Abstract
Purpose
One of the earliest hallmarks of diabetic retinopathy is the loss of retinal pericytes. However, the mechanisms that promote pericyte dropout are unknown. In the present study, we propose a novel pathway in which pericyte apoptosis is mediated by macrophages, TGFβ and pro-apoptotic BIGH3 (TGFβ-induced Gene Human Clone 3) protein.
Patients and methods
To elucidate this pathway, we assayed human retinal pericyte (HRP) apoptosis by TUNEL assay, BIGH3 mRNA expression by qPCR, and BIGH3 protein expression by western blot analysis. HRP were treated with BIGH3 protein, TGFβ1 and TGFβ2 and inhibition assays were carried out by blocking with antibodies against BIGH3. The distribution of BIGH3 and CD68+ macrophages were compared in a post-mortem donor eye with 7-year history of Type II diabetes and histopathogically confirmed non-proliferative diabetic retinopathy (NPDR).
Results
TGFβ induced a significant increase in BIGH3 mRNA and protein expression, and HRP apoptosis. BIGH3 treatment showed HRP undergo apoptosis in a dose-dependent manner. At 5 μg/ml, BIGH3 induced 3.5-times more apoptosis in HRP than in retinal endothelial cells. TGFβ induced apoptosis was inhibited by blocking with antibodies against BIGH3. In an example of NPDR, BIGH3 accumulated within the walls of the inner retina arterioles. Macrophage infiltrates were frequently associated with these vessels and the inner nuclear layer.
Conclusion
Together with our previously published results on macrophage-induced retinal endothelial cell apoptosis, the present study supports a novel inflammatory pathway mediated by macrophages and the BIGH3 protein leading to HRP apoptosis. As shown in human post-mortem globes, these observations are clinically relevant, suggesting a new mechanism underlying pericyte dropout during NPDR.
Introduction
Diabetic retinopathy (DR) is a leading cause of blindness worldwide.1, 2, 3 An early process in the pathogenesis of DR is pericyte dropout, which leads to a cascade of events including microaneurysms, thickening of the basement membrane, increased vascular permeability, hypoxia, and eventually angiogenesis.4 Pericytes share a common basement membrane with endothelial cells and are most prominent in retinal capillaries in comparison with capillaries in other tissues in the body.5 Pericytes closely resemble smooth muscle cells on arterioles and contract for vessel constriction; however, unlike smooth muscle cells, are embedded within the basement membrane.6 Mouse models testing for pericyte deficiencies have shown that DR will develop after 50% of pericyte loss has occurred.7 Endothelial cell-to-pericyte ratio increased from 1:1 in healthy retinas to 2:1 in diabetic retinas of humans.8
Increased inflammation is noted in the early stages of DR, coinciding with the activation of the pro-inflammatory transcription factor NF-κB, which is elevated in both retinal endothelial cells (RECs) and pericytes in response to high glucose.9 Rangasamy et al10 demonstrated that RECs upregulate the expression of CCL2 (chemokine ligand 2) when exposed to high-glucose conditions. Interestingly, the resulting monocyte/macrophage infiltration is dependent on the expression of CCL2.10 Macrophages constitutively secrete transforming growth factor beta-1 (TGFβ1) and increase its secretion in response to inflammation and phagocytosis.11, 12, 13 In an earlier report, we showed macrophage infiltration induces apoptosis of RECs by secreting TGFβ1, which upregulates the gene TGFBI which encodes TGFβ-Induced Gene Human Clone 3 (or BIGH3).14 BIGH3 is an extracellular matrix (ECM) cell-adhesion protein that has known apoptotic functions in corneal epithelial cells, HeLa cells, osteosarcoma cells, and bovine retinal pericytes;14, 15, 16, 17 specifically through the EPDIM and RGD integrin-binding sequences at the C-terminal.16
On the basis of preliminary studies, we hypothesize that, in diabetic conditions, macrophages infiltrate retinal tissue and secrete TGFβ upregulating BIGH3 production and secretion. The BIGH3 protein then causes apoptosis of retinal pericytes in an autocrine manner. This pathway elucidates a novel mechanism of retinal pericyte death, commonly seen as the one of the first morphological changes in the diabetic eye.18 Vascular occlusion and increased permeability follow pericyte death and lead to a high level of vascular endothelial growth factor,19 which promotes retinal neovascularization in later stages of DR.20 Recently, we have shown that culture medium from macrophages grown in diabetic conditions had higher levels of TGFβ, which induces BIGH3 synthesis, secretion, and REC apoptosis.14
Detailed analyses of this pathway and effects of BIGH3 should provide better understanding of events leading to DR and research into therapeutic treatment and prevention of this disease. Presently, treatments of DR include laser photocoagulations of ocular blood vessels and anti-inflammatory steroids.4 Better understanding the role of macrophages, TGFβ, and BIGH3 in the early progression of DR may lead to improved treatments and preventative care.
Materials and methods
Cell culture
Human retinal pericytes (HRP) were obtained, from Lonza Group (Allendale, NJ, USA; Cat No. CC-7131). HRP were maintained in endothelial basal medium-2 (medium with Bullet kit purchased from Lonza Group; Cat No. CC-3202). Rhesus RECs (RhRECs), primary renal proximal tubule epithelial cells, and cell media were described in a previous publication from our laboratories.14, 21
Transforming growth factor β
TGFβ1 and TGFβ2 were purchased from Sigma Aldrich (Cat No: T7039, T2815; St. Louis, MO USA).
Antibodies
BIGH3 bacterial fusion protein was used to immunize New Zealand white rabbits. The resultant anti-BIGH3 antiserum has been previously characterized.16, 21 Pre-immune serum was used as negative control where indicated in the individual experiments. Anti-rat CD68 antibody was obtained from ABD seroTec (Cat No. MCA1957T; Raleigh, NC, USA). Mouse anti-TGFβ1 antibody was purchased from R&D Systems (clone 9016; Minneapolis, MN, USA).
Immunocytochemistry
Immunostaining was carried out as described in an earlier publication.14
TUNEL Staining
To test if the pathway reported in previous publications14, 21 was also a valid pathway for retinal pericytes, HRP were cultured in cell medium containing 5 ng/ml of TGFβ1 or 2, with or without rabbit anti-BIGH3 antiserum (BIGH3 Ab). To determine if BIGH3 could induce HRP apoptosis, cells were treated with increasing concentrations of recombinant BIGH3.21 In one part of this study, cells were incubated with increasing concentrations of BIGH3 for 24 h and TUNEL assays were performed to determine cell death. We next compared the sensitivity of different cell types for BIGH3-induced cell death. HRP, RhRECs and renal proximal tubules epithelial cells (RPTEC) were treated for 24 h with increasing concentration of BIGH3 protein (0–35 μg/ml). Wells of a Lab-Tek 16-well chamber slide from Fisher Scientific (Cat No: 12-565-110N; Waltham, MA, USA) were each seeded with 5 × 103 HRP, RhREC, or RPTEC in 300 μl of MEM. After 24-h incubation, the MEM was aspirated and replaced with either BIGH3 in MEM growth media, BIGH3 Ab, TGFβ 1, 2 or a combination thereof (see the ‘Result' section on treatment paradigm). After incubation, cell media was aspirated and the cells were fixed for 1 h in 4% paraformaldehyde in PBS. After washing with PBS, cells were treated for 2 min with 250 μl sodium citrate buffer containing 0.1% Triton X-100 at 4 °C. TUNEL assay (Roche, Cat No: 11684795910; Indianapolis, IN, USA) and 4',6-diamidino-2-phenylindole (DAPI) staining (Vector Labs; Cat No: H-1500; Burlingame, CA. USA) were performed according to the manufacturer's recommendations. After washing with PBS, cells were sealed with Vectashield. The numbers of stained cells in ten different random fields (× 20 magnification) were counted using a ZEISS Axioplan II fluorescent microscope (Zeiss; Thornwood, NY, USA). A positive control well comprised cells that were pre-treated with camptothecin, an apoptosis inhibitor of DNA enzyme topoisomerase I.
Western blot analysis
Proteins in equal volumes of conditioned media were resolved on reducing 4–10% SDS polyacrylamide gels and transferred to Immobilon-P membrane. The membrane was incubated with BIGH3 antibody (1:5000), washed and then incubated with horseradish peroxidase-labeled goat anti-rabbit IgG (1:3000). The complex was detected using diaminobenzidine. Densitometry analysis was performed by Image J software developed by NIH (Bethesda, MD, USA).
qPCR
We first test this inflammatory pathway by treating HRPs with increasing concentrations of TGFβ1 and TGFβ2. Cells were incubated for 24 h and BIGH3 expression was assayed through qPCR. Total RNA isolated using RNeasy mini kit (Qiagen; Cat No: 74104; Valencia, CA, USA) was reverse-transcribed into cDNA using Taqman Reverse Transcription reagents (Life Technologies, Cat No: 4304134; Grand Island, NY, USA). Quantitative PCR measured BIGH3 mRNA and 18s rRNA using Syber Green PCR Mix (Life Technologies, Cat No: 4309155). BIGH3 primers were: forward 5′-TGGACAGACCCTGGAAACTC-3′ and reverse 5′-GTCTCCCTTCAGGACATCCA-3′. 18s-rRNA primers were forward 5′-AGACCTGGAGCGACTGAAGA-3′ and reverse 5′-AGAAGTGACGCAGCCCTCTA-3′. The comparative Ct (ΔΔCt) method was used to obtain quantitative data of relative gene expression; values were normalized to expression levels of 18s rRNA.
Immunohistochemical distribution of BIGH3 and macrophages in human retina
HRP were stained with phalloidin for actin, DAPI for cell nuclei, and antibodies against BIGH3. The retinal distribution of BIGH3 and macrophage marker, CD68 were characterized in a post-mortem globe with documented non-proliferative DR (NPDR). Unstained formalin-fixed paraffin-embedded sections of the right eye were obtained through the National Disease Research Interchange (NDRI, Philadephia, PA, USA). The 5 μM complete globe sections were oriented through the foveola. Each globe had been harvested and placed in 120 ml 10% of buffered formalin 4 h after death. A full histopathological and clinical summary was prepared by on one of us (FG-F) and is available through the Ocular Pathology NDRI Slide Program (NDRI Donor registry #68373; http://ndriresource.org/Human-Tissue-Services/Eye-Research/5066/).
Results
Cultured HRP synthesize and secrete BIGH3
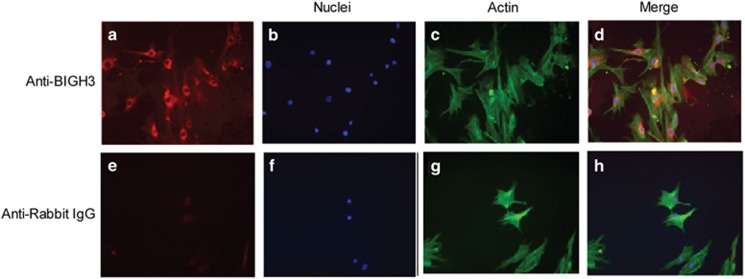
DAPI and phalloidin staining confirm the elongated cell body and normal HRP morphology (Figures 1b and c). Figures 1a and d show that BIGH3 staining is localized inside the cell and in the ECM, confirming protein synthesis and excretion.
Figure 1.
Immunocytochemistry localization of BIGH3 protein in cultured HRP. Primary HRP were cultured in EBM-2 supplemented with 10% FBS and stained with BIGH3 antibody (red, a) or rabbit IgG (e; negative control) followed by DAPI (nuclei; blue, b and f), phalloidin (actin; green, c and g). In d (a–c merged) shows cytosolic and extracellular locations of BIGH3 protein. Images are shown at × 20 magnification.
TGFβ-induced BIGH3 expression and secretion in HRP
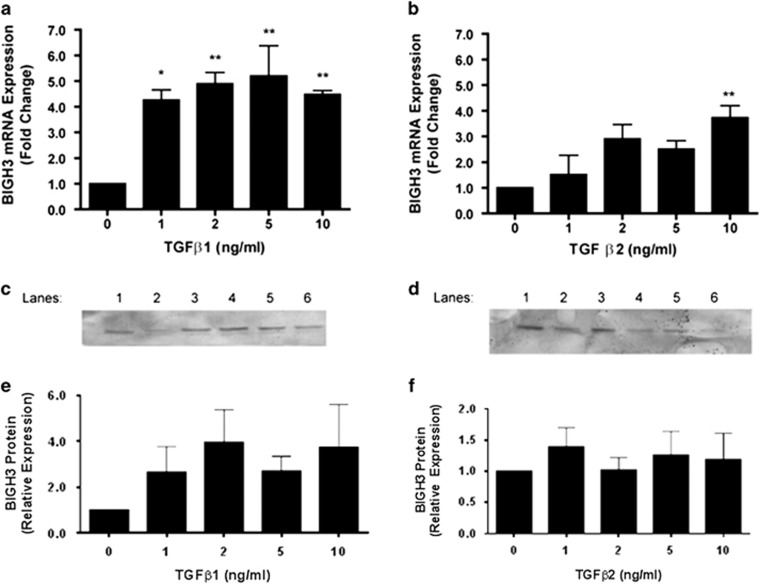
HRP's treated with 1, 2, 5, and 10 ng/ml TGFβ1 all had a statistically significant increase in BIGH3 expression compared with the control (Figure 2a). HRP treated with 10 ng/ml TGFβ2, had a statistically significant increase in BIGH3 expression compared with the control (Figure 2b). The presence of BIGH3 protein from cultured medium was shown in by western blots (Figures 2c and d). BIGH3 was detected in the medium when HRP were treated with 1, 2, 5, and 10 ng/ml TGFβ1 or TGFβ2 (Figures 2e and f).
Figure 2.
TGFβ increased BIGH3 expression in HRP. (a, b) HRP were cultured with increasing concentrations of TGFβ1 or 2 added to conditioned media for 24 h. BIGH3 mRNA expression was measured by qPCR after TGFβ treatment. One-way ANOVA showed significant treatment effects by TGFβ 1 and 2 (F(4,14)=8.083; P<0.01)], and (F(4,14)=4.975; P<0.05). Dunnett's multiple comparison to test differences between groups was significant (*P<0.05, **P<0.01) between BIGH3 mRNA expression and TGFβ1 in all treatment groups (0 ng/ml TGFβ used as control group). Dunnett's test only showed 10 ng/ml of TGFβ2 to have a significant difference from the control group. (c, d) HRP were cultured with either TGFβ1 (c) or TGFβ2 (d) at 1, 2, 5, or 10 ng/ml for 24 h and refreshed media were collected after a 24 h period and probed for BIGH3 protein by western blot. Lane 1, 0.1 μg recombinant BIGH3; TGFβ1 or 2: lane 2, 0 ng/ml; lane 3, 1 ng/ml; lane 4, 2 ng/ml; lane 5, 5 ng/ml; lane 6, 10 ng/ml. (e, f) Densitometry results from c and d, respectively.
BIGH3 antibody blocks TGFβ-induced apoptosis
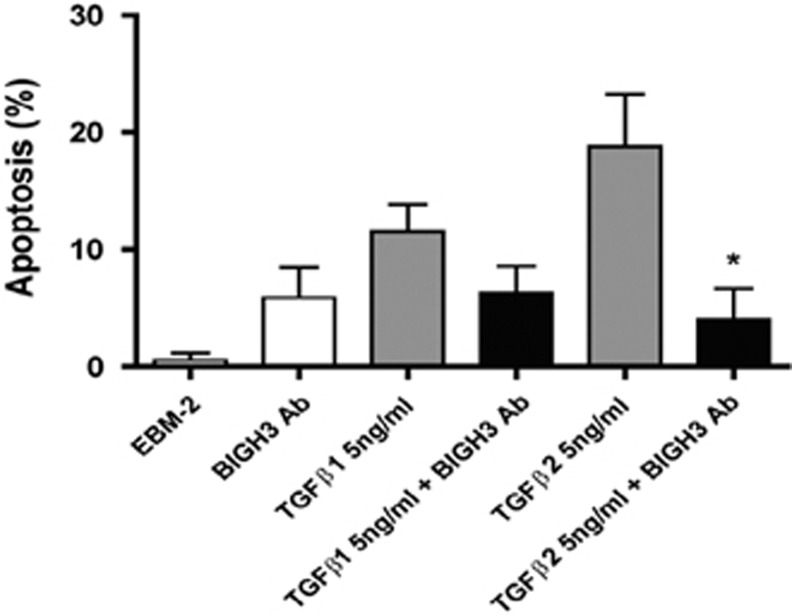
Treatment of retinal pericytes with TGFβ1 and 2 induced 12 and 18% apoptosis in HRPs, respectively. The inhibition with BIGH3 Ab in cells treated with TGFβ1 reduced the level of cell death from 12 to 6% (Figure 3). The inhibition with BIGH3 Ab in cells treated with TGFβ2 significantly reduced the cell death from 18 to 3% (Figure 3). These results demonstrate that TGFβ1- and 2-induced cell death is mediated by BIGH3 in HRPs, as in RECs reported in our previous study.14
Figure 3.
TGFβ-mediated apoptosis mitigated by BIGH3 antibodies. HRP were cultured in EMB-2 for 24 h, media was refreshed with 5 ng/ml of TGFβ1 or TGFβ2, with and without rabbit anti-BIGH3 antiserum (BIGH3 Ab). BIGH3 Ab significantly reduced TGFβ-induced apoptosis (Tukey's multiple comparison test, *P<0.05). One-way ANOVA was significant F(5,27)=5.695; P<0.01.
BIGH3-induced apoptosis of HRPs
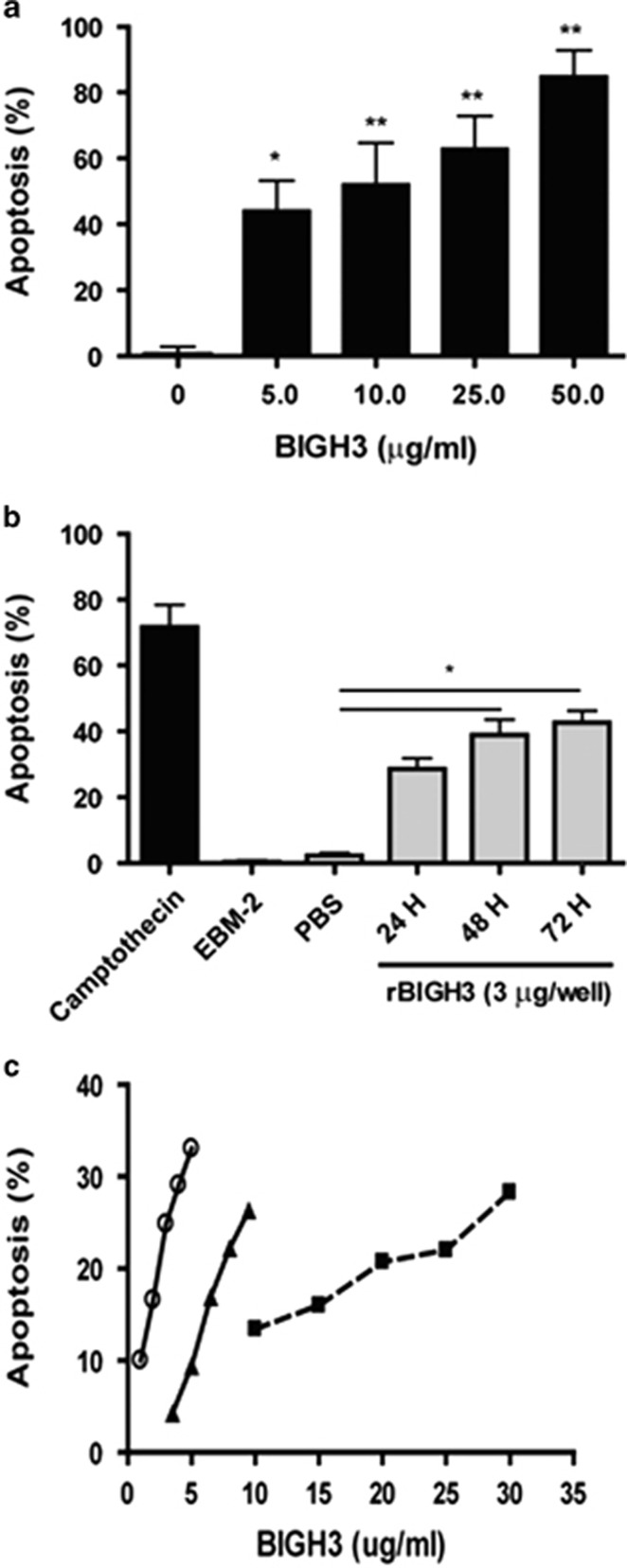
Cells treated with 0, 5, 10, 25, and 50 μg/ml of BIGH3 resulted in an increase in percent apoptosis in a dose-dependent manner (Figure 4a; one-way ANOVA shows a significant dose effect). In addition, BIGH3-induced HRP apoptosis was also determined to be time-dependent within the treatment period of 24 to 72 h (Figure 4b; one-way ANOVA shows a significant time effect).
Figure 4.
BIGH3 induced HRP apoptosis. (a) Dose-dependent increase in apoptosis: HRP were cultured in media containing recombinant BIGH3 protein at increasing concentrations. TUNEL assay was performed following 24 h treatment period, and a dose-dependent relationship existed between BIGH3 amount and percent apoptosis. Results from one-way ANOVA were significant (F(4,18)=9.154; P<0.001). A Dunnett's multiple comparison test was performed using 0 μg/ml as the control (*P<0.05, **P<0.01). (b) Time-dependent increase in apoptosis: cultured HRP were treated with 3 μg/ml of BIGH3 protein for 72 h. TUNEL assay was performed every 24 h, and a time-dependent relationship was observed. One-way ANOVA was significant (F(5,11)=17.54; P<0.01) and Dunnett's test was performed using recombinant BIGH3 vehicle (PBS) as control (*P<0.05). Camptothecin used a positive control (72 h; topoisomerase I inhibitor). (c) Cell type dependency in apoptosis: HRP (open circle), rhesus monkey RECs (closed triangle) and human RPTECs (closed square) were cultured and treated with increasing concentrations of BIGH3 protein. Cell Death was confirmed by TUNEL assay following 24 h treatment period. HRP were most sensitive to low concentration (1–5 μg/ml) of BIGH3 treatment which induced a 25% increase in apoptosis (or 6% increase in apoptosis per unit increase in BIGH3 concentration—μg/ml). RhRECs were less sensitive to BIGH3 treatment (2–10 μg/ml) which induced a 22% increase in cell apoptosis (or 2.5% increase in apoptosis per unit increase in BIGH3 concentration—μg/ml). RPTEC were the least sensitive to BIGH3 treatment (at a higher concentration of 10–30 μg/ml) which induced a 16% increase in cell apoptosis (or 0.8% increase in apoptosis per unit increase in BIGH3 concentration—μg/ml).
High sensitivity of HRP to BIGH3-induced apoptosis
Figure 4c shows that BIGH3 induced a dose-dependent increase of cell apoptosis in all three cell types. Changes in percent apoptosis with increasing BIGH3 concentration varied greatly by cell type. At 5 μg/ml, BIGH3 induced <10% apoptosis in RhREC and 35% in HRP, suggesting a 3.5-fold higher in HRP sensitivity to BIGH3 treatment. At 10 μg/ml, BIGH3 resulted in 27% apoptosis in RhREC and 14% in RPTEC, suggesting that renal epithelial cells were twofold lower in sensitivity to BIGH3-induced apoptosis than RECs. Furthermore, the progressive increase in apoptosis with increasing BIGH3 concentration also varied greatly among cell types. In HRP, apoptosis increased by 16% per unit increase in BIGH3 concentration (at the range of 1–5 μg/ml BIGH3). In comparison, RhREC apoptosis increased by 2.5% per unit increase in BIGH3 concentration (at range of 2–10 μg/ml BIGH3). RPTEC were the least responsive of all three cell types with a rate of 0.8% increase in apoptosis per unit increase in BIGH3 concentration (at the range of 10–30 μg/ml BIGH3, Figure 4c).
Histopathological analysis of retinal BIGH3 expression and macrophage distribution in NPDR
The gross and histological findings in the post-mortem globes from NDRI Donor 68373 were characteristic of NPDR. This Donor, a 68-year old woman with 7-year history of diabetes mellitus, died of complications secondary to renal and congestive heart failure. She was initially diagnosed with type II diabetes at 61 years of age and began to require insulin 4 years later. Her clinical course was complicated by peripheral neuropathy, renal failure requiring dialysis, and coronary artery disease. She received focal laser retinal therapy, bilateral intraocular lens placement, and intravitreal kenalog for diabetic macular edema. Her last A1c measurement at 4 months prior to death was 10.6%. There was no clinical history other retinal disease.
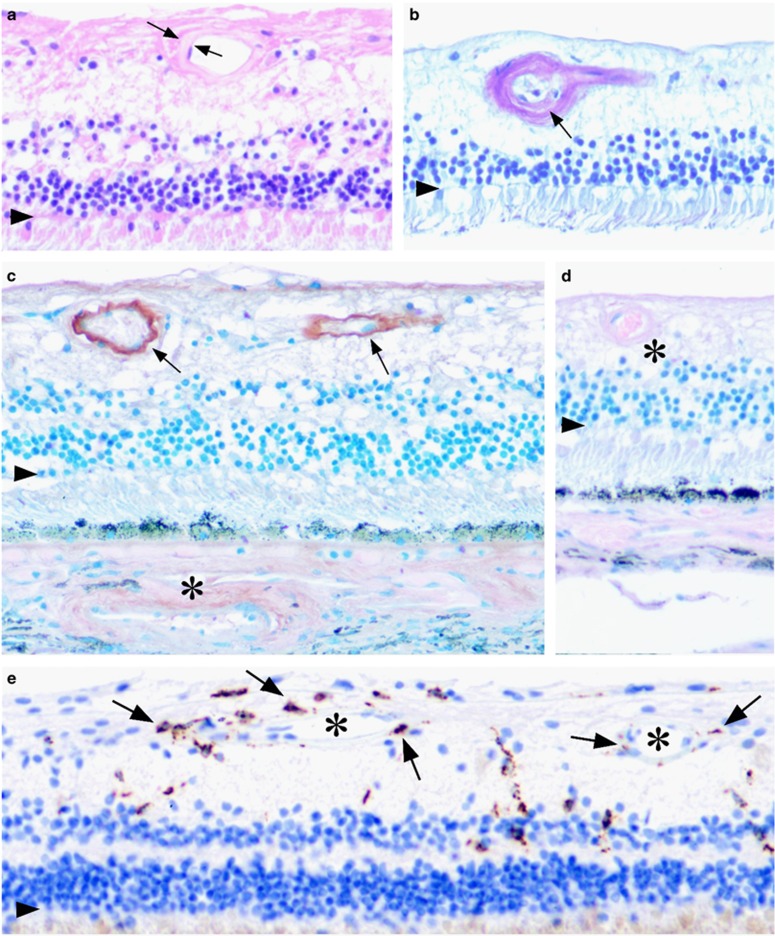
The whole-eye histological sections examined were oriented through the foveola and optic nerve head. The panels presented in Figure 5 correspond to mid-peripheral or perifoveal retina. For orientation an arrowhead in each panel marks the position of the outer limiting membrane. Histological study showed that the eye was free of evidence for age-related macular degeneration, glaucoma, or other retinal disorder. Uniform and diffuse thickening of the ciliary body basement membrane was present as is typically seen in diabetes. There was no evidence for vitreal neovascularization or hemorrhage. Hyaline thickening of the retinal vessels walls and arteriolosclerosis could be appreciated in the hematoxylin and eosin-stained sections and periodic acid schiff preparations (Figures 5a and b). Hyaline choroidopathy was also present. Occasional retinal microaneurysms could be identified in the sections. Focal dot/bot hemorrhages were appreciated macroscopically, and were confirmed histologically (data not illustrated). Taken together these findings are in keeping with early diabetic changes (NPDR).
Figure 5.
Distribution of BIGH3 and macrophage marker, CD68 in the retina of a 68-year old woman with 7-year history of Type II diabetes mellitus who died of renal and congestive heart failure. (a) H&E-stained section showing hyaline thickening of a vessel wall (between arrows). (b) Periodic acid schiff stain showing arteriolosclerosis with early luminal narrowing (arrow). (c) Immunohistochemical localization of BIGH3. The most prominent expression was found in the retinal vessels (arrows) and occasionally in the choroidal vessels (asterisk). Counter stain here and in d was Azure B, which stains melanin metachromatically blue/green allowing the brown reaction product to be clearly distinguished from the retinal pigmented epithelium and choroid melanosomes. (d) Adsorbed control performed by co-incubating the primary antibody with purified recombinant BIGH3 protein. Immunospecific staining was abolished. *Retinal arteriole. (e) Distribution of CD68 expressing macrophages (arrows). The macrophages were often associated with the retinal arterioles and capillaries (asterisk). Each of the panels represents perifoveal/mid-peripheral retina of the right globe photographed at × 200. Arrowhead in each panel marks the outer limiting membrane.
The distribution of BIGH3 and macrophage marker, CD68 were examined by avidn–biotin complex immunohistochemistry (Figures 5c–e). In Figure 5c and d, Azure B was used as the counter stain. Here, melanin granules stain metachromatically green/blue allowing the brown reaction product to be distinguished from retinal pigment epithelium and choroidal melanosomes.22 immunospecific staining for BIGH3 could often be appreciated in the walls of the retinal vessels (arrows, Figure 5c), sometimes in the choroid vessels as well (asterisk). Adsorbed controls performed by co-incubating the primary antibody with purified recombinant BIGH3 protein abolished the immunospecific staining. Macrophages could be identified within the neural retina by their prominent CD68 expression (Figure 5e). These cells were rarely present in the outer nuclear layer, but could be found within the inner nuclear layer and inner plexiform layers. In both of these regions, the macrophages were often associated with the arterioles and capillaries. The vascular BIGH3 deposition and presence of retinal macrophages was not observed in normal human control sections.
Discussion
A study on retinal pericyte dropout is essential to better understand the early development of DR and progression of the disease. In two previous studies, we reported an inflammatory pathway whereby diabetes related macrophage infiltration to the eye and the kidney led to TGFβ and TGFβ-induced BIGH3 protein resulting in apoptosis in RhREC14 and renal proximal tubule epithelial cells (RPTEC21), respectively. However, this macrophage-TGFβ—BIGH3 pathway has not been applied to retinal pericyte apopotsis. Therefore, we hypothesize that a similar pathway also contributes to pericyte dropout which, is an important early event in the development of DR+.
Using immunofluorescence RT/PCR and western blot analysis, we provided strong evidence that HRP in culture synthesize and secret BIGH3 (Figure 1) and that TGFβ (1 and 2) both induced expression (mRNA) and secretion (western blots on conditioned media) of BIGH3 protein (Figure 2). As pointed in our previous publication, both isomers of TGFβ are expressed in the eye, therefore, it is important to compare the efficacy of both TGFβ1 and β2 isomers. Our results show that TGFβ1 seems more effective than TGFB2 in the induction of BIGH3 expression and protein synthesis/secretion. Additional experiments, however, will need to be carried out to further clarify the significance of these two protein isoforms in BIGH3 gene expression and the extent of BIGH3-mediated retinal cell apoptosis.
BIGH3 antiserum significantly reduced the effect of TGFβ-induced HRP apoptosis (Figure 3) strongly implicating that BIGH3 is a key mediator of TGFB-induced apoptosis. This is highly consistent with conclusions in our previous publications14, 21 that TGFβ from macrophage induced the synthesis and release of BIGH3 protein from target cells. The apoptotic BIGH3 protein then acted in an autocrine or paracrine manner to induce cell death.
The mechanism(s) by which retinal pericytes undergo cell death remains unknown, and our data support the notion that exogenously added recombinant BIGH3 protein induced HRP apoptosis in a time- and dose-dependent manner (Figures 4a and b). Importantly, investigating which vascular cell type is more sensitive to BIGH3-mediated cell death would be relevant as it is recognized that pericytes dropout before endothelial cells is an early hallmark of breakdown of the blood-retinal barrier. Our in vitro data showed that HRP are 3.5-fold more sensitive than RhREC to BIGH3-mediated cell death (Figure 4c). Thus raising the possibility that elevated TGFβ and BIGH3 levels in the diabetic retina may contribute to the earlier dropout of pericytes and promote further vascular pathology.
To complement our in vitro findings we sought to examine the retinal expression of BIGH3 and microglia/macrophages in early DR. We selected a NDRI post-mortem Donor who had Type II DM for a relatively short period (7 years) and did not have a clinical history of PDR. A thorough histological evaluation revealed pathological changes characteristic of NPDR.23 No evidence for proliferative retinopathy was found in the eye of this donor, or other retinal disease. BIGH3 was clearly detected in the retinal arterioles (Figure 5). and perivascular microglia/macrophages were also associated within the tissue. Our human histopathological studies are therefore consistent with the hypothesis that monocyte/macrophage-induced BIGH3 expression is an important progressive step in the pathophysiology of pericyte loss. Ongoing studies will extend these observations to additional cases representing the full-spectrum DR.
Han et al17 reported that BIGH3 induced bovine retinal pericyte apoptosis via an RGD sequence within the C-terminus. However, it is not clear if BIGH3-induced HRP apoptosis reported in this study is similarly mediated by RGD, or by EPDIM binding to the laminin receptor (α3β1) as suggested by Zamplia et al.16 Further studies, including the identification of the integrin receptor and the activation of specific pro-caspases will be essential to understand the molecular basis of cell death in this pathway on the development of DR.

Acknowledgments
We thank Robert Moritz and Kalpana Parvathaneni for technical assistance, Priscilla Gonzalez-Fernandez for preparation of the human histological sections, the staff of the National Disease Research Interchange and Lions Eye Banks for efficiently orchestrating the procurement of these valuable globes, and the Donor's family who ultimately made the material available to the biomedical research community. This work was supported by the National Institute on Minority Health and Health Disparities (G12MD007591), the National Heart Lung and Blood Institute (R01HL70963) of the National Institutes of Health, The San Antonio Life Sciences Institute Grant (SALSI) from Texas Higher Education Coordinating Board, National Institutes of Health (R01 EY09412) (FG-F); Merit Review Award 101BX007080 from the Biomedical Laboratory Research and Development Service of the Veterans Affair Office of Research and Development (FG-F); an unrestricted grant from Research to Prevent Blindness to the Department of Ophthalmology at State University of New York at Buffalo; NIH grant U42-OD011158 to the National Disease Research Interchange for the Human Tissue and Organ Research Resource. Research Start-Up Awards from Research Mississippi! and the the VA office of Research Development (FG-F).
The authors declare no conflict of interest.
References
- Geiss LS, Wang J, Cheng YJ, Thompson TJ, Barker L, Li Y et al. Prevalence and incidence trends for diagnosed diabetes among adults aged 20 to 79 years, United States, 1980-2012. JAMA 2014; 312(12): 1218–1226. [DOI] [PubMed] [Google Scholar]
- Owsley C, McGwin G Jr., Lee DJ, Lam BL, Friedman DS, Gower EW et al. Diabetes eye screening in urban settings serving minority populations: detection of diabetic retinopathy and other ocular findings using telemedicine. JAMA Ophthalmol 2015; 133(2): 174–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempen JH, O'Colmain BJ, Leske MC, Haffner SM, Klein R, Moss SE et al. The prevalence of diabetic retinopathy among adults in the United States. Arch Ophthalmol 2004; 122(4): 552–563. [DOI] [PubMed] [Google Scholar]
- Gardner TW, Antonetti DA, Barber AJ, LaNoue KF, Levison SW. Diabetic retinopathy: more than meets the eye. Surv Ophthalmol 2002; 47(Suppl 2): S253–S262. [DOI] [PubMed] [Google Scholar]
- Hall AP. Review of the pericyte during angiogenesis and its role in cancer and diabetic retinopathy. Toxicol Pathol 2006; 34(6): 763–775. [DOI] [PubMed] [Google Scholar]
- Pfister F, Feng Y, vom Hagen F, Hoffmann S, Molema G, Hillebrands JL et al. Pericyte migration: a novel mechanism of pericyte loss in experimental diabetic retinopathy. Diabetes 2008; 57(9): 2495–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enge M, Bjarnegard M, Gerhardt H, Gustafsson E, Kalen M, Asker N et al. Endothelium-specific platelet-derived growth factor-B ablation mimics diabetic retinopathy. EMBO J 2002; 21(16): 4307–4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speiser P, Gittelsohn AM, Patz A. Studies on diabetic retinopathy. 3. Influence of diabetes on intramural pericytes. Arch Ophthalmol 1968; 80(3): 332–337. [DOI] [PubMed] [Google Scholar]
- Kowluru RA, Koppolu P, Chakrabarti S, Chen S. Diabetes-induced activation of nuclear transcriptional factor in the retina, and its inhibition by antioxidants. Free Radic Res 2003; 37(11): 1169–1180. [DOI] [PubMed] [Google Scholar]
- Rangasamy S, McGuire PG, Franco Nitta C, Monickaraj F, Oruganti SR, Das A. Chemokine mediated monocyte trafficking into the retina: role of inflammation in alteration of the blood-retinal barrier in diabetic retinopathy. PLoS One 2014; 9(10): e108508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asmis R, Qiao M, Rossi RR, Cholewa J, Xu L, Asmis LM. Adriamycin promotes macrophage dysfunction in mice. Free Radic Biol Med 2006; 41(1): 165–174. [DOI] [PubMed] [Google Scholar]
- McDonald PP, Fadok VA, Bratton D, Henson PM. Transcriptional and translational regulation of inflammatory mediator production by endogenous TGF-beta in macrophages that have ingested apoptotic cells. J Immunol 1999; 163(11): 6164–6172. [PubMed] [Google Scholar]
- Skonier J, Bennett K, Rothwell V, Kosowski S, Plowman G, Wallace P et al. Beta ig-h3: a transforming growth factor-beta-responsive gene encoding a secreted protein that inhibits cell attachment in vitro and suppresses the growth of CHO cells in nude mice. DNA Cell Biol 1994; 13(6): 571–584. [DOI] [PubMed] [Google Scholar]
- Mondragon AA, Betts-Obregon BS, Moritz RJ, Parvathaneni K, Navarro MM, Kim HS et al. BIGH3 protein and macrophages in retinal endothelial cell apoptosis. Apoptosis 2015; 20(1): 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morand S, Buchillier V, Maurer F, Bonny C, Arsenijevic Y, Munier FL et al. Induction of apoptosis in human corneal and HeLa cells by mutated BIGH3. Invest Ophthalmol Vis Sci 2003; 44(7): 2973–2979. [DOI] [PubMed] [Google Scholar]
- Zamilpa R, Rupaimoole R, Phelix CF, Somaraki-Cormier M, Haskins W, Asmis R et al. C-terminal fragment of transforming growth factor beta-induced protein (TGFBIp) is required for apoptosis in human osteosarcoma cells. Matrix Biol 2009; 28(6): 347–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JH, Ha SW, Lee IK, Kim BW, Kim JG. High glucose-induced apoptosis in bovine retinal pericytes is associated with transforming growth factor beta and betaIG-H3: betaIG-H3 induces apoptosis in retinal pericytes by releasing Arg-Gly-Asp peptides. Clin Experiment Ophthalmol 2010; 38(6): 620–628. [DOI] [PubMed] [Google Scholar]
- Hammes HP. Pericytes and the pathogenesis of diabetic retinopathy. Horm Metab Res 2005; 37(Suppl 1): 39–43. [DOI] [PubMed] [Google Scholar]
- Benjamin LE. Glucose, VEGF-A, and diabetic complications. Am J Pathol 2001; 158(4): 1181–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank RN. Diabetic retinopathy. N Engl J Med 2004; 350(1): 48–58. [DOI] [PubMed] [Google Scholar]
- Moritz RJ, LeBaron RG, Phelix CF, Rupaimoole R, Kim H-S, Tsin AT et al. Macrophages TGF-beta1 and the preapoptotic extracellular matrix protein BIGH3 induce renal cell apoptosis in prediabetic and diabetic conditions. Int J Clin Med 2016; 7: 496–510.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamino H, Tam ST. Immunoperoxidase technique modified by counterstain with azure B as a diagnostic aid in evaluating heavily pigmented melanocytic neoplasms. J Cutan Pathol 1991; 18(6): 436–439. [DOI] [PubMed] [Google Scholar]
- Yanoff M, Sassani JW15 – Diabetes Mellitus. In: Sassani MYW (ed.). Ocular Pathology, 7th edn. W.B. Saunders: London, UK, 2015. pp 527–553.