Abstract.
An ultrahigh-resolution (UHR) data collection mode was enabled on a whole-body, research photon counting detector (PCD) computed tomography system. In this mode, 64 rows of detector pixels were used, which corresponded to a pixel size of at the isocenter. Spatial resolution and image noise were quantitatively assessed for the UHR PCD scan mode, as well as for a commercially available UHR scan mode that uses an energy-integrating detector (EID) and a set of comb filters to decrease the effective detector size. Images of an anthropomorphic lung phantom, cadaveric swine lung, swine heart specimen, and cadaveric human temporal bone were qualitatively assessed. Nearly equivalent spatial resolution was demonstrated by the modulation transfer function measurements: 15.3 and spatial frequencies were achieved at 10% and 2% modulation, respectively, for the PCD system and 14.2 and for the EID system. Noise was 29% lower in the PCD UHR images compared to the EID UHR images, representing a potential dose savings of 50% for equivalent image noise. PCD UHR images from the anthropomorphic phantom and cadaveric specimens showed clear delineation of small structures.
Keywords: computed tomography, spatial resolution, photon counting detector, high-resolution mode
1. Introduction
Computed tomography (CT) has been widely used in the diagnosis of various diseases and in almost all body parts. Some clinical applications, such as lung, vascular, and temporal bone imaging, require excellent spatial resolution to delineate fine anatomical structure and pathology. There are many factors affecting the spatial resolution of CT images, such as the focal spot size, detector pixel size, geometric magnification, and image reconstruction kernel.1,2 Among these factors, detector pixel size plays a critical role in both in-plane and across-plane spatial resolution. For the majority of commercially available CT scanners, effective detector pixel size ranges from 0.5 to 0.625 mm at the isocenter. Such a detector pixel size is sufficient for most clinical applications, such as routine head and body CT exams. However, certain applications, such as lung and temporal bone imaging, can benefit from further spatial resolution improvements.3–6 One approach for improving spatial resolution is to use comb or grid attenuators in front of the detectors to reduce the effective detector aperture.3,7,8 This approach, however, comes with a reduction of dose efficiency as the comb or grid attenuates photons that have already passed through the patient and would otherwise have contributed to the image formation. Another approach that has been investigated on a prototype system is to use a flat-panel detector to provide spatial resolution as fine as .9,10 However, this system has challenges such as inferior contrast-to-noise ratio (CNR) and longer scanning time compared to standard multidetector CT.9,10
Recently, photon counting detector (PCD) technology has attracted attention in x-ray and CT imaging.11–20 PCDs measure individual photons and their associated energy rather than the integrated charge generated by all measured photons, as occurs with an energy-integrating detector (EID). Benefits of PCD compared to EID include reduced or fully eliminated electronic noise, improved CNR, and improved dose efficiency.11–14,18–23 More importantly, the energy discrimination nature of the PCD enables a single-detector, single-source, and single-tube-potential multienergy imaging technique.18,24–30 Since PCDs use a direct conversion technique rather than the indirect conversion technique in EIDs, the requirement of septa between detector pixels can be avoided. Therefore, PCDs do not suffer from a septa or grid-induced reduced fill factor as detector cell size is decreased.31 This may allow smaller detector pixels to be used on PCDs to provide better spatial resolution than EIDs.
The purpose of this study was to evaluate a new ultrahigh-resolution (UHR) imaging technique available on a research PCD-based CT system having smaller detector pixels relative to current EID technology. This was accomplished by quantitatively and qualitatively assessing image quality and by demonstrating the mode’s clinical potential using phantoms and cadaveric specimens.
2. Methods and Materials
2.1. PCD-Based CT Scanner and Ultrahigh-Resolution Mode
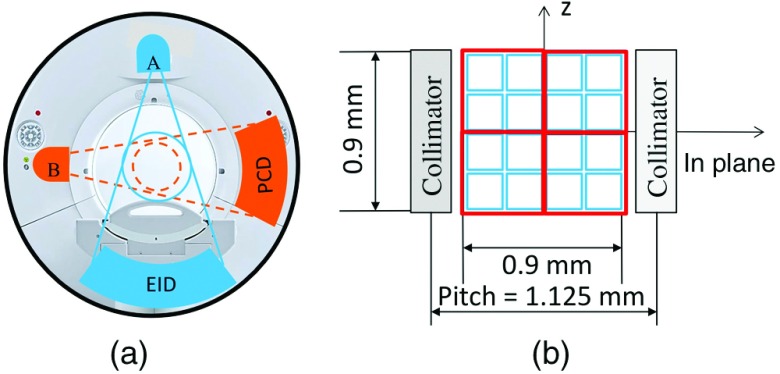
A whole-body research PCD CT scanner has been installed in our lab.20,25,32,33 The research scanner was built based on the second-generation dual-source CT scanner (Definition Flash, Siemens Healthcare, Forchheim, Germany), with the EID subsystem the same as the A system [with 50 cm field of view (FOV)] of the commercial dual-source scanner. The EID detector of the commercial B subsystem (with 33 cm FOV) was replaced with a PCD that covered a 27.5 cm FOV [Fig. 1(a)]. The two subsystems operate independently, providing a platform to investigate both PCD and EID subsystems on the same scanner. The native detector pixel size is for the PCD subsystem. However, the PCD subsystem is usually operated using macropixels () by grouping together native detector pixels [Fig. 1(b)]. The PCD subsystem is capable of acquiring energy selective data with 2 or 4 energy thresholds, consequently yielding 2 or 4 energy bins. Detailed descriptions of the system can be found elsewhere.20,25,32,33 Previous studies using the system demonstrated that the PCD subsystem was capable of providing clinical image quality at clinically realistic levels of x-ray photon flux commensurate with commercial EID systems, and providing improved CNR relative to a state-of-the-art CT scanner using EIDs.23,34
Fig. 1.
(a) A research PCD-based CT system, built based on a second-generation dual-source CT system, consists of an EID and a PCD. (b) Detector configuration of the UHR mode of the PCD, showing both native pixels (blue) and UHR pixels (red).
Recently, a UHR mode was implemented on the PCD subsystem of the research scanner to take advantage of the smaller native detector pixel size, and consequently, to improve spatial resolution to accommodate clinical applications in lung, vascular, musculoskeletal, and temporal bone CT imaging. In this mode, instead of grouping detector pixels together to form a macropixel, the pixel grouping was reduced to , reducing the macropixel size by half [Fig. 1(b)]. This mode used 64 detector rows, each containing 960 pixels of size , which corresponds to at the isocenter. In comparison, the EID system has a detector size of 1.1 mm (fan direction) by 1.1 mm (-direction), which corresponds to at the isocenter. Due to the increased data rate with this mode (4 times of that in macro mode), only one energy threshold was available for each using the early implementation. The energy threshold could be set to any value between 25 and 50 keV. In this study, the threshold was set at 25 keV for all data acquisitions. Therefore, all photons with energy 25 keV or higher were used in the image reconstruction. The same x-ray tubes were used on the EID and PCD subsystems, each equipped with a UHR focal spot of nominal size 0.7 mm (Straton, Siemens Healthcare35). Two scan protocols were available for the PCD UHR mode: (1) a head protocol using a carbon bowtie filter and (2) an abdominal protocol using both the carbon bowtie filter and an aluminum bowtie filter,34 both with a 1 s rotation time. No comb filters were used for the PCD UHR mode, thus ensuring optimal dose efficiency.
2.2. Image Quality Assessment
Various phantoms and tissue specimens were scanned to assess the image quality of the PCD UHR mode. The use of tissue specimens in this study was approved by our Institutional Biospecimen Committee.
To evaluate system performance and to demonstrate the PCD UHR mode’s clinical impact, we performed a series of experiments using physical phantoms and cadaveric specimens. Spatial resolution and image noise were quantitatively assessed, and image quality of anthropomorphic phantoms and cadaveric specimens was qualitatively assessed. All images were reconstructed using a weighted filtered backprojection algorithm.36
2.2.1. Spatial resolution assessment with a wire phantom
Spatial resolution was assessed in terms of modulation transfer function (MTF). A diameter tungsten wire phantom was placed in a 20 cm water tank, with the wire parallel to the long axis of the patient table and approximately 10 mm below the scanner’s isocenter (Fig. 2). The phantom was scanned using both head and body protocols, with 120 kV and 250 mAs for the head protocol and 120 kV and 200 mAs for the body protocol. Images were reconstructed with a 50 mm FOV (smallest available on the scanner), 0.25-mm slice thickness, 0.25-mm increment, and a very sharp kernel (S80, available only in the service mode of operation or on an off-line reconstruction workstation). A two-dimensional point spread function (2-D PSF) was generated based on the CT numbers of the wire images after background subtraction. A one-dimensional line profile was generated by radial averaging the 2-D PSF, from which the MTF curve was calculated by Fourier transform.
Fig. 2.
A diameter tungsten wire phantom (a) was placed in a 20 cm water tank (b) for MTF measurements.
For comparison, the MTF was also measured on a clinical dual-source scanner (Definition Flash, Siemens Healthcare) using that system’s EID UHR mode. The reconstruction kernel used to create PCD UHR images was S80. A specially designed kernel (modified S90) was used to create the EID UHR images. This modified S90 kernel on the EID system was designed to match the spatial resolution of S80 on the PCD subsystem. The matching was tested and verified by MTF measurements acquired using the same wire phantom and setup (Fig. 2).
2.2.2. Image quality of anthropomorphic phantoms and cadaveric specimens
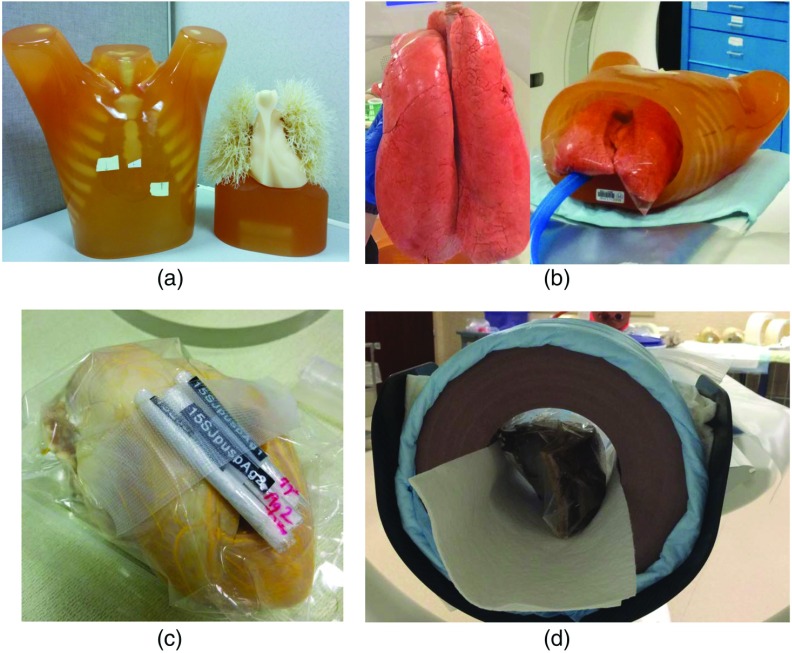
An anthropomorphic lung phantom (LUNGMAN, Kyoto Kagaku, Japan), with incorporated synthetic lung nodules of different sizes and shapes [Fig. 3(a)], was scanned to evaluate the impact of higher resolution on lung imaging. For comparison, the phantom was also scanned on a clinical dual-source scanner (Definition Flash, Siemens Healthcare) using that system’s EID UHR mode, with matched . The matched reconstruction kernels used for MTF measurements were used to create PCD UHR images (S80) and EID UHR images (modified S90). In addition, a swine lung specimen [Fig. 3(b)] was scanned (BioQuest® Inflatable Lungs, eNasco, Fort Atkinson, Wisconsin). These were swine lungs salvaged from a meat plant and specially preserved by the BioFlex process (eNasco). Internal anatomic structures including arteries, veins, bronchioles, alveoli, and bronchi were well preserved. The lungs were placed in the thoracic cavity of the anthropomorphic lung phantom and were inflated using a pump before the scan. Pressure was maintained at the same level during data acquisition to keep the lungs at the same insufflation level. The lung-containing phantom was scanned at 120 kV and 140 mAs using a body protocol, similar to a routine chest CT exam. Images were reconstructed with very sharp kernel (S80), a 0.25-mm slice thickness, and 0.25-mm increment. Images were reviewed by a thoracic radiologist (15 years of experience), with a focus on assessing image quality and potential clinical impact.
Fig. 3.
Phantoms and specimens used in this study: (a) an anthropomorphic lung phantom with synthetic lung nodules; (b) a swine lung specimen inflated and placed in a hollow chest phantom; (c) a swine heart whose vessels were filled with lead-based Microfill®; and (d) a cadaveric temporal bone specimen placed in a 20 cm solid water phantom.
A swine heart [Fig. 3(c)] was placed inside a solid-water annulus of 20 cm diameter and scanned with the PCD subsystem. The heart vessels were filled with lead-based Microfil® (Flow Tech Inc., Carver, Massachusetts) before the scan. The heart was scanned with parameters and a radiation dose matched to clinical cardiac CT scans (120 kV, 200 mA, and 1 s rotation time), with the exception of the longer rotation time, which would typically be less than 0.5 s. PCD UHR images were reconstructed with 0.25-mm slice thickness, 0.25-mm increment, and a very sharp kernel (S80). Images were reviewed by a cardiovascular physician, with special attention paid to examining the intramural coronal arteries.
A cadaveric temporal bone specimen [Fig. 3(d)] was placed inside a 20 cm diameter solid-water annulus and scanned using the PCD UHR mode. For comparison, the specimen was also scanned on the clinical dual-source scanner (Definition Flash, Siemens Healthcare) using its EID UHR mode and a matched . The scan and reconstruction parameters of the two scans are listed in Table 1. The reconstruction FOV used was the same for both the EID and PCD systems, namely 70 mm. For the standard 512 image matrix used in this work, this corresponded to a pixel size of 0.137 mm. Images were subjectively compared by a neuro radiologist (over 20 years of experience), with special focus given to the delineation of fine structures in the temporal bone. Image noise in a uniform region of interest (ROI) was measured and compared between the two systems. Because the slice thickness was 0.6 mm for the EID system and 0.5 mm for the PCD subsystem, noise in the PCD UHR image was first normalized to that of the 0.6 mm image to make a fair comparison. This was achieved based on the relationship that image noise is inversely proportional to the square root of slice thickness, i.e.,
| (1) |
After this normalization, the difference in noise between the PCD UHR and EID UHR images, at matched radiation dose, was calculated
| (2) |
The reduced noise of the PCD UHR mode could also be used to achieve a radiation dose reduction compared to EID UHR if the same image noise was targeted. Since all reconstructions were performed with the same linear algorithm, the amount of dose reduction can be calculated based on the relationship between radiation dose and image noise, i.e.,
| (3) |
To verify the dose reduction estimated in Eq. (3), an anthropomorphic head phantom was scanned on both the EID and PCD systems; the scanning techniques were the same as those used in the cadaveric temporal bone specimen study (Table 1), except that the radiation dose used in PCD was reduced by 50%. The dose reduction was achieved by reducing the effective mAs accordingly (50%). Image noise was then measured and compared between EID and PCD.
Table 1.
Scanning and reconstruction parameters for the temporal bone specimen.
| EID | PCD | |
|---|---|---|
| Scan mode | Spiral/helical | Axial |
| Protocol (bow-tie filter) | Head | Head |
| Tube potential (kV) | 120 | 120 |
| Rotation time (s) | 1 | 1 |
| Effective mAs | 280 | 280 |
| Detector collimation (mm) | ||
| Recon. kernel | Modified S90a | S80 |
| Recon FOV (mm) | 70 | 70 |
| Slice thickness (mm) | 0.6 | 0.5 |
The modified kernel was provided by the manufacturer to best match the S80 kernel on the PCD system.
3. Results
Figure 4(a) shows the MTF curves for the head and body PCD UHR scan modes. The MTF curves are identical and demonstrate a spatial resolution of 15.3 and at 10% and 2% modulation levels, respectively. Figure 4(b) shows the MTF curves of S80 kernel on the PCD subsystem and the modified S90 kernel on the EID system; similar resolution is observed between the two kernels.
Fig. 4.
(a) MTF curves of the UHR mode demonstrate the same spatial resolution for head and body protocols, with a spatial resolution of 15.3 and at 10% and 2% modulation levels, respectively. (b) The MTF curve of the S80 kernel on the PCD system closely matched that of the modified S90 kernel on the EID system (14.2 and at 10% and 2% modulation levels, respectively).
Figure 5 shows images of the anthropomorphic lung phantom scanned using the UHR mode of the PCD subsystem (a–c) and UHR mode of the EID system (d–f). The axial and volume rendered images of the left lung shows clearly the lung vessels [Figs. 5(a) and 5(b)]. The magnified ROIs (c, f) shows better delineation of submillimeter lung vessels using the PCD UHR mode compared to the EID UHR mode.
Fig. 5.
(a, d) Axial and (b, c, e, f) volume rendered images of the anthropomorphic lung phantom scanned using the (a–c) UHR mode of the PCD subsystem and (d–f) UHR mode of the commercial EID system. The zoomed in ROIs (c, f) shows better delineation of submillimeter lung vessels using the PCD subsystem compared to the EID system.
Figure 6 shows axial and maximum intensity projection (MIP) images of the right lung of the anthropomorphic phantom, which show well-defined boundaries of the lower-contrast star-shaped lung nodule (arrow, Fig. 6). Both images show well-defined boundaries of individual structures, which may help with not only qualitative detection tasks, but also quantitative measurements, such as shape and volume, in lung imaging.
Fig. 6.
(a) Axial and (b) MIP PCD UHR images of the right lung show clear boundaries of the low-contrast star-shaped lung nodule (arrow).
Figure 7 shows sample images of the swine lung. Different from the anthropomorphic lung phantom, which essentially has vessels and air, the swine lung images have low attenuating (but not air) parenchyma, which better represents human anatomy. In addition, interlobular septa (arrow) and centrilobular ground-glass opacities (arrow head) surrounding small airways are clearly visible in the lung specimen images, especially within the dependent portions.
Fig. 7.
PCD UHR images of the swine lung at two different locations (a) and (b) show low attenuating parenchyma. Interlobular septa (arrow) and centrilobular ground-glass opacities (arrow head) surrounding small airways are clearly visible in the lung specimen image, especially within the dependent portions.
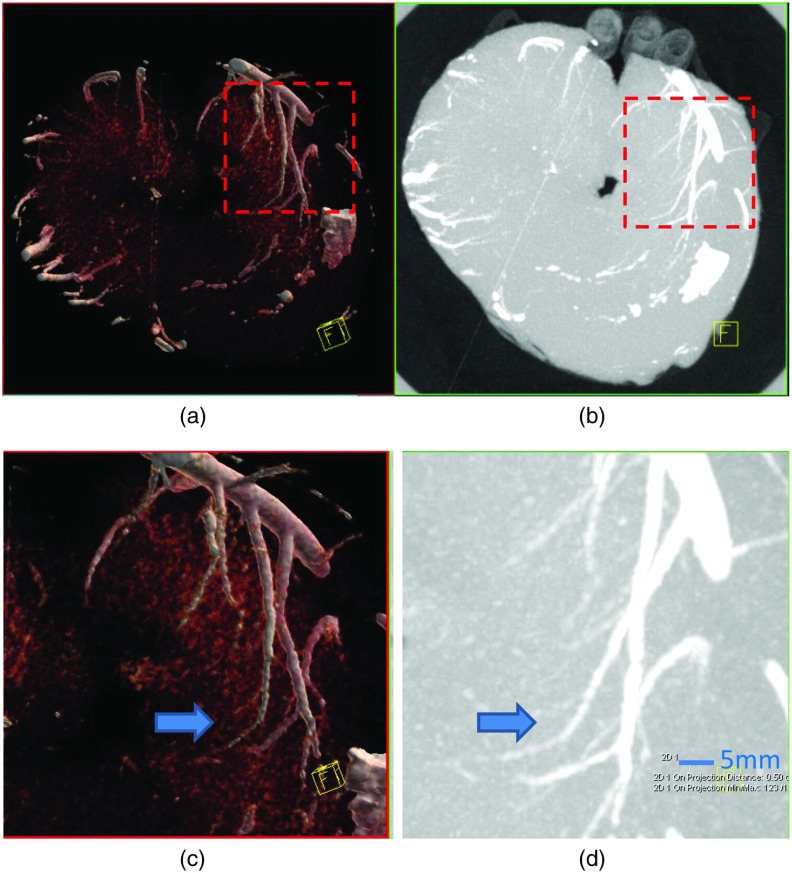
Figure 8 shows volume rendered [Figs. 8(a) and 8(c)] and MIP [Figs. 8(b) and 8(d)] images of the heart specimen. Transmural coronary arteries (arrow), which are much smaller than 1 mm in diameter, are clearly visible in the images with a zoomed ROI [Figs. 8(c) and 8(d)].
Fig. 8.
(a, c) Volume rendered and (b, d) MIP PCD UHR images of the heart specimen. Transmural coronary arteries (arrow), which are much smaller than 1 mm in diameter, are clearly visible in the images, especially with zoomed in ROIs (c, d).
Figures 9 and 10 show sample images of the temporal bone specimen. In both figures, PCD and EID images are shown side by side for comparison. In Fig. 9, the malleus head and incus body are clearly visualized as an “ice cream cone” structure (arrow) on both EID and PCD images (Fig. 9). In Fig. 10, the PCD UHR image [Fig. 10(b)] shows clear delineation of the stapes superstructure (arrow) as a “wishbone” shape. This, however, is not as conspicuous on images from the commercial CT scanner with EID detectors operated in the UHR mode [Fig. 10(a)]. Measurements show image noise of 46.3 hounsfield unit (HU) for the PCD image at 0.5-mm slice thickness and 59.6 HU for the EID image at 0.6-mm slice thickness. The normalized noise of PCD image at 0.6-mm slice thickness, using Eq. (1), is 42.3 HU. This was a 29% reduction in noise for the PCD UHR compared to the EID UHR when data were acquired with matched radiation doses. Using Eq. (3), this could allow for a 50% reduction in dose using PCD compared to EID, if the image noise levels were matched.
Fig. 9.
Images of the temporal bone specimen scanned with (a) EID UHR and (b) PCD UHR modes. The malleus head and incus body are clearly visualized as an “ice cream cone” structure (arrow) on both images, but the PCD image demonstrates modest improvement in delineating the incudomallear joint (arrows). Improved sharpness and lower noise can be appreciated in the PCD image (arrow heads).
Fig. 10.
Images of the temporal bone specimen scanned with (a) EID UHR and (b) PCD UHR. Stapes superstructure was clearly delineated as a “wishbone” structure (arrow) on the PCD image, but was much less conspicuous on the EID image. Measurements of image noise were 59.6 HU for the EID image and 46.3 HU for the PCD image.
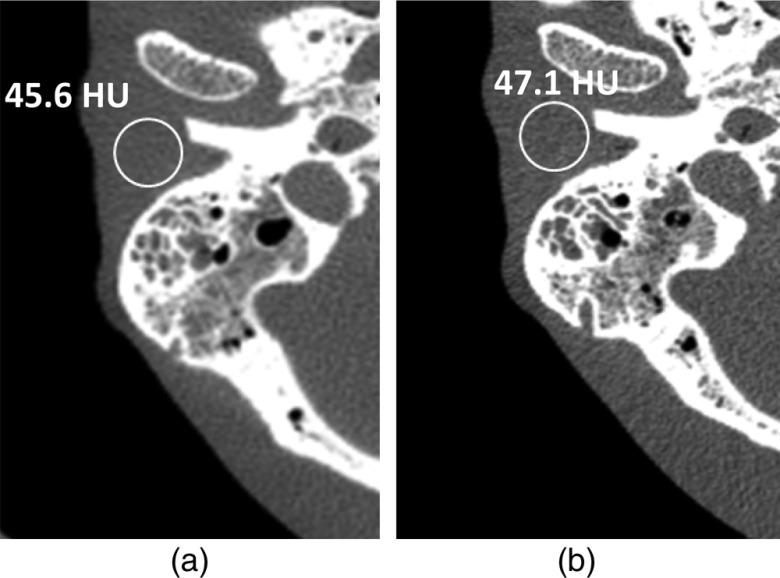
Figure 11 shows sample images of the anthropomorphic head phantom scanned on the EID [Fig. 11(a)] at full dose and PCD [Fig. 11(b)] at 50% dose. Measurements show image noise of 45.6 HU for the EID image at 0.6-mm slice thickness and 47.1 HU for the PCD image at 0.5-mm slice thickness. The normalized noise of the PCD image at 0.6-mm slice thickness, using Eq. (1), was 43.0 HU. This verifies that 50% dose reduction is possible using the PCD system compared to EID system while maintaining similar image noise (45.6 HU for EID and 43.0 HU for PCD).
Fig. 11.
Images of the anthropomorphic phantom scanned on the EID (a) at full dose and PCD (b) at 50% dose. Measurements show image noise of 45.6 HU for the EID image at 0.6-mm slice thickness and 47.1 HU for the PCD image at 0.5-mm slice thickness.
4. Discussions and Conclusions
In this work, we evaluated a new UHR mode on a research PCD CT system using a detector pixel size of at the isocenter. Qualitative and quantitative assessment of image quality in phantoms and specimens demonstrated that the PCD UHR mode had similar spatial resolution compared to the commercial EID UHR scan mode, but had the potential for a 50% reduction in dose for the same image noise. The ultrahigh spatial resolution was demonstrated in the lung and temporal bone, and the potential clinical impact demonstrated in an explanted swine heart. Results of our study showed that when scanning at the same radiation dose, the PCD UHR mode had lower image noise than that of the commercial EID UHR mode using an attenuating postpatient comb filter. The comb filter blocks half of the detector aperture along the fan-angle direction, which effectively reduces the detector size by half along the fan-angle and enables improved spatial resolution. However, the filter also blocks 50% of the photons that have already passed through the patient that would otherwise have contributed to image formation. This results in reduced dose efficiency and consequently higher noise (for the same dose) for exams using the comb filters.3,8 The PCD UHR mode investigated in this study does not have this issue, as no comb filters are used and all photons reaching the detector are used in image formation without losing dose efficiency. Our results demonstrated a 50% improvement in dose efficiency using the PCD UHR mode with matched image noise to that of EID, which would substantially reduce the necessary radiation dose for exams requiring these levels of spatial resolution.
Another factor that affects contrast, noise, and CNR is that the detected signal is energy-weighted with EID systems while the detected signal is photon-count-weighted with PCD systems. In the study by Gutjahr et al.,23 it was found that for the same radiation dose, the noise difference between EID and PCD was minimal. However, iodine contrast and CNR were substantially higher using the PCD system due to the signal weighting difference. Therefore, dose reduction based on matched noise is a relatively conservative estimation. Many clinically important materials, such as bone or iodine, would have higher contrast using a PCD system than using an EID system due to the signal weighting difference. Greater dose reduction would be possible if matched CNR was used as the criteria rather than matched noise. However, the specific amount of dose reduction would depend on the specific material. For simplicity, we only estimated dose reduction for the scenario of matched noise in this study.
It should be noted that the basic relationship between resolution and image noise still holds, i.e., increased spatial resolution comes with increased image noise for the same radiation dose, or increased radiation dose is required for the same image noise.2,37 Spatial resolution and image noise need to be balanced based on the clinical task. For certain clinical applications, such as temporal bone, vascular, musculoskeletal, and lung imaging, delineation of very fine structures, such as small vessels and stress fractures, is needed. For these scenarios, the need for high spatial resolution justifies the increased image noise or even increased radiation dose (to reduce noise) in very sharp CT images. In addition, these exams are associated with high contrast (e.g., bone to air), which can tolerate high noise. To improve the spatial resolution, a comb filter has been applied in front of the standard EID array and this has been adopted in routine clinical practice. We showed in this study that using PCD, similarly high spatial resolution was achieved with reduced image noise (29%) or using reduced radiation dose (50%). Very sharp CT images may not be appropriate for exams aiming to detect low contrast lesions, e.g., liver or pancreatic exams, in which image noise plays the most significant role for accurate lesion detection. In such exams, images with standard resolution should be used (however, those images still could be obtained using a high-resolution scan mode as long as comb filters are not used). We are continuing to investigate potential applications of the high-resolution mode in different clinical areas, with ongoing in vivo studies, which will be reported in the future. Noise reduction techniques, including iterative reconstruction algorithms, have been extensively investigated recently. Applying these techniques to the UHR mode could potentially reduce image noise and increase image quality.
As noted in Sec. 2, the native pixel size of the PCD is , and the regular “macro” mode reads out detector data by binning native detector pixels. The UHR mode investigated in this study was implemented by binning native pixels, which provides an effective detector size of at the isocenter. This represents a factor of 2 reduction of detector size (in one dimension), compared to the standard macro mode. For this detector, it is possible to further reduce the effective detector size by another factor of 2, e.g., at the isocenter, by using the native detector pixels without any binning. However, challenges exist for such a mode, such as the need for high data transfer rates and the trade-off of increased image noise (or dose) for smaller pixels. Compared to standard macro mode, the UHR mode has 4 times more data when using binning instead of binning. Another 4 times more data would be generated if the native detector pixel was used without binning. This increased amount of data imposes a challenge to data transfer systems. Besides directly increasing the data transfer rate, other strategies may be pursued to address this challenge. One option is to use even longer rotation times, when there is no substantial motion of the imaged object, such as in musculoskeletal applications. Another option would be to reduce detector coverage, either in-plane or along the longitudinal direction. This option comes with drawbacks, e.g., potential data truncation or reduced scanning speed. A third option would be to use data compression techniques to compress projection data and to reduce the amount of data that need to be transferred. A recent study showed a factor of 2–3 compression rate was achievable.38 Another major challenge is that smaller detector pixels come with increased image noise for the same radiation dose, or else increased radiation dose is required for the same image noise, as discussed in the previous paragraph.
There are limitations of this study. First of all, this study focused only on phantom and cadaveric specimens, without in vivo patient data. Although the phantom and cadaveric data showed clear benefits of the PCD UHR scan mode, in vivo patient studies are warranted to demonstrate the ultimate benefit of this mode in clinical practice. For the experiment with the heart specimen, lead-based Microfil® instead of regular iodine contrast was used to opacify the vessels. This was intended to fill and opacify microvascular and other spaces of the heart specimen under physiological pressure and with minimal shrinkage so that the specimen could be kept for a long time and used in multiple studies. Patient studies using regular iodine-based contrast are needed to fully evaluate the benefit of this scan mode in vascular imaging. Second, this study focused only on achieving high spatial resolution, without energy specific information. The early implementation of the UHR PCD mode only allowed one energy threshold. This was done to limit the amount of data generated to avoid data transfer issues, as the amount of data generated is proportional to the number of energy thresholds used. Benefits of combining the UHR scan mode with energy specific information is the focus of ongoing work.
In conclusion, we assessed a UHR scan mode on a PCD-based CT scanner, which achieved spatial resolution up to . This high level of spatial resolution was achieved without the use of a dedicated comb or grid attenuator, making the acquisition more dose efficient (50% dose reduction compared to the UHR mode with comb attenuator). Studies using anthropomorphic phantoms and cadaveric specimens demonstrated the potential impact of this imaging mode in lung, temporal bone, and vascular imaging. Other clinical applications that rely on high spatial resolution, such as musculoskeletal imaging, are likely to also benefit from this increased resolution capability.
Acknowledgments
Research reported in this publication was supported by the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health under Award Nos. R01 EB016966 and C06 RR018898, and in collaboration with Siemens Healthcare. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The scanner and algorithm discussed here are not commercially available.
Biographies
Shuai Leng received his BS degree in engineering physics in 2001, his MS degree in engineering physics in 2003, from Tsinghua University, and his PhD in medical physics in 2008 from the University of Wisconsin, Madison, USA. He is an associate professor of medical physics at the Mayo Clinic in Rochester, Minnesota. He has authored over 100 peer-reviewed articles. His research interest is technical development and clinical application of x-ray and CT imaging.
Zhicong Yu received his PhD from the University of Erlangen-Nuremberg, Germany, in 2013. He was a research fellow at Mayo Clinic, Rochester, Minnesota, USA. His research interests include CT/C-arm physics and imaging, with emphasis on photon-counting CT and advanced image reconstruction techniques.
Ahmed Halaweish received his BS degree in electrical engineer in 2005 from South Dakota State University, his PhD in biomedical engineering from the University of Iowa in 2011, and he completed a postdoctoral research fellowship in radiology at the Duke University School of Medicine in 2013. He is currently a staff scientist and R&D collaborations manager for the computed tomography division of Siemens Healthineers. His research interests include preclinical and clinical multimodality CT and MR imaging.
Steffen Kappler received his diploma and PhD in physics from Karlsruhe University, Germany, in 2000 and 2004, respectively. He worked as an assistant lecturer for RWTH Aachen, Germany, from 2005 to 2007. During this time, he was visiting researcher at CERN, Switzerland and FNAL, USA. He joined SIEMENS Healthcare in 2007, where he is responsible for CT technology management. His research interests include detector physics and image reconstruction with special focus on photon counting CT.
Katharina Hahn studied physics at the University of Würzburg, Germany. After receiving her diploma, she joined the group of Frédéric Noo, University of Utah, to work on iterative reconstruction in CT. She joined the physics team of Siemens Healthcare CT in 2014.
Andre Henning studied electrical engineering at the TU Dresden and computer sciences at the FernUniversität Hagen, both Germany. He joined Siemens Healthcare CT Forchheim in 2007 and has been working as engineer and project manager in various innovation fields CT.
Zhoubo Li received his PhD in biomedical engineering from Mayo Graduate School in July 2016. He was a graduate student at Clinical CT Innovation Center at Mayo Clinic, Rochester, Minnesota, USA. His research interests include CT physics, image quality, and dual/multienergy CT applications.
John Lane holds the rank of professor of radiology. He joined the radiology faculty at Mayo Clinic, Rochester, Minnesota, USA, 1999 after having completed his neuroradiology training at Thomas Jefferson University in 1991, served in the US Navy as staff neuroradiologist at the Oakland Naval Hospital from 1991–1995 and practiced in Great Falls, Montana, USA, from 1995–1999. He research and clinical focus is temporal bone imaging.
David L. Levin received his PhD in physiology and pharmacology and his MD degrees from the University of California at San Diego. He is a thoracic radiologist at the Mayo Clinic in Rochester, Minnesota, USA. His radiology residency training and thoracic imaging fellowship were both completed at the University of California at San Francisco. He has held previous faculty positions at the Beth Israel Deaconess Medical Center and UC-San Diego.
Steven Jorgensen received his BSEE from South Dakota School of Mines and Technology in 1979. He is an associate in research and the micro-CT system lead engineer, in Physiological Image Research Lab of the x-ray Imaging Core at the Mayo Clinic College of Medicine, Rochester, Minnesota, USA. Current projects include working with whole-body spectral x-ray imaging with the Mayo’s Computed Tomography Clinical Innovation Center and spectral micro-CT x-ray imaging in the x-ray imaging core.
Erik Ritman is a physiologist who used CT to explore patho-physiological processes in vivo. In the seventies and eighties, he developed and used high-speed volume scanning CT and in the nineties and two thousands on micro-CT. Currently, he is involved in evaluation of a whole-body spectral CT scanner.
Cynthia McCollough received her doctorate from the University of Wisconsin in 1991. She is a professor of radiological physics and biomedical engineering at Mayo Clinic, where she directs the CT Clinical Innovation Center. Her research interests include CT dosimetry, advanced CT technology, and new clinical applications, such as dual-energy and multispectral CT. She is an NIH-funded investigator, and is active in numerous professional organizations. She is a fellow of the AAPM and ACR.
Disclosures
Cynthia McCollough receives research support from Siemens Healthcare. Ahmed Halaweish, Steffen Kappler, Katharina Hahn, and Andre Henning are Siemens employees.
References
- 1.Hsieh J., Computed Tomography: Principles, Design, Artifacts, and Recent Advances, SPIE Press, Bellingham, Washington: (2003). [Google Scholar]
- 2.Kalender W. A., Computed Tomography: Fundamentals, System Technology, Image Quality, Applications, 3rd ed., Publicis, Erlangen, Germany: (2011). [Google Scholar]
- 3.McCollough C. H., et al. , “Spatial resolution improvement and dose reduction potential for inner ear CT imaging using a z-axis deconvolution technique,” Med. Phys. 40(6), 061904 (2013). 10.1118/1.4802730 [DOI] [PubMed] [Google Scholar]
- 4.Whiting B. R., et al. , “Use of computed tomography scans for cochlear implants,” J. Digital Imaging 21(3), 323–328 (2008). 10.1007/s10278-007-9045-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Webb W. R., Muller N. L., Naidich D. P., High-Resolution CT of the Lung, 5th ed., Lippincott Williams & Wilkins, Philadelphia, Pennsylvania: (2014). [Google Scholar]
- 6.Duddalwar V. A., “Multislice CT angiography: a practical guide to CT angiography in vascular imaging and intervention,” Br. J. Radiol. 77(Suppl. 1), S27–S38 (2004). 10.1259/bjr/25652856 [DOI] [PubMed] [Google Scholar]
- 7.Flohr T. G., et al. , “Novel ultrahigh resolution data acquisition and image reconstruction for multi-detector row CT,” Med. Phys. 34(5), 1712–1723 (2007). 10.1118/1.2722872 [DOI] [PubMed] [Google Scholar]
- 8.Leng S., et al. , “Temporal bone CT: improved image quality and potential for decreased radiation dose using an ultra-high-resolution scan mode with an iterative reconstruction algorithm,” Am. J. Neuroradiol. 36(9), 1599–1603 (2015). 10.3174/ajnr.A4338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta R., et al. , “Flat-panel volume CT: fundamental principles, technology, and applications 1,” Radiographics 28(7), 2009–2022 (2008). 10.1148/rg.287085004 [DOI] [PubMed] [Google Scholar]
- 10.Gupta R., et al. , “Ultra-high resolution flat-panel volume CT: fundamental principles, design architecture, and system characterization,” Eur. Radiol. 16(6), 1191–1205 (2006). 10.1007/s00330-006-0156-y [DOI] [PubMed] [Google Scholar]
- 11.Taguchi K., Iwanczyk J. S., “Vision 20/20: single photon counting x-ray detectors in medical imaging,” Med. Phys. 40(10), 100901 (2013). 10.1118/1.4820371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tümer T., et al. , “Preliminary results obtained from a novel CdZnTe pad detector and readout ASIC developed for an automatic baggage inspection system,” in IEEE Nuclear Science Symp. Conf. Record, Vol. 1, pp. 4–36 (2000). 10.1109/NSSMIC.2000.949044 [DOI] [Google Scholar]
- 13.Shikhaliev P. M., “Energy-resolved computed tomography: first experimental results,” Phys. Med. Biol. 53(20), 5595–5613 (2008). 10.1088/0031-9155/53/20/002 [DOI] [PubMed] [Google Scholar]
- 14.Shikhaliev P. M., “Computed tomography with energy-resolved detection: a feasibility study,” Phys. Med. Biol. 53(5), 1475–1495 (2008). 10.1088/0031-9155/53/5/020 [DOI] [PubMed] [Google Scholar]
- 15.Shikhaliev P. M., “Photon counting spectral CT: improved material decomposition with K-edge-filtered x-rays,” Phys. Med. Biol. 57(6), 1595–1615 (2012). 10.1088/0031-9155/57/6/1595 [DOI] [PubMed] [Google Scholar]
- 16.Shikhaliev P. M., Fritz S. G., “Photon counting spectral CT versus conventional CT: comparative evaluation for breast imaging application,” Phys. Med. Biol. 56(7), 1905–1930 (2011). 10.1088/0031-9155/56/7/001 [DOI] [PubMed] [Google Scholar]
- 17.Shikhaliev P. M., Fritz S. G., Chapman J. W., “Photon counting multienergy x-ray imaging: effect of the characteristic x rays on detector performance,” Med. Phys. 36(11), 5107–5119 (2009). 10.1118/1.3245875 [DOI] [PubMed] [Google Scholar]
- 18.Schlomka J. P., et al. , “Experimental feasibility of multi-energy photon-counting K-edge imaging in pre-clinical computed tomography,” Phys. Med. Biol. 53(15), 4031–4047 (2008). 10.1088/0031-9155/53/15/002 [DOI] [PubMed] [Google Scholar]
- 19.Iwanczyk J. S., et al. , “Photon counting energy dispersive detector arrays for x-ray imaging,” IEEE Trans. Biomed. Eng. 56(3), 535–542 (2009). 10.1109/TNS.2009.2013709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kappler S., et al. , “A research prototype system for quantum-counting clinical CT,” Proc. SPIE 7622, 76221Z (2010). 10.1117/12.844238 [DOI] [Google Scholar]
- 21.Persson M., et al. , “Energy-resolved CT imaging with a photon-counting silicon-strip detector,” Phys. Med. Biol. 59(22), 6709–6727 (2014). 10.1088/0022-3727/59/22/6709 [DOI] [PubMed] [Google Scholar]
- 22.Bennett J. R., et al. , “Hybrid spectral micro-CT: system design, implementation, and preliminary results,” IEEE Trans. Biomed. Eng. 61(2), 246–253 (2014). 10.1109/TBME.2013.2279673 [DOI] [PubMed] [Google Scholar]
- 23.Gutjahr R., et al. , “Human imaging with photon counting-based computed tomography at clinical dose levels: contrast-to-noise ratio and cadaver studies,” Invest. Radiol. 51(7), 421–429 (2016). 10.1097/RLI.0000000000000251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roessl E., Proksa R., “K-edge imaging in x-ray computed tomography using multi-bin photon counting detectors,” Phys. Med. Biol. 52(15), 4679–4696 (2007). 10.1088/0031-9155/52/15/020 [DOI] [PubMed] [Google Scholar]
- 25.Kappler S., et al. , “Photon counting CT at elevated x-ray tube currents: contrast stability, image noise and multi-energy performance,” Proc. SPIE 9033, 90331C (2014). 10.1117/12.2043511 [DOI] [Google Scholar]
- 26.Li Z., et al. , “Image-based material decomposition with a general volume constraint for photon-counting CT,” Proc. SPIE 9412, 94120T (2015). 10.1117/12.2082069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faby S., et al. , “Performance of today’s dual energy CT and future multi energy CT in virtual non-contrast imaging and in iodine quantification: a simulation study,” Med. Phys. 42(7), 4349–4366 (2015). 10.1118/1.4922654 [DOI] [PubMed] [Google Scholar]
- 28.Schirra C. O., et al. , “Statistical reconstruction of material decomposed data in spectral CT,” IEEE Trans. Med. Imaging 32(7), 1249–1257 (2013). 10.1109/TMI.2013.2250991 [DOI] [PubMed] [Google Scholar]
- 29.Sawatzky A., et al. , “Proximal ADMM for multi-channel image reconstruction in spectral X-ray CT,” IEEE Trans. Med. Imaging 33(8), 1657–1668 (2014). 10.1109/TMI.2014.2321098 [DOI] [PubMed] [Google Scholar]
- 30.Rigie D. S., La Riviere P. J., “Joint reconstruction of multi-channel, spectral CT data via constrained total nuclear variation minimization,” Phys. Med. Biol. 60(5), 1741–1762 (2015). 10.1088/0031-9155/60/5/1741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pelc N., et al. , “Use of the depth information in edge-on photon counting detectors for improved spectral imaging,” in The 3rd Workshop on Medical Applications of Spectroscopic X-ray Detectors, CERN, Geneva, Switzerland: (2015). [Google Scholar]
- 32.Kappler S., et al. , “First results from a hybrid prototype CT scanner for exploring benefits of quantum-counting in clinical CT,” Proc. SPIE 8313, 83130X (2012). 10.1117/12.911295 [DOI] [Google Scholar]
- 33.Kappler S., et al. , “Multi-energy performance of a research prototype CT scanner with small-pixel counting detector,” Proc. SPIE 8668, 86680O (2013). 10.1117/12.2006747 [DOI] [Google Scholar]
- 34.Yu Z., et al. , “Evaluation of conventional imaging performance in a research whole-body CT system with a photon-counting detector array,” Phys. Med. Biol. 61(4), 1572–1595 (2016). 10.1088/0031-9155/61/4/1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schardt P., et al. , “New x-ray tube performance in computed tomography by introducing the rotating envelope tube technology,” Med. Phys. 31(9), 2699–2706 (2004). 10.1118/1.1783552 [DOI] [PubMed] [Google Scholar]
- 36.Stierstorfer K., et al. , “Weighted FBP—a simple approximate 3D FBP algorithm for multislice spiral CT with good dose usage for arbitrary pitch,” Phys. Med. Biol. 49(11), 2209–2218 (2004). 10.1088/0031-9155/49/11/007 [DOI] [PubMed] [Google Scholar]
- 37.Fuchs T., Kalender W., “On the correlation of pixel noise, spatial resolution and dose in computed tomography: theoretical prediction and verification by simulation and measurement,” Phys. Med. 19(2), 153–164 (2003). 10.1400/19261 [DOI] [Google Scholar]
- 38.Shunhavanich P., Pelc N., “Lossless compression of projection data from photon counting detectors,” Proc. SPIE 9783, 97831J (2016). 10.1117/12.2217231 [DOI] [Google Scholar]