Abstract
The vitally important skin barrier is formed by extensive cross-linking activity of transglutaminases (TGs) during terminal epidermal differentiation. We have previously shown that epidermal deficiency of a disintegrin and metalloproteinase 17 (ADAM17), the principal EGFR ligand sheddase, results in postnatal skin barrier defects in mice due to impeded TG activity. However, the mechanism by which ADAM17/EGFR signalling maintains TG activity during epidermal differentiation remains elusive. Here we demonstrate that ADAM17-dependent EGFR signalling promotes TG activity in keratinocytes committed to terminal differentiation by direct induction of TG1 expression. Restored TG1 expression of EGF-stimulated differentiated Adam17−/− keratinocytes was strongly repressed by inhibitors for PLCγ1 or protein kinase C (PKC) pathways, while treatment with the PKC stimulator 12-O-tetradecanoylphorbol-13-acetate restored TG activity in the epidermis of keratinocyte-specific Adam17−/− (AD17ΔKC) mice. Further investigations emphasized the expression of PKCη, a mediator of TGM1 transcription, to be sensitive to EGFR activation. In agreement, topical skin application of cholesterol sulfate, an activator of PKCη, significantly improved TG activity in epidermis of AD17ΔKC mice. Our results suggest ADAM17/EGFR-driven PLCγ1 and PKC pathways as important promoters of TG1 expression during terminal keratinocyte differentiation. These findings may help to identify new therapeutic targets for inflammatory skin diseases related to epidermal barrier defects.
The multilayered epidermis builds up a barrier that protects the body against transepidermal water loss, foreign substances and microbial invasion1. This skin barrier is very important for the epidermal homeostasis and needs to be continuously renewed and enzymatically modified2. After the basal keratinocytes detach from the underlying basement membrane, they stop to proliferate and become committed to terminal differentiation. During their passage to the skin surface the cells convert into a cornified envelope (CE) that forms the skin barrier. The CE represents an insoluble protein structure that is stabilized by the cross-linking activity of three epidermal transglutaminases (TGs), namely TG1, TG3 and TG53. However, lack of either TG3 or TG5 activity only leads to minor alterations in CE formation and barrier stability4,5, while lack of TG1 activity causes severe skin barrier defects. The crucial role of TG1 during CE formation is demonstrated in patients with nonsense or missense mutations in the TGM1 gene, that led to impaired skin barrier formation, transepidermal water loss and skin inflammation in autosomal recessive lamellar ichthyosis or congenital ichthyosiform erythroderma3,6. However the regulatory mechanisms that control TG activity during skin barrier maintenance remain elusive.
In the epidermis, epidermal growth factor receptor (EGFR) is expressed abundantly in the proliferative basal layer and to a lesser degree in the differentiating suprabasal layers7. It is thought that EGFR signalling in basal keratinocytes mainly supports proliferation and survival but prevents differentiation. Moreover it delays apoptosis during early differentiation in suprabasal keratinocytes that have lost their interaction with the matrix8,9. However, EGFR-ligands are also abundant in differentiated epidermis and there are several evidences that EGFR signalling contributes to terminal keratinocyte differentiation and skin barrier formation10,11,12,13. EGFR deficiency causes defects in hair follicle development and immature epidermal differentiation with inflammatory skin reactions in both mice and humans14,15,16,17. In addition, EGFR inhibitor therapy in cancer patients commonly induces dermatologic side effects including xerotic itchy skin18. Although these data corroborate the relevance of EGFR signalling in skin homeostasis, only little is known about the role of EGFR signalling in skin barrier formation and in suppressing chronic skin inflammation.
ADAM17 (a disintegrin and metalloproteinase 17) or tumor necrosis factor α-converting enzyme (TACE) is a membrane-anchored metalloproteinase that was originally identified to cleave membrane-bound tumor necrosis factor (TNF)-α by a process named as ectodomain shedding19. This protease is also known as crucial upstream regulator of EGFR signalling by shedding of the majority of EGFR ligands20. Mice lacking ADAM17 die at birth due to defects in heart development and show epithelial abnormalities in several organs, such as intestine and skin21. Thereby, Adam17−/− mice nearly phenocopy mice lacking EGFR, or mice lacking the EGFR ligands TGF-α, HB-EGF, or amphiregulin (AREG), indicating an in vivo relevance of ADAM17 in EGFR signalling22.
To investigate the role of ADAM17 and EGFR in skin homeostasis, mice with a conditional keratinocyte-specific deletion were generated. Adam17ΔKC (AD17ΔKC) mice phenocopy EgfrΔKC mice in having an intact skin barrier at birth, but developing a pronounced defect in the skin barrier after the third postnatal week leading to more than 80% lethality. The surviving animals develop chronic dermatitis as adults13,16,23. During the last five years several patients with germline loss of function mutations in ADAM17 or EGFR have been described17,24,25, which developed chronic dermatitis with iterated skin infections, very similar to the phenotype of AD17ΔKC mice, which suggests similar skin barrier defects13,23. Our investigations on AD17ΔKC and EgfrΔKC mice revealed that ADAM17/EGFR axis sustained the CE formation and postnatal skin barrier stability by tightly regulation of the expression and proteolytic processing of several CE components, especially by supporting TG activity13,26. Accordingly, application of EGFR ligand TGF-α to AD17ΔKC mouse skin restored epidermal barrier integrity by stimulating skin TG activity13. However, the ADAM17-driven mechanisms that maintain epidermal TG activity during terminal differentiation are not well understood.
Here we demonstrate that ADAM17-dependent EGFR signalling directly induces TG1 expression in keratinocytes committed to terminal differentiation prominently through phospholipase C γ1 (PLCγ1) and protein kinase C (PKC) pathways. Further investigations identified PKCη expression to be responsive to EGFR activation. In agreement, topical skin application of cholesterol sulfate, an activator of PKCη, significantly improved TG activity in epidermis of AD17ΔKC mice. These findings will help to uncover novel therapeutic strategies for inflammatory skin diseases related to disrupted EGFR signalling.
Results
ADAM17-driven EGFR signalling induces expression and activity of TG1 in terminal differentiating keratinocytes
The TG activity in terminal differentiating keratinocyte is composed of activities derived from TG1, TG3 and TG52. We previously demonstrated that lack of Adam17 in differentiated murine keratinocytes leads to significantly reduced TG activity and decreased expression of TG1 and TG313 as well as reduced Tgm5 transcription (data not shown). These findings suggest that ADAM17 controls TG activity by modulation of all epithelial TGs. However, lack of either TG3 or TG5 activity causes, if at all, only minor barrier defects4,5, while lack or strongly reduced TG1 activity leads to severe barrier defects3,6 as seen in AD17ΔKC mice. Therefore, we focused our investigations on TG1 expression and activity.
For the determination of TG1 activity we analyzed skin cryo sections or culture cells on coverslips in situ using the biotinylated amine donor substrate monodansylcadaverine in a neutral pH 7.4 buffer system, in which TG3 and TG5 are not active5,27. It has been previously demonstrated that this in situ fluorescence technique can discriminate among the activities of TG1 and TG3/TG5 when performed either at neutral pH 7.4 or at basic pH 8.4, respectively. This based on the fact that the catalytic optimum of TG1 lies between pH 7.4 and pH 8.4, while the catalytic optimum for TG3 and TG5 lies at basic pH 8.427,28. To investigate how the ADAM17-dependent EGFR signalling regulates TG activity in keratinocytes committed to differentiation, we used suspension cultures on polyhydroxyethylmethacrylate (polyHEMA)-coated plates10. This system mimicks terminal differentiation of suprabasal epidermal keratinocytes, since it disrupts the cell-extracellular matrix interaction, while maintaining cell-cell interactions. Time-resolved analysis of the TG activity in polyHEMA cultured wild type keratinocytes revealed strong activity after 6 h, which was retained for at least 48 h (Supplementary Fig. S1a). This correlated with a strong increase in transcription of the genes encoding for TG1 or involucrin (Supplementary Fig. S1b). Since permanent cell cycle exit and commitment to terminal differentiation of keratinocytes occurred within the first 24 h of suspension culture29, we have used 24 h suspension culture in our following experiments.
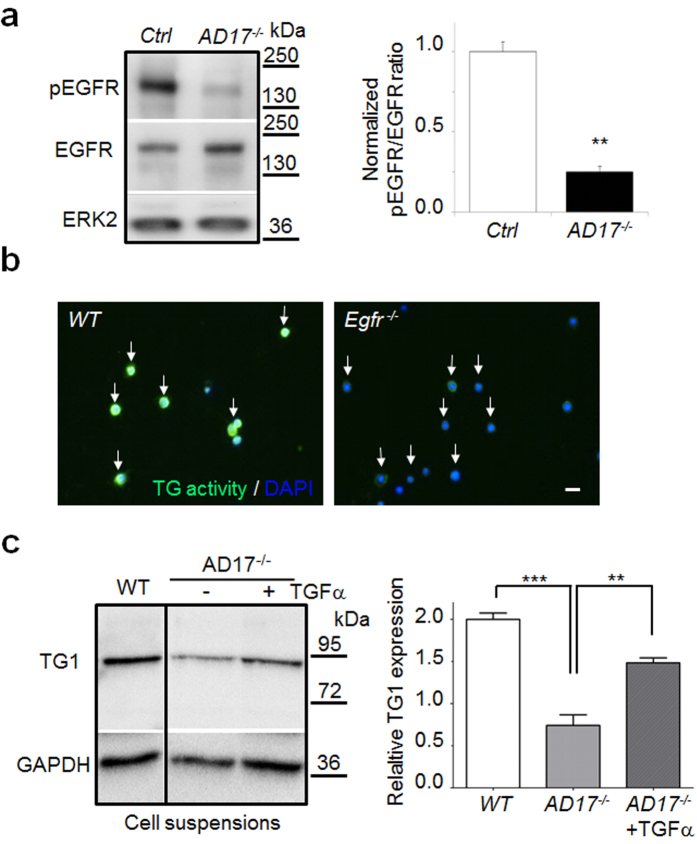
Analysis of EGFR signalling in Adam17−/− keratinocytes by Western blot (WB) revealed about 80% reduced EGFR activation (Fig. 1a). To validate whether the reduced TG activity is caused by lack of EGFR signalling, we analyzed 24 h suspension cultures of Egfr−/− keratinocytes. Indeed, Egfr−/− keratinocytes showed strongly reduced TG activity (Fig. 1b). In addition, the reduced TG1 expression in ECM-disrupted Adam17−/− keratinocytes was nearly restored by supplementation with the EGFR-ligand TGF-α (40 ng/ml) on protein (Fig. 1c) and on RNA level (Supplementary Fig. S2), suggesting that the lack of EGFR activation is the major cause of reduced TG1 expression in Adam17−/− keratinocytes. Since reduced EGFR signalling in the suprabasal Adam17−/− keratinocytes can lead to decreased survival signals and apoptosis30, we analyzed skin sections of 10-day-old AD17ΔKC mice with skin barrier defects and their littermates by TUNEL labelling. No difference in the proportion of TUNEL-positive keratinocytes was detectable (Supplementary Fig. S3), indicating no increase of apoptosis in the epidermis of AD17ΔKC mice. Moreover, supplementation of the EGFR-ligands EGF, TGF-α, and Epiregulin (40 ng/ml) to 24 h Adam17−/− keratinocyte suspension cultures resulted in very similar recovery of TG activity (Supplementary Fig. S4), suggesting coinciding functions among these EGFR-ligands. These results suggest that EGFR signalling accelerates TG activity in differentiating keratinocytes via transcription.
Figure 1. Transglutaminase activity in differentiating mouse keratinocytes is regulated by EGFR signalling.
(a) WB of primary keratinocyte lysates derived from AD17∆KC mice or control littermates (Ctrl) revealed significantly reduced level of activated EGFR (normalized ratio of pEGFR/total EGFR), while ERK 2 protein as control was equal. Graph shows band intensities as mean ± SD, n = 3, **p < 0.01. (b) Suspension cultured Egfr−/− keratinocytes analyzed for TG activity at pH 7.4 by fluorescence microscopy revealed strongly reduced signals (white arrows, scale bar, 20 μm) (representative staining from three independent experiments). (c) WB analysis of mouse AD17−/− keratinocytes with or without stimulation with 40 ng/ml TGF-α after 24 h suspension culture with antibodies directed against TG1. (n = 3 per group). Data as mean ± SEM, **p < 0.01, ***p < 0.001.
PKC regulates TG1 expression downstream of EGFR
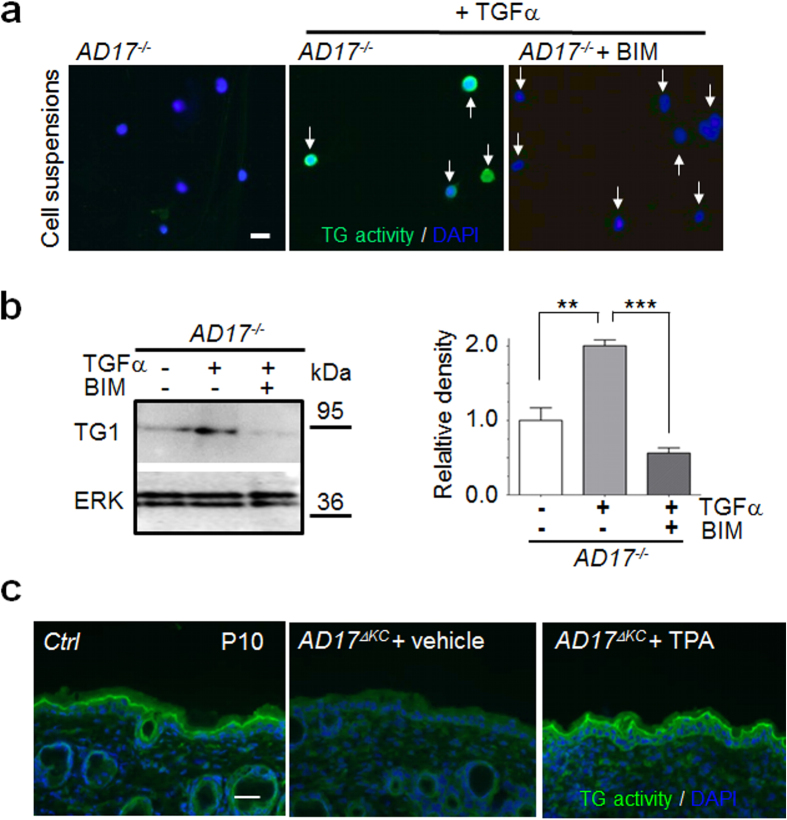
The terminal differentiation of keratinocytes is partly regulated by PKC31. These kinases are thought to play an important role in the modulation of transglutaminase activity32,33,34. However, only little is known about the interactions of EGFR and PKC pathways in keratinocytes committed to terminal differentiation. To analyse the interactions of both pathways, we used TGFα-stimulated differentiated Adam17−/− keratinocytes as a model for EGFR-dependent TG activity. Addition of the general PKC inhibitor bisindolylmaleimide (1 μM) led to strongly reduced TG activity and TG1 expression in TGFα-stimulated differentiated Adam17−/− keratinocytes (Fig. 2a,b), suggesting a connection of both pathways in the regulation of TG expression and that PKCs are localized downstream of ADAM17/EGFR signalling. These results were confirmed by stimulation experiments with the phorbol ester 12-O-tetradecanoylphorbol-13-acetate (TPA), a strong PKC activator. Stimulation of 24 h suspension cultures with 1 μM TPA completely restored the TG activity in differentiating Adam17−/− keratinocytes (Supplementary Fig. S5a). WB analysis revealed significantly increased TG1 expression in these cells (Supplementary Fig. S5b), suggesting a transcriptional regulation. To further validate the in vivo relevance of our results, we used 10 days old keratinocyte-specific Adam17−/− mice (A17ΔKC) with strongly reduced epidermal TG activity13 and topically applied a single dose of 1.5 μg TPA in acetone or acetone vehicle alone (controls) on their back skin. TPA treatment for 6 h resulted in strongly improved TG activity in the epidermis (Fig. 2c). In contrast, TPA treatment for 1 h did not led to improved TG activity (data not shown). In summary, our results demonstrate that the ADAM17/EGFR axis regulates TG1 expression during terminal keratinocyte differentiation via PKCs.
Figure 2. Protein kinase C (PKC) in murine keratinocytes regulates TG1 expression & activity downstream of EGFR in vitro and in vivo.
(a,b) Mouse wild type and Adam17−/− keratinocytes were cultured in suspension for 24 h. Adam17−/− keratinocytes were either treated with 40 ng/ml TGF-α or 40 ng/ml TGF-α and 1 μM bisindolylmaleimide (BIM) and then analyzed by (a) in situ TG activity detection at pH 7.4 by fluorescence microscopy or (b) WB with antibodies against TG1 and ERK as control. The improved TG1 expression and activity of TGF-α stimulated Adam17−/− keratinocytes was strongly inhibited by 1 μM bisindolylmaleimide. Scale bar, 20 μm. Graph shows band intensities as mean ± SEM, n = 3, **p < 0.01, ***p < 0.001. (c) The phorbol ester TPA was topically applied on the shaved skin surface of 10 days old AD17ΔKC mice (P10) and the skin was analyzed for in situ TG activity at pH 7.4 after 6 h. Scale bar, 50 μm. TPA treatment resulted in strongly improved TG activity (representative staining from three independent experiments).
ADAM17/EGFR axis induces TG activity mainly through PLCγ1 and PKC pathways
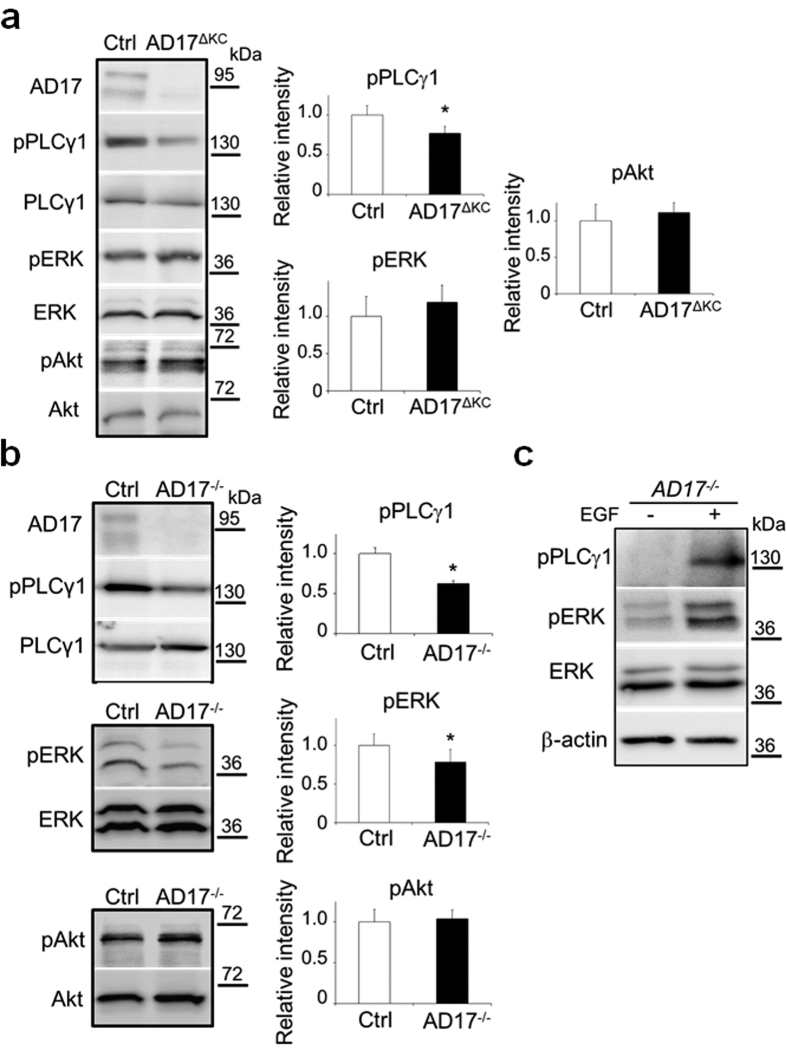
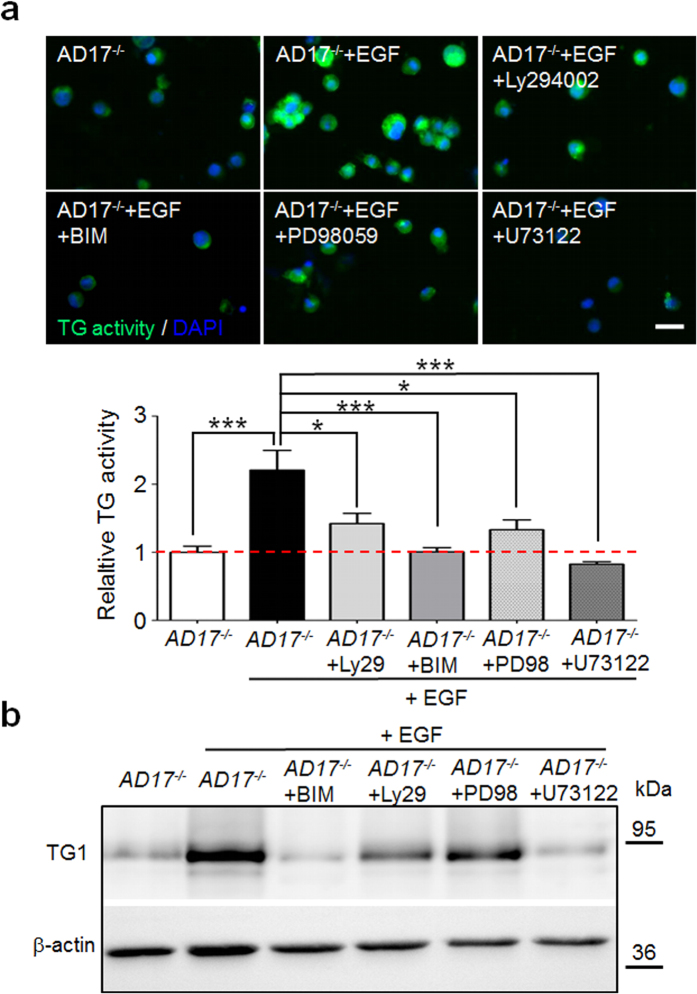
To identify the pathways by which the ADAM17/EGFR axis regulates TG activity in keratinocytes during terminal differentiation we have analyzed the MAPK, PI3K/Akt and PLC/PKC signalling as well known EGFR downstream pathways9,35. WB analysis of lysates of epidermis splits derived from 10 day old AD17ΔKC mice with strongly decreased TG activity13 revealed significant reduced activation of the PLCγ1 pathway, but no changes in ERK1/2 and Akt activation (Fig. 3a). The reduction in PLCγ1 signalling was verified as a cell-autonomous mechanism, since it was also detected in differentiation-committed Adam17−/− keratinocytes (Fig. 3b). In addition, we also saw reduced ERK1/2 signalling in these cells (Fig. 3b), suggesting that the ERK pathway might be involved as well. The PLCγ1 and the ERK1/2 signalling was strongly induced by EGF stimulation of 24 h Adam17−/− keratinocyte suspension cultures, validating the responsiveness of both pathways in differentiation-committed keratinocytes (Fig. 3c). Using EGF-stimulated differentiated Adam17−/− keratinocytes as a model for EGFR dependent TG activity, the addition of either the PLC inhibitor U73122 or the general PKC inhibitors bisindolylmaleimide or Gö6983 (data not shown) strongly repressed TG activity. However, the addition of the selective PI3K inhibitor Ly294002 or the selective MEK1 and MEK2 inhibitor PD98059 led to partial inhibition of TG activity in these cells (Fig. 4a). Further analysis of TG1 expression of those cells by WB revealed very similar results (Fig. 4b). In conclusion, these results suggest that especially the PLCγ1 and PKC pathways are involved in the regulation of TG activity in differentiation-committed keratinocytes.
Figure 3. The PLCγ1 pathway is downregulated in murine Adam17−/− keratinocytes.
(a,b) Representative WBs of (a) epidermal splits derived from P10 AD17∆KC or wild type mice or (b) 24 h suspension cultured wild type and Adam17−/− keratinocytes for activation of PLCγ1, ERK and Akt pathways. The graphs on the right show the mean ± SD of the relative intensities of pPLCγ1/PLCγ1, pERK/ERK and pAkt/Akt (n = 3), *p < 0.05. The activation of the PLCγ1 pathway was significantly reduced in AD17∆KC epidermis as well as Adam17−/− keratinocytes. (c) After suspension culture for 24 h, Adam17−/− keratinocytes were treated with 40 ng/ml EGF or vehicle for additional 30 min and analyzed by WB for activation of PLCγ1 and ERK. Both, pPLCγ1 and pERK were strongly increased by EGF stimulation (representative blots of three independent experiments).
Figure 4. EGFR induced TG activity in murine Adam17−/− keratinocytes is mainly driven by PLCγ1 and PKC signalling.
(a) Murine Adam17−/− keratinocytes were cultured in suspension on poly-HEMA for 24 h in the presence of 40 ng/ml EGF and either DMSO (vehicle control), 50 μM Ly294002, 1 μM bisindolylmaleimide (BIM), 50 μM PD98059, or 5 μM U73122. Afterwards, the keratinocytes were analyzed for TG activity at pH 7.4 by fluorescence microscopy. The addition of either the PLC inhibitor U73122 or the general PKC inhibitor bisindolylmaleimide completely decreased EGF-induced TG activity in Adam17−/− keratinocytes, while addition of the selective PI3K inhibitor Ly294002 or the selective MEK1 and MEK2 inhibitor PD98059 caused partially inhibition. (n = 4). Scale bar, 20 μm. Graph shows quantitative analysis of fluorescence intensities: mean ± SEM, *p < 0.05, ***p < 0.001. (b) WB analysis of cell lysates from the in (a) treated Adam17−/− keratinocytes with antibodies against TG1. β-actin was used as loading control (representative blots of two experiments).
Expression of PKCη, but not PKCδ is responsive to EGFR/PLCγ1 signalling
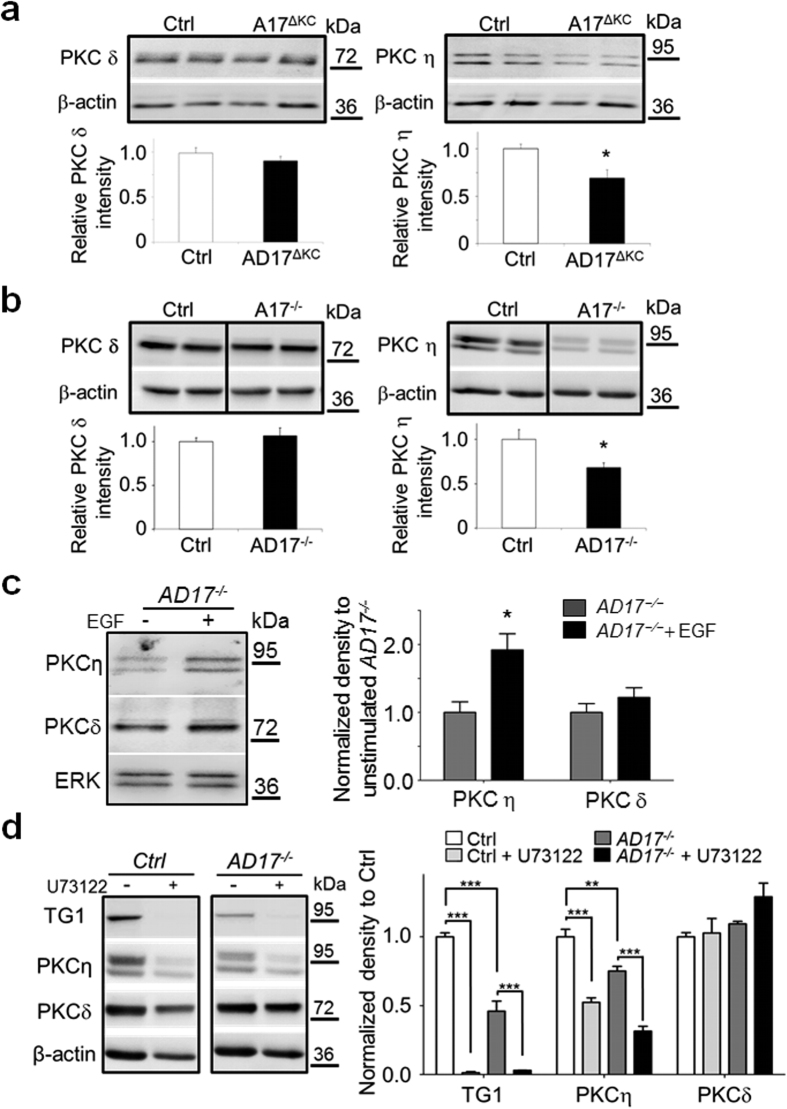
The expression of three protein kinase C members, namely PKCα, PKCδ and PKCη, has been linked to epidermal terminal differentiation36,37. However, only PKCδ and PKCη seem to be involved in the induction of TG1 activity and expression37. To further understand which of the epidermal PKC isoforms are directly affected by absence of ADAM17/EGFR signalling, we investigated their expression in AD17ΔKC versus wild type mice by WB analysis. Interestingly, we found significantly reduced expression of PKCη in Adam17−/− epidermal splits as well as in ECM-disrupted differentiated Adam17−/− keratinocytes. In contrast, no changes were observed for PKCδ (Fig. 5a,b) or PKCα (data not shown). The reduced PKCη expression in differentiation-committed Adam17−/− keratinocytes was significantly induced by stimulation with EGF, while PKCδ expression was not affected (Fig. 5c). This result was also seen in wild type keratinocytes (Supplementary Fig. S6), indicating that PKCη expression is modulated by EGFR signalling. The linkage of PLCγ1 signalling and PKCη expression was assessed by treatment of wild type or Adam17−/− keratinocyte suspensions with the PLC inhibitor U73122. Supplementation of 5 μM U73122 led to dramatically decreased protein expression of PKCη and TG1 in wild type and Adam17−/− keratinocytes, while the expression PKCδ was not altered (Fig. 5d). To further confirm the role of PKCη in the modulation of TGs, wild type ECM-disrupted keratinocytes were cultivated for 24 h in the presents of selective inhibitors for either PKCδ or PKCη. The selective myristoylated pseudosubstrate inhibitor (Myr-TRKRQRAMRRRVHQING-OH) for PKCη caused a significant reduction in TG activity. In contrast, rottlerin, a selective PKCδ inhibitor, had no effect on TG activity (Supplementary Fig. S7). Taken together, these results suggest that EGFR/PLCγ1 signalling is involved in the induction of PKCη expression, which in turn leads to increased TG1 expression and activity.
Figure 5. Expression of PKCη but not PKCδ is enhanced by EGFR-PLCγ1 signalling in murine epidermis.
(a,b) WB analysis of (a) epidermal splits derived from P10 AD17∆KC or wild type mice or (b) lysates derived from 24 h suspension cultured mouse wild type and Adam17−/− keratinocytes with antibodies against PKC isoforms eta or delta, and β-actin as loading control. The graphs below show the mean ± SD of the signal intensities (n = 5), *p < 0.05; **p < 0.01. The expression of PKCη was significantly reduced in Adam17−/− keratinocytes in vivo and in vitro. (c) Adam17−/− keratinocytes were suspension-cultured on poly-HEMA with or without 40 ng/ml EGF for 24 h and further analyzed by WB for PKC isoforms eta or delta and ERK as loading control. Graph on the right shows quantitative analysis of band intensities as mean ± SEM, *p < 0.05. PKCη expression in Adam17−/− keratinocytes was significantly induced by stimulation with EGF, while PKCδ expression was unchanged. (d) Wild type or Adam17−/− keratinocytes were suspension-cultured for 24 h with or without addition of 5 μM U73122 and further analyzed by WB for TG1, PKC isoforms eta or delta. Antibodies against β-actin were used as loading control. Graph on the right shows quantitative analysis of band intensities normalized to controls (n = 3): mean ± SEM, **p < 0.01; ***p < 0.001. The supplementation of 5 μM U73122 led to dramatically decreased protein expression of PKCη and TG1 in wild type and Adam17−/− keratinocytes, while the expression PKCδ was not affected. Full-length blots of (b) and (d) are presented in Supplementary Fig. S8.
Cholesterol-sulfate supplementation restores TG activity in Adam17 −/− keratinocytes in vitro and in vivo
The membrane lipid cholesterol sulfate is thought to play an important role in the physiological induction of epidermal terminal differentiation and skin barrier formation. Cholesterol sulfate is constitutively produced in the suprabasal epidermal layers where it progressively accumulates during terminal differentiation. It has previously been shown in vitro that stimulation of adhesive keratinocytes with cholesterol sulfate leads to increased expression of terminal differentiation markers, including TG1. This effect is mediated via PKC activity, in which cholesterol sulfate acts as a more specific activator of novel PKCs, especially PKCη38,39,40.
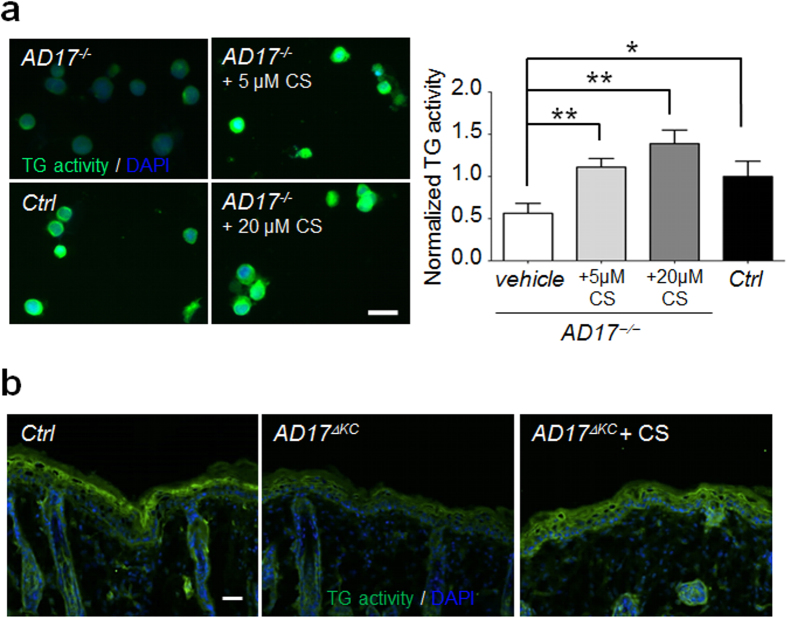
To investigate whether cholesterol sulfate can increase TG activity in Adam17−/− keratinocytes, we analyzed either the addition of 5 or 20 μM cholesterol-3-sulfate to suspension cultures. Stimulation of 24 h suspension cultures completely restored the TG activity in the Adam17−/− keratinocytes in a dose dependent manner (Fig. 6a). However, WB analysis revealed no increase in PKCη expression in these cells (data not shown), suggesting an induction in kinase activity. To further validate the in vivo relevance of our results, we treated 10 days old AD17ΔKC mice by a single topically application of 100 μg cholesterol-3-sulfate or acetone vehicle (controls) on their back skin. Cholesterol sulfate treatment for 6 h was sufficient to restore the TG activity in the epidermis of AD17ΔKC mice (Fig. 6b), indicating a therapeutic value of local cholesterol sulfate administration.
Figure 6. Cholesterol sulfate supplementation increased TG activity in murine keratinocytes in vivo and in vitro.
(a) Mouse Adam17−/− keratinocytes were cultured in suspension and stimulated with either 5 μM or 20 μM cholesterol-3-sultate for 24 h. Analysis of TG activity at pH 7.4 by fluorescence microscopy. TG activity of wild type keratinocytes is shown as control. n = 3. Scale bar, 20 μm. Graph shows fluorescence intensities as mean ± SEM, **p < 0.01, ***p < 0.001. Cholesterol sulfate stimulation resulted in a dose-dependent increase of TG activity in differentiating Adam17−/− keratinocytes. (b) Cholesterol sulfate (100 μg/20 μl) or 20 μl acetone-vehicle (control) was topically applied on the shaved skin surface of 10 days old AD17ΔKC mice (P10) and the skin was analyzed for in situ TG activity at pH 7.4 after 6 h. Untreated skin of wild type mice was used as positive control. Fluorescence microscopy analysis revealed strongly improved TG activity by cholesterol sulfate treatment (representative images of three independant experiments). Scale bar, 50 μm.
Reduced TG activity in ADAM17-deficient human skin
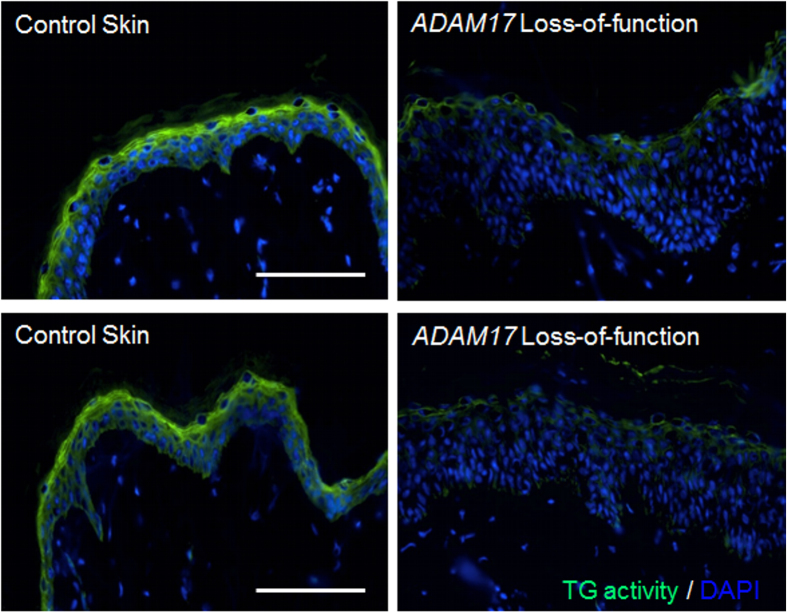
After the discovery of the first two ADAM17 deficient patients with inflammatory skin and bowel disease in 201124, additional pediatric patients with deficiencies in ADAM17 or EGFR were described17,25. All of these patients suffered of skin inflammation and iterated skin infections, very similar to the phenotype of keratinocyte-specific Adam17−/− mice, which suggests similar skin barrier defects13,23. However, the TG activity in the skin of these patients was not evaluated. Thus, we analyzed skin cryo sections derived from an ADAM17-deficient patient or healthy donors by in situ immunofluorescence microscopy using biotinylated monodansylcadaverine at neutral pH 7.4. As shown in Fig. 7, the TG activity in the stratum granulosum and stratum corneum of the epidermis was strongly reduced in ADAM17 deficient skin compared to control skin. Therefore, we conclude that the mechanisms of the loss of transglutaminase activity due to ADAM17-deficiency are very similar in human and mouse skin.
Figure 7. Lack of ADAM17 in humans is associated with decreased epidermal TG activity.
In situ detection of TG activity in skin sections at pH 7.4 derived from an ADAM17 deficient patient24 and healthy volunteers by fluorescence microscopy. The TG activity in the stratum granulosum and stratum corneum of the epidermis was strongly reduced in ADAM17 deficient skin compared to control skin (representative images of three independant experiments). Scale bars, 100 μm.
Discussion
Using epidermis-specific conditional Adam17 or Egfr knockout mice, we have previously shown that lack of ADAM17-dependent EGFR ligand shedding in keratinocytes leads to postnatal skin barrier defects due to decreased TG activity13,26. Here, we provide new insights into the downstream mechanisms and demonstrate that ADAM17-driven EGFR signalling directly promotes TG1 expression and activity in keratinocytes committed to differentiation mainly via PLCγ1 and PKC pathways. At first glance our results seem to be in direct conflict with previously published data, which showed EGFR-dependent repression of TGM1 transcription in keratinocytes due to induced production of the transcription factor homeobox protein A7 (HOXA7)41. However, these results derived from adherent, proliferative cells that strongly express HOXA7, while its expression is lost in terminal differentiated keratinocytes due to enhanced PKC signalling41. Thus, our results emphasize the difference in the biological effect of EGFR signalling in delaminated terminal differentiating keratinocytes, where it promotes terminal differentiation via induction of TG1 expression and activity. In agreement, a constitutive increase of ADAM17-driven EGFR signalling was observed in tylosis (TOC) keratinocytes42. TOC is an autosomal dominant syndrome with focal palmoplantar keratoderma and esophageal cancer due to autosomal dominant iRHOM2 mutations43. TOC skin also shows epidermal hyperproliferation and hyperkeratosis with elevated epidermal TG activity42.
EGFR signalling in basal membrane anchored keratinocytes promotes proliferation and survival via the ERK and Akt pathways, but not via PLCγ19. In contrast, PLCγ1 signalling is proposed to lead to terminal keratinocyte differentiation through activation of PKCs44,45. Our data provides the novel finding that the ADAM17/EGFR axis in terminal differentiating keratinocytes mainly promotes PLCγ1 signalling. Although PLCγ1 activation by direct membrane recruitment to EGFR has been shown in several cell systems46,47, no such activation has been demonstrated so far in keratinocytes48. The increased cholesterol production and membrane integration during terminal keratinocyte differentiation seems to have an important impact on the morphological cell changes and the lipid raft-mediated signalling49. Therefore it is very likely that this increase in lipid microdomains leads to enriched EGFR cell surface localization in differentiation-committed keratinocytes, which in turn facilitates cell surface recruitment of PLCγ1 and EGFR/PLCγ1 signalling50. Upon membrane recruitment and activation, PLCγ1 catalyses the hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2) to inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG), which both act as second messengers in the activation of PKCs in terminal differentiation44. Thus, our data suggest that EGFR signalling stabilizes terminal differentiation by maintenance of PKC activity in differentiation-committed keratinocytes via PLCγ1 activation.
In vitro studies revealed that lack of EGFR/ERK signalling in suspension cultured keratinocytes for more than 24 h can lead to apoptosis due to the loss of basement membrane interactions30, which could be the reason for decreased activity and expression of TG1 in Adam17−/− keratinocytes. Although we used a different suspension culture system than the authors and cultured the cells for a maximum of 24 h under EGF supplementation, we saw reduced ERK activation in Adam17−/− keratinocytes. However, we neither detected decreased ERK signalling nor increased apoptosis as determined by TUNEL labelling in the epidermis of AD17ΔKC mice, which questions the in vivo relevance of the above findings. Furthermore, it has been demonstrated that PKCs can be involved in the activation of ERK signalling51,52. Thus, reduced PKC signalling might cause decreased ERK activation in Adam17−/− keratinocytes.
Our results suggest that ADAM17-driven EGFR signalling in differentiated keratinocytes acts in two different ways to induce PKC signalling and promote TG1 activity. Firstly, it activates PLCγ1 that produces second messengers for the activation of keratinocyte-derived PKC isoforms, namely the classical calcium- and diacylglycerol-dependent PKCα and the novel calcium-independent but diacylglycerol-dependent PKCδ, PKCε and PKCη31. Secondly, it selectively induces the expression of PKCη. This result is of particular interest, since only little is known about the transcriptional control of the novel PKCs during terminal differentiation. The induction of PKCη expression is probably driven via EGFR/PLCγ1 signalling, since PKCη expression was highly sensitive to PLCγ1 inhibition. However, it can not be ruled out that other signalling pathways are involved as well. PKCη is strongly linked to terminal keratinocyte differentiation, since it is selectively expressed in suprabasal epidermal layers and its viral overexpression in adherent human keratinocytes caused growth inhibition and induction of TG1 expression and activity37,39,53. However, PKCη activity seems not to be crucial for epidermal differentiation in normal skin and is most likely replaceable by other PKC isoforms, since PKCη null mice showed no defects in skin architecture and development54.
Taken together, our data suggest ADAM17/EGFR-driven PLCγ1 and PKC pathways as important promoters of TG1 expression during terminal keratinocyte differentiation and skin barrier formation. It further provides evidence that topical skin application of TPA or cholesterol sulfate can restore the TG activity in the epidermis of AD17ΔKC mice, which should have a beneficial effect on the skin barrier function. In contrast to the tumor-promoting phorbol ester TPA, that activates almost all PKCs, cholesterol sulfate is a more specific activator of novel PKCs, especially PKCη, and rather acts as an anticarcinogenic component38,39,40. Thus, cholesterol sulfate seems to have therapeutic value for the local treatment of disrupted EGFR signalling caused skin barrier defects in atopic dermatitis55 or EGFR inhibitor-treated cancer patients18.
Methods
Material
The PI3K inhibitor Ly294002 was purchased from Merck Millipore (Germany) and the ERK1/2 inhibitor PD98059 derived from Cell signalling technology (Germany). The general PKC inhibitors Bisindolylmaleimide and Gö6983 were obtained from Sigma-Aldrich (Germany) and Merck Millipore (Germany), respectively. Rottlerin, a selective inhibitor of PKCδ was obtained from Sigma-Aldrich (Germany) and the PKCη pseudosubstrate inhibitor, myristoylated (Myr-TRKRQRAMRRRVHQING-OH) was bought from Merck Millipore. Recombinant murine EGF, human TGF-α and human Epiregulin were obtained from Peprotech (Germany).
Animals
The generation of Adam17flox/flox Krt14-Cre (AD17ΔKC) and Egfrflox/flox Krt14-Cre (EgfrΔKC) mice has been described previously13. All mice were of mixed genetic background (129 Sv, C57BL/6), and all comparisons were between littermates. In experiments, mice received a single 100 μl application of either 1.5 μg 12-O-tetradecanoylphorbol-13-acetate (TPA, Sigma-Aldrich) or 100 μg cholesterol 3-sulfate (Sigma-Aldrich) in acetone or acetone vehicle on the shaved back skin. The mice were maintained in the Center for Experimental Models and Transgenic Service (CEMT) of the Medical Center University of Freiburg, and all experiments were performed according to the guidelines of the German Animal Welfare association and approved by the Regierungspräsidium Freiburg (G-11/98 and G-15/145).
Primary keratinocyte preparation and cultivation
Keratinocytes were isolated from the skin of neonatal AD17ΔKC mice and their wild type littermates as described previously56. The primary cells were cultured in defined serum-free keratinocyte growth medium (CellnTec, Switzerland) supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin (Invitrogene). The cells were maintained at 37 °C, 5% CO2 and 95% humidity. Keratinocytes derived from passages 1–2 were used for the experiments.
Keratinocyte suspension culture
Suspension culture on poly (2-hydroxyethyl methacrylate (HEMA))–coated plates was performed as previously described10. 6-well or 12-well plates were coated with either 10 mg or 4 mg poly-HEMA (Sigma-Aldrich) respectively, followed by extensive PBS washes. 1 × 106 or 0,5 × 106 cells in keratinocyte growth medium (CellnTec, Switzerland) were added to each coated 6-well or 12-well respectively and incubated in a humidified incubator with 5% CO2 in air at 37 °C for 24 h. EGFR stimulations were performed with either 30 ng/ml EGF, TGFα or Epiregulin. In some experiments, the cell suspensions were supplied with 500 nM GÖ 6983, 1 μM bisindolylmaleimide, 50 μM Ly294002, 50 μM PD98059, or 5 μM U73122 for 24 h. Cell suspensions were harvested by centrifugation and either used for preparation of cell lysates or detection of TG activity. For in vitro TG activity detection, 24-h suspension-cultured keratinocytes were attached on gelatin-coated coverslips for 1 h and TG activity was determined as described below.
Quantitative RT-PCR analysis
Total RNA from suspension-cultured Adam17−/− and wild type keratinocytes was extracted using RNeasy kit (Qiagen). 1 μg of total RNA was reverse transcribed using a First Strand cDNA Synthesis kit (Fermentas). Relative quantification of gene expression was performed by real-time quantitative PCR using iQ SYBR-Green Supermix on the CFX96TM C1000TM Thermal Cycler (Bio-Rad, Germany) following the manufacturer’s protocols. The used primer sequences for Tgm1, Adam17, Egfr, Inv, Krt14 and Gapdh were described previously13. Relative expression was normalized for levels of Gapdh.
Transglutaminase activity assay
For in situ detection of TG activity in skin sections or cells, the biotinylated amine donor substrate monodansylcadaverine (MDC) was used as described earlier13. 5 μm thick cryostat sections were air dried and preincubated with 1% BSA in 0.1 M Tris-HCl, pH 7.4 for 30 min at room temperature. The sections were then incubated for 45 to 60 min with 100 μM MDC, 5 mM CaCl2, 0.1 M Tris-HCl, pH 7.4. After stopping the reaction with 10 mM EDTA and extensive PBS washing, the sections were stained with Streptavidin-conjugated Alexa Fluor 488 (Invitrogen) and DAPI-supplemented mounting medium.
Immunoblotting
For WB analysis dispase-separated epidermis splits or cells were homogenized in 50 mM Tris-HCl, pH 8.0, 0.15 M NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate supplemented with 2 mM EDTA, 5 mM 1,10- ortho-phenanthroline (Sigma-Aldrich) and protease inhibitor cocktail set III (Calbiochem) as described previously13. Total protein content was determined using the BCATM protein assay kit (Invitrogen). 30 μg of protein was separated and transferred either onto nitrocellulose or PVDF membranes. Protein detection on membranes was performed with subsequent primary antibodies: rabbit anti-PKCη, rabbit anti PKCδ, rabbit anti-transglutaminase 1 (TG1), (Santa Cruz Biotechnology), rabbit anti-PLCγ1, rabbit anti-phospho PLC-γ1, rabbit anti-EGFR (Cell Signaling), rabbit anti–phospho EGFR (pY1068, clone EP774Y; Epitomics Inc.), rabbit anti-ERK1/2, mouse anti-β-actin (Sigma-Aldrich) and mouse anti-GAPDH (Invitrogen). Visualization was performed with secondary horseradish peroxidase labeled goat anti-mouse IgG (Merck) or horseradish peroxidase labeled goat anti-rabbit IgG (KPL) and Amersham ECL prime western blotting detection reagent as described by the manufacturer (GE Healthcare).
TUNEL assay
The fluorometric DeadEnd™TUNEL System (Promega) was used to analyze apoptotisis in paraffin skin sections (5 μm) of AD17ΔKC mice and their wild type littermates according the manufacturer´s recommendations.
Patient material
Skin samples were obtained from a 17-year-old male ADAM17-deficient patient and healthy volunteers undergoing cosmetic surgery after informed consent and in adherence to the declaration of Helsinki principles. Clinical details of the ADAM17-deficient patient were described elsewhere24. The study was approved by the ethics committee of the Queen Mary University of London.
Statistics
The data are present as mean ± SEM. Data of two groups were analyzed for significance using the unpaired Student´s t test and differences are considered to be statistically significant at P < 0.05.
Additional Information
How to cite this article: Wolf, C. et al. ADAM17/EGFR axis promotes transglutaminase-dependent skin barrier formation through phospholipase C γ1 and protein kinase C pathways. Sci. Rep. 6, 39780; doi: 10.1038/srep39780 (2016).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
This work was supported by the German Research Foundation DFG (SFB 850/B6) and by the Fritz-Thyssen foundation (Az.10.14.2.150) to C.-W.F and the Medical Research Council (MR/L010402/1) to D.P.K. We thank M. Hartmann for technical assistance.
The authors declare no competing financial interests.
Author Contributions C.-W.F. conceived the study. Y.Q., C.W., M.B., D.K. and C.-W.F. performed experiments and/or analyzed data. C.W., Y.Q., D.K. and C.-W.F. wrote the manuscript. All authors have read and approved the final manuscript.
01/23/2017
A correction has been published and is appended to both the HTML and PDF versions of this paper. The error has been fixed in the paper.
References
- Proksch E., Brandner J. M. & Jensen J. M. The skin: an indispensable barrier. Exp Dermatol 17, 1063–72 (2008). [DOI] [PubMed] [Google Scholar]
- Eckhart L., Lippens S., Tschachler E. & Declercq W. Cell death by cornification. Biochim Biophys Acta 1833, 3471–80 (2013). [DOI] [PubMed] [Google Scholar]
- Candi E. Schmidt R. & Melino G. The cornified envelope: a model of cell death in the skin. Nat Rev Mol Cell Biol 6, 328–340 (2005). [DOI] [PubMed] [Google Scholar]
- John S. et al. Epidermal transglutaminase (TGase 3) is required for proper hair development, but not the formation of the epidermal barrier. PLoS One 7, e34252 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigors M. et al. TGM5 mutations impact epidermal differentiation in acral peeling skin syndrome. J Invest Dermatol 132, 2422–9 (2012). [DOI] [PubMed] [Google Scholar]
- Russell L. J. et al. Mutations in the gene for transglutaminase 1 in autosomal recessive lamellar ichthyosis. Nat Genet 9, 279–83 (1995). [DOI] [PubMed] [Google Scholar]
- Piepkorn M., Predd H., Underwood R. & Cook P. Proliferation-differentiation relationships in the expression of heparin-binding epidermal growth factor-related factors and erbB receptors by normal and psoriatic human keratinocytes. Arch Dermatol Res 295, 93–101 (2003) [DOI] [PubMed] [Google Scholar]
- Pastore S. & Mascia F. Novel acquisitions on the immunoprotective roles of the EGF receptor in the skin. Expert Rev Dermatol 3, 525–527 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M. R., Werner S., Paus R. & Wolf E. Beyond wavy hairs: the epidermal growth factor receptor and its ligands in skin biology and pathology. Am J Pathol 173, 14–24 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakita H. & Takigawa M. Activation of epidermal growth factor receptor promotes late terminal differentiation of cell-matrix interaction-disrupted keratinocytes. J Biol Chem 274, 37285–91 (1999). [DOI] [PubMed] [Google Scholar]
- Denning M. F. et al. Cross-talk between epidermal growth factor receptor and protein kinase C during calcium-induced differentiation of keratinocytes. Exp Dermatol 9, 192–9 (2000). [DOI] [PubMed] [Google Scholar]
- Cheng X. et al. TRP channel regulates EGFR signaling in hair morphogenesis and skin barrier formation. Cell 141, 331–43 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzke C. W. et al. Epidermal ADAM17 maintains the skin barrier by regulating EGFR ligand-dependent terminal keratinocyte differentiation. J Exp Med 209, 1105–19 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miettinen P. J. et al. Epithelial immaturity and multiorgan failure in mice lacking epidermal growth factor receptor. Nature 376, 337–341 (1995). [DOI] [PubMed] [Google Scholar]
- Sibilia M. et al. Mice humanised for the EGF receptor display hypomorphic phenotypes in skin, bone and heart. Development 130, 4515–25 (2003). [DOI] [PubMed] [Google Scholar]
- Lichtenberger B. M. et al. Epidermal EGFR controls cutaneous host defense and prevents inflammation. Sci Transl Med 5, 199ra111 (2013). [DOI] [PubMed] [Google Scholar]
- Campbell P. et al. Epithelial inflammation resulting from an inherited loss-of-function mutation in EGFR. J Invest Derm 134, 2570–2578, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanprapaph K., Vachiramon V. & Rattanakaemakorn P. Epidermal growth factor receptor inhibitors: a review of cutaneous adverse events and management. Dermatology research and practice 2014, 734249 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black R. A. et al. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature 385, 729–33 (1997). [DOI] [PubMed] [Google Scholar]
- Sahin U. et al. Distinct roles for ADAM10 and ADAM17 in ectodomain shedding of six EGFR ligands. J Cell Biol 164, 769–79 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peschon J. J. et al. An essential role for ectodomain shedding in mammalian development. Science 282, 1281–4 (1998). [DOI] [PubMed] [Google Scholar]
- Blobel C. P., ADAMs: key components in EGFR signalling and development. Nature reviews. Molecular cell biology 6, 32–43 (2005). [DOI] [PubMed] [Google Scholar]
- Murthy A. et al. Notch activation by the metalloproteinase ADAM17 regulates myeloproliferation and atopic barrier immunity by suppressing epithelial cytokine synthesis. Immunity 36, 105–19 (2012). [DOI] [PubMed] [Google Scholar]
- Blaydon D. C. et al. Inflammatory skin and bowel disease linked to ADAM17 deletion. N Engl J Med 365, 1502–8 (2011). [DOI] [PubMed] [Google Scholar]
- Tsukerman P. et al. Cytokine secretion and NK cell activity in human ADAM17 deficiency. Oncotarget 6, 44151–60 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tholen S. et al. Skin Barrier Defects Caused by Keratinocyte-Specific Deletion of ADAM17 or EGFR Are Based on Highly Similar Proteome and Degradome Alterations. J Proteome Res 15, 1402–17 (2016). [DOI] [PubMed] [Google Scholar]
- Raghunath M. et al. A novel in situ method for the detection of deficient transglutaminase activity in the skin. Arch Dermatol Res 290, 621–7 (1998). [DOI] [PubMed] [Google Scholar]
- Candi E. et al. Transglutaminase 5 cross-links loricrin, involucrin, and small proline-rich proteins in vitro. J Biol Chem 276, 35014–23 (2001). [DOI] [PubMed] [Google Scholar]
- Nishi K., Inoue H., Schnier J. B. & Rice R. H. Cyclin D1 downregulation is important for permanent cell cycle exit and initiation of differentiation induced by anchorage-deprivation in human keratinocytes. J Cell Biochem 106, 63–72 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost M., Huggett T. M., Kari C. & Rodeck U. Matrix-independent survival of human keratinocytes through an EGF receptor/MAPK-kinase-dependent pathway. Mol Biol Cell 12, 1519–27 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denning M. F. Epidermal keratinocytes: regulation of multiple cell phenotypes by multiple protein kinase C isoforms. Int J Biochem Cell Biol 36, 1141–6 (2004). [DOI] [PubMed] [Google Scholar]
- Długosz A. A. & Yuspa S. H. Protein kinase C regulates keratinocyte transglutaminase (TGK) gene expression in cultured primary mouse epidermal keratinocytes induced to terminally differentiate by calcium. J Invest Dermatol 102, 409–14 (1994). [DOI] [PubMed] [Google Scholar]
- Tibudan S. S., Wang Y. & Denning M. F. Activation of protein kinase C triggers irreversible cell cycle withdrawal in human keratinocytes. J Invest Dermatol. 119, 1282–9 (2002). [DOI] [PubMed] [Google Scholar]
- Adhikary G., Chew Y. C., Reece E. A. & Eckert R. L. PKC-delta and -eta, MEKK-1, MEK-6, MEK-3, and p38-delta are essential mediators of the response of normal human epidermal keratinocytes to differentiating agents. J Invest Dermatol. 130, 2017–30 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- Wieduwilt M. J. & Moasser M. M. The epidermal growth factor receptor family: Biology driving targeted therapeutics. Cellular and molecular life sciences: CMLS. 65, 1566–1584 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo H. R. et al. PKCalpha induces differentiation through ERK1/2 phosphorylation in mouse keratinocytes. Exp Mol Med 36, 292–9 (2004). [DOI] [PubMed] [Google Scholar]
- Ohba M. et al. Induction of differentiation in normal human keratinocytes by adenovirus-mediated introduction of the eta and delta isoforms of protein kinase C. Mol Cell Biol. 18, 5199–207 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strott C. A. & Higashi Y. Cholesterol sulfate in human physiology: what’s it all about? J Lipid Res 44, 1268–78 (2003). [DOI] [PubMed] [Google Scholar]
- Kashiwagi M., Ohba M., Chida K. & Kuroki T. Protein kinase C eta (PKC eta): its involvement in keratinocyte differentiation. J Biochem 132, 853–7 (2002). [DOI] [PubMed] [Google Scholar]
- Kawabe S. et al. Cholesterol sulfate activates transcription of transglutaminase 1 gene in normal human keratinocytes. J Invest Dermatol 111, 1098–102 (1998). [DOI] [PubMed] [Google Scholar]
- La Celle P. T. & Polakowska R. R. Human homeobox HOXA7 regulates keratinocyte transglutaminase type 1 and inhibits differentiation. J Biol Chem 276, 32844–53 (2001). [DOI] [PubMed] [Google Scholar]
- Brooke M. A. et al. iRHOM2-dependent regulation of ADAM17 in cutaneous disease and epidermal barrier function. Hum Mol Genet 23, 4064–76 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaydon D. C. et al. RHBDF2 mutations are associated with tylosis, a familial esophageal cancer syndrome. Am J Hum Genet 90, 340–6 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M. J., Kim E., Ryu S. H. & Suh P. G. The mechanism of phospholipase C-gamma1 regulation. Exp Mol Med 32, 101–109 (2000). [DOI] [PubMed] [Google Scholar]
- Xie Z., Singleton P. A., Bourguignon L. Y. & Bikle D. D. Calcium-induced humankeratinocyte differentiation requires src- and fyn-mediated phosphatidylinositol 3-kinase-dependent activation of phospholipase C-gamma1. Mol Biol Cell 16, 3236–46 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugh J. M., Schooler K., Wells A., Wiley H. S. & Lauffenburger D. A. Effect of epidermal growth factor receptor internalization on regulation of the phospholipase C-gamma1 signaling pathway. J Biol Chem 274, 8958–65 (1999). [DOI] [PubMed] [Google Scholar]
- Gierschik P., Buehler A. & Walliser C. Activated PLCγ Breaking Loose. Structure 20, 1989–90 (2012). [DOI] [PubMed] [Google Scholar]
- Reynolds N. J. et al. Differential induction of phosphatidylcholine hydrolysis, diacylglycerol formation and protein kinase C activation by epidermal growth factor and transforming growth factor-alpha in normal human skin fibroblasts and keratinocytes. Biochem J 294, 535–44. Erratum in: Biochem J 295, 903 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spörl F. et al. Real-time monitoring of membrane cholesterol reveals new insights into epidermal differentiation. J Invest Dermatol 130, 1268–78 (2010). [DOI] [PubMed] [Google Scholar]
- Hur E. M. et al. Sensitization of epidermal growth factor-induced signaling by bradykinin is mediated by c-Src. Implications for a role of lipid microdomains. J Biol Chem 279, 5852–60 (2004). [DOI] [PubMed] [Google Scholar]
- Toker A. Signaling through protein kinase C. Front Biosci 3, D1134–47 (1998). [DOI] [PubMed] [Google Scholar]
- Mitev V., Le Panse R., Coulomb B., Miteva L. & Houdebine L. M. Epidermal growth factor stimulates mitogen-activated protein kinase by a PKC-dependent pathway in human keratinocytes. Biochem Biophys Res Commun 208, 245–52 (1995). [DOI] [PubMed] [Google Scholar]
- Ueda E. et al. The eta isoform of protein kinase C mediates transcriptional activation of the human transglutaminase 1 gene. J Biol Chem 271, 9790–4 (1996). [DOI] [PubMed] [Google Scholar]
- Chida K. et al. Disruption of protein kinase Ceta results in impairment of wound healing and enhancement of tumor formation in mouse skin carcinogenesis. Cancer Res 63, 2404–8 (2003). [PubMed] [Google Scholar]
- Sääf A. et al. Characterization of EGFR and ErbB2 expression in atopic dermatitis patients. Arch Dermatol Res 304, 773–80 (2012). [DOI] [PubMed] [Google Scholar]
- Franzke C. W., Bruckner-Tuderman L. & Blobel C. P. Shedding of collagen XVII/BP180 in skin depends on both ADAM10 and ADAM9. J Biol Chem 284, 23386–96 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.