minutes, rinsed with water and mounted. Hepatic glycogen content was assessed in 8-µm liver sections that were embedded in paraffin and fixed in 10% buffered formaldehyde solution. Sections were periodic acid-Schiff (PAS)-stained, according to the manufacturer’s instruction (Sigma-Aldrich, St Louis, MO, USA) and counterstained with hematoxylin (Sigma-Aldrich, St Louis, MO, USA). Total hepatocyte cell area was quantified in hematoxylin eosin-stained liver sections. The cell areas in the photomicrographs were measured using the software ImageJ (version 1.37, National Institutes of Health, Bethesda, MD). All the histology images were taken using a light microscope coupled with a digital image acquisition system (Nikon Eclipse E600, Kawasaki, Kanagawa, Japan).
Immunofluorescence
Frozen sections of 8-µm were rehydrated, blocked with 5% normal goat serum (NGS) and incubated with mouse anti-Ki-67 (1:100, Abcam) or rabbit anti-CCND1 (1:100, Cell Signaling). Controls without primary antibodies were revealed with Alexa-597 goat-anti-mouse IgG (1:1000, Invitrogen). Samples were analyzed with a fluorescence microscope (Nikon Eclipse E600, Kawasaki, Kanagawa, Japan). The cells were counterstained with DAPI to visualize the nuclei.
Western blot analysis
Tissue lysates were prepared in a lysis buffer (Tris–HCl 20 mM pH 7.4 containing 1% Triton X-100, 0.1% SDS, 50 mM NaCl, 2.5 mM EDTA, 1 mM Na4P2O7 10H2O, 20 mM NaF, 1 mM Na3VO4, 2 mM Pefabloc and Complete® from Roche). Proteins were separated on a 7.5% SDS-polyacrylamide gel (Mini Protean III, BioRad, Richmond, Ca) and transferred for 2 hours at 4°C to nitrocellulose membranes of 0,45 µm (Transblot Transfer Medium, BioRad, Richmond, CA) that were stained with Ponceau-S red as a control for protein loading. The membranes were incubated at 4°C overnight with the following antibodies; rabbit anti-FoxO1 (1:1000, Cell Signaling), rabbit anti-phospho-FoxO1 (Ser256) (1:1000, Cell Signaling), rabbit anti-Phospho-Akt (Ser473) (1:1000, Cell Signaling), mouse anti-Akt1 (1:1000, Cell Signaling), anti Rabbit-Akt2 (1:1000, Cell Signaling), rabbit anti-Akt (1:5000, Cell signaling), rabbit anti-CCND1 (1:1000, Cell Signaling), mouse anti-PCNA (1:1000, Sigma), HSP90 and Tubulin. Next, the membranes were incubated with a donkey ECL-anti-rabbit IgG or sheep ECL-anti-mouse IgG peroxidase-conjugated secondary at 1:5000 dilution (GE Healthcare) for 1 hour at room temperature. The bands were visualized using LuminataTM Forte Western HRP Substrate (Millipore) and ImageQuantTM LAS 4000 (GE Healthcare). Alternatively, the membranes were incubated with secondary fluorescently-labeled antibodies (Molecular Probes, Invitrogen) and proteins bands were visualized using Odyssey Infrared Imaging System (LI-COR Biotecnology). Densitometry analysis of the gels was performed using the imaging software Image J [version 1.37, National Institutes of Health, Bethesda, MD (NIH)].
Biochemical assays
For glucose measurement, 1–2 mm of tissue was cut from the tail tip of mice with a scalpel early in the morning. Then blood was obtained by direct flow, collected in test strips and directly measured in a glucometer (Accu-Chek Active; Roche Diagnostics, Manheim, Germany).
Lipidomics
Livers (50 mg) were homogenized in 500 µL of PBS, and lipids were extracted from 100 µL of the homogenate in the presence of internal standards for each lipid class. The different lipid classes were quantified from the chloroform extracts using shotgun lipidomics based on class separation by MS/MS specific methods, as previously described (25).
Statistics
In the case of homoscedasticity and normally distributed data (assessed by Shapiro-Wilk test) groups were compared using two-sided Student's t-test or ANOVA (analysis of variance) for independent samples. For other types of data, the two-sided Student's t-test Mann–Whitney U test or the Kruskal–Wallis test was used. Tukey's test (with ANOVA) or Dunn's test (with Kruskal-Wallis) were used as post hoc test to perform pairwise comparisons. Survival curves after PH were generated using the product limit method of Kaplan and Meier. The survival curves were compared using the log-rang test. Differences were considered to be significant at a P value <0.05. Data are presented as the mean ± standard error of the mean (SEM).
Study approval
All animal experiments were approved by the Institutional Animal Care Use Committee of New York University Medical Center, the University of Pennsylvania IACUC and the Investigation and Ethics Committees of the Hospital Clinic.
RESULTS
Hepatic Akt1 and Akt2 deficiency results in impaired liver regeneration and increased mortality after partial hepatectomy
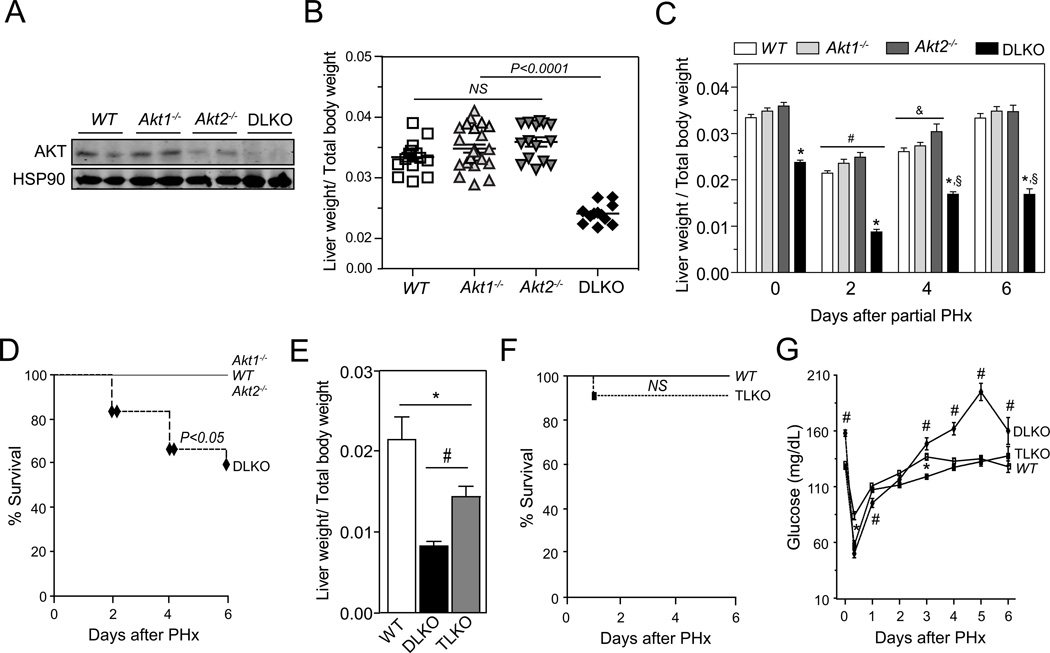
To study the contribution of Akt1 and Akt2 during liver regeneration, we performed two-thirds partial hepatectomy (PH) in wild-type (WT), Akt1−/−, Akt2−/− and DLKO mice. Liver specific mice deficient in Akt1 and Akt2 (DLKO) were generating by breeding Akt2 loxP/loxP with Akt1loxP/loxP mice and injecting them with an adeno-associated virus expressing Cre recombinase under the control of the thyroxine-binding globulin promoter (AAV8-Tgb-Cre), which led to the liver –specific deletion of Akt1 and Akt2 (referred to here as DLKO). Western blot analysis confirmed the efficient deletion of Akt1 and Akt2 in the liver from the DLKO mice 2 weeks after virus injection (Fig. 1A). Morphologically, the livers from Akt1−/− and DLKO mice are smaller than the livers from WT and Akt2−/− mice (data not shown). Despite the difference in liver size, the liver to body-weight ratio found in Akt1−/− and Akt2−/− mice was similar to the WT mice (Fig. 1B). However, the double mutant mice presented a marked decrease of the liver/body weight ratio compared to the other groups of mice, suggesting that Akt is required for physiological liver growth (Fig. 1B). To study the contribution of Akt1 and Akt2 during liver regeneration, we performed two-thirds PH in WT, Akt1−/−, Akt2−/− and DLKO mice. Mice were sacrificed 2, 4, and 6 days after PH and the wet liver remnant weight, together with the total body weight, was used to calculate the hepatic regenerative rate. WT, Akt1−/− and Akt2−/− mice showed a significant and progressive increase in the hepatic regenerative index (from 2–2.5 % at second day to ~3.4% at sixth day) after PH, consistent with what was expected for an efficient liver regeneration. In contrast, DLKO mice showed an increase in the regenerative index early after PH that was interrupted after day 4 without further progression (Fig. 1C). This abnormality observed in the DLKO mice was associated with a marked increase in mortality, compared with WT, Akt1−/− and Akt2−/− mice, that was evident from day 2 after PH (Fig. 1D).
Figure 1. FoxO1 genetic deletion rescue the impaired liver regeneration and increased mortality observed in DLKO mice showed after PH.
(A) Representative Western blot analysis of Akt (total Akt) in liver samples isolated from WT, Akt1−/−, Akt2−/− and DLKO mice. (B) Scatter plot of the hepatic regenerative index (Liver weight/Total body weight) obtained in WT, Akt1−/−, Akt2−/− and DLKO mice in basal conditions before PH. The central horizontal line in each group represents the mean ± S.E.M. (C) Liver to body weight ratio of WT, Akt1−/−, Akt2−/− and DLKO mice at the indicated time points before and after PH. Due to the mortality associated with the DLKO condition, the n varies from 2 to 10 among the different experimental groups. *P<0.01 vs WT, Akt1−/− and Akt2−/−; #P<0.01 vs the same experimental group on day 0, 4 and 6; &P<0.05 vs the same experimental group on day 0 and 6; §P<0.01 vs the same experimental group on day 0 and 2. (D) Survival curves from WT, Akt1−/−, Akt2−/− and DLKO mice after PH were generated using the product limit method of Kaplan and Meier. The survival curves representing the WT, Akt1−/−, Akt2−/− and DLKO (n=10–12) mice were compared using the log-rang test. Black diamonds denote animals that died at follow-up. (E) Liver to body weight ratio of WT, DLKO and TLKO mice two days after PH. *P<0.05; #P<0.01..(F) Survival curves from WT and TLKO mice after PH were generated using the product limit method of Kaplan and Meier. The survival curves representing the WT (n=10) and the TLKO (n=10) mice were compared using the log-rang test. Black squares denote animals that died at follow-up. (G) Blood glucose concentration was measured in WT, DLKO and TLKO before and after PH. Data are expressed as mean ± S.E.M., (n=10). *P<0.05 compared with WT mice at the same time points, #P<0.05 compared with WT and TLKO mice at the same time points.
Liver-specific FoxO1 deletion improved survival rates and liver function in DLKO mice after PH
The FoxO1 transcription factor is a downstream target of Akt and considerable data support a major role of this signaling pathway on the glucoregulation (25, 26). This information points to FoxO1 as a potential mediator of the impaired hepatic regeneration observed in DLKO mice. Thus, we determined the contribution of FoxO1 during hepatic regeneration in mice lacking Akt1, Akt2 and FoxO1 (TLKO). To this end, WT, DLKO and TLKO mice were partially hepatectomized and sacrificed at 0, 2, 4, and 6 days after surgery. DLKO and TLKO mice showed no significant differences in the liver to body weight ratio before PH. At the second day post-hepatectomy, the TLKO mice showed a significant increase in the hepatic regenerative index, compared with the DLKO mice (Fig. 1E). Despite this improvement in liver regeneration, liver/total body weight values were remained lower in the TLKO mice compared to WT mice (Fig. 1E). After 4 days post-hepatectomy, the DLKO and the TLKO mice showed similar regenerative indexes. However, TLKO mice showed a survival rate comparable to that observed in WT mice at 6 days post-hepatectomy (Fig. 1F). This remarkable result contrasts with the high mortality observed in the hepatectomized DLKO group (Fig. 1D) and pointed to the activation of FoxO1 as the mechanism through which hepatic Akt deficiency increases mortality after PH.
After PH, the remnant liver has to compensate the functionality of the resected hepatic mass. This is achieved through both hepatocellular hypertrophy and hyperplasia that helps maintaining key processes such as glucose homeostasis. Failure to do so may result in liver failure and death (1, 2, 8). We expected the high survival rate observed in TLKO mice to be accompanied by an improved control of glucose homeostasis, compared with DLKO. To verify this assumption we measured glycemia and hepatic glycogen storage in WT, DLKO and TLKO mice. Before the PH was performed, DLKO mice showed significant higher levels of blood glucose concentration compared with WT mice (Fig. 1G; 0 days after PH). We have previously described this impaired regulation of the glucose homeostasis in DLKO mice in response to fasting and feeding conditions(25). Under these experimental conditions, we demonstrated the occurrence of hyperglycemia, insulin resistance and lower levels of glycogen content in the DLKO(25). After PH, WT mice developed early hypoglycemia and this situation was normalized after 72 hours post-PH, compared with its basal level. DLKO mice also developed a marked hypoglycemia starting at 12 hours after PH. In addition, DLKO mice showed lower levels of blood glucose concentration at 12 and 24 hours after PH, compared with WT mice. DLKO mice that survived after this period of time displayed a glycemic profile significantly different from the WT mice and characterized by a severe hyperglycemia after 72 hours. In TLKO livers, despite the lack of Akt, the liver adapts appropriately to maintain the euglycemia in basal conditions (Fig. 1G; 0 days after PH). In addition, TLKO mice normalized their glucose profile after PH, and reversed the hyperinsulinemia associated with the liver-specific Akt1 and Akt2 deficiency at later times after PH (Fig. 1G). We next measured hepatic glycogen content in liver samples isolated from WT, DLKO and TLKO mice before and after PH. We found that the three experimental groups showed similar levels of hepatic glycogen before PH (Fig. S1). Glycogen storage diminished drastically in all groups of mice at day 2 post-hepatectomy (Fig. S1). This phenomenon is likely due to fasting that experienced the mice after surgery and the need of the remnant liver to maintaining euglycemia, despite the loss of hepatic mass. WT and TLKO recovered their baseline levels of glycogen at day 6 (Fig. S1). By contrast, hepatic glycogen was barely present in DLKO mice after 6 of PH (Fig. S1). Thus, loss of hepatic FoxO1 activity allows improved glucose metabolism in Akt-deficient hepatectomized livers.
Loss of FoxO1 activity restored hepatocellular hyperplasia in Akt-deficient regenerative livers
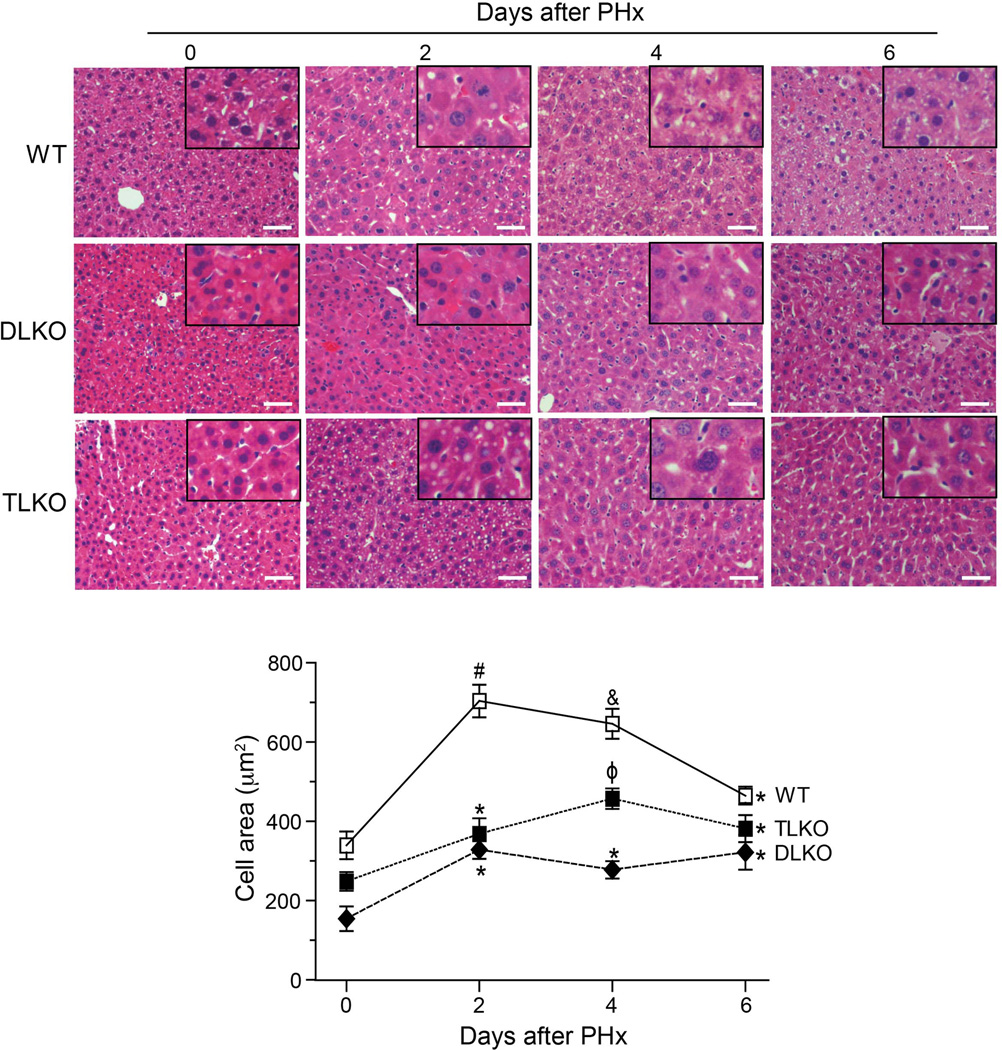
To further understand the mechanism by which FoxO1 deficiency improves survival and liver function in Akt-null livers, we quantified hepatocellular hypertrophy and hyperplasia following PH. To this end, we measured the cellular area of hepatocytes in basal conditions and at different times following PH. Histological examination of livers in basal conditions revealed that loss of Akt1 and Akt2 in livers was associated with a significant decrease in hepatocyte area (Fig. 2, quantified in the bottom panel). The differences in cell size were also apparent in the TLKO mice although to a less extent (Fig. 2, quantified in the bottom panel). Two days after PH, WT mice showed a significant increase in hepatocyte cell area. This hepatocellular hypertrophy decreased in the following days and reached levels similar to baseline values at day 6 post-hepatectomy (Fig. 2, quantified in the bottom panel). By contrast, DLKO and TLKO mice showed a less pronounced hypertrophic response significantly lower than WT mice (Fig. 2, quantified in the bottom panel). FoxO1 deficiency in TLKO mice partially correct the poor hypertrophy observed in the context of dual Akt1/Akt2 loss. However, the maximum increase in cell area observed in TLKO mice was far lower than the cell area quantified in WT mice at day 2 and 4.
Figure 2. Morphometric analysis of hepatocyte cell area in response to partial hepatectomy.
Representative H&E-stained sections of livers isolated from WT, DLKO and TLKO mice before (T=0) and after PH (2, 4 and 6 days). Morphometric analysis of cell area was analyzed as described in Material and Methods. Original magnification ×200. The bottom graph shows the computed-assisted quantification of hepatocyte cell area (mean) before and after PH. Mean ± S.E.M is shown. *P<0.05 vs. 0 days of the same experimental group, #P<0.001 vs. 0, 4 and 6 days of the same experimental group, &P<0.05 vs. 0 and 6 days of the same experimental group and φP<0.001 vs. 0 and 2 days of the same experimental group. The n varies from 2 to 10 among the different experimental groups due to the mortality associated with the DLKO condition.
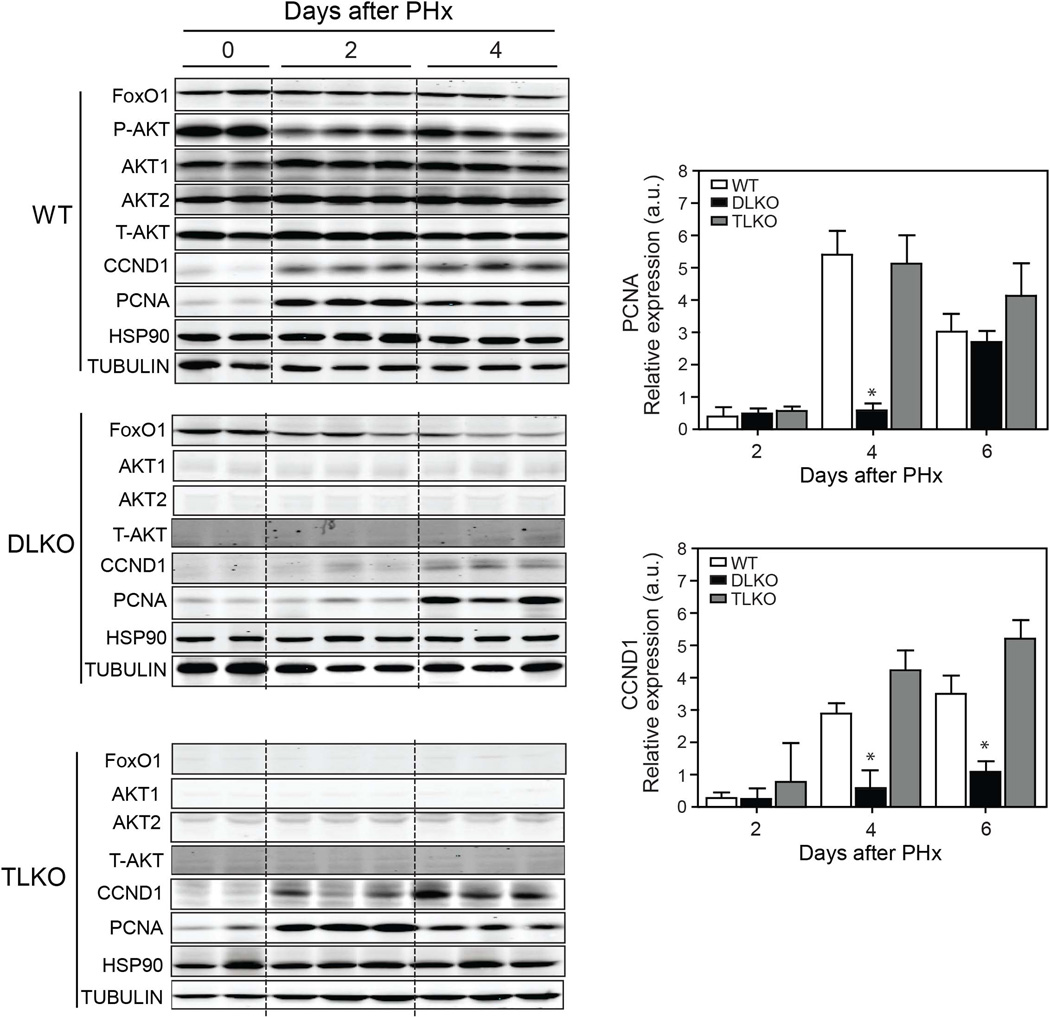
As described above, FoxO1 deficiency does not dramatically alter hepatocyte cell area in DLKO mice after PH. Therefore, other mechanisms different from cellular hypertrophy may account for the improvement in hepatic regeneration and survival observed in TLKO mice. Therefore, we studied hepatocellular hyperplasia by measuring the expression of cell cycle markers. Western blot analysis confirmed efficient deletion of Akt1, Akt2 and FoxO1 in livers of DLKO and TLKO, respectively (Fig. 3). WT mice showed a significant decrease of Akt phosphorylation at the S473 phosphorylation site after surgery. However, detectable levels of Akt phosphorylation are maintained during the post-hepatectomy period and are slightly increased at day 4, compared with day 2 post-hepatectomy (Fig. 3). This result suggests that despite the initial reduction in Akt phosphorylation, likely due to post-surgical fasting, Akt activity is needed for the whole regenerative process. CCND1 and PCNA expression was significantly increased in WT mice after 2 days of performing PH, compared with basal condition (Fig. 3, quantified in right panels). In contrast, CCND1 and PCNA expression was markedly attenuated in DLKO after PH (Fig. 3, quantified in right panels). In this experimental condition the expression of CCND1 was absent after 2 days post-hepatectomy and barely present at 4 days post-hepatectomy. The expression profile of PCNA, a cell proliferating marker, in DLKO also differed from WT mice and was characterized by a delay in its expression. While the PCNA expression in DLKO occurred on day 4 post-hepatectomy, we found a significant expression of this marker in WT mice 2 days after PH (Fig. 3). We also assessed the presence of immunoreactivity for CCND1 and Ki-67 in all the experimental groups before and after PH. The results showed that both CCND1 and Ki-67 expression were absent in the liver from the experimental groups studied before PH (Fig. 4A and Fig. S2A). Additionally, cell death (measured by TUNEL) was undetectable in the WT, DLKO or TLKO livers before PH (Fig. S2B).
Figure 3. Loss of FoxO1 normalized the expression of CCND1 and PCNA in Akt-deficient regenerative livers.
Western blot analysis of indicated proteins in livers lysated from WT, DLKO and TLKO before (T=0 days) and after PH (T=2 and 4 days). HSP90 and tubulin were used as loading controls. The right panels show the densitometry analysis of hepatic CCND1 and PCNA expression in WT, DLKO and TLKO before and after PH. Results are represented as mean ± S.E.M. *P<0.05 vs. WT at the same day.
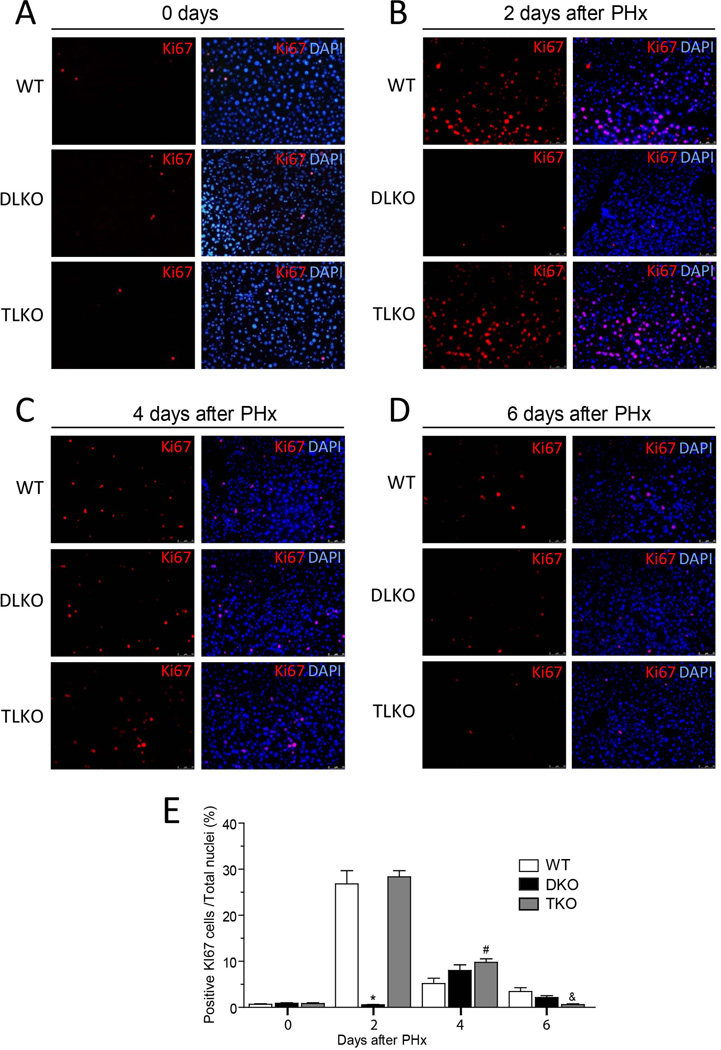
Figure 4. Genetic ablation of FoxO1 restored cell proliferation in Akt-deficient regenerative livers.
(A–D) Representative immunofluorescence pictures for the Ki-67 positive cells in WT, DLKO and TLKO livers at 0 (A), 2 (B), 4 (C) and 6 (D) days following PH. Merged images on right panels show co-localization of Ki-67 (red) and nuclear DNA (DAPI, blue). Original magnification 100×, (n=2 to 10). The bottom graph (E) shows the computed-assisted quantification of positive Ki-67 cells / total nuclei at different times following PH. Mean ± S.E.M is shown. *P<0.001 vs. WT and TLKO at the same day, #P<0.01 vs. WT at the same day, &P<0.05 vs. WT and DLKO at the same day.
The reduced expression of CCND1 and PCNA observed in DLKO mice correlated with an impaired hepatocellular proliferation that was quantified by Ki-67 immunostaining (Fig. 4). WT mice showed a significant population of Ki-67 positive cells at day 2 post-hepatectomy (Fig. 4B, upper panels and quantified in 4E). Thereafter, this immunostaining declined markedly. This result is in agreement with similar reports that describe an early proliferation of hepatocyte after liver resection (1, 2). In contrast, DLKO mice showed barely detectable Ki-67 immunostaining at day 2 after PH (Fig. 4B, middle panels and quantified in 4E). In the DLKO mice that survive after PH, Ki-67 immunostaining increased slightly after 4 days (Fig. 4C, middle panels and quantified in 4E) but overall, DLKO mice showed a dramatic decrease of hepatic cellular proliferation, compared with WT mice. Of note, FoxO1 deficiency in the context of dual loss of Akt1/Akt2 (TLKO mice) normalized the expression of CCND1 and PCNA (Fig. 3, quantified in right panels) and restored hepatocellular proliferation to levels comparable to that of WT (Fig. 4A–D), bottom panels and quantified in 4E). Similarly, we found a marked increase expression of proliferative markers (PCNA, Ki-67 and CCND1) in mouse primary hepatocytes treated with a FoxO1 specific inhibitor (AS1842856) or in combination with the Akt1/Akt2 inhibitor (A6730) (Fig. S3A, quantification is shown in bottom panels). As expected, hepatic cellular density was significantly higher in cells treated with Akt and FoxO1 inhibitors compared to cells treated with Akt inhibitor alone (Fig. S3B). Taken together, these in vivo and in vitro results strongly suggests that Akt allows cell proliferation in regenerative livers through the suppression of FoxO1 activity.
Genetic ablation of FoxO1 restored hepatic lipid accumulation and nearly normalized the hepatic lipidome in Akt-deficient regenerative livers
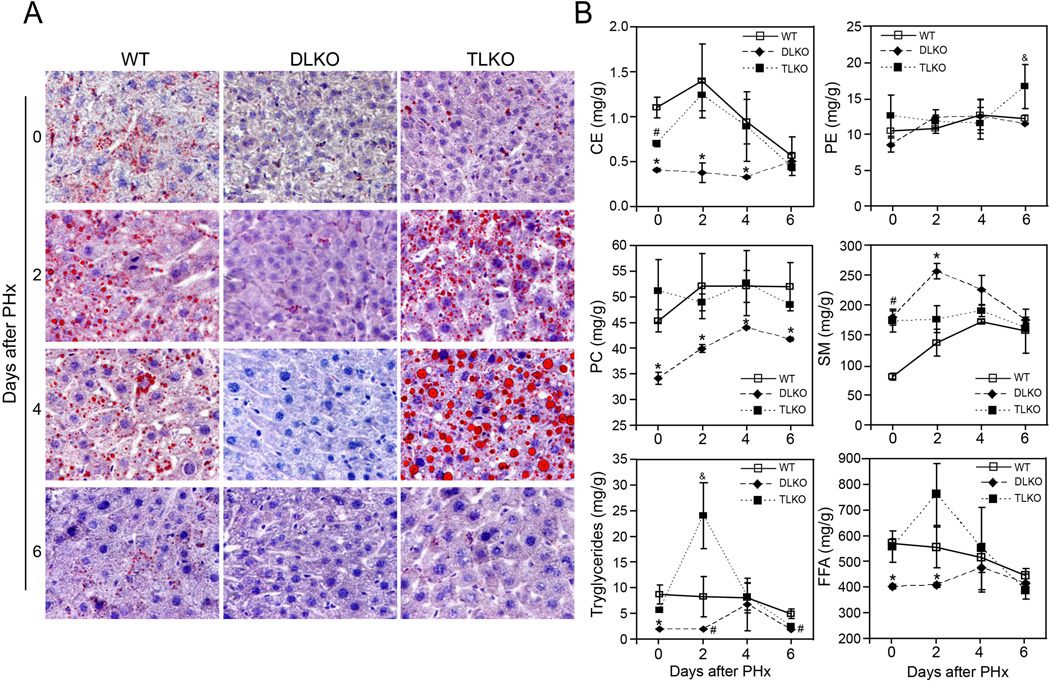
Early stages of liver regeneration are characterized by the formation of hepatocellular lipid droplets and changes in the hepatic lipidome. Several preclinical studies showed that alterations of these features impair hepatocellular proliferation during liver regeneration (27). To explore whether the hepatic loss of Akt may additionally affects lipid metabolism, we characterized both the hepatic lipid accumulation and the lipidomic profile of livers from WT, DLKO and TLKO mice before and after PH. Oil-red O staining of liver sections from WT and TLKO mice displayed modest accumulation of lipids before PH (Fig. 5A). As a response to PH, WT mice showed a significant steatosis characterized by the presence of microvesicular lipid droplets. This accumulation of lipids was evident as early as 2–4 days post-partial hepatectomy and declined thereafter. In contrast, DLKO mice failed to accumulate lipid droplets under normal physiological conditions or following PH. Interestingly, TLKO mice re-established lipid droplet formation in Akt1 and Akt2 deficient livers. At day 2 post-hepatectomy, the livers from TLKO mice displayed microvesicular steatosis that was comparable to the levels found in WT mice. At day 4 post-hepatectomy, the microvesicular steatosis became significant macrovesicular steatosis, which was characterized by more and larger lipid droplet accumulation, compared with WT condition at the same time point. Finally, the transient steatosis observed in both WT and TLKO resolved at day 6 post-hepatectomy (Fig. 5A).
Figure 5. Genetic ablation of FoxO1 in Akt deficient livers restored lipid droplet formation and nearly normalized the hepatic lipidome after partial hepatectomy.
(A) Representative histological panels of Oil red O-staining (red) in liver sections from WT, DLKO and TLKO mice before and after 2, 4 and 6 days of PH. Original magnification 200×, n= 3 to 5 animals. (B) Hepatic content of cholesterol ester (CE), phosphatidylethanolamine (PE), phosphatidylcholine (PC), sphingomyelin (SM), triglycerides and free fatty acids (FFA) in liver tissue isolated from WT, DLKO and TLKO mice. Zero-h data was obtained prior PH. Mean ± S.E.M is shown. *P<0.05 vs. WT and TLKO at the same time points, #P<0.01 vs. WT at the same time points, &P<0.05 vs. WT and DLKO at the same time points. The n varies from 2 to 10 among the different experimental groups due to the mortality associated with the DLKO condition.
We further assessed the lipid profile in all groups of mice before and after PH. As shown in Fig. 5B, the hepatic lipidome of DLKO differed significantly from WT mice before and after PH. Before PH, DLKO mice showed marked alterations in the concentration of cholesterol esters (CE), phosphatidylcholine (PC), sphingomyelin (SM), triglycerides and free fatty acids (FFA), compared with WT animals. This data suggest that the normal activity of hepatic Akt is required to maintain the lipid metabolism balance in normal conditions. After PH, WT mice showed a significant increase in CE, triglycerides and FFA that reached a maximum concentration at 2 days and declined thereafter (Fig. 5B). These changes agreed with the temporal distribution of lipid droplets observed in Fig. 5A and are consistent with the concept that lipid droplets are lipid structures enriched in cholesterol esters and triglycerides (27, 28). By contrast, the concentration of CE, triglycerides, (TG) and FFA remained significantly lower in DLKO mice compared with WT mice, without major changes throughout the hepatic regeneration. As well as cholesterol, another major structural component of cellular membranes whose concentration diminished in the liver of DLKO mice was PC (Fig. 5B). This finding is relevant considering that decrease of PC in lipid droplets from regenerative livers has previously been associated with quiescent hepatocytes (29). The increased in SM concentration was the only upward trend observed in DLKO mice early after PH, which almost double the concentration observed in WT mice at day 2 (Fig. 5B). Consistent with the Oil-red O staining experiments, genetic ablation of Foxo1 in Akt deficient mice tended to normalized the hepatic lipidome before and after PH (Fig. 5B). Comparing hepatectomized TLKO and WT mice, we only observed differences in the concentration of TG and phosphatidylethanolamine (PE) at days 2 and 6 post-hepatectomy, respectively (Fig. 5B). The 2.5-fold increase in triglycerides observed in TLKO mice, compared with WT, may be responsible for the increase in macrovesicular steatosis that we detected in this experimental group. We also detected an increase of FFA concentration in TLKO mice at day 2, although these values did not reach statistical significance (Fig. 5B).
DISCUSSION
Our study identifies Akt-FoxO1 as a major signaling pathway for controlling liver regeneration. Using a triple liver conditional knockout mouse model, we demonstrate that Akt-FoxO1 regulates a number of physiological processes associated with liver regeneration including cell proliferation and glucose and lipid metabolism.
Akt/PKB protein is a serine/threonine kinase that is activated in response to various stimuli, such as growth factors and cytokines. Upon the recruitment of Akt to the plasma membrane, its Ser473 and Thr308 amino acid residues are phosphorylated by mTORC2 complex and PDK1, respectively (30, 31). In this state of phosphorylation, Akt acquires serine/threonine kinase activity against proteins containing the RxRxxS/T motif. This Akt-dependent phosphorylation causes the inhibition or activation of key regulatory proteins that control such cellular processes as cell growth (inhibition of PRAS40 and TSC2), apoptosis (inhibition of GSK3, FOX transcription factors, BAD and Caspase-9 and activation of MDM2), metabolism (inhibition of PRAS40, TSC2, GSK3 and FOX transcription factors), proliferation (inhibition of TSC2, PRAS40, p27, GSK3, FOX transcription factors and activation of MDM2) (10) and angiogenesis (activation of EDG1 and eNOS) (32, 33).
Previous reports have demonstrated the hepatoprotective properties of Akt in experimental models of cirrhosis and ischemia-reperfusion injury (34–37). However, its role in liver regeneration has only been investigated by correlation or through non-specific inhibitors that may affect other key molecular mediators. For instance, the majority of these studies aimed at evaluating the phosphorylation kinetics of Akt after PH. These studies agree that Akt is markedly activated early after the surgical procedure (16–18). In addition, the treatment of partially hepatectomized mice with LY294002 (38), wortmannin, small interfering RNA targeting PI3-K (20), Rapamycin (39) or PTEN inhibitors (40) impaired liver regeneration in response to PH.
Non-specific genetic-targeting of the Akt signaling pathway also suggests the participation of this serine/threonine kinase in liver regeneration. Haga et al. (19) showed that liver-specific PDK1 knockout mice presented a poor liver regeneration, impaired hepatocyte hypertrophy and high mortality rate after PH. Interestingly, PDK1 deficient mice maintain hepatocellular proliferation after PH to values comparable to those of WT mice. Although this study point to a major function of PDK1 in the hepatic regenerative process, it does not allow drawing firm conclusions about the role of Akt in liver regeneration. One of the reasons that support this assertion is that PDK1 also targets other key mediators that may affect cell hypertrophy and division, such as S6K, RSK and some PKC isoforms (41, 42). The authors tried to overcome this limitation using a “pif-pocket” mutant of PDK1, which allows PDK1 to signal exclusively to Akt. Under this experimental setting, adenoviral transduction of the active pif-pocket mutant of PDK1 restored hepatocellular hypertrophy in partially hepatectomyzed PDK1 null mice. The main limitation of this approach is that adenoviral treatment does not guaranty high transduction efficiency of the PDK1 mutant into the liver. Therefore, Akt activity would be restored only in a little percentage of hepatocytes, while the full deficiency of PDK1 would be maintained in the rest. In addition, other phosphorylation sites than Thr308 may regulate Akt activity under different biological contexts. For example, phosphorylation in Akt Ser473 by mTORC2, DNA-dependent protein kinase, integrin-linked kinase or mitogen-activated protein kinase-activated protein kinase-2 is required for full activation (30, 43). AktS477/T479 phosphorylation triggered by cyclin-dependent kinase 2/cyclinA, mTORC2 or DNA-PK is essential for Akt activation (44). Akt phosphorylation in Tyr315, Tyr326 (by Src-related tyrosine kinases) (45) and Tyr176 (by Ack1) (46) play also a role in Akt activation. It remains unclear the hierarchy of these phosphorylations residues in Akt activation. However, and considering the above, we cannot rule out the possibility that any residual activity of Akt may be present in the PDK1 mice.
One of the reasons that may explain the lack of studies assessing specifically the role of Akt in hepatic regeneration is the existence of different Akt isoforms. Although these Akt isoforms share high sequence homology and some functional redundancy, emerging evidence suggests distinct biological functions for each isoform. Therefore, it is necessary to perform analytic and systematic approaches to unveil the functional differences and overlaps of the Akt family members during liver regeneration. Specific gene-disruption has been a valuable tool to discern some of the pathways involved in tissue regeneration. In our study, we used this strategy and we investigated the role of Akt in liver regeneration by performing PH in mice with Akt1, Akt2, dual Akt1/Akt2 or triple Akt1/Akt2/FoxO1 hepatic deficiency. Our results showed that Akt1 and Akt2 act redundantly during liver regeneration, as neither Akt1 nor Akt2 showed any regenerative impairment alter PH. The role of Akt during hepatocellular proliferation only becomes apparent after dual hepatic suppression of Akt1 and Akt2. This results contrast with the publications that suggest distinct roles for each Akt isoform. In this context, we recently published that despite the existence of isoform-substrate specificity for Akt in endothelial cells, overexpression of Akt2 can bypass the loss of Akt1 and phosphorylates Akt1 targets (13). These observations suggest that in the context of single hepatic deficiency of Akt1 or Akt2, overexpression of the remaining Akt isoform would be sufficient to enable the regeneration of the liver.
A novel contribution of our study is that Akt activity is required for both hepatocellular hypertrophy and proliferation. In the context of hypertrophy, the cell area of the DLKO hepatocytes was significantly diminished in basal conditions and may account for the significant reduction of the “liver weight / total body weight” ratio observed in DLKO livers before PH. This observation is in agreement with previous reports showing impaired hepatocellular hypertrophy in partially hepatectomized PDK1 knockout mice and general growth defect in Akt1-null mice (19, 47, 48). However, we also showed that hepatic dual Akt1/Akt2 deficiency dramatically affects hepatocellular proliferation. More importantly, the triple hepatic deficiency of FoxO1, Akt1 and Akt2 recovered the hepatocellular mitotic response induced by PH in Akt deficient mice, but failed to significantly increase cellular hypertrophy. The results obtained using partially hepatectomized TLKO mice and primary hepatocytes treated with the AS1842856 inhibitor, indicate that Akt mediates cell proliferation through FoxO1 inhibition. By contrast, FoxO1 activity is not directly involved in hepatocellular hypertrophy; suggesting that other downstream Akt targets, such as PRAS40 and TSC2, may be responsible for cell growth after PH. Consistent with this notion, “liver weight/total body weight” ratio of TLKO mice remained lower than wild-type mice despite the recovery in cell proliferation and the normalization of the survival rate of partially hepatectomyzed TLKO.
The FoxO subclass of forkhead-box transcripition factors is constituted by FoxO1, FoxO3, FoxO4 and FoxO6. The FoxO members are evolutionary conserved and despite their diversification they share in common that all of them are downstream effectors of Akt. Akt inhibits FoxO1 by direct phosphorylation on the residues T24, S253 and S316 (mouse sequence). This posttranslational modification excludes FoxO1 from the nucleus and makes the protein more susceptible to ubiquitination and proteasome degradation (49–51). FoxO1 regulate a significant number of cellular processes including the regulation of the cell cycle machinery (49, 52). In the present study, hepatic Akt deficiency was associated with barely detectable levels of CCND1 expression and impaired hepatocellular proliferation after PH. Interestingly; FoxO1 deficiency was able to restore CCND1 expression in the same Akt deficient context. This finding is relevant considering that D-type Cyclins are expressed early in the cells in response to mitogenic signals and that they play a necessary role allowing G1/S phase transition through the inactivation of the S-phase repressor pRb (53). We also showed that the pharmacological inhibition of FoxO1 in primary hepatocytes was associated with overexpression of CCND1. Our results are in agreement with other studies where the authors demonstrated that overexpression or conditional activation of FoxO proteins downregulated the expression of D-type Cyclins (54). Additionally, we showed that primary hepatocytes treated in vitro with AS1842856 up-regulated PCNA and Ki-67 expression. Therefore, we cannot rule out the possibility that other components of the cell cycle machinery regulated by FoxO --such as p21, p27, CCND1, p130, cyclin G2 and polo-like kinases-- may also contribute to the reestablishment of the cell proliferation observed in TLKO mice.
Partially hepatectomized DLKO mice show a profound metabolic imbalance characterized by hyperglycemia and the lack of both glycogen and lipid droplets in hepatocytes. Strikingly, all these deficiencies are restored in TLKO mice. In the context of the glucose metabolism, our results reproduce what have already been reported by others (25). In brief, mice with hepatic deletion of Akt1 and Akt2 are glucose intolerant and insulin resistant and these defects are normalized upon liver–specific deletion of FoxO1. The predominant mechanism behind this phenotype is sustained on the reported ability of FoxO1 to activate the transcription of rate-limiting enzymes of gluconeogenesis, such as glucose-6-phosphatase and phosphoenolpyruvate carboxykinase (55, 56). Therefore, the activation of FoxO1 in an Akt deficient context would increase the rate of gluconeogenesis and glycogenolysis, which is concordant to what we found in the DLKO mice after PH. More intriguingly is the effect of hepatic Akt deficiency in lipid metabolism before and after PH. In this context, DLKO mice failed to accumulate lipid droplets following PH. Lipid droplets are mainly composed of triacylglycerol and cholesteryl esters (28). Consistent with this notion, the hepatic lipidome of DLKO mice showed a lack of CE accumulation throughout all the experimental points before and after PH and a significant decrease of triglyceride levels at 0 and 2 days post-hepatectomy. Notably, liver–specific deletion of FoxO1 in Akt deficient mice corrected these lipid abnormalities. Several studies may provide insight into the observed relationship between FoxO1 activation and lipid-cholesterol metabolism. For instance, AFPCre-FoxO1loxP/loxP mice that were fasted overnight and then refed displayed increased hepatic TG levels compared to controls (57). A second study shows that FoxO1 inhibits the expression of the sterol element binding protein 1 (SREBP1), a transcriptional factor that regulates hepatic lipogenesis, thereby reducing hepatic lipid synthesis. Moreover, FoxO1 might influence hepatic lipid droplet accumulation by increasing the expression of microsomal triglyceride transfer protein (MTP), which is a protein required for assembly of very-low density lipoprotein (VLDL) (58). In this regard, Kamagate et al demonstrated that mice in a fed state downregulate MTP expression through Akt-mediated FoxO1 inactivation (59). This signaling cascade resulted in reduced VLDL formation and, therefore, accumulation of lipid droplets into hepatocytes. These observations could explain the phenotype observed in DLKO and TLKO mice. However, further studies would be needed to better understand the pathophysiology of lipid abnormalities in partially hepatectomized DLKO mice. Nevertheless, our results unravel a major role of the Akt/FoxO1 pathway as a major regulator during liver regeneration.
Supplementary Material
Acknowledgments
FINANCIAL SUPPORT
This work was supported by Grants SAF2013-41840-R from the Ministerio de Economía y Competitividad (to MM-R), Grant PI14/00962 (to JB and LB), Fundacio La Marato de TV3-2013 (Marato 120930 to WJ), National Institutes of Health (R01HL107953, and R01HL106063 to CF-H; R01HL105945 to YS; R01DK56886 to MJB; R01HL107794 to AB; 1F31AG043318 to LG) and the Foundation Leducq Transatlantic Network of Excellence in Cardiovascular Research (to CFH). Ciberehd is funded by Instituto de Salud Carlos III.
REFERENCES
- 1.Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43:S45–S53. doi: 10.1002/hep.20969. [DOI] [PubMed] [Google Scholar]
- 2.Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276:60–66. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- 3.Higgins GK, Johnson D., Jr Detection of Antihypertensive Agents in Urine. Trans Assoc Life Insur Med Dir Am. 1964;48:186–190. [PubMed] [Google Scholar]
- 4.Bismuth H, Houssin D, Ornowski J, Meriggi F. Liver resections in cirrhotic patients: a Western experience. World J Surg. 1986;10:311–317. doi: 10.1007/BF01658152. [DOI] [PubMed] [Google Scholar]
- 5.Olthoff KM, Emond JC, Shearon TH, Everson G, Baker TB, Fisher RA, Freise CE, et al. Liver regeneration after living donor transplantation: adult-to-adult living donor liver transplantation cohort study. Liver Transpl. 2015;21:79–88. doi: 10.1002/lt.23966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruix J, Gores G, Mazzaferro V. Authors' response to the letter: Liver resection for patients with hepatocellular carcinoma and macrovascular invasion, multiple tumours or portal hypertension by Zhong et al. Gut. 2015;64:522. doi: 10.1136/gutjnl-2014-308381. [DOI] [PubMed] [Google Scholar]
- 7.Wong TC, Lo CM. Resection strategies for hepatocellular carcinoma. Semin Liver Dis. 2013;33:273–281. doi: 10.1055/s-0033-1351782. [DOI] [PubMed] [Google Scholar]
- 8.Taub R. Liver regeneration: from myth to mechanism. Nat Rev Mol Cell Biol. 2004;5:836–847. doi: 10.1038/nrm1489. [DOI] [PubMed] [Google Scholar]
- 9.Michalopoulos GK. Liver regeneration. J Cell Physiol. 2007;213:286–300. doi: 10.1002/jcp.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang ZZ, Tschopp O, Hemmings-Mieszczak M, Feng J, Brodbeck D, Perentes E, Hemmings BA. Protein kinase B alpha/Akt1 regulates placental development and fetal growth. J Biol Chem. 2003;278:32124–32131. doi: 10.1074/jbc.M302847200. [DOI] [PubMed] [Google Scholar]
- 12.Di Lorenzo A, Fernandez-Hernando C, Cirino G, Sessa WC. Akt1 is critical for acute inflammation and histamine-mediated vascular leakage. Proc Natl Acad Sci U S A. 2009;106:14552–14557. doi: 10.1073/pnas.0904073106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee MY, Luciano AK, Ackah E, Rodriguez-Vita J, Bancroft TA, Eichmann A, Simons M, et al. Endothelial Akt1 mediates angiogenesis by phosphorylating multiple angiogenic substrates. Proc Natl Acad Sci U S A. 2014;111:12865–12870. doi: 10.1073/pnas.1408472111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cho H, Mu J, Kim JK, Thorvaldsen JL, Chu Q, Crenshaw EB, 3rd, Kaestner KH, et al. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta) Science. 2001;292:1728–1731. doi: 10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
- 15.Easton RM, Cho H, Roovers K, Shineman DW, Mizrahi M, Forman MS, Lee VM, et al. Role for Akt3/protein kinase Bgamma in attainment of normal brain size. Mol Cell Biol. 2005;25:1869–1878. doi: 10.1128/MCB.25.5.1869-1878.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hong F, Nguyen VA, Shen X, Kunos G, Gao B. Rapid activation of protein kinase B/Akt has a key role in antiapoptotic signaling during liver regeneration. Biochem Biophys Res Commun. 2000;279:974–979. doi: 10.1006/bbrc.2000.4044. [DOI] [PubMed] [Google Scholar]
- 17.Liu HX, Fang Y, Hu Y, Gonzalez FJ, Fang J, Wan YJ. PPARbeta Regulates Liver Regeneration by Modulating Akt and E2f Signaling. PLoS One. 2013;8:e65644. doi: 10.1371/journal.pone.0065644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marshall KM, He S, Zhong Z, Atkinson C, Tomlinson S. Dissecting the complement pathway in hepatic injury and regeneration with a novel protective strategy. J Exp Med. 2014;211:1793–1805. doi: 10.1084/jem.20131902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haga S, Ozaki M, Inoue H, Okamoto Y, Ogawa W, Takeda K, Akira S, et al. The survival pathways phosphatidylinositol-3 kinase (PI3-K)/phosphoinositide-dependent protein kinase 1 (PDK1)/Akt modulate liver regeneration through hepatocyte size rather than proliferation. Hepatology. 2009;49:204–214. doi: 10.1002/hep.22583. [DOI] [PubMed] [Google Scholar]
- 20.Jackson LN, Larson SD, Silva SR, Rychahou PG, Chen LA, Qiu S, Rajaraman S, et al. PI3K/Akt activation is critical for early hepatic regeneration after partial hepatectomy. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1401–G1410. doi: 10.1152/ajpgi.00062.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mullany LK, Nelsen CJ, Hanse EA, Goggin MM, Anttila CK, Peterson M, Bitterman PB, et al. Akt-mediated liver growth promotes induction of cyclin E through a novel translational mechanism and a p21-mediated cell cycle arrest. J Biol Chem. 2007;282:21244–21252. doi: 10.1074/jbc.M702110200. [DOI] [PubMed] [Google Scholar]
- 22.Wan M, Easton RM, Gleason CE, Monks BR, Ueki K, Kahn CR, Birnbaum MJ. Loss of Akt1 in mice increases energy expenditure and protects against diet-induced obesity. Mol Cell Biol. 2012;32:96–106. doi: 10.1128/MCB.05806-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leavens KF, Easton RM, Shulman GI, Previs SF, Birnbaum MJ. Akt2 is required for hepatic lipid accumulation in models of insulin resistance. Cell Metab. 2009;10:405–418. doi: 10.1016/j.cmet.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsumoto M, Pocai A, Rossetti L, Depinho RA, Accili D. Impaired regulation of hepatic glucose production in mice lacking the forkhead transcription factor Foxo1 in liver. Cell Metab. 2007;6:208–216. doi: 10.1016/j.cmet.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 25.Lu M, Wan M, Leavens KF, Chu Q, Monks BR, Fernandez S, Ahima RS, et al. Insulin regulates liver metabolism in vivo in the absence of hepatic Akt and Foxo1. Nat Med. 2012;18:388–395. doi: 10.1038/nm.2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gross DN, Wan M, Birnbaum MJ. The role of FOXO in the regulation of metabolism. Curr Diab Rep. 2009;9:208–214. doi: 10.1007/s11892-009-0034-5. [DOI] [PubMed] [Google Scholar]
- 27.Pauta M, Rotllan N, Vales F, Fernandez-Hernando A, Allen RM, Ford DA, Mari M, et al. Impaired liver regeneration in Ldlr−/− mice is associated with an altered hepatic profile of cytokines, growth factors, and lipids. J Hepatol. 2013;59:731–737. doi: 10.1016/j.jhep.2013.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin S, Parton RG. Lipid droplets: a unified view of a dynamic organelle. Nat Rev Mol Cell Biol. 2006;7:373–378. doi: 10.1038/nrm1912. [DOI] [PubMed] [Google Scholar]
- 29.Garcia-Arcos I, Gonzalez-Kother P, Aspichueta P, Rueda Y, Ochoa B, Fresnedo O. Lipid analysis reveals quiescent and regenerating liver-specific populations of lipid droplets. Lipids. 2010;45:1101–1108. doi: 10.1007/s11745-010-3492-2. [DOI] [PubMed] [Google Scholar]
- 30.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 31.Stephens L, Anderson K, Stokoe D, Erdjument-Bromage H, Painter GF, Holmes AB, Gaffney PR, et al. Protein kinase B kinases that mediate phosphatidylinositol 3,4,5-trisphosphate-dependent activation of protein kinase B. Science. 1998;279:710–714. doi: 10.1126/science.279.5351.710. [DOI] [PubMed] [Google Scholar]
- 32.Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, et al. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee MJ, Thangada S, Paik JH, Sapkota GP, Ancellin N, Chae SS, Wu M, et al. Akt-mediated phosphorylation of the G protein-coupled receptor EDG-1 is required for endothelial cell chemotaxis. Mol Cell. 2001;8:693–704. doi: 10.1016/s1097-2765(01)00324-0. [DOI] [PubMed] [Google Scholar]
- 34.Harada N, Hatano E, Koizumi N, Nitta T, Yoshida M, Yamamoto N, Brenner DA, et al. Akt activation protects rat liver from ischemia/reperfusion injury. J Surg Res. 2004;121:159–170. doi: 10.1016/j.jss.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 35.Morales-Ruiz M, Cejudo-Martin P, Fernandez-Varo G, Tugues S, Ros J, Angeli P, Rivera F, et al. Transduction of the liver with activated Akt normalizes portal pressure in cirrhotic rats. Gastroenterology. 2003;125:522–531. doi: 10.1016/s0016-5085(03)00909-0. [DOI] [PubMed] [Google Scholar]
- 36.Morales-Ruiz M, Fondevila C, Munoz-Luque J, Tugues S, Rodriguez-Laiz G, Cejudo-Martin P, Romero JM, et al. Gene transduction of an active mutant of akt exerts cytoprotection and reduces graft injury after liver transplantation. Am J Transplant. 2007;7:769–778. doi: 10.1111/j.1600-6143.2006.01720.x. [DOI] [PubMed] [Google Scholar]
- 37.Liu S, Premont RT, Kontos CD, Zhu S, Rockey DC. A crucial role for GRK2 in regulation of endothelial cell nitric oxide synthase function in portal hypertension. Nat Med. 2005;11:952–958. doi: 10.1038/nm1289. [DOI] [PubMed] [Google Scholar]
- 38.Ping C, Lin Z, Jiming D, Jin Z, Ying L, Shigang D, Hongtao Y, et al. The phosphoinositide 3-kinase/Akt-signal pathway mediates proliferation and secretory function of hepatic sinusoidal endothelial cells in rats after partial hepatectomy. Biochem Biophys Res Commun. 2006;342:887–893. doi: 10.1016/j.bbrc.2006.02.034. [DOI] [PubMed] [Google Scholar]
- 39.Palmes D, Zibert A, Budny T, Bahde R, Minin E, Kebschull L, Holzen J, et al. Impact of rapamycin on liver regeneration. Virchows Arch. 2008;452:545–557. doi: 10.1007/s00428-008-0604-y. [DOI] [PubMed] [Google Scholar]
- 40.Yan-nan B, Zhao-yan Y, Li-xi L, jiang Y, Qing-jie X, Yong Z. MicroRNA-21 accelerates hepatocyte proliferation in vitro via PI3K/Akt signaling by targeting PTEN. Biochem Biophys Res Commun. 2014;443:802–807. doi: 10.1016/j.bbrc.2013.12.047. [DOI] [PubMed] [Google Scholar]
- 41.Mackay HJ, Twelves CJ. Targeting the protein kinase C family: are we there yet? Nat Rev Cancer. 2007;7:554–562. doi: 10.1038/nrc2168. [DOI] [PubMed] [Google Scholar]
- 42.Mora A, Komander D, van Aalten DM, Alessi DR. PDK1, the master regulator of AGC kinase signal transduction. Semin Cell Dev Biol. 2004;15:161–170. doi: 10.1016/j.semcdb.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 43.Dong LQ, Liu F. PDK2: the missing piece in the receptor tyrosine kinase signaling pathway puzzle. Am J Physiol Endocrinol Metab. 2005;289:E187–E196. doi: 10.1152/ajpendo.00011.2005. [DOI] [PubMed] [Google Scholar]
- 44.Liu P, Begley M, Michowski W, Inuzuka H, Ginzberg M, Gao D, Tsou P, et al. Cell-cycle-regulated activation of Akt kinase by phosphorylation at its carboxyl terminus. Nature. 2014;508:541–545. doi: 10.1038/nature13079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen R, Kim O, Yang J, Sato K, Eisenmann KM, McCarthy J, Chen H, et al. Regulation of Akt/PKB activation by tyrosine phosphorylation. J Biol Chem. 2001;276:31858–31862. doi: 10.1074/jbc.C100271200. [DOI] [PubMed] [Google Scholar]
- 46.Mahajan K, Coppola D, Challa S, Fang B, Chen YA, Zhu W, Lopez AS, et al. Ack1 mediated AKT/PKB tyrosine 176 phosphorylation regulates its activation. PLoS One. 2010;5:e9646. doi: 10.1371/journal.pone.0009646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen WS, Xu PZ, Gottlob K, Chen ML, Sokol K, Shiyanova T, Roninson I, et al. Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene. Genes Dev. 2001;15:2203–2208. doi: 10.1101/gad.913901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cho H, Thorvaldsen JL, Chu Q, Feng F, Birnbaum MJ. Akt1/PKBalpha is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J Biol Chem. 2001;276:38349–38352. doi: 10.1074/jbc.C100462200. [DOI] [PubMed] [Google Scholar]
- 49.Birkenkamp KU, Coffer PJ. FOXO transcription factors as regulators of immune homeostasis: molecules to die for? J Immunol. 2003;171:1623–1629. doi: 10.4049/jimmunol.171.4.1623. [DOI] [PubMed] [Google Scholar]
- 50.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 51.Kops GJ, de Ruiter ND, De Vries-Smits AM, Powell DR, Bos JL, Burgering BM. Direct control of the Forkhead transcription factor AFX by protein kinase B. Nature. 1999;398:630–634. doi: 10.1038/19328. [DOI] [PubMed] [Google Scholar]
- 52.Accili D, Arden KC. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell. 2004;117:421–426. doi: 10.1016/s0092-8674(04)00452-0. [DOI] [PubMed] [Google Scholar]
- 53.Ekholm SV, Reed SI. Regulation of G(1) cyclin-dependent kinases in the mammalian cell cycle. Curr Opin Cell Biol. 2000;12:676–684. doi: 10.1016/s0955-0674(00)00151-4. [DOI] [PubMed] [Google Scholar]
- 54.Schmidt M, Fernandez de Mattos S, van der Horst A, Klompmaker R, Kops GJ, Lam EW, Burgering BM, et al. Cell cycle inhibition by FoxO forkhead transcription factors involves downregulation of cyclin D. Mol Cell Biol. 2002;22:7842–7852. doi: 10.1128/MCB.22.22.7842-7852.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu Y, Dentin R, Chen D, Hedrick S, Ravnskjaer K, Schenk S, Milne J, et al. A fasting inducible switch modulates gluconeogenesis via activator/coactivator exchange. Nature. 2008;456:269–273. doi: 10.1038/nature07349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Puigserver P, Rhee J, Donovan J, Walkey CJ, Yoon JC, Oriente F, Kitamura Y, et al. Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1alpha interaction. Nature. 2003;423:550–555. doi: 10.1038/nature01667. [DOI] [PubMed] [Google Scholar]
- 57.Wan M, Leavens KF, Saleh D, Easton RM, Guertin DA, Peterson TR, Kaestner KH, et al. Postprandial hepatic lipid metabolism requires signaling through Akt2 independent of the transcription factors FoxA2, FoxO1, and SREBP1c. Cell Metab. 2011;14:516–527. doi: 10.1016/j.cmet.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hussain MM, Shi J, Dreizen P. Microsomal triglyceride transfer protein and its role in apoB-lipoprotein assembly. J Lipid Res. 2003;44:22–32. doi: 10.1194/jlr.r200014-jlr200. [DOI] [PubMed] [Google Scholar]
- 59.Kamagate A, Qu S, Perdomo G, Su D, Kim DH, Slusher S, Meseck M, et al. FoxO1 mediates insulin-dependent regulation of hepatic VLDL production in mice. J Clin Invest. 2008;118:2347–2364. doi: 10.1172/JCI32914. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.