Abstract
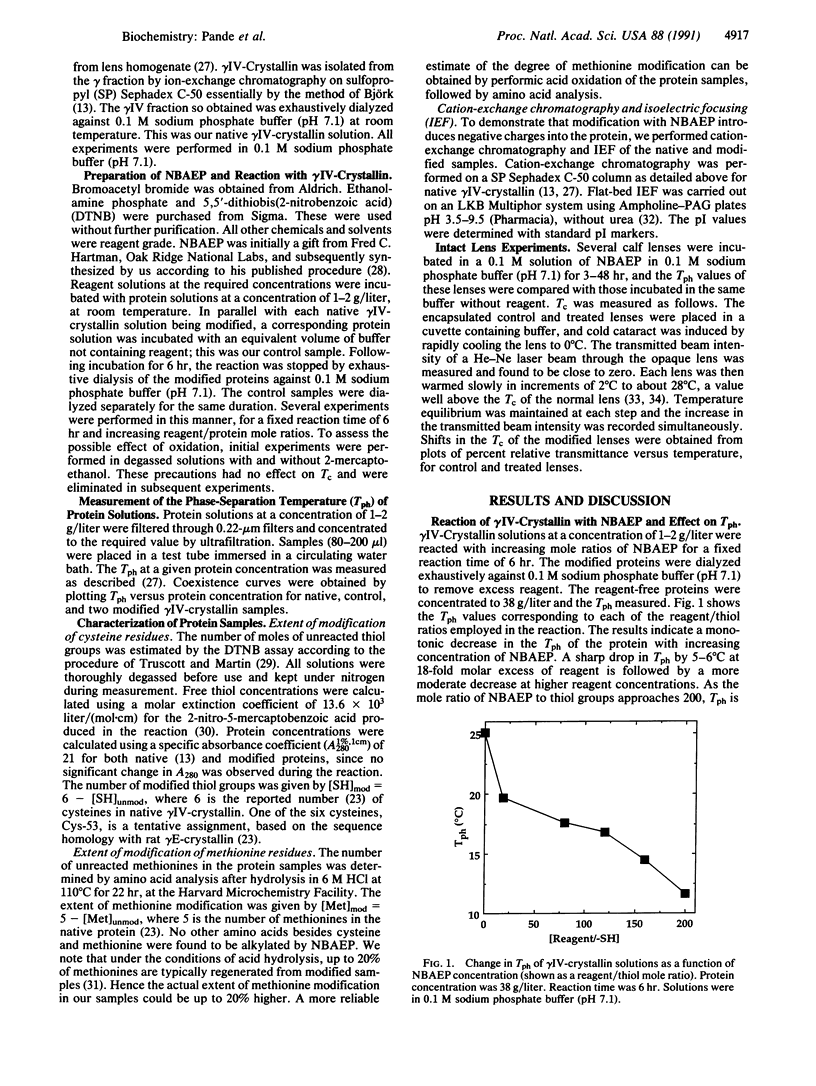
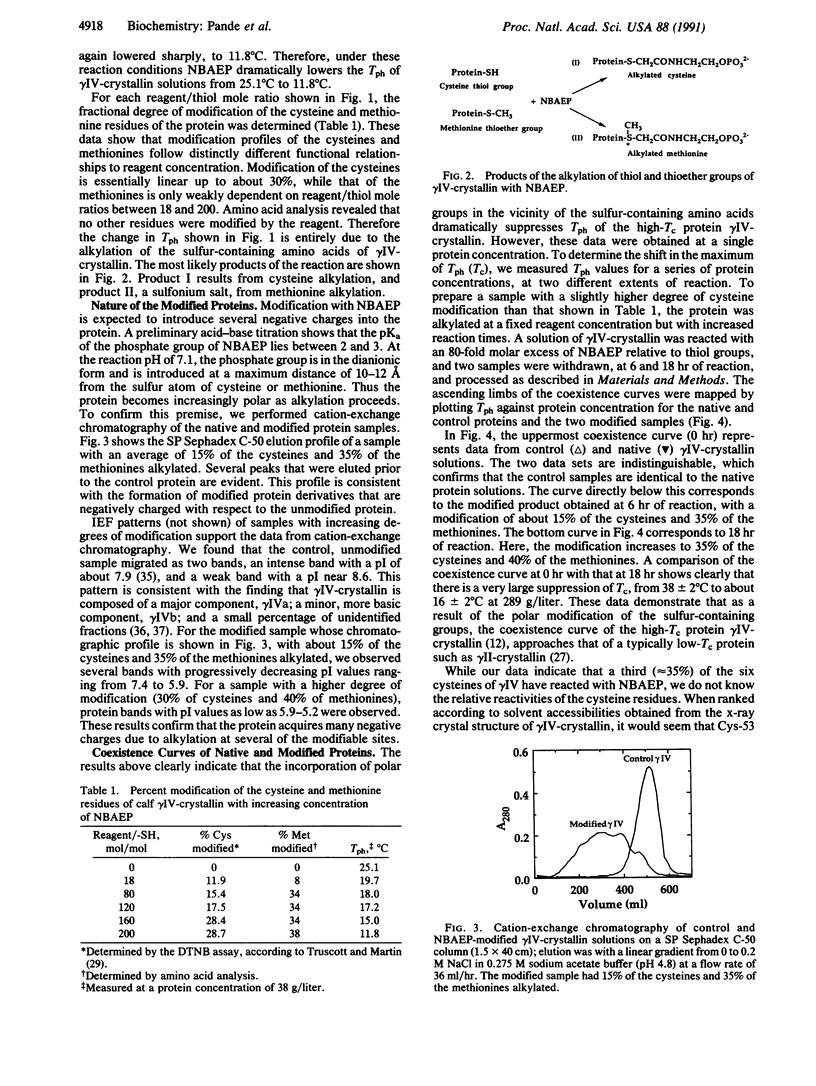
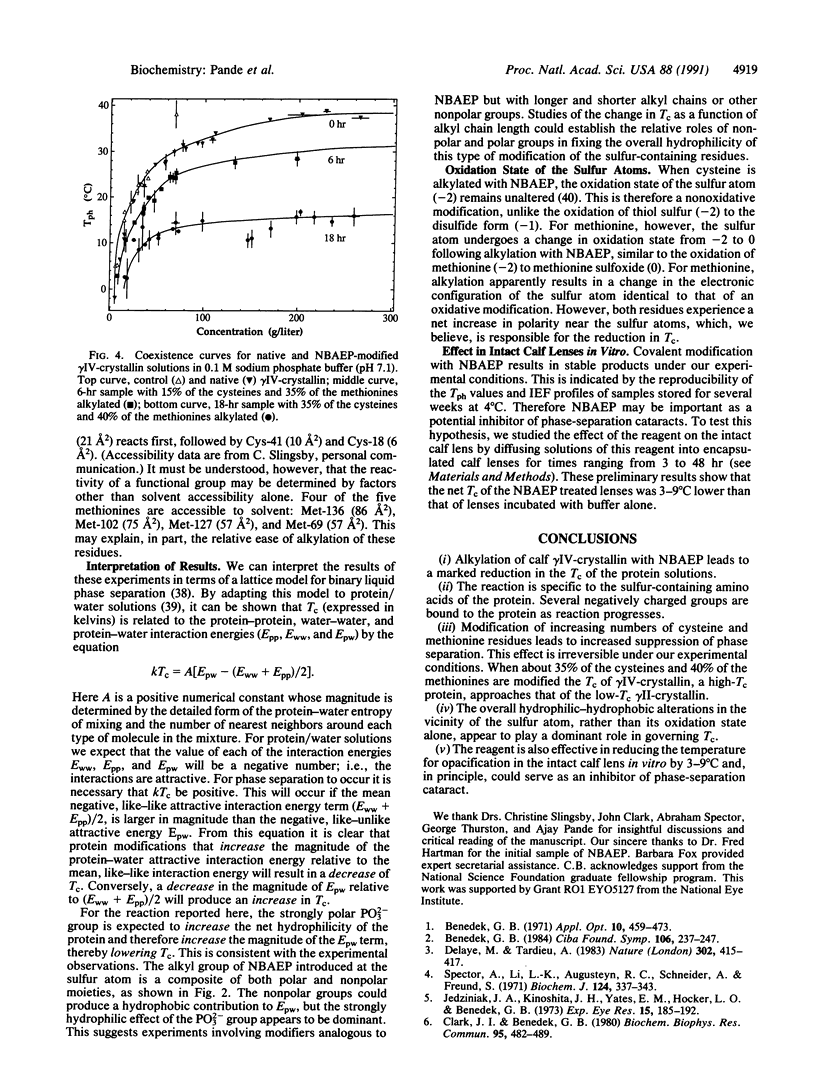
The calf lens protein gamma IV-crystallin, a strong determinant of the net phase-separation temperature of the lens, was chemically modified with N-bromoacetylethanolamine phosphate. The phase-separation temperatures of solutions of the modified protein were measured and found to be dramatically reduced with respect to those of the native protein. At neutral pH the reagent alkylates only the cysteine and methionine residues and introduces a doubly charged phosphate anion at a maximum distance of 10-12 A from the sulfur atoms. At a protein concentration of 38 g/liter, and with 30% of the cysteines and 40% of the methionines alkylated, the phase-separation temperature is lowered from approximately 25 +/- 2 degrees C to approximately 12 +/- 2 degrees C. The ascending limbs of the coexistence curves for the native and modified proteins were determined at two different degrees of modification. The coexistence curve of the protein with 35% of the cysteines and 40% of the methionines modified shows that as protein concentration approaches the critical concentration of 289 g/liter, there is a much larger suppression of the critical temperature, from approximately 38 +/- 2 degrees C in the native protein to approximately 16 +/- 2 degrees C. Incubation of intact calf lenses in vitro with the reagent results in the suppression of the phase-separation temperature by 3-9 degrees C. These results are consistent with the view that the observed suppression in the critical temperature is due to an increase in the hydrophilicity of the protein in the vicinity of the sulfur-containing residues.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benedek G. B. The molecular basis of cataract formation. Ciba Found Symp. 1984;106:237–247. doi: 10.1002/9780470720875.ch14. [DOI] [PubMed] [Google Scholar]
- Björk I. Studies on gamma-crystallin from calf lens. 3. Comparison of the main protein components by peptide mapping. Exp Eye Res. 1970 Jan;9(1):152–157. doi: 10.1016/s0014-4835(70)80070-7. [DOI] [PubMed] [Google Scholar]
- Clark J. I., Benedek G. B. Phase diagram for cell cytoplasm from the calf lens. Biochem Biophys Res Commun. 1980 Jul 16;95(1):482–489. doi: 10.1016/0006-291x(80)90763-9. [DOI] [PubMed] [Google Scholar]
- Clark J. I., Carper D. Phase separation in lens cytoplasm is genetically linked to cataract formation in the Philly mouse. Proc Natl Acad Sci U S A. 1987 Jan;84(1):122–125. doi: 10.1073/pnas.84.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark J. I., Giblin F. J., Reddy V. N., Benedek G. B. Phase separation of X-irradiated lenses of rabbit. Invest Ophthalmol Vis Sci. 1982 Feb;22(2):186–190. [PubMed] [Google Scholar]
- Delaye M., Clark J. I., Benedek G. B. Identification of the scattering elements responsible for lens opacification in cold cataracts. Biophys J. 1982 Mar;37(3):647–656. [PMC free article] [PubMed] [Google Scholar]
- Delaye M., Tardieu A. Short-range order of crystallin proteins accounts for eye lens transparency. 1983 Mar 31-Apr 6Nature. 302(5907):415–417. doi: 10.1038/302415a0. [DOI] [PubMed] [Google Scholar]
- Driessen H. P., Herbrink P., Bloemendal H., de Jong W. W. Primary structure of the bovine beta-crystallin Bp chain. Internal duplication and homology with gamma-crystallin. Eur J Biochem. 1981 Dec;121(1):83–91. doi: 10.1111/j.1432-1033.1981.tb06433.x. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- GUNDLACH H. G., MOORE S., STEIN W. H. The reaction of iodoacetate with methionine. J Biol Chem. 1959 Jul;234(7):1761–1764. [PubMed] [Google Scholar]
- Garner M. H., Spector A. Selective oxidation of cysteine and methionine in normal and senile cataractous lenses. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1274–1277. doi: 10.1073/pnas.77.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman F. C., Norton I. L. Detection of an essential sulfhydryl group in phosphoglycerate mutase with an affinity-labeling reagent. J Biol Chem. 1976 Aug 10;251(15):4565–4569. [PubMed] [Google Scholar]
- Hartman F. C., Suh B., Welch M. H., Barker R. Inactivation of class I fructose diphosphate aldolases by the substrate analog N-bromoacetylethanolamine phosphate. J Biol Chem. 1973 Dec 10;248(23):8233–8239. [PubMed] [Google Scholar]
- Ishimoto C., Goalwin P. W., Sun S. T., Nishio I., Tanaka T. Cytoplasmic phase separation in formation of galactosemic cataract in lenses of young rats. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4414–4416. doi: 10.1073/pnas.76.9.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedziniak J. A., Kinoshita J. H., Yates E. M., Hocker L. O., Benedek G. B. On the presence and mechanism of formation of heavy molecular weight aggregates in human normal and cataractous lenses. Exp Eye Res. 1973 Feb;15(2):185–192. doi: 10.1016/0014-4835(73)90118-8. [DOI] [PubMed] [Google Scholar]
- McDermott M. J., Gawinowicz-Kolks M. A., Chiesa R., Spector A. The disulfide content of calf gamma-crystallin. Arch Biochem Biophys. 1988 May 1;262(2):609–619. doi: 10.1016/0003-9861(88)90413-4. [DOI] [PubMed] [Google Scholar]
- Schloss J. V., Stringer C. D., Hartman F. C. Identification of essential lysyl and cysteinyl residues in spinach ribulosebisphosphate carboxylase/oxygenase modified by the affinity label N-bromoacetylethanolamine phosphate. J Biol Chem. 1978 Aug 25;253(16):5707–5711. [PubMed] [Google Scholar]
- Siezen R. J., Coppin C. M., Benedek G. B. Permanent suppression of phase separation cataract in calf lens using amine modification agents. Biochem Biophys Res Commun. 1985 Nov 27;133(1):239–247. doi: 10.1016/0006-291x(85)91867-4. [DOI] [PubMed] [Google Scholar]
- Siezen R. J., Fisch M. R., Slingsby C., Benedek G. B. Opacification of gamma-crystallin solutions from calf lens in relation to cold cataract formation. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1701–1705. doi: 10.1073/pnas.82.6.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siezen R. J., Kaplan E. D., Anello R. D. Superior resolution of gamma-crystallins from microdissected eye lens by cation-exchange high-performance liquid chromatography. Biochem Biophys Res Commun. 1985 Feb 28;127(1):153–160. doi: 10.1016/s0006-291x(85)80138-8. [DOI] [PubMed] [Google Scholar]
- Slingsby C., Miller L. R. Purification and crystallization of mammalian lens gamma-crystallins. Exp Eye Res. 1983 Nov;37(5):517–530. doi: 10.1016/0014-4835(83)90028-3. [DOI] [PubMed] [Google Scholar]
- Spector A., Garner M. H., Garner W. H., Roy D., Farnsworth P., Shyne S. An extrinsic membrane polypeptide associated with high-molecular-weight protein aggregates in human cataract. Science. 1979 Jun 22;204(4399):1323–1326. doi: 10.1126/science.377484. [DOI] [PubMed] [Google Scholar]
- Spector A., Li L. K., Augusteyn R. C., Schneider A., Freund T. -Crystallin. The isolation and characterization of distinct macromolecular fractions. Biochem J. 1971 Sep;124(2):337–343. doi: 10.1042/bj1240337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector A., Roy D. Disulfide-linked high molecular weight protein associated with human cataract. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3244–3248. doi: 10.1073/pnas.75.7.3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Ishimoto C., Chylack L. T., Jr Phase separation of a protein-water mixture in cold cataract in the young rat lens. Science. 1977 Sep 2;197(4307):1010–1012. doi: 10.1126/science.887936. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Rubin S., Sun S. T., Nishio I., Tung W. H., Chylack L. T., Jr Phase separation in rat lenses cultured in low glucose media. Invest Ophthalmol Vis Sci. 1983 Apr;24(4):522–525. [PubMed] [Google Scholar]
- Thomson J. A., Schurtenberger P., Thurston G. M., Benedek G. B. Binary liquid phase separation and critical phenomena in a protein/water solution. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7079–7083. doi: 10.1073/pnas.84.20.7079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truscott R. J., Martin F. The reaction of proteins with 3-hydroxyanthranilic acid as a possible model for senile nuclear cataract in man. Exp Eye Res. 1989 Dec;49(6):927–940. doi: 10.1016/s0014-4835(89)80017-x. [DOI] [PubMed] [Google Scholar]
- White H. E., Driessen H. P., Slingsby C., Moss D. S., Lindley P. F. Packing interactions in the eye-lens. Structural analysis, internal symmetry and lattice interactions of bovine gamma IVa-crystallin. J Mol Biol. 1989 May 5;207(1):217–235. doi: 10.1016/0022-2836(89)90452-x. [DOI] [PubMed] [Google Scholar]
- Wistow G. J., Piatigorsky J. Lens crystallins: the evolution and expression of proteins for a highly specialized tissue. Annu Rev Biochem. 1988;57:479–504. doi: 10.1146/annurev.bi.57.070188.002403. [DOI] [PubMed] [Google Scholar]


