Abstract
BACKGROUND AND PURPOSE:
Outcomes following hematopoietic stem cell transplantation for higher risk childhood-onset cerebral adrenoleukodystrophy are variable. We explored whether a brain MR imaging gadolinium intensity scoring system improves prediction of neurologic outcome.
MATERIALS AND METHODS:
We developed a 4-point scale of gadolinium intensity relative to the choroid plexus: 0 = no enhancement; 1 = hypointense; 2 = isointense; 3 = hyperintense. The interobserver concordance of the scale was assessed on 30 randomly chosen studies. Scores were generated for 64 evaluable patients and compared with CSF chitotriosidase levels, a known inflammatory marker correlating with outcomes following transplantation. For 25 evaluable higher risk patients (Loes ≥10), the gadolinium intensity score was compared with longer term posttransplantation clinical change.
RESULTS:
The gadolinium intensity scoring system showed good interobserver reproducibility (κ = 0.72). Of 64 evaluable boys, the score positively correlated with average concomitant CSF chitotriosidase activity in nanograms/milliliter/hour: 0: 2717, n = 5; 1: 3218, n = 13; 2: 6497, n = 23; and 3: 12,030, n = 23 (P < .01). For 25 evaluable higher risk patients, more intense pretransplantation brain MR imaging gadolinium enhancement predicted greater average loss on the adrenoleukodystrophy neurologic function scale following transplantation: 0/1: adrenoleukodystrophy neurologic function scale score difference = 4.3, n = 7; 2/3: adrenoleukodystrophy neurologic function scale score difference = 10.4, n = 18 (P = .05).
CONCLUSIONS:
Gadolinium enhancement intensity on brain MR imaging can be scored simply and reproducibly for cerebral adrenoleukodystrophy. The enhancement score significantly correlates with chitotriosidase. In boys with higher risk cerebral disease (Loes ≥10), the enhancement score itself predicts neurologic outcome following treatment. Such data may help guide treatment decisions for clinicians and families.
Adrenoleukodystrophy (ALD) is an X-linked peroxisomal disorder affecting approximately 1 in 21,000 males. Mediated by elevated concentrations of very long chain fatty acids, the disease may manifest as central nervous system demyelination, primary hypoadrenalism, and/or primary hypogonadism. The disease results from pathogenic mutations in the peroxisomal transporter ABCD1 gene, but genotype does not predict the presentation, and different presentations may occur within the same family.1,2
In 35% of affected males, cerebral involvement (cerebral adrenoleukodystrophy [cALD]) begins in childhood. This devastating phenotype is characterized by rapidly progressive central nervous demyelination and, if untreated, usually death within years of onset of clinical signs and symptoms.3 Postmortem analyses of affected brains have implicated mononuclear inflammatory mechanisms.4–6 Radiographic changes generally precede clinical neurologic disease by several years in childhood cALD and are characterized by symmetric, expanding white matter lesions.7 Consistent with known neuroinflammatory histopathology, gadolinium enhancement is typically observed near the leading edge of active demyelination and, when present, strongly predicts disease progression.8,9 The cALD brain MR imaging severity scale of Loes et al10 is commonly used to quantify radiographic disease burden in adrenoleukodystrophy. Increasing Loes scores denote accumulating white matter disease and atrophy in defined neuroanatomic or functional regions: periventricular/subcortical areas (parieto-occipital, anterior-temporal, and frontal), corpus callosum, visual and auditory pathways, frontopontine-corticospinal projection fibers (internal capsule and brain stem), basal ganglia, cerebellum, and anterior thalamus. For patients with ALD with no cerebral involvement, the Loes score is by definition zero, while maximal cerebral involvement on the scale (Loes = 34) correlates with profound neurologic impairment.10
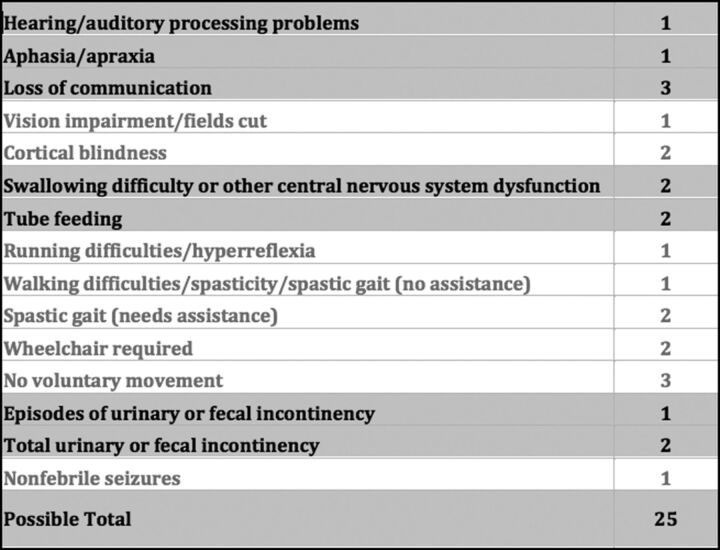
Although experimental gene therapy trials are currently underway, allogeneic hematopoietic stem cell transplantation (HSCT) remains the standard therapy to arrest cerebral disease progression in cALD.3 Most important, long-term functional outcome analyses have demonstrated critical dependence on cerebral white matter disease burden as measured by the Loes score at the time of transplantation.11 Most patients with Loes of <10 are lacking signs of cerebral disease as measured on the ALD neurologic function scale (NFS, Fig 1)12 and are seriously considered for HSCT. For these standard-risk patients with cALD (Loes <10 at HSCT), gross neurologic function following transplantation nearly uniformly remains the same on the NFS. However, outcomes after transplantation for higher risk disease (Loes ≥10 at HSCT) are considerably more difficult to prognosticate and range from mild clinical progression to profound devastation.13 For such higher risk patients, the absolute pretransplantation Loes score alone does not predict neurologic outcome.
Fig 1.
The cerebral adrenoleukodystrophy neurologic function scale used to evaluate gross clinical neurologic status for the higher risk cALD cohort pretransplantation and at most recent posttransplantation follow-up. Note that a score of zero denotes absence of clinical signs of cerebral disease. Maximal signs within a domain score the total of all grades within that domain (for example, a patient with “total urinary or fecal incontinency” scores 3, for the sum of episodes of incontinency [n = 1] and total incontinency [n = 2]).
Despite effort aimed at early detection, many boys still arrive at ALD diagnosis because of neurologic impairment from higher risk cALD (Loes ≥10). In these cases, clinicians and families face difficult decisions regarding the use of HSCT, a procedure that carries significant risk of injury and mortality.14 Therefore, additional prognostic indicators are sought of likely benefit from HSCT in these higher risk patients with cALD, who already demonstrate extensive cerebral white matter disease burden at diagnosis.
In previous reports, a strong correlation between pretransplantation chitotriosidase enzyme activity (CHIT, elaborated by activated monocytes) in plasma and CSF and clinical neurologic change at 1 year following HSCT for cALD have been observed.15 In that analysis, higher CSF CHIT in the pretransplantation setting strongly correlated with clinical neurologic worsening for a nonstratified cALD cohort (standard and higher risk patients combined).
We have anecdotally observed a possible correlation between the intensity of gadolinium enhancement on pre-HSCT brain MR imaging and clinical neurologic outcomes following HSCT in higher risk cALD (Loes ≥10). In this single-institution study, we established a simple gadolinium intensity scoring (GIS) system for cALD brain MR imaging and applied it to a large patient cohort. We explored the reproducibility of the GIS and its correlation with concomitant CSF CHIT. Finally, we analyzed the pretransplantation GIS predictive value for posttransplantation neurologic outcomes in higher risk cALD.
Materials and Methods
Cohort Identification and CSF Chitotriosidase Activity Determination
All patients confirmed to have ALD by diagnostic plasma very long chain fatty acid profile and who underwent evaluation at the University of Minnesota after January 1, 2000, were considered for this retrospective study. CSF CHIT activity and CHIT genotypes were determined by methods previously described.15
Because 1 aim of our analysis was to assess a correlation between GIS and CSF CHIT in cALD regardless of how limited or extensive white matter disease may be, all patients with cALD, regardless of Loes score, were included for analysis of GIS and CSF CHIT correlation if they had the following: 1) an untreated MR imaging evaluable for GIS (pretransplantation, if the patient proceeded to HSCT), and 2) concomitant CSF CHIT data. For patients genotypically determined to be heterozygous CHIT null (CHITWT/CHIT0, seen in approximately 35% of the general population), CSF CHIT activity reported for this study was set to twice that measured in the assay. Patients were excluded for analysis of GIS and CSF CHIT correlation for the following reasons: 1) They were genotypically homozygous CHIT null (CHIT0/CHIT0, seen in approximately 5% of the general population), because these patients are not capable of producing the enzyme; or 2) they did not demonstrate MR imaging evidence of cerebral disease (Loes = 0).
Because previous reports have shown standard-risk patients with cALD (Loes <10 at the time of HSCT) to demonstrate no-to-minimal worsening in posttransplantation general clinical neurologic function,13 only higher risk patients with cALD (Loes ≥10 at the time of transplantation) were considered for retrospective analysis of the correlation between pretransplantation GIS and neurologic function outcomes posttransplantation. These higher risk patients were included in this study if the following conditions were met: 1) a pretransplantation brain MR imaging was evaluable for GIS; 2) the pretransplantation Loes score was ≥10; and 3) robust donor hematopoietic engraftment (≥80%) was achieved following transplantation. Patients were excluded if death resulted from transplant-related complications.
All patients receiving transplants were treated on protocols approved by the institutional review board and following the provision of informed consent. For each patient, the best available allograft according to standard institutional guidelines was chosen. The transplant conditioning regimen was dependent on the appropriate protocol available to the patient at the time of transplantation. Antimicrobial prophylaxis and therapy, graft-versus-host disease prophylaxis, and blood product supportive care followed standard institutional guidelines.
ALD MR Imaging Severity (Loes) Score and Gadolinium Intensity Score Assignment
Brain MR imaging studies included in this analysis were prospectively assigned a severity score according to the Loes system by a single neuroradiologist (D.R.N.) who was blinded to CHIT data and neurologic outcomes.10
Gadolinium intensity scores were determined from 3D-T1-weighted MPRAGE (TR, 1900 ms; TE, 2.19 ms; TI, 900 ms; 1 average; flip angle, 9°; section thickness, 0.9 mm; voxel size, 0.9 × 0.9 × 0.9 mm; matrix, 256 × 256) images obtained approximately 5 minutes following intravenous dosing of either Magnevist (gadopentetate dimeglumine; Bayer HealthCare Pharmaceuticals, Wayne, New Jersey), 0.1 mmol/kg, or Gadavist (gadobutrol; Bayer Schering Pharma, Berlin, Germany), 0.05 mmol/kg.
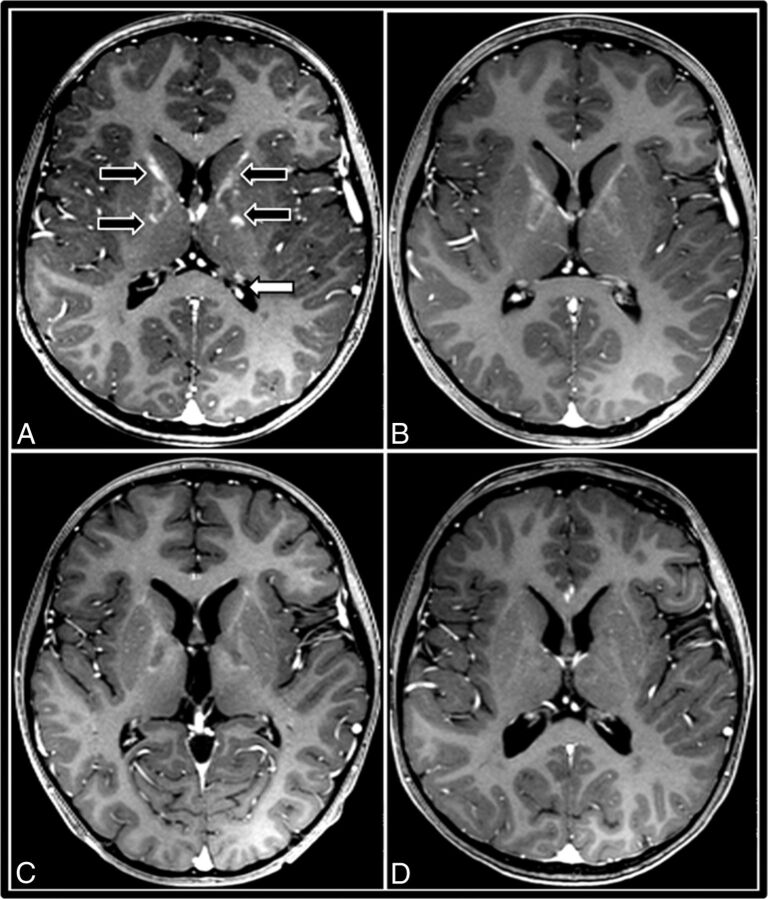
Because gadolinium enhancement intensity may vary due to interscan differences in timing of the IV contrast bolus, we developed the GIS so that the enhancement intensity would be internally controlled. Each brain MR imaging was assigned a GIS as follows: 0 = no contrast enhancement; 1 = maximal lesion enhancement less than that of the choroid plexus; 2 = maximal lesion enhancement of equal intensity to that of the choroid plexus; 3 = maximal lesion enhancement more intense than that of the choroid plexus (Fig 2). All GISs used for analysis were assigned by a single neuroradiologist (D.R.N.) who was blinded to CHIT data and neurologic outcomes.
Fig 2.
A gadolinium intensity scoring system for cerebral adrenoleukodystrophy demonstrated by successive postcontrast T1WI of a 7-year-old boy with ALD primarily involving the internal capsule. This patient exhibits all 4 grades of GIS, from initial intense contrast enhancement progressing to nonenhancement. A, Pre-HSCT MR imaging demonstrates GIS = 3 in the internal capsules (black arrows) with maximal lesion enhancement hyperintense to the choroid plexus (white arrow). B, Thirty days post-HSCT, the GIS is 2, because maximal lesion enhancement equal to the choroid plexus is observed. C, Later, MR imaging exhibits GIS = 1 with maximal lesion enhancement hypointense to the choroid plexus. D, Ninety days after successful HSCT, resolution of contrast enhancement is seen (GIS = 0).
The Fleiss κ was used to assess interrater agreement among 3 neuroradiologists (D.R.N., J.B.R, R.S.G.) on 30 randomly selected scans. Good interobserver reliability was observed (κ = 0.72).16
Neurologic Function Scale Score Assignment
For higher risk patients with cALD (Loes ≥10 at HSCT) who had undergone transplantation, clinical cerebral disease severity was scored immediately before HSCT and at the latest posttransplantation follow-up by using the ALD NFS (Fig 1).12 NFS assignment was performed from retrospective review of clinical notes by a single investigator (W.P.M.) who was blinded to radiographic GIS, Loes, and CHIT data. Change in NFS (ΔNFS) was defined as the difference between the NFS at the most recent follow-up and the baseline NFS obtained immediately before HSCT. For patients who died of cALD disease progression following HSCT, the most recent NFS was set to the maximum (25) at the time of death.
Study Objectives and Statistical Analyses
The main objective of this retrospective cohort study was to determine, in all evaluable patients with cALD, whether brain MR imaging gadolinium intensity correlates with CSF CHIT, an inflammatory biomarker shown to be associated with neurologic function at 1 year following transplantation for cALD. A secondary objective was to determine whether pretransplantation GIS correlates with long-term change in neurologic function (ΔNFS) in higher risk patients with cALD (Loes ≥10) undergoing HSCT.
Comparisons of mean CSF CHIT activity levels across each cALD GIS were made by the Kruskal-Wallis test. Comparison of mean CSF CHIT activity by the GIS group in higher risk patients with cALD (Loes ≥10) was performed with the t test by using the Welch correction for disparate variance. Comparison of change in the NFS by the GIS group in higher risk patients with cALD (Loes ≥10) was performed with the t test.
Results
Brain MR Imaging Gadolinium Intensity Score Correlates Strongly with CSF Chitotriosidase Activity in cALD
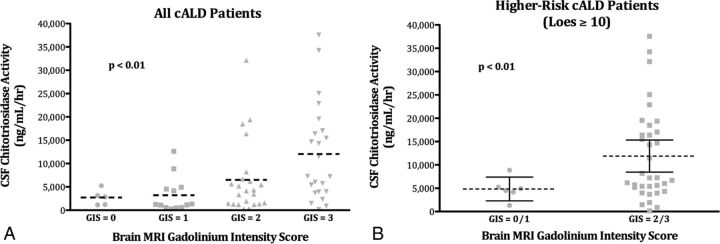
Sixty-four boys with cALD were evaluable for simultaneous CSF CHIT and brain MR imaging GIS. Very few patients (n = 5) demonstrated no gadolinium enhancement (GIS = 0). The remaining cohort (n = 59) was distributed relatively equally among nonzero GIS. For the entire group, mean CSF CHIT varied significantly between GIS cohorts (Fig 3). GIS, mean CSF CHIT activity (nanogram/milliliter/hour), and number of patients observed were as follows: GIS = 0, 2717, n = 5; GIS = 1, 3218, n = 13; GIS = 2, 6497, n = 23; and GIS = 3, 12,030, n = 23 (P < .01, difference in mean CHIT across GIS groups).
Fig 3.
CSF chitotriosidase activity correlates with the brain MR imaging gadolinium intensity score in cALD. A, The mean value of CSF CHIT activity (dashed line) by GIS for the entire evaluable cALD cohort (n = 64). B, The mean value of CSF CHIT activity (dashed line) and 95% confidence intervals (solid bars) by GIS for patients with higher risk cALD (Loes ≥10; n = 40).
When one considers higher risk patients with cALD only (Loes ≥10), CSF CHIT varied significantly by GIS (Fig 3). Because of low patient numbers in this cohort subset, a binary GIS status was assigned. Patients with low GIS (1/2, n = 6) demonstrated a mean CHIT of 4844 ng/mL/h (95% CI, 2429–7393 ng/mL/h), while those with high GIS (2/3, n = 34) showed a mean CHIT of 11,892 ng/mL/h (95% CI, 8461–15,322 ng/mL/h; P < .01, difference in mean CHIT).
Pretransplant Brain MR Imaging Gadolinium Intensity Score Correlates with Longer Term Clinical Neurologic Outcome following HSCT in Higher Risk Patients with cALD
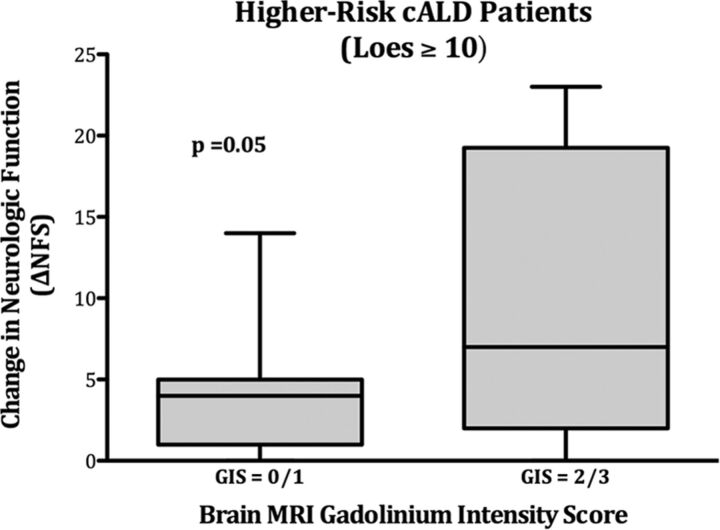
Twenty-five patients with higher risk cALD (Loes ≥10 at HSCT) and near-complete donor hematopoietic engraftment following HSCT (>80% at most recent follow-up) were evaluable for long-term change in neurologic function (ΔNFS) based on pretransplantation brain MR imaging GIS (Fig 4). Seven patients with low GISs (0/1) demonstrated a mean ΔNFS of 4.3 at an average posttransplantation follow-up of 3 years. Eighteen patients with high GISs (2/3) experienced a mean ΔNFS of 10.4 at an average posttransplantation follow-up of 1 year 10 months (P = .05, difference in mean ΔNFS).
Fig 4.
Change in the neurologic function score after HSCT correlates with the pretransplantation MR imaging gadolinium intensity score in higher risk cALD. ΔNFS by pretransplant brain MR imaging GIS status is shown for patients with higher risk cALD (Loes ≥10 at transplantation). Seven patients had low GIS status (0/1), while 18 had high GIS status (2/3) on pretransplantation MR imaging. Solid rectangles show the first quartile (bottom), median (midline), and third quartile (top) results; whiskers define range.
The distribution of transplantation, demographic, and Loes characteristics between the 2 groups is shown in the Table.
Demographic, transplant, and Loes characteristics in higher risk patients with cALD (Loes ≥10) analyzed for neurologic function change by pretransplantation gadolinium intensity score
| GIS 0/1 (n = 7) | GIS 2/3 (n = 18) | Difference (95% CI) | |
|---|---|---|---|
| Age at HSCT (yr) | |||
| Mean | 10.1 | 9.0 | 1.2 (−1.9–4.2) |
| IQR | 8.1–13.3 | 6.8–10.0 | |
| Pre-HSCT Loes | |||
| Mean | 12.6 | 14.0 | −1.5 (−3.3–0.4) |
| IQR | 11–14 | 11–16 | |
| Donor chimerism (%)a | |||
| Mean | 98.7 | 97.1 | 1.6 (−2.1–5.3) |
| IQR | 100–100 | 92–100 |
Note:—IQR indicates interquartile range.
Donor chimerism reflects the percentage of donor hematopoietic engraftment at the most recent follow-up.
Discussion
Currently, allogeneic hematopoietic stem cell transplantation is considered the best treatment for “standard-risk” childhood cerebral adrenoleukodystrophy (Loes brain MR imaging severity score <10).3,11,13 In these children with relatively low-level cerebral white matter involvement at the time of HSCT, gross neurologic function on the ALD NFS is generally normal before transplantation and remains largely unchanged afterward. However, a significant proportion of patients with cALD present at diagnosis with advanced, often-symptomatic cerebral disease. Owing to greater white matter involvement (Loes ≥10), these patients with cALD are considered at higher risk for poor neurologic outcomes after transplantation.
In a recent analysis, 30 consecutive higher risk patients (Loes ≥10) were reported to have a median ΔNFS of 7.5 (interquartile range, 4–19; range, 0–23) when analyzed at a median of 2.1 years after HSCT. Such highly variable and unpredictable neurologic outcomes (ranging from mild, if any, changes to significant neurologic impairment) make for difficult decisions by both clinicians and families, especially because no other effective therapies for higher risk cALD currently exist. Moreover, while large analyses have shown consistently favorable neurologic outcomes for patients with a pretransplantation Loes <10, the absolute pre-HSCT Loes score within the higher risk cALD group (pretransplant Loes ≥10) has not been proved soley prognostic.13 Therefore, additional predictive biomarkers for this challenging patient subset are sought.
Recently, a tight correlation between pretransplantation CHIT activity in CSF and neurologic outcomes at 1 year following HSCT for all cALD has been reported.15 Because CHIT is elaborated by activated monocytes and macrophages, this marker may quantify active neuroinflammation in patients with cALD. However, CHIT assay is not readily available to most clinicians when assessing boys with cALD. Furthermore, baseline CHIT has not yet been shown within the higher risk cALD subset to be a clear predictor of neurologic function after HSCT.
Radiographic strategies for the quantification of neuroinflammation are limited. While the Loes score is an objective measurement of brain regions affected by demyelination, it does not reflect the volume of brain involved, and it does not quantify contrast enhancement. Practical volumetric analysis of the affected white matter or of areas involved by inflammation is not easily performed with standard clinical PACSs from most vendors. Therefore, we developed a simple scoring system based on the maximal intensity of enhancement observed on each cALD brain MR imaging, positing a correlation between the degree of enhancement and active neuroinflammation. Because observer judgment of MR imaging gadolinium enhancement intensity is fundamentally relative, our system incorporates intrascan intensity in the highly vascular choroid plexus as a normalizing reference. Indeed, we found the scoring system to have high interobserver reproducibility among neuroradiologists.
Using this scale, we showed that higher gadolinium enhancement intensity scores on the untreated cALD brain MR imaging positively correlated with concomitant CSF CHIT. Furthermore, for patients with higher risk cALD at the time of HSCT (Loes ≥10), we observed an association between higher pretransplantation GISs and worse longer term neurologic functional outcomes. There were relatively fewer higher risk patients with cALD (Loes ≥10) in our evaluable cohort who had lower GIS status at baseline (4% with GIS = 0%; and 24% with GIS = 1). However, our findings suggest significantly better neurologic outcomes for this group deemed at higher risk by the Loes score (ΔNFS of only 4.3 versus ΔNFS of 10.4 for higher risk patients with GIS = 2/3) at a long-term posttransplantation follow-up. Although the mechanism of action of HSCT for cALD is not well-understood, it may, in part, exert its desired effect (arresting further myelin loss) by quelling neuroinflammation. In the higher risk patients with cALD (Loes ≥10), the baseline absolute Loes score—which tallies discrete regions within the cerebrum, brain stem, and cerebellum with radiographically evident demyelination—has not been observed to independently correlate with posttransplantation neurologic outcome. However, the Loes system neither accounts for nor attempts to quantify radiographic evidence of neuroinflammation. In fact, among evaluable higher risk patients with cALD in this current analysis (Loes ≥10), gadolinium enhancement intensity as measured by GIS did not correlate with absolute Loes scores (data not shown). Therefore, biomarkers that do address this component of active cALD may add prognostic value.
A hindrance to better understanding of HSCT for cALD is the relative rarity of the disorder. Although this cohort is considered large in the field, our study is limited due to few evaluable patients. In particular, confidence in the utility of the pretransplantation GIS to predict neurologic outcomes following HSCT for higher risk cALD (Loes ≥10) would be greater if more such patients were evaluable. Ultimately, a matrix of various “measures” of cerebral disease burden before HSCT (clinical, neuropsychologic, tissue biomarkers, Loes severity, and gadolinium intensity status) may better predict likely longer term outcomes for this challenging cALD population.
Conclusions
Brain MR imaging gadolinium enhancement in cALD can be quantified with a simple, reproducible scoring system. When applied to MR imaging studies of untreated boys, the gadolinium intensity score shows a strong positive correlation with the activity of cerebrospinal chitotriosidase, an enzyme elaborated by activated monocytes and previously shown to correlate with neurologic function at 1 year posttransplantation in all patients with cALD undergoing HSCT. In higher risk patients with cALD (Loes ≥10), a subset for whom prediction of neurologic outcomes following HSCT has been difficult, the baseline brain MR imaging gadolinium intensity status appears to significantly predict long-term neurologic functional change. Although this study was performed by using a dedicated 3T MR imaging protocol with fixed T1WI parameters, we believe this method will likely yield useful information regardless of scanner manufacturer, field strength, or sequence parameters. These findings may help to inform clinician and parental decision-making for higher risk patients with cALD who seek transplantation intervention.
ABBREVIATIONS:
- ALD
adrenoleukodystrophy
- cALD
cerebral adrenoleukodystrophy
- CHIT
chitotriosidase
- ΔNFS
change in NFS score
- GIS
brain MRI gadolinium intensity scale score
- HSCT
hematopoietic stem cell transplantation
- NFS
adrenoleukodystrophy neurologic function scale score
Footnotes
Disclosures: Ryan M. Shanley—UNRELATED: Grants/Grants Pending: National Institutes of Health.* Gerald V. Raymond—UNRELATED: Consultancy: Department of Health and Human Services; Expert Testimony: various medicolegal cases; Grants/Grants Pending: Patient-Centered Outcomes Research Institute.* Paul J. Orchard—UNRELATED: Other: Phase 2/3 Study of the Efficacy and Safety of Hematopoietic Stem Cells Transduced With Lenti-D Lentiviral Vector for the Treatment of Childhood Cerebral Adrenoleukodystrophy,* Comments: Our institution is participating in a clinical trial of gene therapy for this disease. The company sponsoring the trial is Bluebird Bio. As Principal Investigator of the study, I have some salary support for the study. However, the gene therapy study does not support the investigations reported in this article. *Money paid to the institution.
References
- 1. Engelen M, Kemp S, de Visser M, et al. X-linked adrenoleukodystrophy (X-ALD): clinical presentation and guidelines for diagnosis, follow-up and management. Orphanet J Rare Dis 2012;7:51 10.1186/1750-1172-7-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Moser H, Raymond G, Dubey P. Adrenoleukodystrophy: new approaches to a neurodegenerative disease. JAMA 2005;294:3131–34 10.1001/jama.294.24.3131 [DOI] [PubMed] [Google Scholar]
- 3. Mahmood A, Raymond G, Dubey P, et al. Survival analysis of haematopoietic cell transplantation for childhood cerebral X-linked adrenoleukodystrophy: a comparison study. Lancet Neurol 2007;6:687–92 10.1016/S1474-4422(07)70177-1 [DOI] [PubMed] [Google Scholar]
- 4. Powers JM, Pei Z, Heinzer AK, et al. Adreno-leukodystrophy: oxidative stress of mice and men. J Neuropathol Exp Neurol 2005;64:1067–79 10.1097/01.jnen.0000190064.28559.a4 [DOI] [PubMed] [Google Scholar]
- 5. Powers JM, Liu Y, Moser AB, et al. The inflammatory myelinopathy of adreno-leukodystrophy: cells, effector molecules, and pathogenetic implications. J Neuropathol Exp Neurol 1992;51:630–43 10.1097/00005072-199211000-00007 [DOI] [PubMed] [Google Scholar]
- 6. Berger J, Forss-Petter S, Eichler FS. Pathophysiology of X-linked adrenoleukodystrophy. Biochimie 2014;98:135–42 10.1016/j.biochi.2013.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moser HW, Raymond GV, Lu SE, et al. Follow-up of 89 asymptomatic patients with adrenoleukodystrophy treated with Lorenzo's oil. Arch Neurol 2005;62:1073–80 10.1001/archneur.62.7.1073 [DOI] [PubMed] [Google Scholar]
- 8. Melhem ER, Loes DJ, Georgiades CS, et al. X-linked adrenoleukodystrophy: the role of contrast-enhanced MR imaging in predicting disease progression. AJNR Am J Neuroradiol 2000;21:839–44 [PMC free article] [PubMed] [Google Scholar]
- 9. Loes DJ, Fatemi A, Melhem ER, et al. Analysis of MRI patterns aids prediction of progression in X-linked adrenoleukodystrophy. Neurology 2003;61:369–74 10.1212/01.WNL.0000079050.91337.83 [DOI] [PubMed] [Google Scholar]
- 10. Loes DJ, Hite S, Moser H, et al. Adrenoleukodystrophy: a scoring method for brain MR observations. AJNR Am J Neuroradiol 1994;15:1761–66 [PMC free article] [PubMed] [Google Scholar]
- 11. Peters C, Charnas LR, Tan Y, et al. Cerebral X-linked adrenoleukodystrophy: the international hematopoietic cell transplantation experience from 1982 to 1999. Blood 2004;104:881–88 10.1182/blood-2003-10-3402 [DOI] [PubMed] [Google Scholar]
- 12. Moser HW, Raymond GV, Koehler W, et al. Evaluation of the preventive effect of glyceryl trioleate-trierucate (“Lorenzo's oil”) therapy in X-linked adrenoleukodystrophy: results of two concurrent trials. Adv Exp Med Biol 2003;544:369–87 10.1007/978-1-4419-9072-3-47 [DOI] [PubMed] [Google Scholar]
- 13. Miller WP, Rothman SM, Nascene D, et al. Outcomes after allogeneic hematopoietic cell transplantation for childhood cerebral adrenoleukodystrophy: the largest single-institution cohort report. Blood 2011;118:1971–78 10.1182/blood-2011-01-329235 [DOI] [PubMed] [Google Scholar]
- 14. Appelbaum FR, Thomas E. Thomas' Hematopoietic Cell Transplantation: Stem Cell Transplantation. Oxford: Wiley-Blackwell; 2009 [Google Scholar]
- 15. Orchard PJ, Lund T, Miller W, et al. Chitotriosidase as a biomarker of cerebral adrenoleukodystrophy. J Neuroinflammation 2011;8:144 10.1186/1742-2094-8-144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fleiss JL, Levin B, Paik MC. Statistical Methods for Rates and Proportions. New York: John Wiley & Sons; 2003 [Google Scholar]