Abstract
Previous observational studies have suggested a potential relationship between osteoarthritis (OA) and the risk of cardiovascular disease (CVD), with conflicting results. We aimed to provide a systematic and quantitative summary of the association between OA and the risk of CVD. We searched Medline and EMBASE to retrieve prospective and retrospective studies that reported risk estimates of the association between OA status and CVD risk. Pooled estimates were calculated by a random effects model. The search yielded 15 articles including a total of 358,944 participants, including 80,911 OA patients and 29,213 CVD patients. Overall, the risk of CVD was significantly increased by 24% (RR: 1.24, 95% CI: 1.12 to 1.37, P < 0.001) in patients with OA compared with the general population, with no significant publication bias. Furthermore, sensitivity analysis indicated that our results were robust and were not influenced by any one study. In conclusion, this meta-analysis provides strong evidence that OA is a significant risk factor for CVD.
Cardiovascular disease (CVD), such as ischemic heart disease (IHD), congestive heart failure (CHF), transient ischemic attacks (TIA), and stroke, is a leading cause of morbidity and mortality in the general population worldwide. According to the World Health Organization, 17.5 million people die each year from CVD, constituting approximately 30% of all deaths worldwide1. Cardiovascular diseases therefore place a great burden on people, the economy, and society in general. However, cardiovascular disorders are largely preventable. The identification of new cardiovascular risk factors and interventions to modify these factors is of great importance for addressing the current epidemic.
Osteoarthritis (OA) is also a major cause of morbidity and healthcare expenditures and affects approximately 15% of the population2. By age 65, 80% of the population has radiographic evidence of OA, and 60% are experiencing symptoms of OA3.
Recent epidemiological studies have suggested a potential relationship between OA and CVD, with conflicting results. Whereas Jonsson et al. found a linear association between the severity of hand OA and atherosclerosis in the AGES Reykjavik study4, Haugen et al. concluded that symptomatic hand OA but not radiographic hand OA was associated with an increased risk of coronary heart disease events using the data from the Framingham Heart Study5. Moreover, the results from the Rotterdam Study indicated that disability, not OA, predicted cardiovascular disease6. This topic remains controversial, and the meta-analysis presented here provides a valid and up-to-date summary of the relevant literature. We aimed to determine whether OA patients are at increased risk of CVD. We also evaluated whether this association differs by type of OA or CVD.
Results
Literature search
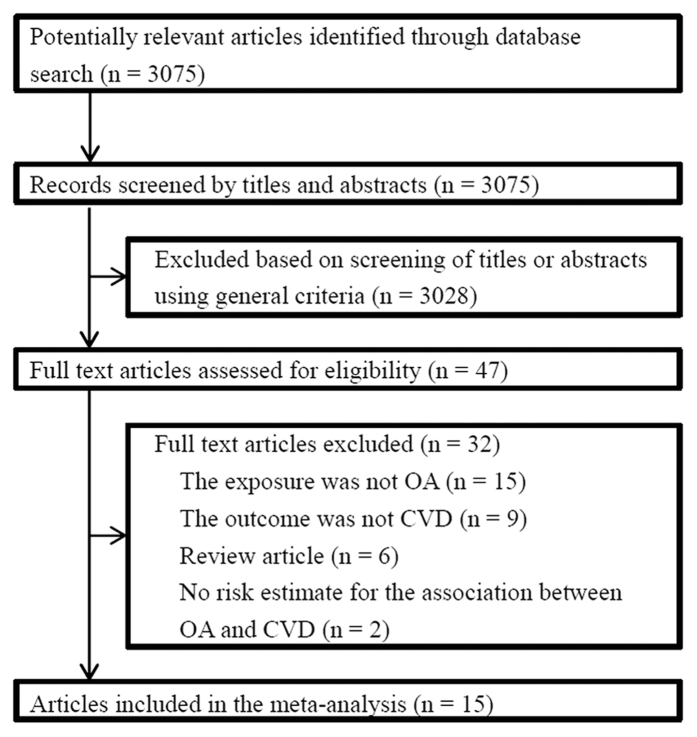
Overall, 3,075 references were initially identified. After the initial screening of titles and abstracts, a total of 3,028 articles were excluded, leaving 47 articles for retrieval. Full text assessment of these articles resulted in 15 eligible articles that met our inclusion criteria4,5,6,7,8,9,10,11,12,13,14,15,16,17,18. The total number of participants included was 358,944, with 80,911 OA patients and 29,213 CVD patients. Figure 1 displays the process of selection of studies.
Figure 1. Literature Search for the Meta-analysis.
Study characteristics
Table 1 shows the characteristics of the 15 included articles. The articles included in our systematic review were quite heterogeneous. Five were retrospective studies4,9,12,14,16, and 10 were prospective studies5,6,7,8,10,11,13,15,17,18. The studies were conducted in the United States5,7,10,14, Europe4,6,8,9,11,12,18, Canada15,16, and Japan17. Thirteen studies included men and women4,5,6,8,9,10,12,13,14,15,16,17,18, and four reported results separately by sex4,8,13,16; the other two studies included only women7,11.
Table 1. Characteristics of the included studies.
| Study | Design | Country | Cohort | CVD confirmation | Adjustment |
|---|---|---|---|---|---|
| Kadam et al.9 | case-control | England and Wales | Morbidity Statistics in General Practice | medical record | Age, sex, social class, and number of other broad disease groups for which subjects consulted |
| Nielen et al.12 | cross-sectional | Netherlands | The Netherlands Information Network of General Practice | medical record | Age, gender, hypertension and hypercholesterolemia |
| Jonsson et al.4 | case-control | Iceland | AGES Reykjavik study | question | Age, smoking, cholesterol, triglycerides, body mass index, pulse pressure and statin use |
| Ong et al.14 | cross-sectional | U.S. | NHANES 1999–2008 | question | Age, gender, race/ethnicity, and survey period |
| Haara et al.8 | cohort | Finland | medical record | Age, education, history of workload, smoking, and body mass index | |
| Kishimoto et al.10 | cohort | U.S. | The Honolulu Heart Program | medical record | Age, BMI, physical activity index, hypertension, diabetes mellitus, HDL cholesterol, total cholesterol, smoking status, fibrinogen, alcohol intake, and ASA and/or NSAID use |
| Hoeven et al.6 | cohort | Netherlands | The Rotterdam Study | medical record | Age, sex, body mass index, diabetes, hypertension, total cholesterol/HDL cholesterol ratio and smoking |
| Tsuboi et al.17 | cohort | Japan | medical record | Age, gender, BMI, and lifestyle (smoking, drinking, and exercise habits) | |
| Barbour et al.7 | cohort | U.S. | Study of Osteoporotic Fractures | medical record | Age, body mass index, education, smoking, health status, diabetes, and stroke |
| Haugen et al.5 | cohort | U.S. | The Framingham Heart Study | medical record | Age, sex, cohort, BMI, total cholesterol: HDL ratio, current lipid-lowering treatment, increased blood pressure, current antihypertensive treatment, elevated fasting or non-fasting blood glucose, current antidiabetic treatment (oral or insulin), current use of NSAIDs, daily use of aspirin, current/previous smoking, alcohol use |
| Kluzek et al.11 | cohort | UK | The Chingford study | medical record | Age, smoking, total cholesterol, HDL-cholesterol, systolic BP and BP medication, occupation, BMI, HRT use, past physical activity, current/previous CVD disease, non-ASA NSAIDs and glucose levels |
| Rahman et al.16 | cross-sectional | Canada | Medical Services Plan | medical record | Age, sex, income, education, body mass index, physical activity, smoking, fruit and vegetable consumption, pain medication use, chronic obstructive pulmonary disease, hypertension and diabetes |
| Nuesch et al.13 | cohort | England | The Somerset and Avon Survey of Health | medical record | No adjustment |
| Rahman et al.15 | cohort | Canadian | Canadian Community Health Survey | medical record | Age, sex, family history, high cholesterol, high blood pressure, diabetes mellitus, high body mass index (BMI), smoking, and diet |
| Veronese et al.18 | cohort | Italy | Progetto Veneto Anziani | medical record | Age; gender; waste-to-hip ratio; education level; presence at baseline of diabetes, hypertension, atrial fibrillation, chronic obstructive pulmonary disease; use at baseline of aspirin, anti-hypertensives, NSAIDSs; number of medications; smoking status; activities of daily living, mini-mental state, geriatric depression scale scores; glycosylated hemoglobin, total cholesterol, serum uric acid, estimated glomerular filtration rate, erythrocytes sedimentation rate; ankle brachial index; short physical performance battery and handgrip strength |
Features of exposure varied across studies. Although 12 studies ascertained OA by either radiographic results or medical records4,5,6,7,8,9,11,12,13,17,18, the other three used questionnaires to confirm OA status10,14,16. The studies focused on different sites of OA, including the hand4,6,8,11,18, knee6,11,17,18, or hip6,7,18.
The method of outcome ascertainment varied across articles. Although the majority of articles ascertained CVD by medical records, three articles used questionnaires to ascertain CVD4,14,16. Two articles6,12 reported an overall risk outcome, 35,14,18 reported both an overall risk outcome and separately for different outcomes, 67,8,10,11,13,17 reported risk estimate for one specific outcome. The others4,9,15,16 reported risk estimates separately for different outcomes; and in this case, the risk estimate for the most prevalent type of outcome serves as a surrogate for CVD risk estimate in the pooled analysis.
Although the included articles were quite heterogeneous, the inter-reviewer reliability for data extraction was almost perfect (kappa ranged 0.99–1.00). All studies were rated as medium to high quality.
Systematic review of evidence
Fifteen articles provided 19 risk estimates of the association between OA and CVD risk. Of these 19 estimates, 11 reported that OA was associated with a significantly increased risk of CVD, five that OA was associated with a non-significantly increased CVD risk, and three that OA was associated with a non-significantly decreased CVD risk. No study included in this systematic review reported that OA was associated with a significantly decreased risk of CVD.
For the non-parametric sign test, we considered whether studies reported an increased or decreased risk of CVD associated with OA, regardless of significance level or magnitude of effect. Overall, 16 risk estimates found that OA was associated with an increased risk of CVD, and three found that OA was associated with a decreased risk of CVD. The sign test (P = 0.004) rejected the null hypothesis of equal CVD risk in patients with and without OA.
Meta-analysis
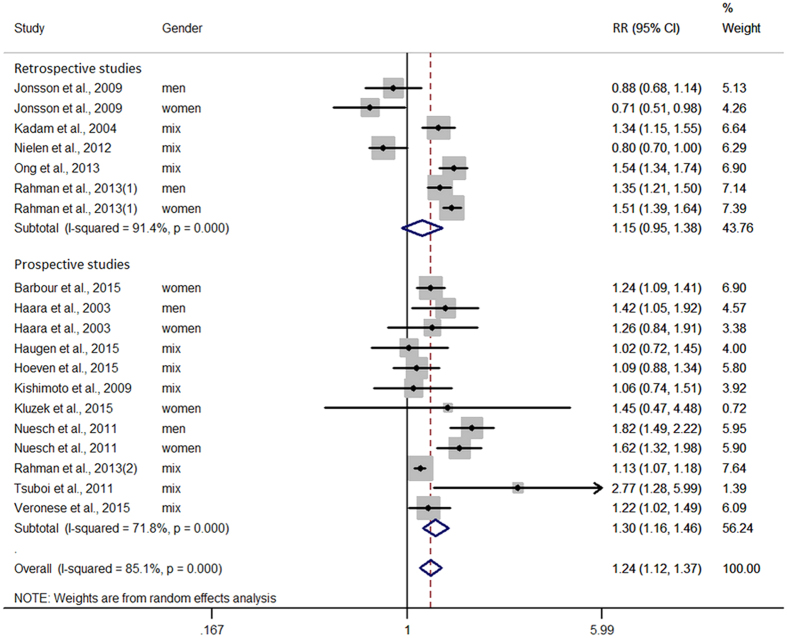
Figure 2 displays the results of the meta-analysis of the 15 articles. A high level of between-study heterogeneity was detected, with I2 = 85.1%. Overall, the risk of CVD was significantly increased by 24% (RR: 1.24, 95% CI: 1.12 to 1.37, P < 0.001) in patients with OA compared with the general population. No significant publication bias was observed according to the Egger test (P = 0.659) or funnel plot (Supplementary Figure S1).
Figure 2. Association between OA and CVD risk.
Estimates are derived from random effects. Dots indicate relative risks. Horizontal lines indicate 95% confidence intervals for relative risks. Diamonds represent pooled relative risk estimates with 95% confidence intervals.
A sensitivity analysis was performed to evaluate the impact of additional study characteristics on the pooled RR. First, we calculated separate estimates by study design and found that the prospective studies (12 estimates) had a pooled RR of 1.30 (95% CI: 1.16 to 1.46, P < 0.001) and the retrospective studies (seven estimates) had a pooled RR of 1.15 (95% CI: 0.95 to 1.38, P = 0.147).
Second, we excluded studies that ascertained OA or CVD status by questionnaire. The remaining studies had a pooled RR of 1.26 (95% CI: 1.12 to 1.42, P < 0.001). Finally, we excluded individual study estimates one at a time to examine the influence of each study on the overall RR. We found that the omission of any one study did not appreciably change the pooled RR (Supplementary Figure S2).
Subgroup analysis and meta-regression analysis
When subgroups were stratified by study design, we found a significant effect of prospective studies (RR: 1.30, 95% CI: 1.16 to 1.46, P < 0.001) and a non-significant effect of retrospective studies (RR: 1.15, 95% CI: 0.95 to 1.38, P = 0.147). The stratified analysis according to OA site included 3 subgroups, hand (RR: 1.03, 95% CI: 0.85 to 1.25, P = 0.749), knee (RR: 1.30, 95% CI: 1.00 to 1.69, P = 0.047) and hip (RR: 1.23, 95% CI: 1.11 to 1.38, P < 0.001). When studies were stratified according to confirmation methods for OA, we found a RR of 1.47 (95% CI: 0.91 to 2.39, P = 0.118) for clinical OA and a RR of 1.23 (95% CI: 1.02 to 1.48, P = 0.028) for radiographic OA. When studies were stratified according to different CVD types, we found significant results for the IHD (RR: 1.33, 95% CI: 1.20 to 1.46, P < 0.001), CHF (RR: 1.40, 95% CI: 1.13 to 1.73, P = 0.002) and cardiovascular death (RR: 1.53, 95% CI: 1.27 to 1.84, P < 0.001) subgroups, whereas a non-significant result was found for the stroke subgroup (RR: 1.11, 95% CI: 0.96 to 1.29, P = 0.16). Finally, we considered whether subgroup analysis reported a higher or lower CVD risk associated with OA, regardless of significance level. The results indicated that all subgroup estimates found that OA was associated with an increased risk of CVD. The results of the subgroup analyses are summarized in Table 2.
Table 2. Results for subgroup analyses.
| Subgroup | Included study | OA patients | CVD patients | Total participants | RR (95% CI) | P |
|---|---|---|---|---|---|---|
| Study design | ||||||
| Retrospective | Refs 4,9,12,14,16 | 61,779 | 15,662 | 284,358 | 1.15 (0.95–1.38) | 0.147 |
| Prospective | Refs 5, 6, 7, 8,10,11,13,15,17,18 | 19,132 | 13,551 | 74,586 | 1.30 (1.16–1.46) | <0.001 |
| OA site | ||||||
| Hand | Refs 4,6,8,11,18 | 6,587 | 3,256 | 15,728 | 1.03 (0.85–1.25) | 0.749 |
| Knee | Refs 6,11,17,18 | 1,593 | 2,106 | 7,796 | 1.30 (1.00–1.69) | 0.047 |
| Hip | Refs 6,7,18 | 1,374 | 3,956 | 13,968 | 1.23 (1.11–1.38) | <0.001 |
| OA confirmation | ||||||
| Radiographic | Refs 4, 5, 6, 7, 8,11,13,17 | 8,321 | 5,116 | 25,362 | 1.23 (1.02–1.48) | 0.028 |
| Clinical | Refs 5,6,11 | 579 | 1,309 | 6,037 | 1.47 (0.91–2.39) | 0.118 |
| Outcome type | ||||||
| IHD | Refs 4,5,9,10,14, 15, 16,18 | 71,207 | 8,480 | 177,253 | 1.33 (1.20–1.46) | <0.001 |
| Stroke | Refs 4,5,14, 15, 16,18 | 59,429 | 3,469 | 151,321 | 1.11 (0.96–1.29) | 0.16 |
| CHF | Refs 5,9,14,15,18 | 27,516 | 3,389 | 87,500 | 1.40 (1.13–1.73) | 0.002 |
| Cardiovascular death | Refs 7,8,11,13,17,18 | 5,143 | 2,978 | 16,109 | 1.53 (1.27–1.84) | <0.001 |
The impact of age and follow up time on the pooled result was explored by meta-regression analysis. As illustrated in Supplementary Figures S3, S4 and S5, there was no significant impact of age and follow up time on the pooled result.
Discussion
This is the most comprehensive systematic review and meta-analysis of published observational studies assessing the relationship between OA and CVD risk. The results of our meta-analysis demonstrate that OA is associated with a significantly increased risk of CVD. Furthermore, this association was robust across sensitivity analyses that accounted for the influence of each individual study.
The underlying mechanisms behind the observed association between OA and CVD risk remain unclear, but several factors may account for this relationship. First, the two diseases have some shared risk factors. Epidemiological studies have provided evidence for an association between OA and most of the traditional cardiovascular risk factors, including hypertension19,20, diabetes21, hypercholesterolemia22, and obesity23. Second, the most commonly prescribed drugs to relieve pain in OA patients are non-steroidal anti-inflammatory drugs (NSAIDs), and NSAIDs have been related to an increased risk of vascular events24. Third, OA patients are less physically active because of severe pain in the joints compared with the general population, particularly those with knee or hip OA. Physical inactivity is among the leading risk factors for CVD25. Finally, the most important pathological features of CVD include arterial thickening, stiffness, and atherosclerosis, which contribute to inadequate tissue perforation (ischemia). Ischemia of the bone decreases cartilage nutrition and induces multiple bone infarcts, which are characteristics of advanced OA26,27. This effect of ischemia of the bone is one potential explanation of the interrelationship between OA and CVD.
Potential limitations of this meta-analysis arise from the unavailability of individual participant data from the included studies. For instance, Jonsson et al.4 reported that the intake of polyunsaturated fatty acids was significantly more frequent in the OA group than in the control group, which potentially explained the inverse association between OA and cardiovascular events; Nielen et al.12 reported that the mean age of OA patients was significantly higher than that of controls, and thus it is likely that the OA patients with the highest risks had already died, resulting in an underrepresentation of the prevalence rate of CVD in OA patients. With more information, we would be able to analyze the associations between different types of OA and certain types of outcomes as well as better control confounding factors. To overcome this limitation, we performed subgroup analyses when possible. As a significant association was observed in most subgroups, the lack of individual participant data was not a serious limitation. The non-significant results observed in several subgroups can be partially ascribed to the 2 articles mentioned above, which did not control confounders well. Our meta-analysis was based on studies that varied in many ways (study design, population sample, adjustment for confounders, and different ascertainment methods for exposure and outcome), which may be considered another limitation. However, we adopted appropriate meta-analytic techniques with random-effect models, which enabled us to account for these differences.
The strengths of this study include the comprehensive and systematic review of the literature. Study selection, data extraction, and quality assessment were performed independently by two authors according to predesigned criteria to minimize bias and transcription errors. We included both prospective and retrospective studies that used large sample sizes, which increased the statistical power to detect potential associations. Compared to the previous meta-analysis on this topic28, we incorporated 3 times more articles (15 versus 5) and approximately 2 times more participants (358,944 versus 177,214) in the statistical analysis. Furthermore, the included studies had generally satisfactory designs, methods, and outcomes, and the study quality was medium to high. Finally, the consistency of the evidence overall supports a real association between OA and CVD risk.
Because OA is a very common health condition, an association between OA and CVD would be important from a public health perspective. In the general population, middle-aged people may consider screening for OA status as well as traditional cardiovascular risk factors to enable early intervention to reduce future CVD events. Patients with OA should pay more attenuation to their CVD risk. Among clinicians, cardiovascular risk must be taken into account when prescribing any non-steroidal anti-inflammatory drug for OA patients.
In conclusion, this meta-analysis provides strong evidence that OA is a significant risk factor for CVD. Given the high prevalence and incidence of OA and CVD in the general population, the observed relationship between OA and CVD has clinical and public health importance.
Methods
Search strategy
We carried out a meta-analysis of studies that evaluated the association between osteoarthritis and cardiovascular diseases in adults. We followed the quality of reporting of meta-analysis guidelines (the PRISMA statement) for performing and reporting the present meta-analysis (Supplementary Tables S1 and S2)29. Between March 2016 and May 2016, we searched Medline and EMBASE to retrieve relevant studies. The following search terms were used in different combinations: osteoarthritis, cardiovascular disease, coronary artery disease, coronary heart disease, ischemic heart disease, myocardial infarction, congestive heart failure, transient ischemic attack, and stroke. Further information was identified through a manual search of references from the extracted papers.
Study selection
The eligibility of studies was assessed through a three-step process. First, two independent reviewers performed an initial screening of all titles and abstracts according to the following criteria: (1) original research articles were included, and other types of articles, including reviews, editors, commentaries and meta-analyses, were excluded; (2) population-based association studies reporting the relationship between OA and CVD risk were included, and articles focused on other exposure or outcomes were excluded. The full texts of all potentially relevant articles were then reviewed, and studies were included if they met the following criteria: (1) predefined diagnosis criteria for both OA and CVDs; (2) reported risk ratio (RR), hazards ratio (HR), or odds ratio (OR) estimates and 95% CIs describing the relationship between OA and risk of CVDs; (3) inclusion of a non-exposed group in prospective studies or a control group in retrospective studies. Finally, discrepancies were resolved by consensus or consultation with a third reviewer.
Data extraction and quality assessment
We designed a standardized data collection form to extract information. Two reviewers independently performed the extraction of data. We adopted OR for retrospective studies and HR and RR for prospective studies as a measure of the association. The following characteristics were recorded in the data abstraction form: study name, authors, year of publication, residential region, type of study, source of the study population, OA and CVD definition, sample size, and adjusted confounding factors.
After the data extraction procedure, the two forms from the two reviewers were compared in a point-by-point manner, and the degree of agreement between the reviewers was assessed using kappa statistics. Any discrepancies were resolved by consensus with a third investigator while referring to the original article.
Finally, we assessed study quality according to the Newcastle–Ottawa quality assessment scale, which was recommended by the Cochrane guidelines. Details of the scoring system and the quality score of each study are listed in Supplementary Tables S3 and S4.
Statistical analysis
For each study, we extracted the estimate and 95% confidence interval for the association result. The ORs in retrospective studies were converted to RRs for meta-analysis (RR = OR/([1 − pRef] + [pRef * OR]), where pRef is the prevalence of CVD in the control group30. We then performed a non-parametric sign test of the extracted estimates with a null hypothesis of “no additional increased risk of CVD in OA patients”; we considered whether studies reported a higher or lower CVD risk associated with OA, regardless of significance level or magnitude of effect.
We calculated a pooled RR estimate across all studies using a random-effects model that assumes that individual studies estimate different association effects. The random-effects model was adopted because it is probably the most conservative analysis to account for variance within and between studies.
The between-study heterogeneity was assessed by means of the I2 statistic, calculated from the Q statistic31. We considered the result for heterogeneity significant at P < 0.10 (two-sided) for the Q statistic. I2 > 75% was considered a high level of heterogeneity.
Publication bias was assessed by visually examining the asymmetry of a funnel plot in which the log estimates were plotted against their standard errors. Furthermore, we also employed an Egger regression test in our analysis to calculate two-tailed P values for quantifying publication bias32. To test the robustness of our findings, we performed a multiple-step sensitivity analysis by important study quality components and by omitting each estimate one at a time.
To explore the influence of potential sources of heterogeneity on the pooled estimate, we carried out subgroup analyses by important study characteristics, including study design (prospective or retrospective), OA sites (hand, knee or hip), OA types (radiographic or clinical), and outcomes (ischemic heart disease, stroke, congestive heart failure, or cardiovascular death). The impact of mean age and follow up time on the pooled result was explored by meta-regression. Analyses were performed with Stata Version 12.0 (StataCorp LP, College Station, TX).
Additional Information
How to cite this article: Wang, H. et al. Osteoarthritis and the risk of cardiovascular disease: a meta-analysis of observational studies. Sci. Rep. 6, 39672; doi: 10.1038/srep39672 (2016).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
The English language in our text was edited for natural and correct English by American Journal Experts (http://www.aje.com/). Thanks for their efforts in improving the quality of our work.
Footnotes
Author Contributions All authors contributed to data collection and wrote the manuscript. XH and DL drafted the study protocol. HW, JB and DL extracted data. HW and JB performed the analyses. HW, JB and BH drafted the paper. All authors critically reviewed the paper. HW and DL had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. HW and DL are the guarantors of the paper.
References
- Mortality, G. B. D. & Causes of Death, C. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 385, 117–171, doi: 10.1016/S0140-6736(14)61682-2 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson V. L. & Hunter D. J. The epidemiology of osteoarthritis. Best practice & research. Clinical rheumatology 28, 5–15, doi: 10.1016/j.berh.2014.01.004 (2014). [DOI] [PubMed] [Google Scholar]
- Green G. A. Understanding NSAIDs: from aspirin to COX-2. Clinical cornerstone 3, 50–60 (2001). [DOI] [PubMed] [Google Scholar]
- Jonsson H. et al. Hand osteoarthritis in older women is associated with carotid and coronary atherosclerosis: the AGES Reykjavik study. Annals of the rheumatic diseases 68, 1696–1700, doi: 10.1136/ard.2008.096289 (2009). [DOI] [PubMed] [Google Scholar]
- Haugen I. K. et al. Hand osteoarthritis in relation to mortality and incidence of cardiovascular disease: data from the Framingham heart study. Annals of the rheumatic diseases 74, 74–81, doi: 10.1136/annrheumdis-2013-203789 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeven T. A. et al. Disability and not osteoarthritis predicts cardiovascular disease: a prospective population-based cohort study. Annals of the rheumatic diseases 74, 752–756, doi: 10.1136/annrheumdis-2013-204388 (2015). [DOI] [PubMed] [Google Scholar]
- Barbour K. E. et al. Hip Osteoarthritis and the Risk of All-Cause and Disease-Specific Mortality in Older Women: A Population-Based Cohort Study. Arthritis & rheumatology 67, 1798–1805, doi: 10.1002/art.39113 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haara M. M. et al. Osteoarthritis of finger joints in Finns aged 30 or over: prevalence, determinants, and association with mortality. Annals of the rheumatic diseases 62, 151–158 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadam U. T., Jordan K. & Croft P. R. Clinical comorbidity in patients with osteoarthritis: a case-control study of general practice consulters in England and Wales. Annals of the rheumatic diseases 63, 408–414 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto M. et al. Arthritis as a risk factor for incident coronary heart disease in elderly Japanese-American males - the Honolulu Heart Program. Bulletin of the NYU hospital for joint diseases 67, 230–235 (2009). [PubMed] [Google Scholar]
- Kluzek S. et al. Painful knee but not hand osteoarthritis is an independent predictor of mortality over 23 years follow-up of a population-based cohort of middle-aged women. Annals of the rheumatic diseases, doi: 10.1136/annrheumdis-2015-208056 (2015). [DOI] [PubMed] [Google Scholar]
- Nielen M. M. et al. Cardiovascular disease prevalence in patients with inflammatory arthritis, diabetes mellitus and osteoarthritis: a cross-sectional study in primary care. BMC musculoskeletal disorders 13, 150, doi: 10.1186/1471-2474-13-150 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuesch E. et al. All cause and disease specific mortality in patients with knee or hip osteoarthritis: population based cohort study. Bmj 342, d1165, doi: 10.1136/bmj.d1165 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong K. L., Wu B. J., Cheung B. M., Barter P. J. & Rye K. A. Arthritis: its prevalence, risk factors, and association with cardiovascular diseases in the United States, 1999 to 2008. Annals of epidemiology 23, 80–86, doi: 10.1016/j.annepidem.2012.11.008 (2013). [DOI] [PubMed] [Google Scholar]
- Rahman M. M., Kopec J. A., Anis A. H., Cibere J. & Goldsmith C. H. Risk of cardiovascular disease in patients with osteoarthritis: a prospective longitudinal study. Arthritis care & research 65, 1951–1958, doi: 10.1002/acr.22092 (2013). [DOI] [PubMed] [Google Scholar]
- Rahman M. M., Kopec J. A., Cibere J., Goldsmith C. H. & Anis A. H. The relationship between osteoarthritis and cardiovascular disease in a population health survey: a cross-sectional study. BMJ open 3, doi: 10.1136/bmjopen-2013-002624 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi M. et al. Do musculoskeletal degenerative diseases affect mortality and cause of death after 10 years in Japan? Journal of bone and mineral metabolism 29, 217–223, doi: 10.1007/s00774-010-0214-z (2011). [DOI] [PubMed] [Google Scholar]
- Veronese N. et al. Association of Osteoarthritis With Increased Risk of Cardiovascular Diseases in the Elderly: Findings From the Progetto Veneto Anziano Study Cohort. Arthritis & rheumatology 68, 1136–1144, doi: 10.1002/art.39564 (2016). [DOI] [PubMed] [Google Scholar]
- Puenpatom R. A. & Victor T. W. Increased prevalence of metabolic syndrome in individuals with osteoarthritis: an analysis of NHANES III data. Postgraduate medicine 121, 9–20, doi: 10.3810/pgm.2009.11.2073 (2009). [DOI] [PubMed] [Google Scholar]
- Conaghan P. G., Vanharanta H. & Dieppe P. A. Is progressive osteoarthritis an atheromatous vascular disease? Annals of the rheumatic diseases 64, 1539–1541, doi: 10.1136/ard.2005.039263 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louati K., Vidal C., Berenbaum F. & Sellam J. Association between diabetes mellitus and osteoarthritis: systematic literature review and meta-analysis. RMD open 1, e000077, doi: 10.1136/rmdopen-2015-000077 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart D. J., Doyle D. V. & Spector T. D. Association between metabolic factors and knee osteoarthritis in women: the Chingford Study. The Journal of rheumatology 22, 1118–1123 (1995). [PubMed] [Google Scholar]
- Yusuf E. et al. Association between weight or body mass index and hand osteoarthritis: a systematic review. Annals of the rheumatic diseases 69, 761–765, doi: 10.1136/ard.2008.106930 (2010). [DOI] [PubMed] [Google Scholar]
- Trelle S. et al. Cardiovascular safety of non-steroidal anti-inflammatory drugs: network meta-analysis. Bmj 342, c7086, doi: 10.1136/bmj.c7086 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignarro L. J., Balestrieri M. L. & Napoli C. Nutrition, physical activity, and cardiovascular disease: an update. Cardiovascular research 73, 326–340, doi: 10.1016/j.cardiores.2006.06.030 (2007). [DOI] [PubMed] [Google Scholar]
- Cheras P. A., Freemont A. J. & Sikorski J. M. Intraosseous thrombosis in ischemic necrosis of bone and osteoarthritis. Osteoarthritis and cartilage/OARS, Osteoarthritis Research Society 1, 219–232 (1993). [DOI] [PubMed] [Google Scholar]
- Bullough P. G. & DiCarlo E. F. Subchondral avascular necrosis: a common cause of arthritis. Annals of the rheumatic diseases 49, 412–420 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A. J., Stubbs B., Mamas M. A., Myint P. K. & Smith T. O. Association between osteoarthritis and cardiovascular disease: Systematic review and meta-analysis. European journal of preventive cardiology 23, 938–946, doi: 10.1177/2047487315610663 (2016). [DOI] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D. G. & Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS medicine 6, e1000097, doi: 10.1371/journal.pmed.1000097 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J. & Yu K. F. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. Jama 280, 1690–1691 (1998). [DOI] [PubMed] [Google Scholar]
- Higgins J. P. & Thompson S. G. Quantifying heterogeneity in a meta-analysis. Statistics in medicine 21, 1539–1558, doi: 10.1002/sim.1186 (2002). [DOI] [PubMed] [Google Scholar]
- Egger M., Davey Smith G., Schneider M. & Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj 315, 629–634 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.