Abstract
Purpose
To determine the preliminary efficacy and safety of off-label dexamethasone implant for treatment of recurrent cystoid macular edema (CME) secondary to Irvine–Gass syndrome (IGS).
Patients and methods
This study was set in Raghudeep Eye Clinic, Ahmedabad and LV Prasad Eye Institute, Hyderabad (India). It is a Prospective Case Series. Prospective case series comprising of patients with uncomplicated pseudophakia and CME due to IGS who recurred after one course of topical steroids with NSAIDS and a sub-Tenon corticosteroid injection. A complete ocular and systemic exam, fluorescein angiography, and central subfield thickness (CST) on optical coherence tomography scans were performed. Follow-up visits were on days 1, 15, and 30 and then monthly for a year. Appropriate statistical analysis was done. The primary outcome measure was the change in CDVA at months 1, 6, and 12. Secondary outcome measures were recurrence of CME and complications if any as noted at months 1, 2, 6, and 12.
Results
About 27 patients (27 eyes) with 16 males were included. Median age: 63.24±5.62 years. At 1 month, the CDVA improved to 0.04±0.02 (20/25) logMAR from 0.52±0.12 logMAR (20/70) (P=0.001) with a reduction in CST from 454.2±45.3 to 218.32±38.15 microns(P=0.013). The CDVA was 0.04±0.03 logMAR(P<0.001) at month 6 and 0.05±0.02 logMAR(P<0.001) at month 12. The CST was 221±35.2 microns (P=0.013) at month 6 and 214±43.34 microns (P=0.0124) at month 12. All improvements were maintained for a year. Only one patient required a second injection. No complications were noted.
Conclusion
The implant is safe and effective for the treatment of recurrent CME due to IGS.
Introduction
Pseudophakic cystoid macular edema due to Irvine–Gass syndrome1, 2, 3, 4, 5 remains an important cause of decreased vision following uncomplicated cataract surgery and intraocular lens (IOL) implantation. The most accepted patho-physiologic mechanism for PCME is increased inflammation,1, 2, 3, 4, 5 although vitreous disturbance6 and hypotony1, 2 may account for a sizeable proportion of cases. It typically presents 4–12 weeks after cataract surgery, and often resolves spontaneously.4 PCME or Irvine–Gass syndrome develops independently of co-morbidities such as diabetes mellitus, vein occlusion, or uveitis.1, 2, 3, 4, 5
Prophylaxis is suggested for in situations where patients who are prone to develop cystoid macular edema following cataract surgery, such as patients with uveitic cataract7 and those with diabetes.8, 9 In cases that do not resolve spontaneously, a course of topical steroids and non-steroidal anti-inflammatory agents (NSAIDS) helps in the resolution of a large proportion of cases.10 A certain subset of patients, however, does not respond to topical therapy alone. A step ladder approach for the management of these patients is proposed and includes oral carbonic anhydrase inhibitor therapy,11 peribulbar12 or intravitreal steroids,13 anti-VEGF agents,14 immunomodulators15, 16 and, in cases secondary to vitreous traction, Nd: Yag laser vitreolysis,17 vitrectomy,18 or other surgical approaches. There is, however, no standardized protocol prescribed for its management, probably because of a lack of randomized trials.
A dexamethasone implant19, 20 (Ozurdex, Allergan Inc., New Jersey, CA, USA) has been recently approved for the treatment of intermediate and posterior uveitis, as well as macular edema secondary to diabetes mellitus or branch vein occlusions. It appears to have a better safety profile when compared with other corticosteroids used locally19, 20, 21 and it has been found to be safe and effective in the treatment of the aforementioned conditions. There is published literature available on the use of this implant in recalcitrant cystoid macular edema secondary to the Irvine–Gass syndrome mainly in the form of case reports and retrospective case series.22, 23, 24, 25 However, there is no prospective study on the use of this implant in either recalcitrant or recurrent cystoid macular edema secondary to Irvine–Gass syndrome in patients with uncomplicated pseudophakia.
We aimed to obtain data on the efficacy and safety of the dexamethasone implant (0.7 mg) in the treatment of recurrent cystoid macular edema due to Irvine–Gass syndrome in patients with uncomplicated pseudophakia(off-label use).
Materials and methods
This prospective case series included adult patients, with senile cataract (age >40 years), who underwent uncomplicated phacoemulsification with posterior chamber IOL implantation.
Study population
For inclusion, patients were required to have: Symptomatic PCME consistent with the diagnosis of Irvine–Gass syndrome that had recurred after at least one course of topical steroids with NSAIDs and one sub-Tenon triamcinolone acetonide injection. Additional therapy prior to inclusion was permissible and could consist of any of the following: (1) a repeat course of topical steroids combined with NSAIDS (2) posterior sub-tenon or intravitreal triamcinolone acetonide (3) A course of oral carbonic anhydrase inhibitors. The patients could only be included once at least 3 months had elapsed after the last corticosteroid injection.
Resolution and recurrence of CME
Resolution of cystoid macular edema was defined as the complete disappearance of retinal cysts, retinal thickening, and subretinal fluid (if present) along with a restoration of the normal foveal contour and a reduction of central subfield thickness (CST) to 250 microns or less as seen on OCT and clinical examination, and associated with an improvement in CDVA to 20/25 (0.1 logMAR) or better. Recurrence was defined as the re-appearance of retinal thickening, cysts and/or subretinal fluid after complete resolution of the CME with therapy associated with a drop in CDVA to 20/40 (0.3 logMAR) or worse, with or without metamorphopsia.
Exclusion criteria
Patients excluded were patients (1) who were known steroid responders (2) who had PCME secondary to obvious causes such as persistent vitreomacular traction or ocular hypotony (3) confounding co-morbidities such as uveitis, diabetes retinopathy, and/or vein occlusions (4) patients who had already received an intravitreal dexamethasone implant for CME secondary to Irvine–Gass syndrome, and (5) a baseline VA worse than 20/200(1 logMAR). Informed consent for the procedure was obtained from patients prior to intervention. The study was approved by the institutional review board and adhered to the tenets of Helsinki.
Examination procedure
A thorough history was obtained from all patients and a comprehensive ocular and systemic examination performed. Data noted included demographics, the corrected distance visual acuity (CDVA) measured on an ETDRS chart, details of the anterior and posterior segment examination including the intraocular pressure measured using applanation tonometry. The diagnosis was based on clinical examination, fundus fluorescein angiography, and optical coherence tomography scans.
Fundus fluorescein angiography was performed using the Zeiss Fundus Camera (Visucam 500, Jena, Germany) with informed consent. Cystoid macular edema was identified by the typical ‘flower-petal' appearance observed in the late phase. Care was taken to rule out other disorders.
The OCT scan was used to document retinal thickening, cysts, and subretinal fluid in these patients and was a part of the diagnostic criteria. The CST was measured using a standardized technique using a spectral domain ocular coherence tomography scan and volume scans were performed (OCT, Zeiss Meditec, Erlangen, Germany) in accordance with past literature.19, 20, 22, 23
Off-label intravitreal dexamethasone implant injection
The conjunctival cul-de-sac was anesthetized using proparacaine hydrochloride (0.5%) eye drops thrice, 10 min apart. A drop of povidone iodine (5%) was instilled in the cul-de-sac 10 min prior to the procedure. The injection was carried out in the operating room using aseptic measures and a standardized surgical technique. The implant (0.7 mg) was injected in the infero-temporal quadrant 3.5 mm from the limbus. The site was massaged using a sterile scleral indenter and a drop of povidone iodine (5%) instilled in the conjunctival cul-de-sac at the end of the procedure. Post operatively the patient was given antibiotic eye drops (ofloxacin 0.3%) four times a day for a week.
Postoperative clinical examination was done on days 1, 15, and 30 and then monthly for a year, unless complications necessitated a more frequent follow-up. The CDVA, IOP, and a complete ocular examination along with the OCT raster scan was performed at each visit. The central foveal thickness was measured at each visit, and the change in thickness, if any was noted. An IOP rise of 6 mm Hg or more from baseline and/or an absolute IOP value of 24 mm Hg or more was defined as an IOP spike.
Outcome measures
The change in CDVA at the 1-month, 6-month, and 1-year follow-up was the primary outcome measure. The change in central foveal thickness at 1 month, 6 months and 1 year, recurrence rate and complications, if any were noted as the secondary outcome measures. Analyses were performed at months 1, 6, and 12. IOP measurements were additionally analyzed at month 2. Thus visits at months 1, 6, and 12 (and IOP measurements at month 2) were analyzed, but patients were followed up at least every month, and monitored both for improvement (or deterioration as the case may be) and to look for complications.
Statistical analysis
The repeated measures ANOVA test was used to assess the significance of the change in CDVA, the IOP, and CST over months 1, 6, and 12. Change in IOP from baseline, if any was also noted, at month 2. Analyses were performed using the Statistical Package for Social Sciences (Version 20.0, Chicago, IL, USA). Statistical significance was set at P<0.05.
Results
A total of 33 patients (33 eyes) were found to be eligible for the study in the stated period of which 27 patients completed the 1-year follow-up, and these 27 patients (27 eyes) form the basis for our analysis.
Demographics
About 16 of these 27 patients were males. The median age range was 63.24±5.62 years with a range of 50–74 years. None of the patients had co-existent systemic disease that could confound the diagnosis or the response to therapy. None of the patients had any other ocular and/or systemic disease that could confound the results.
All patients had undergone uneventful phacoemulsification with posterior chamber IOL implantation (in the bag). The median duration from cataract surgery to first episode of CME was 6.12±2.72 weeks. All patients had one course of topical therapy with prednisolone acetate and ketorolac eye drops for the treatment of symptomatic CME due to IGS and had responded to it, but had a recurrence following cessation of therapy. Following the course of topical therapy, all patients had at least one sub-Tenon injection of triamcinolone acetonide (dose 40 mg), and responded favorably to it, but had a recurrence once the effect had worn off. Five patients had received a second posterior sub-Tenon injection of 40 mg triamcinolone acetonide prior to inclusion. None of the patients had an IOP spike noted prior to inclusion. The median duration of CME after the last recurrence was 7.25±1.2 weeks (range 4–12 weeks) prior to inclusion. All patients received the intravitreal dexamethasone implant as stated earlier.
The change in CDVA (including the approximate Snellen's equivalent), CST, and IOP over the course of follow-up is outlined in Table 1. Significant improvements were seen in CDVA and CST, with no significant change in IOP. Only one patient had a recurrence of CME, 3 months after the dexamethasone implant injection; he required a second dexamethasone implant injection for recurrent PCME. He experienced no further recurrences, and maintained a vision of 0.04 logMAR (approximately Snellens equivalent of 20/25) till the end of follow-up. Subset analysis of patients who had received a second sub-Tenon injection showed that they responded equally well to therapy, and that none of them had a recurrence till the end of follow-up.
Table 1. Assessment of change in CDVA, CST, and IOP from baseline at months 1, 6, and 12 after injection of dexamethasone implant.
| Parameter | Baseline values (range) (Snellena) | Month 1 | Month 6 | Month 12 |
|---|---|---|---|---|
| P-value from baseline | P-value from baseline | P-value from baseline | ||
| CDVA (logMAR) | 0.52±0.12 (0.2–0.78) (20/70) | 0.04±0.02 (20/25) | 0.04±0.03 (20/25) | 0.05±0.02 (20/25) |
| P<0.001 | P<0.001 | P<0.001 | ||
| CST thickness (microns) | 454.2±45.3 (358–587) | 218.32±38.15 | 221±35.2 | 214±43.34 |
| P=0.013 | P=0.013 | P=0.0124 | ||
| IOPb (mm Hg) | 14.4±4.32 (8–19) | 16.4±4.4 | 14.8±5 | 13.54±4.2 |
| P=0.56 | P=0.48 | P=0.6 |
Abbreviations: CDVA, corrected distance visual acuity; CST, central subfield thickness; IOP, intraocular pressure.
Snellen's equivalent is for mean logMAR.
The median IOP at 2 months was 15.2±3.72 mm Hg (P=0.59).
None of the patients developed an IOP spike till the end of the follow-up. None of the patients had any adverse event till the end of the follow-up period. None of the patients required additional therapy till the end of the follow-up period (except for the one patient mentioned earlier). Eventually, at the 1-year follow-up, all patients had a final visual acuity of 20/25 or better (Figure 1).
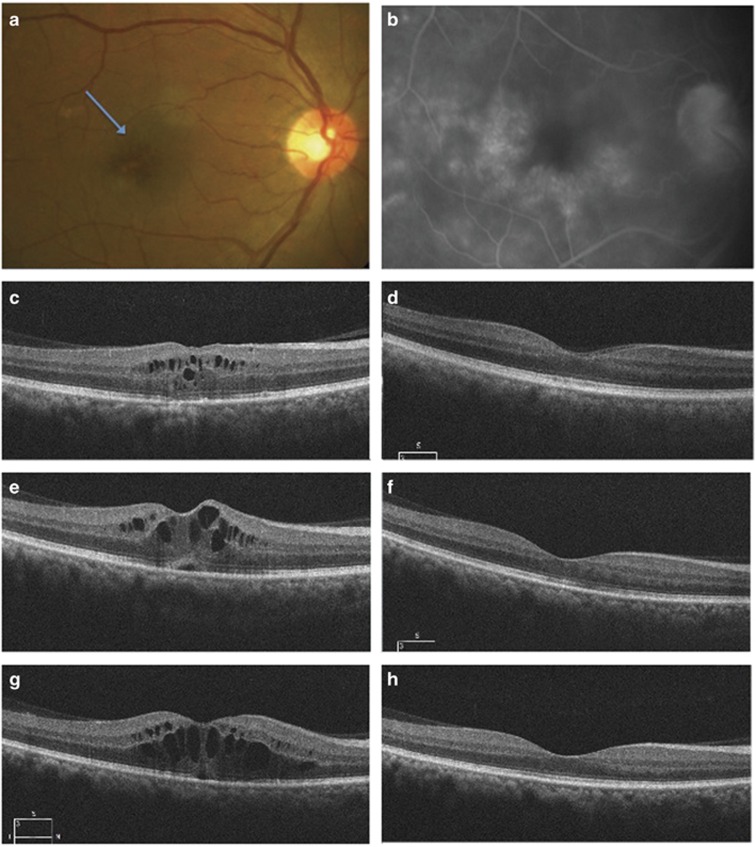
Figure 1.
(a) The clinical fundus photograph and (b) the late phase of fluorescein angiogram of a 64-year-old male who underwent uneventful phacoemulsification and developed CME (blue arrow, a) due to IGS 5 weeks postoperatively. His vision was 20/40 and had associated metamorphopsia. OCT (c) showed retinal thickening and cysts with some loss of foveal contour. A course of topical steroids and NSAIDS helped resolve the edema (d) with CDVA improving to 20/20. One month later, he came back with a drop in CDVA(6/18) and metamorphopsia. The OCT (e) shows retinal thickening, cystic changes, and subretinal fluid. A sub-tenon injection was administered and it helped resolve the CME (f) with vision restored to 20/20, but it recurred 2.5 months after the injection. (g) The patient received an intravitreal dexamethasone implant 5 weeks after the recurrence, and the CME had resolved at the 1-month follow-up (h) with no recurrence till the end of follow-up.
Discussion
Our study shows good results through the use of the intravitreal dexamethasone implant (Ozurdex, Allergan Inc.) in patients who have had an otherwise uncomplicated postoperative course following cataract surgery in terms of resolution of CME due to IGS as well as prevention of its recurrence. All patients had at least two episodes of recurrence but demonstrated a subjective and objective improvement without complications after the injection both in terms of visual gain and resolution of the cystoid macular edema on clinical examination, as well as OCT scans. Only one patient required a second injection.
The Irvine–Gass syndrome1, 2, 3, 4, 5 is a poorly understood entity, and spontaneous resolution (which is a common end point) can take from 2 months to a year. Observation or repeat courses of topical therapy may be troublesome for professionally active individuals.
There have been numerous retrospective reports22, 23, 24, 25 on the use of the intravitreal dexamethasone implant for recalcitrant or refractory cystoid macular edema. Refractory CME is defined in these studies as persistent CME with foveal thickness more than 250 μm and intraretinal cystic changes revealed by spectral domain optical coherence tomography, lasting for at least 90 days after the initiation of treatment.23 Subset analysis in one of largest trials on this implant26 found it to be effective in the treatment of Irvine–Gass syndrome with an acceptable safety profile. Two retrospective series22, 23 comprising 9 and 11 eyes, respectively, reported good results in patients who had several attempts at treatment. Treatment administered in this series prior to treatment with the implant included topical steroids and NSAIDs, triamcinolone acetonide intravitreally or sub-Tenon's, bevacizumab, and oral carbonic anhydrase inhibitors. The CME had persisted in both for more than 6 months. The results were favorable both in terms of safety and efficacy till the end of the follow-up period, which in both series was 6 months. The duration of refractory CME in these studies is comparable to the median time lapse between cataract surgery and first dexamethasone implant injection.
Corticosteroids other than this implant have been used in the past, with the optimal route of corticosteroids delivery a matter of debate. The sub-Tenon route is considered equal to the retrobulbar route in terms of efficacy and safety too27 in patients with pseudophakic CME. Also, the sub-Tenon route is generally considered safe with respect to IOP.28 A study29 that looked at IOP elevation following administration of triamcinolone acetonide through various routes found that the intravitreal group had a higher incidence of IOP rise beyond 30 mm Hg. Other studies,27, 28 as already mentioned did not find a significant IOP rise with the use of triamcinolone acetonide irrespective of the route used. There is in general, a concern over a higher incidence of IOP elevation following intravitreal use of triamcinolone acetonide.30 The implant is considered safer than other corticosteroids for ocular use.21 Also, a retrospective comparative case series31 found the dexamethasone implant superior to sub-tenon corticosteroids in the treatment of inflammatory cystoid macular edema, including CME, due to Irvine–Gass syndrome both in terms of efficacy and safety. Most studies on the role of corticosteroids in refractory CME due to IGS provide a follow-up of 6 months. The intravitreal dexamethasone implant (Ozurdex, Allergan Inc.) remains to date the only steroid preparation approved for intraocular use albeit for diseases other than the IGS. Our study underscores the safety and efficacy of the dexamethasone implant as noticed in the aforementioned study;31 however, we cannot comment on its superiority to other corticosteroid preparations as we had no comparative arm.
Our study is limited by the lack of a comparative arm and the relatively small number of patients. The sample size is probably too small to find out any adverse effects such as endophthalmitis, retinal detachment, and vitreous hemorrhage. It deals with an important problem and one that can be frustrating for the cataract surgeon who might feel robbed of success after a perfectly executed cataract surgery. Often, it resolves spontaneously but there are situations wherein it may be vital to the patient's interests to have the CME resolve expeditiously. The intravitreal dexamethasone implant may help achieve rapid resolution of the CME without recurrence, and given its relatively better safety profile, reduce complications. Overall, to the best of our knowledge, this is the first study that explores the outcome of recurrent CME due to IGS prospectively and for a follow-up period of 1 year.
In conclusion, the intravitreal dexamethasone implant is a valid treatment option for patients with recurrent pseudophakic CME secondary to Irvine–Gass syndrome who have been treated with topical therapy and a posterior sub-Tenon triamcinolone acetonide injection. It seems to improve visual acuity, reduces CST and is effective in preventing recurrences with a good safety profile upto a year. Further randomized trials can look at its efficacy and safety vis-à-vis triamcinolone acetonide and/or other treatment modalities such as anti-VEGF agents or oral carbonic anhydrase inhibitors.
The authors declare no conflict of interest.
Footnotes
Presented as a free paper at the ASCRS Meet April 2015, San Diego, CA, USA.
References
- Lobo C. Pseudophakic cystoid macular edema. Ophthalmologica 2012; 227: 61–67. [DOI] [PubMed] [Google Scholar]
- Joussen A, Wolfensberger T. Mechanisms of macular edema and therapeutic approaches In Ryan SJ (ed) Retina, 5th edn. Elsevier: New York, NY, USA, 2013, pp 590–594.
- Bhattacherjee P. The role of arachidonate metabolites in ocular inflammation. Prog Clin Biol Res 1989; 312: 211–227. [PubMed] [Google Scholar]
- Flach AJ. The incidence, pathogenesis and treatment of cystoid macular edema following cataract surgery. Trans Am Ophthalmol Soc 1998; 96: 557–634. [PMC free article] [PubMed] [Google Scholar]
- Bergman M, Laatikainen L. Cystoid macular oedema after complicated cataract surgery and implantation of an anterior chamber lens. Acta Ophthalmol (Copenh) 1994; 72: 178–180. [DOI] [PubMed] [Google Scholar]
- Frost NA, Sparrow JM, Strong NP, Rosenthal AR. Vitreous loss in planned extracapsular cataract extraction does lead to a poorer visual outcome. Eye 1995; 9: 446–451. [DOI] [PubMed] [Google Scholar]
- Yilmaz T, Cordero-Coma M, Gallagher MJ. Ketorolac therapy for the prevention of acute pseudophakic cystoid macular edema: a systematic review. Eye 2012; 26: 252–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosker M, Sungur G, Celik T, Unlu N, Simsek S. Phacoemulsification with intraocular lens implantation in patients with anterior uveitis. J Cataract Refract Surg 2013; 39: 1002–1007. [DOI] [PubMed] [Google Scholar]
- Lara Smalling A, Cakiner-Egilmez T. Diabetes and cataract surgery: preoperative risk factors and positive nursing interventions. Insight 2014; 39: 18–20. [PubMed] [Google Scholar]
- Elsawy MF, Badawi N, Khairy HA. Prophylactic postoperative ketorolac improves outcomes in diabetic patients assigned for cataract surgery. Clin Ophthalmol 2013; 7: 1245–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehelsta H, Jampol L. Pharmacologic therapy of pseudophakic cystoid macular edema: 2010 update. Retina 2011; 31: 4–12. [DOI] [PubMed] [Google Scholar]
- Lafranco Dafflon M, Tran VT, Guex-Crosier Y, Herbort CP. Posterior sub-tenon's steroid injections for the treatment of posterior ocular inflammation: indications, efficacy and side effects. Graefes Arch Clin Exp Ophthalmol 1999; 237: 289–295. [DOI] [PubMed] [Google Scholar]
- Jonas JB, Kreissing I, Segenring RF. Intravitreal triamcinolone acetonide for pseudophakic cystoid macular edema. Am J Ophthalmol 2003; 136: 384–386. [DOI] [PubMed] [Google Scholar]
- Spitzer MS, Ziemssen F, Yoeruek E, Petermeier K, Aisenbrey S, Szurman P. Efficacy of intravitreal bevacizumab in treating postoperative pseudophakic cystoid macular edema. J Cataract Refract Surg 2008; 34: 70–75. [DOI] [PubMed] [Google Scholar]
- Deuter CM, Gelisken F, Stublger N, Zierhut M, Doycheva D. Successful treatment of chronic pseudophakic macular edema (Irvine-Gass syndrome) with interferon alpha: a report of three cases. Ocul Immunol Inflamm 2011; 19: 216–218. [DOI] [PubMed] [Google Scholar]
- Wu L, Arevalo JF, Hernandez-Bogantes E, Roca JA. Intravitreal infliximab for refractory pseudophakic cystoid macular edema: results of the Pan-American Collaborative Retina Study Group. Int Opthalmol 2012; 32: 235–243. [DOI] [PubMed] [Google Scholar]
- Steinert RF, Wasson P. Neodynium:YAG laser anterior vitreolysis for Irvine-Gass cystoid macular edema. J Cataract Refract Surg 1989; 15: 304–307. [DOI] [PubMed] [Google Scholar]
- Harbour JW, Smiddy WE, Rubsamen PE, Murray TG, Davis JL, Flynn HW Jr. Pars plana vitrectomy for chronic pseudophakic cystoid macular edema. Am J Ophthalmol 1995; 120: 302–307. [DOI] [PubMed] [Google Scholar]
- Kuppermann BD, Blumenkranz MS, Haller JA, Williams GA, Weinberg DV, Chou C et al. Randomized controlled study of an intravitreous dexamethasone drug delivery system in patients with persistent macular edema. Arch Ophthalmol 2007; 125: 309–317. [DOI] [PubMed] [Google Scholar]
- Boyer D, Yoon Y, Belfort R, Bandello F, Maturi RK, Augustin AJ et al. MEAD Study Group. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology 2014; 121(10): 1904–1914. [DOI] [PubMed] [Google Scholar]
- Taylor SR, Isa H, Joshi L, Lightman S. New developments in corticosteroid therapy for uveitis. Ophthalmologica 2010; 224(Suppl 1): 46–53. [DOI] [PubMed] [Google Scholar]
- Medeiros M, Navarro R, Arumí J, Mateo C, Corcóstegui B. Dexamethasone intravitreal implant for treatment of patients with recalcitrant macular edema resulting from Irvine-Gass syndrome. Inv Ophthalmol Vis Sci 2013; 54(5): 3320–3324. [DOI] [PubMed] [Google Scholar]
- Al Zamil W. Short-term safety and efficacy of intravitreal 0.7-mg dexamethasone implants for pseudophakic cystoid macular edema. Saudi J Ophthalmol 2015; 29(2): 130–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer L, Schönfeld C. Cystoid macular edema after complicated cataract surgery resolved by an intravitreal dexamethasone 0.7-mg implant. Case Rep Ophthalmol 2011; 2(3): 319–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brynskov T, Laugesen CS, Halborg J, Kemp H, Sorenson TL. Longstanding refractory pseudophakic cystoid macular edema resolved using intravitreal 0.7mg dexamethasone implants. Clin Ophthalmol 2013; 7: 1171–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GA, Haller JA, Kuppermann BD, Blumenkranz MS, Weinberg DV, Chou C et al. Dexamethasone DDS Phase II Study Group. Dexamethasone posterior-segment drug delivery system in the treatment of macular edema resulting from uveitis or Irvine-Gass syndrome. Am J Ophthalmol 2009; 147: 1048–1054, 1054.e1–e2. [DOI] [PubMed] [Google Scholar]
- Thach AB, Dugel PU, Flindall RJ, Sipperley JO, Sneed SR. A comparison of retrobulbar versus sub-Tenon's corticosteroid therapy for cystoid macular edema refractory to topical medications. Ophthalmology 1997; 104(12): 2003–2008. [DOI] [PubMed] [Google Scholar]
- Mueller AJ, Jian G, Banker AS, Rahhal FM, Capparelli E, Freeman WR. The effect of deep posterior subtenon injection of corticosteroids on intraocular pressure. Am J Ophthalmol 1998; 125(2): 158–163. [DOI] [PubMed] [Google Scholar]
- Hirano Y, Ito T, Nozaki M, Yasukawa T, Sakurai E, Yoshida M et al. Intraocular pressure elevation following triamcinolone acetonide administration as related to administration routes. Jpn J Ophthalmol 2009; 53(5): 519–522. [DOI] [PubMed] [Google Scholar]
- Bucolo C, Grosso G, Drago V, Gagliano C. Intravitreal triamcinolone acetonide in the treatment of ophthalmic inflammatory diseases with macular edema: a meta-analysis study. J Ocul Pharmacol Ther 2015; 31(4): 228–240. [DOI] [PubMed] [Google Scholar]
- Errera M. Dexamethasone implant versus posterior sub-tenon triamcinolone acetonide for treatment of macular edema and/or vitritis in intraocular inflammatory diseases. Retrospective case series. Acta Ophthalmologica 2014; 92(S253): 1–3. [Google Scholar]