Abstract
Because latent form of matrix metallopeptidase-9 (proMMP9) levels are positively related to blastocyst development, it was hypothesized that addition during maturation may improve development of heat-stressed oocytes. To test hypothesis, 0, 30 or 300 ng/ml human proMMP9 (hMMP9) was added at 18 h of in vitro maturation (hIVM) to cumulus-oocyte complexes matured at 38.5 or 41.0ºC (first 12 h only). Heat stress decreased 24 hIVM proMMP9 levels only in 0 and 30 ng/ml groups and increased progesterone in 0 and 300 ng/ml hMMP9 groups. Heat stress decreased cleavage and blastocyst development. Independent of maturation temperature, hMMP9 at 18 hIVM decreased blastocyst development. In a second study, cumulus-oocyte complexes were matured for 24 h at 38.5 or 41.0ºC (HS first 12 h only) with 0 or 300 ng/ml hMMP9 added at 12 hIVM. Without hMMP9, heat stress decreased 24 hIVM proMMP9 levels and increased progesterone production. Addition of 300 ng/ml of hMMP9 produced equivalent levels of proMMP9 at 24 hIVM (271 vs. 279 ± 77 for 38.5ºC and 41.0ºC treated oocytes, respectively). Heat stress did not affect ability of oocytes to cleave but reduced blastocyst development. Independent of temperature, hMMP9 decreased cleavage and blastocyst development. In summary, hMMP9 supplementation during IVM did not improve development of heat-stressed oocytes even when it was added for the entire maturation period. At doses tested, hMMP9 appeared detrimental to development when supplemented during the last 12 or 6 h of oocyte maturation.
Keywords: Cumulus cells, Heat stress, Matrix metallopeptidase-9 (MMP9), Oocyte maturation, Progesterone
When the effects of heat stress to compromise developmental competence of maturing oocytes are most pronounced [1, 2], bovine oocytes are intimately associated with cumulus cells [3]. Some of the negative effects of heat stress may be mediated through functional changes in the cumulus cells because the application of a physiologically-relevant heat stress during first 12 h of in vitro maturation (hIVM) altered the abundance of several developmentally-important cumulus-derived transcripts [4]. Notably, elevating the temperature at the beginning of maturation resulted in reduced mRNA expression of matrix metallopeptidase-9 (MMP9) in cumulus as early as 6 hIVM [4]. This translated into heat-induced reductions in secretion of the latent form of MMP9 (proMMP9) between 12 and 18 hIVM [4].
MMP9, a zinc-dependent gelatinase, has been demonstrated to be positively associated with developmental competence of the oocyte. For instance, Mmp9-knockout mice have impaired fertility; specifically reduced litter size and increased number of infertile breeding pairs [5]. In bovine granulosa cells, the presence of MMP9 transcripts was indicative that the follicle of origin contained an oocyte that would develop into a blastocyst stage embryo after IVF [6]. Rispoli et al. [4] showed that concentrations of proMMP9 derived from bovine cumulus-oocyte complexes after 24 hIVM were correlated with the subsequent rate of blastocyst development. Furthermore, levels of proMMP9 were greater in preovulatory follicles from which resulted successful fertilization [7], implantation and pregnancy [8, 9] of human oocytes after IVF treatment.
The proMMP9 transforms from a 92 kDa glycosylated prometalloenzyme to an active form through a cascade of proteolytic steps involving other MMPs, tissue- and urokinases-type plasminogen activators, etc. (reviewed by [10]). The active form of MMP9 not only degrades extracellular components such as collagens, but also has the capacity to activate epidermal growth factor-like (EGF-like) ligands (e.g., amphiregulin and epiregulin; [11]). These EGF-like ligands have been demonstrated to stimulate meiotic maturation [12, 13], increase metabolism [14] and improve developmental competence [15] of cumulus enclosed oocytes. Interestingly, exposing cumulus-oocyte complexes to heat stress during first 12 h of maturation consistently reduced secretion of proMMP9 concurrent with decreased embryo development after IVF [4]. We hypothesized that heat-induced reductions in MMP9 during oocyte maturation may explain some of the reductions in blastocyst development. To test this hypothesis, we examined the benefit of supplementing proMMP9 during maturation to alleviate the negative effects of heat stress on developmental competence of the bovine oocyte. To provide a more precise test of hypothesis, a specific aim was to add human proMMP9 (hMMP9) to heat-stressed cumulus-oocyte complexes at a dose sufficient to produce equivalent levels to those matured under thermoneutral conditions.
Materials and Methods
Materials
Reagents were purchased from Sigma Chemical Co. (St. Louis, Missouri, USA) unless otherwise noted. All gelatin zymography gels and buffers were prepared according to Toth and Fridman [16]. Coomassie Brilliant Blue and glycine were purchased from MP Biomedicals (Santa Ana, California, USA).
In vitro production of embryos
In vitro maturation, fertilization, and embryo culture were performed as previously described [17]. Unless otherwise specified, groups of 36 ± 5.6 cumulus-oocyte complexes per well of 500 µl maturation medium were matured for 24 h at either 38.5 (control) or 41.0ºC (heat stress; first 12 h only, then at 38.5ºC thereafter). After 24 hIVM, conditioned maturation medium was harvested for assessment of MMP9 and progesterone levels prior to subjecting oocytes to IVF. The ability of putative zygotes to cleave beyond the one cell stage was assessed at approximately 72 h post IVF (hpi). Development to blastocyst stage embryos was assessed at approximately 210 hpi; stage and quality scores were assigned as per Schrock et al. [18]. Blastocyst stage embryos were fixed using 3% paraformaldehyde and then stained with 0.5 µg/ml Hoechst 33342 to enumerate nuclei using a Nikon Eclipse TE300 (UV-2A filter: ex330–380 nm, em400−420 nm; Nikon Instruments, Melville, NY, USA).
Assessment of MMP9 by gelatin zymography
Gelatin zymography was used to assess the levels of MMP9 in conditioned maturation medium as per Rispoli et al. [4] with minor modifications. Protein concentration of maturation medium was determined using Coomassie Plus (Bradford) Assay Kit (Thermo Scientific, Rockford, IL, USA); then samples were mixed with Tris-glycine SDS sample buffer to achieve a 1 µg/µl concentration. Gelatin-impregnated 7.5% polyacrylamide gels were loaded with 20 µg of protein per sample and run at 125 V for 3 h prior to development at 37.0ºC for 18 h. Gels were stained in Coomassie for 2 h at room temperature and then destained for 4 h. Dried gels were scanned and analyzed according to Leber and Balkwill [19] using ImageJ software (ver. 1.45s; U.S. National Institutes of Health).
Progesterone radioimmunoassay
Progesterone concentrations in conditioned maturation medium were determined using a commercially-available solid phase radioimmunoassay kit (Coat-A-Count; Siemens Medical Solutions Diagnostic, Los Angeles, CA) after diluting samples at a 1:8 ratio with unconditioned media. Assay sensitivity was 0.02 ng/ml. Average intra- and inter-assay CVs were 5.35% and 5.39%, respectively.
Experimental design
Experiment 1 – Effects of adding hMMP9 during last 6 hIVM on development of control and heat-stressed cumulus-oocyte complexes: In a previous study, differences in proMMP9 levels between control and heat-stressed cumulus-oocyte complexes were not detected until 18 hIVM [4]. Thus for experiment one, control and heat-stressed cumulus-oocytes complexes were supplemented with hMMP9 at 18 hIVM. Previous efforts in our laboratory estimated the average amount of proMMP9 after 24 hIVM to be ~60 ng/ml in conditioned media from control oocytes (n = 9; standard curve of hMMP9 on zymogram; CV = 16%). Since heat stress exposure consistently reduced proMMP9 levels by ~50% [4], 30 ng/ml was chosen as an initial supplemental dose for comparison to an additional dose which was ~10-fold higher. To this end, supplemental doses for experiment one consisted of 0 (diluent), 30 or 300 ng/ml hMMP9, resulting in a 2 × 3 factorial arrangement of treatments. This experiment was replicated on twelve different occasions using a total of 4,336 oocytes. For this experiment, hMMP9 added to maturation media was either recombinant human proMMP9 (protein consisting of proform of human MMP9 enzyme with C-terminal polyhistidine tag) sourced from Sino Biological (PBS diluent; Beijing, P. R. China; four replicates) or native human proMMP9 diluted in PBS with 50% glycerol and 250 mM NaCl (Abcam, Cambridge, MA, USA; eight replicates).
Experiment 2 – Impact of adding hMMP9 during last 12 hIVM on development of control and heat-stressed cumulus-oocyte complexes: Because differences in proMMP9 levels between control and heat-stressed cumulus-oocyte complexes may be occurring between 12 and 18 hIVM [4], a second experiment was performed where control and heat-stressed cumulus-oocytes complexes were supplemented with hMMP9 at 12 hIVM. Addition of hMMP9 occurred at the time when heat-stressed oocytes were transferred from 41 to 38.5ºC (12 hIVM). To this end, maturation medium of control and heat-stressed cumulus-oocyte complexes was supplemented with 0 (diluent) or 300 ng/ml human MMP9 (hMMP9), resulting in a 2 × 2 factorial arrangement of treatments. Preference for utilizing 300 versus 30 ng/mL for this experiment was based on results from experiment one documenting equivalence of MMP9 levels in the medium of cumulus-oocytes complexes matured under control or heat-stressed conditions. Experiment two was replicated on ten different occasions using recombinant hMMP9 (Sino Biological) on a total of 3,966 oocytes.
Experiment 3 – Impact of adding hMMP9 during 24 hIVM on development of control and heat-stressed cumulus-oocyte complexes: Because effects begin while cumulus-oocytes are exposed to heat stress, [4], a third experiment was performed where control and heat-stressed cumulus-oocytes complexes were supplemented with hMMP9 at the beginning of in vitro maturation (0 hIVM). To this end, maturation medium of control and heat-stressed cumulus-oocyte complexes was supplemented with 0 (diluent) or 300 ng/ml human MMP9 (hMMP9), resulting in a 2 × 2 factorial arrangement of treatments. Experiment three was replicated on three different occasions using recombinant hMMP9 (Sino Biological) on a total of 1,008 oocytes.
Statistical analyses
Data were analyzed as a randomized block design, blocking on replicate, using generalized linear models (PROC GLIMMIX) in SAS (9.3, SAS Inst., Cary, NC, USA). Fixed effects included maturation temperature, hMMP9 dose, and the interaction of maturation temperature × hMMP9 dose. Experimental unit was defined as a plate containing well(s) of oocytes. Treatment differences were determined using protected least significant differences and reported as least squares means ± SEM using the inverse link option.
For the second and third studies, an equivalence test was used to further examine the proportion of putative zygotes that developed to the blastocyst stage for groups treated with 0 or 300 ng/ml hMMP9. Equivalence between 0 and 300 ng/ml hMMP9 blastocyst development means was set at 6% (two one-sided t tests). In order for two means to be considered equivalent (P ≤ 0.05), means and their confidence intervals must be within 6% of each other.
To examine proportional relationship between the heat-induced changes in proMMP9 and progesterone levels, percent differences between thermoneutral and heat stress levels per cumulus-oocyte complex were calculated for only samples without hMMP9 addition (diluent) for each replicate of both experiments. To increase the power of this analysis, seven replicates from Rispoli et al. [4] were included in the correlation. A Pearson correlation of MMP9 percent differences and progesterone percent differences was conducted without blocking on replicate (PROC CORR).
Results
Experiment 1: Effects of adding hMMP9 during last 6 hIVM on development of control and heat-stressed cumulus-oocyte complexes
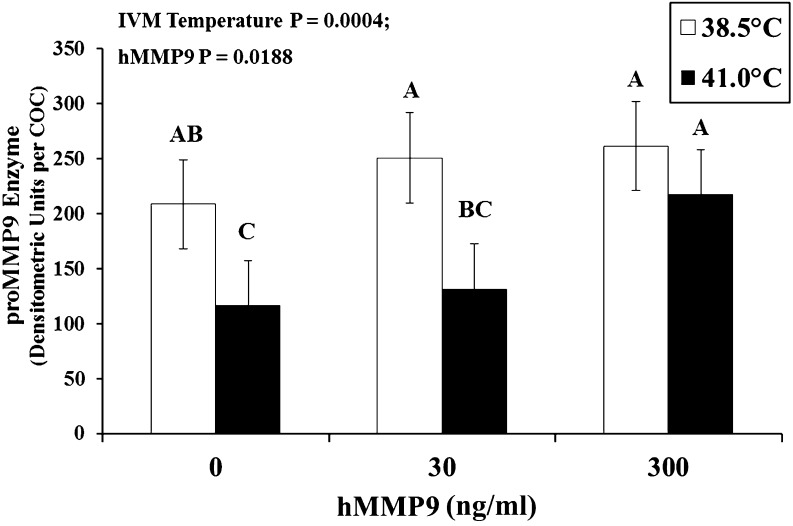
Elevated temperature during the first 12 h of oocyte maturation reduced proMMP9 levels at 24 hIVM in 0 and 30 ng/ml hMMP9 groups, whereas addition of 300 ng/ml hMMP9 resulted in equivalent levels between the heat stress and control groups (P = 0.0004; Fig. 1). Heat stress decreased the ability of putative zygotes to cleave and develop further to the 8 to 16-cell stage (P < 0.0001; Table 1). Heat stress also decreased the proportion of putative zygotes that developed to blastocyst stage (P < 0.0001), yet did not affect stage or number of nuclei per each blastocyst (Table 1). Quality of blastocysts was similar between IVM temperature groups (1.78 vs. 1.73 ± 0.06 for 38.5 vs. 41.0 C, respectively; P = 0.5422).
Fig. 1.
Levels of latent matrix metallopeptidase-9 (proMMP9) per cumulus-oocyte complex (COC) at 24 h of in vitro maturation (hIVM) after supplementing with human proMMP9 (hMMP9) at 18 hIVM. Cumulus-oocyte complexes were matured at 38.5 or 41.0ºC (first 12 h only and then transferred to 38.5ºC). ABC Denotes means differ.
Table 1. Cleavage and blastocyst development after supplementing hMMP9 at 18 h of oocyte maturation.
| Effect | No. PZs | Cleavage (69.94 ± 6.77 hpi) |
Blastocyst development (211.37 ± 1.25 hpi) |
|||
| % Cleaved of PZs |
% 8 to 16-cell of cleaved |
% Blastocysts of PZs |
Stage | Nuclei | ||
| Maturation temperature (ºC) | ||||||
| 38.5 | 1642 | 69.76A | 72.72A | 26.83 A | 7.22 | 140.71 |
| 41.0 | 1723 | 60.76B | 62.27B | 16.35 B | 7.14 | 136.23 |
| SEM | 1.7 | 3.1 | 2.0 | 0.05 | 5.4 | |
| P-value | < 0.0001 | < 0.0001 | < 0.0001 | 0.0926 | 0.3798 | |
| hMMP9 (ng/ml) | ||||||
| 0 (Diluent) | 1166 | 67.23 | 69.00 | 24.07 A | 7.15 | 131.24 |
| 30 | 1026 | 64.45 | 67.57 | 19.39 B | 7.20 | 143.07 |
| 300 | 1173 | 64.48 | 66.56 | 20.10 B | 7.18 | 141.09 |
| SEM | 1.9 | 3.3 | 2.1 | 0.05 | 6.0 | |
| P-value | 0.2916 | 0.6079 | 0.0220 | 0.6884 | 0.1334 | |
| Maturation temperature × hMMP9 | ||||||
| 38.5ºC × 0 ng/mL (Diluent) | 580 | 70.33 | 74.83 | 30.17 | 7.17 | 135.74 |
| 41.0ºC × 0 ng/ml (Diluent) | 586 | 63.97 | 62.49 | 18.86 | 7.14 | 126.75 |
| 38.5ºC × 30 ng/ml | 485 | 71.64 | 72.30 | 26.41 | 7.27 | 148.21 |
| 41.0ºC × 30 ng/ml | 541 | 56.55 | 62.45 | 13.88 | 7.13 | 137.93 |
| 38.5ºC × 300 ng/ml | 557 | 67.20 | 70.95 | 24.11 | 7.21 | 138.17 |
| 41.0ºC × 300 ng/ml | 596 | 61.65 | 61.85 | 16.62 | 7.15 | 144.00 |
| SEM | 2.4 | 3.7 | 2.4 | 0.07 | 7.4 | |
| P-value | 0.0513 | 0.7364 | 0.3189 | 0.5486 | 0.3544 | |
hMMP9 = human matrix metallopeptidase-9; hpi = hours post in vitro fertilization; PZs = Putative Zygotes. AB Least squares means within a column differ.
Supplementing with hMMP9 during the last 6 hIVM did not affect the ability of putative zygotes to cleave or reach the 8 to 16-cell stage, but did reduce their ability to progress to the blastocyst stage (P = 0.0220; Table 1). Addition of hMMP9 did not affect stage or number of nuclei per blastocyst (P > 0.13), but the lower dose of hMMP may have affected quality (1.62 vs. 1.83 and 1.82 ± 0.07 for 30 ng/ml vs. 0 and 300 ng/ml hMMP, respectively; P = 0.0396). When replicates with recombinant hMMP9 were compared to those with native hMMP9, no apparent differences were noted (data not shown).
Experiment 2: Impact of adding hMMP9 during last 12 hIVM on development of control and heat-stressed cumulus-oocyte complexes
A priori comparison of 38.5 vs. 41.0ºC diluent groups (0 ng/ml hMMP9) revealed a heat-induced decrease in proMMP9 production from cumulus-oocyte complexes (75 vs. 42 ± 14 arbitrary units for 38.5ºC and 41.0ºC, respectively; P = 0.0097). When 300 ng/ml hMMP9 was added at 12 hIVM, proMMP9 levels for both treatment groups were equivalent at 24 hIVM (271 vs. 279 ± 77 for 38.5ºC and 41.0ºC, respectively; P = 0.8732).
Elevated temperature during the first 12 hIVM had no effect on the overall ability of putative zygotes to cleave after IVF, but reduced progression to the 8 to 16-cell stage (P = 0.0006) and development to the blastocyst stage (P = 0.0062; Table 2). Heat stress exposure did not affect stage or number of nuclei per blastocyst (Table 2). Quality of blastocysts was similar between IVM temperature groups (1.86 vs. 1.86 ± 0.05 for 38.5 vs. 41.0 C, respectively; P = 0.9855). Preliminary efforts examined the effect of diluent (PBS; Sino Biological) compared to controls and found no difference in subsequent embryo development (data not shown). The presence of hMMP9 during the last 12 hIVM decreased the ability of putative zygotes to cleave (P = 0.0157) without affecting ability of cleaved embryos to progress to the 8 to 16-cell stage. The proportion of putative zygotes that developed to blastocyst stage tended to be less when hMMP9 was present in maturation medium during last 12 hIVM (P = 0.0823; Table 2). An equivalence test confirmed blastocyst development from putative zygotes in 300 ng/ml hMMP9 groups was not equivalent to diluent groups (P = 0.0922; means are considered equal when P ≤ 0.05). Addition of hMMP9 at 12 h of oocyte maturation did not affect blastocyst stage or number of nuclei per blastocyst (Table 2), nor was the quality of the resulting blastocyst stage embryos affected (1.86 vs. 1.85 ± 0.05 for 0 vs. 300 ng/ml hMMP, respectively; P = 0.8982).
Table 2. Cleavage and blastocyst development after supplementing hMMP9 at 12 h of oocyte maturation.
| Effect | No. PZs | Cleavage (72.32 ± 0.62 hpi) |
Blastocyst development (210.68 ± 0.81 hpi) |
|||
| % Cleaved of PZs |
% 8 to 16-cell of cleaved |
% Blastocysts of PZs |
Stage | Nuclei | ||
| Maturation temperature (ºC) | ||||||
| 38.5 | 1677 | 66.77 | 59.56 A | 25.31 A | 7.16 | 137.65 |
| 41.0 | 1633 | 66.55 | 51.01 B | 19.74 B | 7.19 | 133.99 |
| SEM | 1.6 | 3.2 | 1.7 | 0.06 | 6.3 | |
| P-value | 0.8940 | 0.0006 | 0.0062 | 0.5886 | 0.4238 | |
| hMMP9 (ng/ml) | ||||||
| 0 (Diluent) | 1655 | 68.76 A | 56.90 | 24.12 | 7.15 | 139.37 |
| 300 | 1655 | 64.50 B | 53.74 | 20.77 | 7.20 | 132.27 |
| SEM | 1.6 | 3.2 | 1.7 | 0.06 | 6.3 | |
| P-value | 0.0157 | 0.1561 | 0.0823 | 0.4332 | 0.1200 | |
| Maturation temperature × hMMP9 | ||||||
| 38.5ºC × 0 ng/ml (Diluent) | 855 | 69.40 | 60.20 | 27.85 | 7.17 | 143.72 |
| 41.0ºC × 0 ng/ml (Diluent) | 800 | 68.11 | 53.53 | 20.75 | 7.13 | 135.02 |
| 38.5ºC × 300 ng/ml | 822 | 64.04 | 58.92 | 22.93 | 7.14 | 131.58 |
| 41.0ºC × 300 ng/ml | 833 | 64.95 | 48.48 | 18.77 | 7.26 | 132.96 |
| SEM | 2.0 | 3.5 | 2.2 | 0.08 | 7.0 | |
| P-value | 0.5054 | 0.4018 | 0.5318 | 0.2142 | 0.2638 | |
hMMP9 = human matrix metallopeptidase-9; hpi = hours post in vitro fertilization; PZs = Putative Zygotes. AB Least squares means within a column differ.
Experiment 3: Impact of adding hMMP9 during 24 hIVM on development of control and heat-stressed cumulus-oocyte complexes
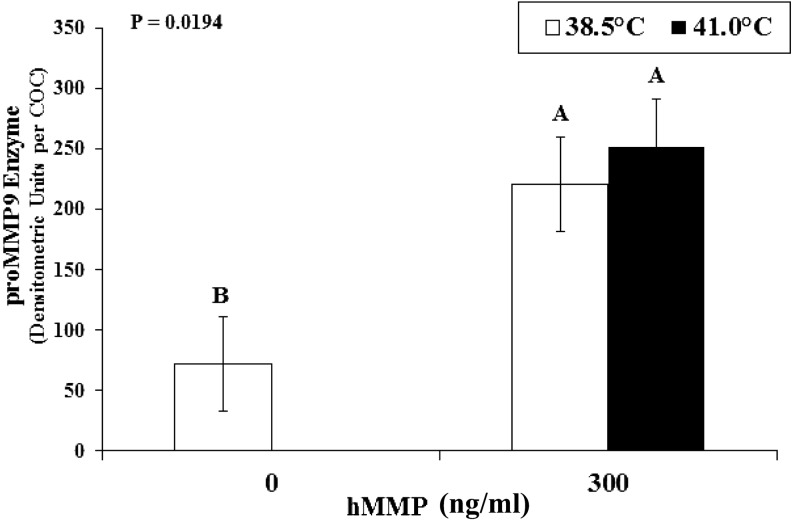
When 300 ng/ml hMMP9 was added at 0 hIVM, the proMMP9 levels at 24 hIVM from control and heat-stressed groups were equivalent (P = 0.5245), yet higher compared to COCs matured at 38.5ºC with 0 ng/ml hMMP (Fig. 2; P = 0.0194). Levels of proMMP9 in media from COCs matured at 41.0ºC with diluent were indistinguishable from background on zymograms.
Fig. 2.
Levels of latent matrix metallopeptidase-9 (proMMP9) per cumulus-oocyte complex (COC) at 24 h of in vitro maturation (hIVM) after supplementing with human proMMP9 (hMMP9) at 0 hIVM. Cumulus-oocyte complexes were matured at 38.5 or 41.0ºC (first 12 h only and then transferred to 38.5ºC). AB Denotes means differ (P = 0.0194). Data from COCs matured at 41.0 with diluent are absent as were indistinguishable from background.
Elevated temperature during the first 12 h of maturation had no effect on the ability of putative zygotes to cleave (P = 0.2246). However, heat stress decreased development further to the 8 to 16-cell (P = 0.0246) and blastocyst stages (P = 0.0305) without affecting stage or number of nuclei per each blastocyst (P > 0.32; Table 3). Also quality of blastocysts was similar between IVM temperature groups (1.95 vs. 2.89 ± 0.58 for 38.5 vs. 41.0ºC, respectively; P = 0.2836).
Table 3. Cleavage and blastocyst development after supplementing hMMP9 at 0 h of oocyte maturation.
| Effect | Cleavage (73.5 ± 1.6 hpi) |
Blastocyst development (211.2 ± 0.65 hpi) |
||||
| % Cleaved of PZs |
% 8 to 16-cell of cleaved |
% Blastocysts of PZs |
Stage | Nuclei | ||
| Maturation temperature (ºC) | ||||||
| 38.5 | 76.62 | 52.57 A | 26.86 A | 7.26 | 149.73 | |
| 41.0 | 72.64 | 40.86 B | 21.86 B | 7.12 | 122.92 | |
| SEM | 4.1 | 2.8 | 2.5 | 0.17 | 17.6 | |
| P-value | 0.2246 | 0.0246 | 0.0305 | 0.5142 | 0.3231 | |
| hMMP9 (ng/ml) | ||||||
| 0 (Diluent) | 74.69 | 46.91 | 25.77 | 7.30 | 147.52 | |
| 300 | 74.67 | 46.42 | 22.95 | 7.08 | 125.13 | |
| SEM | 4.1 | 2.8 | 2.5 | 0.17 | 17.6 | |
| P-value | 0.9949 | 0.9048 | 0.1633 | 0.3397 | 0.4034 | |
| Maturation temperature × hMMP9 | ||||||
| 38.5ºC × 0 ng/ml (Diluent) | 76.54 | 52.44 | 28.91 | 7.20 | 148.63 | |
| 41.0ºC × 0 ng/ml (Diluent) | 72.76 | 41.46 | 22.63 | 7.40 | 146.40 | |
| 38.5ºC × 300 ng/ml | 76.71 | 52.69 | 24.81 | 7.32 | 150.83 | |
| 41.0ºC × 300 ng/ml | 72.52 | 40.26 | 21.08 | 6.84 | 99.44 | |
| SEM | 4.6 | 3.9 | 2.8 | 0.23 | 24.9 | |
| P-value | 0.9469 | 0.8560 | 0.5014 | 0.1531 | 0.3618 | |
hMMP9 = human matrix metallopeptidase-9; hpi = hours post in vitro fertilization; PZs = Putative Zygotes. AB Least squares means within a column differ.
Supplementing with hMMP9 for the entire 24 hIVM did not affect the ability of putative zygotes to cleave or reach the 8 to 16-cell stage (P > 0.9; Table 3). An equivalence test confirmed blastocyst development from putative zygotes in 300 ng/ml hMMP9 groups was not equivalent to diluent groups (P = 0.0918; means are considered equal when P ≤ 0.05). The addition of hMMP9 did not affect blastocyst stage or number of nuclei per blastocyst (P > 0.34; Table 3). Also the quality of the blastocysts that developed did not differed whether or not hMMP9 was present during entire maturation period (2.09 vs. 2.76 ± 0.58 for 0 vs. 300 ng/ml hMMP, respectively; P = 0.4361).
Relationship between heat-induced changes in levels of MMP9 and progesterone
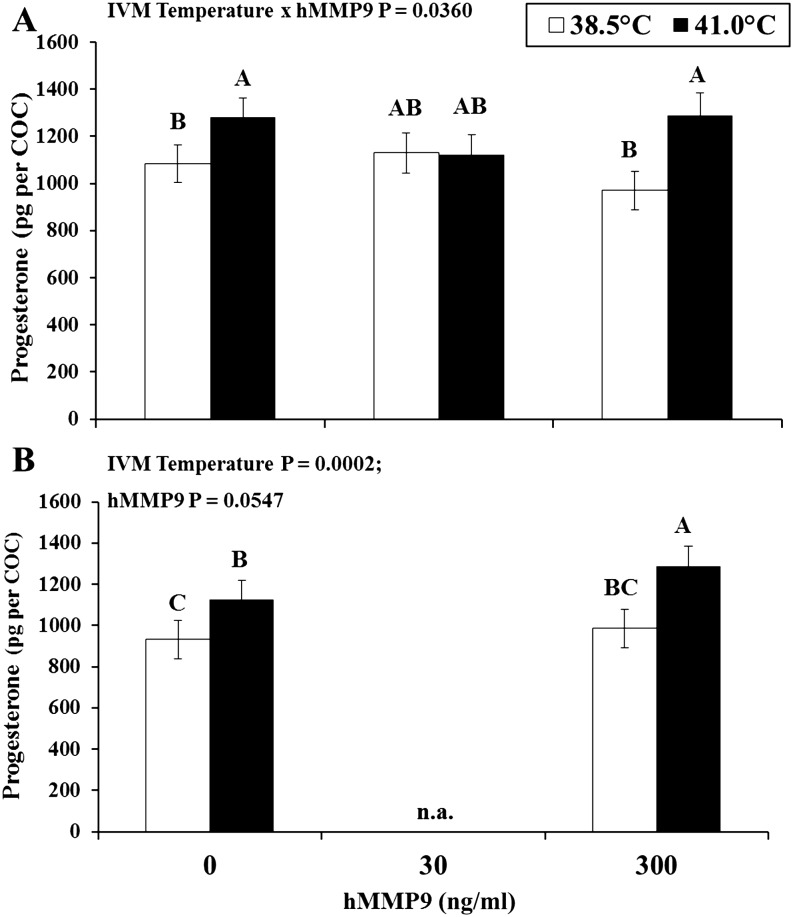
Heat exposure increased progesterone production during the 24 h of maturation whether or not 300 ng/ml of hMMP9 was present in medium during the last 6 (hMMP9 × temperature interaction P = 0.039; Fig. 3, panel A) or 12 h of maturation (IVM temperature P = 0.0002; Fig. 3, panel B). To begin efforts towards examining the relationship between MMP9 and progesterone levels in the context of heat stress effects, data from samples without hMMP9 addition were combined from Rispoli et al. [4], Experiment 1 and Experiment 2. Relevant for this analysis, exposure to a heat stress of 41 C during first 12 h of IVM on average resulted in a 44% decrease in secretion of proMMP9 (224 vs. 126 ± 29 units per cumulus-oocyte complex for control and heat stress, respectively; P < 0.0001) and 20% increase in amount of progesterone produced from cumulus-oocyte complexes (883.22 vs. 1055.92 ± 68.97 pg per cumulus-oocyte complex for control and heat stress, respectively; P < 0.0001). Further analysis of the heat stress effects (percent difference of change from control values) revealed an inverse relationship between changes in MMP9 and progesterone levels (r = –0.37, P = 0.0690). In other words, heat-induced decreases in MMP9 levels were coincident with heat-induced increases in progesterone production.
Fig. 3.
Levels of progesterone per cumulus-oocyte complex (COC) at 24 h of in vitro maturation (hIVM). Recombinant human proMMP9 (hMMP9) was supplemented during the last 6 (experiment 1; A) or 12 (experiment 2; B) hours of maturation to cumulus-oocyte complexes matured at 38.5 or 41.0ºC (first 12 h only and then transferred to 38.5ºC). ABC Different letters denote statistical difference within an experiment. n.a., not applicable.
Discussion
When heat stress effects during the first 12 hours of oocyte maturation were sufficient to impair blastocyst development, proMMP9 levels at 24 hIVM resulting from cumulus-oocyte complexes experiencing heat stress were reduced in greater than 90% of the experimental replicates performed. The high degree of repeatability of this heat stress effect along with data generated by others showing that higher proMMP9 levels in preovulatory follicles were positively related to successful fertilization [7], implantation and pregnancy [8, 9] of human oocytes after IVF treatment warranted efforts described herein to examine the potential benefit of supplementing an exogenous source of proMMP9 during maturation to improve development of heat-stressed oocytes. To this end, the addition of 300 ng/ml prevented heat-induced reductions in proMMP9 levels and provided a more precise test of the hypothesis. However, preventing the heat-induced reductions in MMP9 through supplementation was not sufficient to improve development of heat-stressed oocytes.
Exogenous hMMP9, at the doses tested, appears detrimental especially when examining potential of oocytes to develop to the blastocyst stage when supplementation occurred during the last 6 or 12 h of in vitro maturation. These results at first glance appear inconsistent with previous findings of our laboratory and others demonstrating that the amount of proMMP9 secreted per cumulus-oocyte complex into the maturation medium or the follicular fluid was positively associated with the developmental competence of oocytes after in vitro [4, 6] and in vivo maturation [7,8,9]. In the current study, however, the maturation medium surrounding control and heat-stressed cumulus-oocyte complexes was supplemented with an exogenous source of proMMP9, effectively elevating proMMP9 levels beyond what cumulus-oocyte complexes would otherwise produce during maturation. While important for testing hypothesis, there are several instances appearing in the scientific literature where higher levels of MMP9 are not associated with positive outcomes. The highest amount of MMP9 was detected in bovine follicular fluid from cystic or atretic follicles collected at various stages of the estrous cycle [20, 21]. Higher levels of MMP9 were noted in the follicular fluid from women with polycystic ovary syndrome at time of oocyte recovery [22]. In cases of tubal factor infertility and endometriosis, intrafollicular MMP9 activity was highest in the antral follicles of human IVF patients with endometriosis [23]. Additional effort to examine oocyte stage and embryo quality, showed that increased levels of MMP9 and MMP2 along with decreased levels of TIMP-1 were associated with a decrease in metaphase II oocytes and good quality embryos [23]. Although oocyte maturity was not examined in our study, results of others highlighting negative consequences in circumstances where above normal levels of MMP9 are noted support the notion that exogenous supplementation above and beyond what would normally be produced by the cumulus-oocyte complex may result in some level of dysfunction sufficient to compromise development competence. In other words, it may be possible for MMP9 to have different roles depending on various physiological conditions.
Detrimental consequences were most notable when maturation medium was supplemented during the last 6 or 12 h of in vitro maturation. This is the time period when the oocyte is challenged with progressing from metaphase I to metaphase II while numerous other changes are occurring within the cytoplasm (e.g., translocation of cortical granules, organelles, changes in transcriptome profile, etc.; [24]); associated cumulus are also undergoing final stages of expansion. Although a specific role for MMP9 during any aspect of maturation has not yet been ascribed, the numerous changes occurring during this time period likely contribute to increasing sensitivity of the cumulus-oocyte complex to an overabundance of MMP9 resulting from supplementation during these time periods. Interestingly, when MMP9 was added at the beginning of maturation, detrimental blastocyst development was no longer observed. Disparity in developmental consequences related to the timing of supplementation is difficult to explain but may be related to the fact that cumulus-oocyte complexes at the beginning of maturation (i.e., germinal vesicle (GV) stage) are not actively producing proMMP9. Previous efforts of our lab and others failed to detect MMP9 transcripts in the cumulus of GV-stage oocytes [4, 25]. Mindful of this, MMP9 supplementation at the beginning of maturation may not be as problematic as in other stages because GV-stage cumulus oocyte complex may not provide necessary substrates for activity. Consequences of supplementing at this time period or others on endogenous production is not known.
Nonetheless, negative consequences of adding hMMP9 were not likely due to source-origin of hMMP9. Effects on embryo development were similar whether supplementing with recombinant or native derived hMMP9. Furthermore, the human proMMP9 amino acid sequence is 90% homologous (HomoloGene, NCBI) to the bovine and has been shown to interact with bovine proteins. Bigg et al. [26] demonstrated that native bovine-derived collagen type III can be cleaved by recombinant hMMP9 after activation with p-aminophenylmercuric acetate. Also, bovine bone slices incubated with activated hMMP9 resulted in increased collagen I degradation [27]. Activation was not performed prior to supplementation with the latent form of hMMP9 herein because bovine cumulus-oocyte complexes produce known activators of proMMP9 such as the inducible and endothelial isoforms of nitric oxide synthase [28] and tissue- and urokinases-type plasminogen activators [29].
Application of a physiologically-relevant heat stress during oocyte maturation not only decreased MMP9 production, but also increased progesterone production which is consistent with previous efforts [4]. This inverse relationship is divergent from reports derived by others suggesting that MMP2/9 may be required for progesterone biosynthesis [30,31,32,33]. These studies, performed on mural granulosa cells or intact follicles, showed that MMP2/9 activation of EGF-like ligands was required for LH-induced steroidogenesis [30,31,32,33]. Our studies, however, examined the responsiveness of the cumulus-oocyte complex after removal from an antral follicle. This cumulus-oocyte complex has low expression of LH receptors [34, 35] and reduced amounts of EGF-like ligands [36]. Bovine cumulus-oocyte complexes may be similar to cultured Leydig cells which do not appear to require EGF-like ligands or MMP2/9 for steroidogenesis [30]. In support of this notion, when bovine cumulus-oocytes were matured in the presence of 300 ng/ml hMMP9 for the entire maturation period at a thermoneutral temperature, no impact on progesterone levels were observed by our laboratory [37].
Whether heat-induced increases in progesterone production are impacting MMP9 secretion during in vitro maturation remains unclear. In other reproductive cell types, increasing progesterone levels inhibited the production of MMP9, specifically in human endometrium explants [38, 39], human trophoblast cells [40] and rabbit cervical fibroblasts [41]. Our laboratory reported that heat-induced increases in progesterone levels from cumulus-oocyte complexes are evident by 12 hIVM thereby preceding the heat-induced decreases in MMP9 levels which are not evident until approximately 18 hIVM [4]. It is interesting to note that the consequences of adding progesterone during meiotic maturation under thermoneutral conditions [42, 43] parallel those observed after heat stress exposure (i.e., similar cleavage rates but reduced blastocyst development). Thus, heat-induced increases in progesterone production may have superceded any of the potential benefits of supplementing maturation medium with hMMP9 to improve development of heat-stressed oocytes.
In summary, application of a physiologically-relevant heat stress during oocyte maturation decreased MMP9 levels. Supplementation of maturation medium with hMMP9 while effective for preventing heat-induced reductions in MMP9 levels was not effective at the doses tested to improve development of heat-stressed oocytes. Despite positive associations in the literature between MMP9 levels in human follicular fluid and pregnancy after in vitro fertilization [8, 9], detrimental effects at doses tested emphasize the importance of further investigation before use during in vitro maturation of human oocytes.
Acknowledgments
This research was supported in part by the state of Tennessee through UT AgResearch, East Tennessee Research and Education Center, the Department of Animal Science, and the USDA National Institute of Food and Agriculture, Hatch Project No. 227701.
References
- 1.Putney DJ, Mullins S, Thatcher WW, Drost M, Gross TS. Embryonic development in superovulated dairy cattle exposed to elevated ambient temperatures between the onset of estrus and insemination. Anim Reprod Sci 1989; 19: 37–51. [DOI] [PubMed] [Google Scholar]
- 2.Edwards JL, Hansen PJ. Elevated temperature increases heat shock protein 70 synthesis in bovine two-cell embryos and compromises function of maturing oocytes. Biol Reprod 1996; 55: 341–346. [DOI] [PubMed] [Google Scholar]
- 3.Thomas RE, Armstrong DT, Gilchrist RB. Bovine cumulus cell-oocyte gap junctional communication during in vitro maturation in response to manipulation of cell-specific cyclic adenosine 3′,5′-monophosophate levels. Biol Reprod 2004; 70: 548–556. [DOI] [PubMed] [Google Scholar]
- 4.Rispoli LA, Payton RR, Gondro C, Saxton AM, Nagle KA, Jenkins BW, Schrick FN, Edwards JL. Heat stress effects on the cumulus cells surrounding the bovine oocyte during maturation: altered matrix metallopeptidase 9 and progesterone production. Reproduction 2013; 146: 193–207. [DOI] [PubMed] [Google Scholar]
- 5.Dubois B, Arnold B, Opdenakker G. Gelatinase B deficiency impairs reproduction. J Clin Invest 2000; 106: 627–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robert C, Gagné D, Bousquet D, Barnes FL, Sirard MA. Differential display and suppressive subtractive hybridization used to identify granulosa cell messenger rna associated with bovine oocyte developmental competence. Biol Reprod 2001; 64: 1812–1820. [DOI] [PubMed] [Google Scholar]
- 7.Bilen E, Tola EN, Oral B, Doguç DK, Günyeli I, Köse SA, Ilhan I. Do follicular fluid gelatinase levels affect fertilization rates and oocyte quality? Arch Gynecol Obstet 2014; 290: 1265–1271. [DOI] [PubMed] [Google Scholar]
- 8.Lee DM, Lee TK, Song HB, Kim CH. The expression of matrix metalloproteinase-9 in human follicular fluid is associated with in vitro fertilisation pregnancy. BJOG 2005; 112: 946–951. [DOI] [PubMed] [Google Scholar]
- 9.Horka P, Malickova K, Jarosova R, Janatkova I, Zima T, Kalousova M. Matrix metalloproteinases in serum and the follicular fluid of women treated by in vitro fertilization. J Assist Reprod Genet 2012; 29: 1207–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van den Steen PE, Dubois B, Nelissen I, Rudd PM, Dwek RA, Opdenakker G. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9). Crit Rev Biochem Mol Biol 2002; 37: 375–536. [DOI] [PubMed] [Google Scholar]
- 11.Roelle S, Grosse R, Aigner A, Krell HW, Czubayko F, Gudermann T. Matrix metalloproteinases 2 and 9 mediate epidermal growth factor receptor transactivation by gonadotropin-releasing hormone. J Biol Chem 2003; 278: 47307–47318. [DOI] [PubMed] [Google Scholar]
- 12.Ashkenazi H, Cao X, Motola S, Popliker M, Conti M, Tsafriri A. Epidermal growth factor family members: endogenous mediators of the ovulatory response. Endocrinology 2005; 146: 77–84. [DOI] [PubMed] [Google Scholar]
- 13.Romero S, Smitz J. Epiregulin can effectively mature isolated cumulus-oocyte complexes, but fails as a substitute for the hCG/epidermal growth factor stimulus on cultured follicles. Reproduction 2009; 137: 997–1005. [DOI] [PubMed] [Google Scholar]
- 14.Richani D, Sutton-McDowall ML, Frank LA, Gilchrist RB, Thompson JG. Effect of epidermal growth factor-like peptides on the metabolism of in vitro- matured mouse oocytes and cumulus cells. Biol Reprod 2014; 90: 49. [DOI] [PubMed] [Google Scholar]
- 15.Procházka R, Petlach M, Nagyová E, Nemcová L. Effect of epidermal growth factor-like peptides on pig cumulus cell expansion, oocyte maturation, and acquisition of developmental competence in vitro: comparison with gonadotropins. Reproduction 2011; 141: 425–435. [DOI] [PubMed] [Google Scholar]
- 16.Toth M, Fridman R. Assessment of gelatinases (MMP-2 and MMP-9) by gelatin zymography. In: Brooks SA, Schumacher U (eds.), Metastasis Research Protocols. Totowa, NJ: Humana Press; 2001: 163−174. [DOI] [PMC free article] [PubMed]
- 17.Rispoli LA, Lawrence JL, Payton RR, Saxton AM, Schrock GE, Schrick FN, Middlebrooks BW, Dunlap JR, Parrish JJ, Edwards JL. Disparate consequences of heat stress exposure during meiotic maturation: embryo development after chemical activation vs fertilization of bovine oocytes. Reproduction 2011; 142: 831–843. [DOI] [PubMed] [Google Scholar]
- 18.Schrock GE, Saxton AM, Schrick FN, Edwards JL. Early in vitro fertilization improves development of bovine ova heat stressed during in vitro maturation. J Dairy Sci 2007; 90: 4297–4303. [DOI] [PubMed] [Google Scholar]
- 19.Leber TM, Balkwill FR. Zymography: a single-step staining method for quantitation of proteolytic activity on substrate gels. Anal Biochem 1997; 249: 24–28. [DOI] [PubMed] [Google Scholar]
- 20.Khandoker MA, Imai K, Takahashi T, Hashizume K. Role of gelatinase on follicular atresia in the bovine ovary. Biol Reprod 2001; 65: 726–732. [DOI] [PubMed] [Google Scholar]
- 21.Imai K, Khandoker MA, Yonai M, Takahashi T, Sato T, Ito A, Hasegawa Y, Hashizume K. Matrix metalloproteinases-2 and -9 activities in bovine follicular fluid of different-sized follicles: relationship to intra-follicular inhibin and steroid concentrations. Domest Anim Endocrinol 2003; 24: 171–183. [DOI] [PubMed] [Google Scholar]
- 22.Shalev E, Goldman S, Ben-Shlomo I. The balance between MMP-9 and MMP-2 and their tissue inhibitor (TIMP)-1 in luteinized granulosa cells: comparison between women with PCOS and normal ovulatory women. Mol Hum Reprod 2001; 7: 325–331. [DOI] [PubMed] [Google Scholar]
- 23.Singh AK, Chattopadhyay R, Chakravarty B, Chaudhury K. Altered circulating levels of matrix metalloproteinases 2 and 9 and their inhibitors and effect of progesterone supplementation in women with endometriosis undergoing in vitro fertilization. Fertil Steril 2013; 100: 127–134.e1. [DOI] [PubMed] [Google Scholar]
- 24.Coticchio G, Dal Canto M, Mignini Renzini M, Guglielmo MC, Brambillasca F, Turchi D, Novara PV, Fadini R. Oocyte maturation: gamete-somatic cells interactions, meiotic resumption, cytoskeletal dynamics and cytoplasmic reorganization. Hum Reprod Update 2015; 21: 427–454. [DOI] [PubMed] [Google Scholar]
- 25.Regassa A, Rings F, Hoelker M, Cinar U, Tholen E, Looft C, Schellander K, Tesfaye D. Transcriptome dynamics and molecular cross-talk between bovine oocyte and its companion cumulus cells. BMC Genomics 2011; 12: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bigg HF, Rowan AD, Barker MD, Cawston TE. Activity of matrix metalloproteinase-9 against native collagen types I and III. FEBS J 2007; 274: 1246–1255. [DOI] [PubMed] [Google Scholar]
- 27.Parikka V, Lehenkari P, Sassi M-L, Halleen J, Risteli J, Härkönen P, Väänänen HK. Estrogen reduces the depth of resorption pits by disturbing the organic bone matrix degradation activity of mature osteoclasts. Endocrinology 2001; 142: 5371–5378. [DOI] [PubMed] [Google Scholar]
- 28.Tesfaye D, Kadanga A, Rings F, Bauch K, Jennen D, Nganvongpanit K, Hölker M, Tholen E, Ponsuksili S, Wimmers K, Montag M, Gilles M, Kirfel G, Herzog V, Schellander K. The effect of nitric oxide inhibition and temporal expression patterns of the mRNA and protein products of nitric oxide synthase genes during in vitro development of bovine pre-implantation embryos. Reprod Domest Anim 2006; 41: 501–509. [DOI] [PubMed] [Google Scholar]
- 29.Park K-W, Choi S-H, Song X-X, Funahashi H, Niwa K. Production of plasminogen activators (PAs) in bovine cumulus-oocyte complexes during maturation in vitro: effects of epidermal growth factor on production of PAs in oocytes and cumulus cells. Biol Reprod 1999; 61: 298–304. [DOI] [PubMed] [Google Scholar]
- 30.Light A, Hammes SR. LH-Induced steroidogenesis in the mouse ovary, but not testis, requires matrix metalloproteinase 2- and 9-mediated cleavage of upregulated EGF receptor ligands. Biol Reprod 2015; 93: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Motola S, Cao X, Ashkenazi H, Popliker M, Tsafriri A. GnRH actions on rat preovulatory follicles are mediated by paracrine EGF-like factors. Mol Reprod Dev 2006; 73: 1271–1276. [DOI] [PubMed] [Google Scholar]
- 32.Jamnongjit M, Gill A, Hammes SR. Epidermal growth factor receptor signaling is required for normal ovarian steroidogenesis and oocyte maturation. Proc Natl Acad Sci USA 2005; 102: 16257–16262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carbajal L, Biswas A, Niswander LM, Prizant H, Hammes SR. GPCR/EGFR cross talk is conserved in gonadal and adrenal steroidogenesis but is uniquely regulated by matrix metalloproteinases 2 and 9 in the ovary. Mol Endocrinol 2011; 25: 1055–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yung Y, Aviel-Ronen S, Maman E, Rubinstein N, Avivi C, Orvieto R, Hourvitz A. Localization of luteinizing hormone receptor protein in the human ovary. Mol Hum Reprod 2014; 20: 844–849. [DOI] [PubMed] [Google Scholar]
- 35.Peng XR, Hsueh AJ, LaPolt PS, Bjersing L, Ny T. Localization of luteinizing hormone receptor messenger ribonucleic acid expression in ovarian cell types during follicle development and ovulation. Endocrinology 1991; 129: 3200–3207. [DOI] [PubMed] [Google Scholar]
- 36.Richani D, Ritter LJ, Thompson JG, Gilchrist RB. Mode of oocyte maturation affects EGF-like peptide function and oocyte competence. Mol Hum Reprod 2013; 19: 500–509. [DOI] [PubMed] [Google Scholar]
- 37.Goodwin MR. Impact of matrix metallopeptidase-9 supplementation during in vitro maturation of bovine oocytes. M.S. Thesis, University of Tennessee, 2014. http://trace.tennessee.edu/utk_gradthes/2818.
- 38.Marbaix E, Donnez J, Courtoy PJ, Eeckhout Y. Progesterone regulates the activity of collagenase and related gelatinases A and B in human endometrial explants. Proc Natl Acad Sci USA 1992; 89: 11789–11793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cornet PB, Picquet C, Lemoine P, Osteen KG, Bruner-Tran KL, Tabibzadeh S, Courtoy PJ, Eeckhout Y, Marbaix E, Henriet P. Regulation and function of LEFTY-A/EBAF in the human endometrium. mRNA expression during the menstrual cycle, control by progesterone, and effect on matrix metalloprotineases. J Biol Chem 2002; 277: 42496–42504. [DOI] [PubMed] [Google Scholar]
- 40.Shimonovitz S, Hurwitz A, Hochner-Celnikier D, Dushnik M, Anteby E, Yagel S. Expression of gelatinase B by trophoblast cells: down-regulation by progesterone. Am J Obstet Gynecol 1998; 178: 457–461. [DOI] [PubMed] [Google Scholar]
- 41.Imada K, Ito A, Sato T, Namiki M, Nagase H, Mori Y. Hormonal regulation of matrix metalloproteinase 9/gelatinase B gene expression in rabbit uterine cervical fibroblasts. Biol Reprod 1997; 56: 575–580. [DOI] [PubMed] [Google Scholar]
- 42.Silva CC, Knight PG. Effects of androgens, progesterone and their antagonists on the developmental competence of in vitro matured bovine oocytes. J Reprod Fertil 2000; 119: 261–269. [PubMed] [Google Scholar]
- 43.Schlüter N, Kassens A, Stinshoff H, Knauer K, Wilkening S, Wrenzycki C. Progesterone concentrations during IVM affect bovine oocyte quality at the molecular level. Reprod Fertil Dev 2014; 26: 171. [Google Scholar]