Abstract
Scutellaria baicalensis has been effectively used in Chinese traditional medicine to prevent miscarriages. However, little information is available on its mechanism of action. This study is designed specifically to reveal how baicalin, the main effective ingredient of S. baicalensis, improves developmental competence of embryos in vitro, using the mouse as a model. Mouse pronuclear embryos were cultured in KSOM medium supplemented with (0, 2, 4 and 8 μg/ml) baicalin. The results demonstrated that in vitro culture conditions significantly decreased the blastocyst developmental rate and blastocyst quality, possibly due to increased cellular stress and apoptosis. Baicalin (4 µg/ml) significantly increased 2- and 4-cell cleavage rates, morula developmental rate, and blastocyst developmental rate and cell number of in vitro-cultured mouse embryos. Moreover, baicalin increased the expression of Gja1, Cdh1, Bcl-2, and Dnmt3a genes, decreased the expression of Dnmt1 gene, and decreased cellular stress and apoptosis as it decreased the expression of HSP70, CASP3, and BAX and increased BCL-2 expression in blastocysts cultured in vitro. In conclusion, baicalin improves developmental competence of in vitro-cultured mouse embryos through inhibition of cellular apoptosis and HSP70 expression, and improvement of DNA methylation.
Keywords: Apoptosis, Baicalin, DNA methylation, HSP70, Mouse embryo
In vitro culture of preimplantation embryos is an essential step in assisted reproductive technology (ART) for both human and animals [1, 2], which is now considered to be a part of mainstream medical practice. However, the birth rate of offspring following ART still lags behind that of their in vivo counterparts, with only a certain percentage of in vitro-cultured (IVC) embryos being capable of establishing pregnancy after their transfer into recipients [3]. Moreover, the in vitro culture environment is known to determine embryo quality [4], with the latter being the main reason for decreased developmental competence of IVC embryos after transplantation [5]. Therefore, ongoing efforts have focused on modifying culture conditions to get high-quality embryos, and evaluating cultured embryos in terms of morphology and gene expression.
Scutellaria baicalensis as a traditional Chinese herbal medicine is used as an anti-abortive, anti-inflammatory, and anti-bacterial drug [6] for the treatment of pregnant women [7, 8]. Baicalin, a monomer of flavonoids, extracted from dried roots of S. baicalensis [9], also shows anti-abortive properties as it modulates the Th1/Th2 cytokine balance, promotes mouse embryo implantation [10], and protects pregnant mice from abortion induced by lipopolysaccharide [11]. Additionally, baicalin is the active ingredient in Shuanghuanglian oral liquid, an antipyretic detoxicant widely used for pregnant women and animals in China [12]. Although it has been clinically shown that baicalin can help maintain pregnancy in both humans [8] and mice [13], little information has been presented to explain how baicalin enhances developmental competence of mouse embryos in vitro.
It is well documented that gene expression of the embryo can be altered by the culture conditions, which in turn affects embryo development [5, 14]. Previously, it has been reported that in vitro culture conditions can lead to increased incidence of cellular stress and apoptosis [15, 16] and induce aberrant DNA methylation in mouse embryos [17, 18], both of which may result in decreased blastocyst quality and even affect the viability of offspring after transfer into a surrogate. Gene expression analysis, a preferred method over conventional criteria like embryo morphology and developmental rates [19], has commonly been used to assess embryo quality and to optimize in vitro culture conditions [20]. Therefore, in this study, a group of marker genes related to cellular stress and apoptosis, and DNA methylation were measured to explore the regulatory mechanism of baicalin in developmental competence of mouse embryos in vitro.
Materials and Methods
Reagents and animals
All chemicals used in this study were purchased from Sigma Chemical Co. (St. Louis, MO, USA) unless otherwise stated. Baicalin (concentration ≥ 98%) was bought from the National Pharmaceutical Engineering Center (Jiangxi, China). Both, pregnant mare serum gonadotropin (PMSG) and human chorionic gonadotropin (hCG), were obtained from Ningbo Sansheng Pharmaceutical (Ningbo, China). Goat polyclonal anti-HSP70 (sc-1060), rabbit polyclonal anti-BCL-2 (sc-492), and anti-BAX (sc-526) antibodies were obtained from Santa Cruz Biotechnology (USA), while rabbit polyclonal anti- Caspase-3 (ab-90437) antibodies were obtained from Abcam (Cambridge, UK). Secondary polyclonal anti-goat and anti-rabbit fluorescein isothiocyanate-conjugated antibodies were purchased from Jackson ImmunoResearch Laboratories (USA). Sexually mature Kunming mice of both sexes (5–6 weeks old, with average body weight of 25 g) were purchased from the Experimental Animal Center of Shandong University. The mice were housed under a light-dark cycle of 12/12 h at approximately 23ºC with food and water provided ad libitum, after purchase. All mouse manipulations were performed with the approval of the Animal Care and Ethics Committee of Qingdao Agricultural University.
Embryo collection and culture
Female mice were injected intraperitoneally with 10 IU of PMSG, followed by 10 IU hCG 48 h later, for superovulation and were allowed to mate overnight. After 40, 64, 72, and 96 h post-hCG injection, the embryos in their 2- and 4-cell, morula and blastocyst stage, respectively, were flushed directly from the oviducts, and used as the in vivo control group. All embryos were rinsed with phosphate-buffered saline (PBS) and stored at –80ºC.
Superovulation of mice was conducted as described previously, and pronuclear embryos flushed from the oviducts, after 28 h post-hCG injection, were treated with hyaluronidase (1 mg/ml) to remove cumulus cells and were washed three times with PBS for subsequent culture in vitro. Pronuclear embryos were cultured in KSOM culture medium, and used as the in vitro-cultured control group (n = 246).
Baicalin was dissolved in KSOM culture medium. The pronuclear embryos cultured in KSOM medium supplemented with 2 (n = 204), 4 (n = 210), and 8 μg/ml (n = 201) of baicalin, respectively, for 96 h up to the blastocyst stage were used for the baicalin treatment groups. Each of the above-mentioned groups’ pronuclear embryos were cultured in 50 μl KSOM culture medium droplets, covered with mineral oil and cultured in vitro at 37ºC, 5.0% CO2, and 100% saturated humidity. The developmental rates for each stage of the embryos in each group were recorded every 24 h.
Blastocyst cell count
Blastocysts (n = 45) from each group were fixed in 4% (w/v) paraformaldehyde at room temperature (20–25ºC) for 30 min and washed with PBS containing 0.4% polyvinyl alcohol (PBS-PVA) three times, and permeabilized with PVA-PBS containing 1% TritonX-100 at room temperature for 40 min and washed with PVA-PBS three times. Then, the nuclei of blastocysts were stained with propidium iodide (PI, 10 μg/ml) in PVA-PBS at 37ºC for 10 min and the number of cells were counted using a fluorescence microscope.
Quantitative PCR
The total RNA was extracted from 50 blastocysts using RNeasy Micro Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. The purity and quantity of extracted RNA were determined using Biophotometer Plus (Eppendorf, Hamburg, Germany). The synthesis of cDNA was performed with 500 ng total RNA using First Strand cDNA Synthesis Kit (Fermentas, Waltham, USA). The levels of mRNA were measured by real-time PCR using SYBR Green I and the Light Cycler480 System (Roche Diagnostics, Basel, Switzerland). Each PCR mixture consisted of 2 µl of cDNA, 10 µl of SYBR Green PCR Master Mix (Roche), 1 µl of sense primer, and 1 µl of antisense primer (Sangon Biotech, Shanghai, China, Table 1) in a final volume of 20 µl. They were then subjected to the following conditions: 95ºC for 10 min, followed by 45 cycles at 95ºC for 10 sec, 60ºC for 30 sec, 72ºC for 60 sec in 96-well optical reaction plates (Roche), and the melting curves were analyzed at 65ºC and 97ºC after 45 cycles. Transcript levels presented for each gene were normalized to GAPDH levels, and the 2−ΔΔCt method was used to calculate mRNA levels, as reported by Schmittgen [21].
Table 1. Primer sequences used for the q-PCR assay.
| Gene | Primer sequences | PCR product size (bp) | GenBank [No.] |
| CDH1 | AACCCAAGCACGTATCAGGG | 142 | NM_009864.2 |
| ACTGCTGGTCAGGATCGTTG | |||
| GJA1 | CCCACCTTTGTGTCTTCCAT | 151 | NM_010288.3 |
| TTGCCTCCCTGATGCTAACT | |||
| HSP70 | TGTGTCGGGTCCTTCAGAG | 130 | NM_010479.2 |
| CACCTCCAAGTTCACCAACC | |||
| BAX | CGTGGTTGCCCTCTTCTACT | 110 | XM_006540584.1 |
| CACGGAGGAAGTCCAGTGTC | |||
| BCL-2 | CGACTTCTTCAGCATCAGGA | 103 | NM_009741.4 |
| TGAGCCACAGGGAGGTTCT | |||
| DNMT1 | TGGTGTTGTCTACCGACTGG | 118 | NM_001199431.1 |
| CAGGGTCTCGTTCACAGGAT | |||
| DNMT3a | TCCAAGACACCGCTAAGGTT | 129 | NM_007872.4 |
| TGAATCCCTACCAGCAAAGG | |||
| GAPDH | ACGGCACAGTCAAGGCAGAG | 183 | NM_008084 |
| GTGATGGCGTGGACAGTGGT |
Apoptosis assays
Blastocysts (n = 30) from the in vitro baicalin-treated and in vivo groups were stained with Hoechst 33342 (10 ng/ml) for 10 min and then washed three times in PBS before being mounted and examined using a fluorescence microscope.
Immunostaining
Blastocysts were fixed in 4% paraformaldehyde for 30 min and were permeabilized with PBS containing 1% Triton X-100 for 40 min at 25°C. They were then incubated in PBS containing 1.0% bovine serum albumin at 37°C for 1 h. The blastocysts were subsequently incubated overnight at 4°C with primary antibody (1:100 dilution) against HSP70, BAX, BCL-2, and CASP3. They were further incubated with secondary polyclonal anti-goat or anti-rabbit IgG (1:400 dilution) at 25°C for 1 h. The blastocysts were finally stained with PI at 37°C for 10 min, mounted onto slides, and examined using a confocal microscope.
Statistical analysis
All data were presented as mean ± standard deviation (SD) of at least three independent experiments. Differences between groups were evaluated by one-way ANOVA, followed by Tukey and Dunnett’s t-test using Graphpad Prism 5.01 (GraphPad Software). A difference at P < 0.05 was considered statistically significant.
Results
Effects of baicalin on the development of mouse embryos in vitro
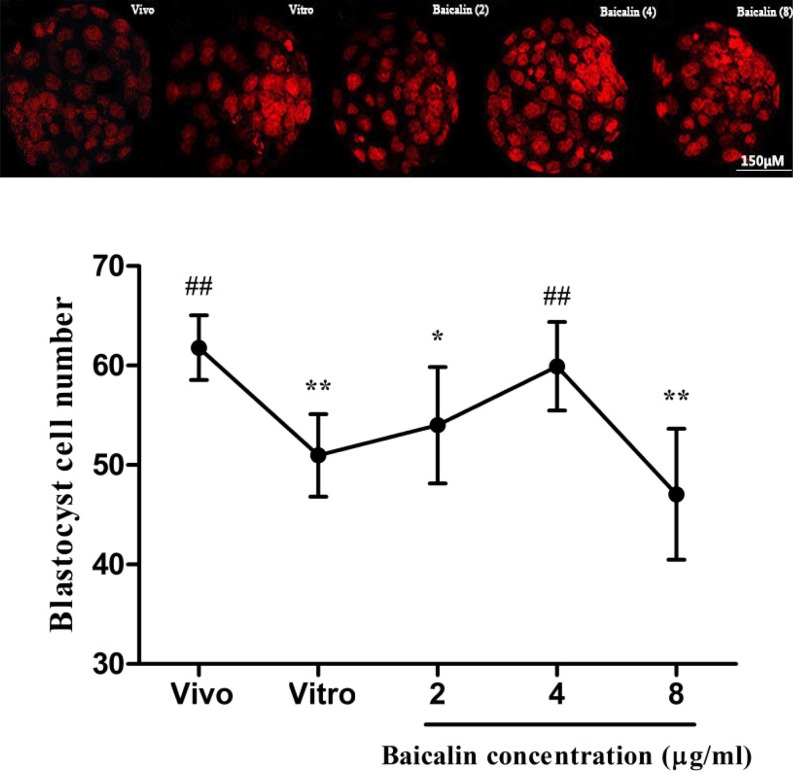
We observed that IVC embryos exhibited lower blastocyst developmental rates compared to the in vivo control group; however, baicalin increased these rates in IVC embryos as compared to the in vitro control group. The percentage of embryos that developed to the 2-cell stage were 92.6, 95.7, and 80.5% for the 2-, 4-, and 8-µg/ml baicalin treatment groups, respectively, compared to 89.6% in the in vitro control group (0.0 µg/ml baicalin; Table 2). The percentage of embryos that developed to the 4-cell stage were significantly higher (P < 0.01) in the 2- and 4- µg/ml baicalin treatment groups and lower (P < 0.01) in the 8-µg/ml baicalin treatment group, compared to the in vitro control group (Table 2). Both morula and blastocyst developmental rates were significantly higher (P < 0.01) in 2-µg/ml and 4-µg/ml baicalin treatment group compared to the in vitro control group. Meanwhile, the blastocyst developmental rate in the 4-µg/ml baicalin group was significantly higher (P < 0.01) than that in the 2-µg/ml baicalin group (Table 2). Furthermore, the number of blastocyst cells in the 4-µg/ml baicalin treatment group (59.93%) was significantly (P < 0.01) higher than those in vitro control group (50.98%) (Fig. 1). Based on the above results, the dose of 4 µg/ml baicalin was used for subsequent experiments.
Table 2. Effect of different concentrations of baicalin on the development of mouse embryos in vitro.
| Baicalin (µg/ml) | No. of 1-cell embryos | 2-cell rate (%) | 4-cell rate (%) | Morula rate (%) | Blastocyst rate (%) |
| In vivo control | – | 97 ± 1.19 Aa | 96 ± 2.43 A | 94.7 ± 3.87 A | 90.3 ± 5.07 A |
| 0 | 82 | 89.6 ± 4.56 Bb | 82.1 ± 3.87 B | 70.1 ± 2.05 Ba | 53.7 ± 3.45 Ba |
| 2 | 68 | 92.6 ± 2.87 BCc | 86.2 ± 3.36 B | 80.4 ± 4.74 C | 63.8 ± 2.56 C |
| 4 | 70 | 95.7 ± 4.40 ACa | 92.9 ± 3.98 A | 88.6 ± 5.32 D | 78.6 ± 4.08 D |
| 8 | 67 | 80.5 ± 3.34 D | 72.0 ± 5.08 C | 68.3 ± 4.00 Ea | 50.0 ± 4.32 Ea |
Different uppercase or lowercase letters in a column represent significant differences of P < 0.01 and P < 0.05, respectively.
Fig. 1.
Effect of different concentrations of baicalin on cell number of blastocyst in vitro. The nuclei of the blastocysts were stained with PI and the cell numbers were counted using a fluorescence microscope. * P < 0.05 vs. in vivo control group and ** P < 0.01 vs. in vivo control group; # P < 0.05 vs. IVC group and ## P < 0.01 vs. IVC group.
Effect of baicalin on gene expression levels of developmentally important genes in mouse embryos
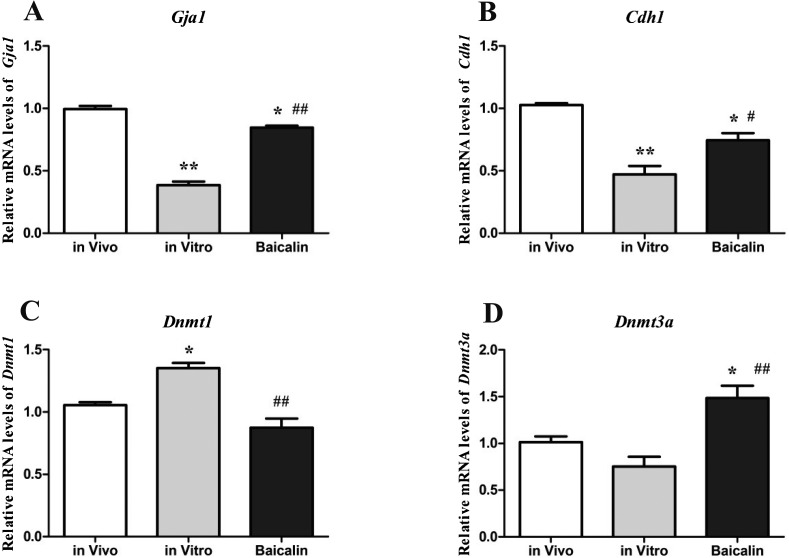
We observed that the mRNA expression levels of both, gap junction protein alpha 1 (Gja1) (Fig. 2-A) and E-cadherin (Cdh1) (Fig. 2-B), in IVC blastocysts were significantly decreased (P < 0.01) compared to the in vivo control group. However, their mRNA expression levels were significantly increased (P < 0.01 for Gja1 and P < 0.05 for Cdh1) in the baicalin-treated blastocysts compared to the in vitro control group.
Fig. 2.
Developmentally important genes measured by q-PCR. Expressions of Gja1, Cdh1, Dnmt1 and Dnmt3a genes in mouse blastocysts from the in vitro, baicalin-treated, and in vivo groups were measured to examine the effect of baicalin on mouse blastocyst quality. * P < 0.05 vs. in vivo control group and ** P < 0.01 vs. in vivo control group; # P < 0.05 vs. IVC group and ## P < 0.01 vs. IVC group.
The mRNA expression level of DNA methyltransferases 1 (Dnmt1) (Fig. 2-C) in IVC blastocysts was significantly increased (P < 0.05) compared to the in vivo control group. Moreover, its expression was significantly decreased (P < 0.01) in the baicalin-treated blastocysts compared to the in vitro control group. However, DNA methyltransferases 3a (Dnmt3a) mRNA expression (Fig. 2-D) in IVC control blastocysts was lower (P > 0.05) compared to the in vivo control group, while it was higher (P < 0.01) in baicalin-treated blastocysts compared to the in vitro control group and the in vivo control group (P < 0.05).
Effect of baicalin on cellular stress in mouse embryos
Heat Shock Protein 70 (Hsp70) mRNA and protein levels were determined by qPCR and immunostaining, respectively, to investigate the effect of baicalin on cellular stress in mouse blastocysts in vitro. We observed that mRNA expression of Hsp70 in IVC blastocysts was significantly increased (P < 0.01) compared to the in vivo control group, while it was significantly decreased (P < 0.01) in the baicalin-treated blastocysts compared to the in vitro control group. Consistent with the data on mRNA expression, weaker expression of HSP70 protein was observed in the baicalin-treated blastocysts than the in vitro control group (Fig. 3).
Fig. 3.
Relative Hsp70 gene and its protein (by immunostaining) expressions in mouse blastocysts (n = 30) from the in vitro, baicalin-treated, and in vivo groups. * P < 0.05 vs. in vivo control group and ** P < 0.01 vs. in vivo control group; # P < 0.05 vs. IVC group and ## P < 0.01 vs. IVC group.
Effect of baicalin on cellular apoptosis in mouse embryos
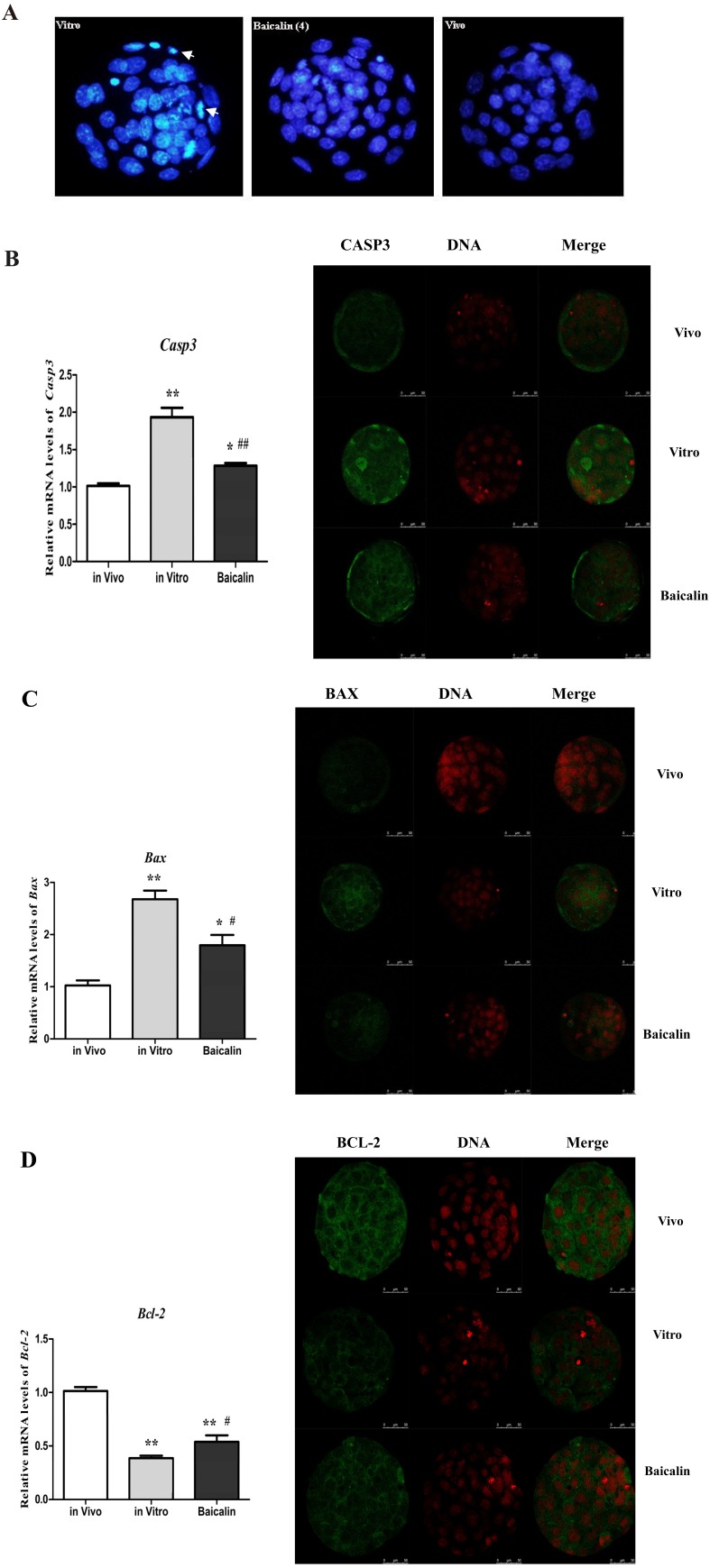
The blastocyst nuclei, stained with high-density fluorescence, showed fragmented or condensed morphology (Fig. 4-A, left) suggesting apoptosis. Baicalin-treated blastocysts had fewer apoptotic nuclei compared to the IVC blastocysts, while the in vivo group had the least. Cysteinyl aspartate specific protease-3 (Casp3), B-cell lymphoma protein 2 (Bcl-2), and BCL-2-associated X protein (Bax) mRNA and protein levels were determined by qPCR and immunostaining, respectively, to investigate the effect of baicalin on cellular apoptosis in mouse blastocysts in vitro. We found that the mRNA expression levels of Casp3 (Fig. 4-B) and Bax (Fig. 4-C) in IVC blastocysts were significantly increased (P < 0.01) compared to the in vivo control group. However, they were significantly decreased (P < 0.05) in the baicalin-treated blastocysts compared to the in vitro control group. Moreover, Bcl-2 mRNA expression of IVC control blastocysts was significantly decreased (P < 0.01) compared to the in vivo control group, while, its expression was significantly increased (P < 0.05) in the baicalin-treated blastocysts compared to the in vitro control group (Fig. 4-D). Consistent with the data on mRNA expression, weaker protein expression of CASP3 (Fig. 4-B) and BAX (Fig. 4-C) were observed in the baicalin-treated blastocysts than in the in vitro control group, while BCL-2 protein expression (Fig. 4-D) was higher in the baicalin-treated blastocysts compared to the in vitro control group.
Fig. 4.
Effect of baicalin on cellular apoptosis in mouse embryos. The nuclei of blastocysts (n = 30) were stained with Hoechst 33342 to examine cell apoptosis (Bar = 100 μm). Blastocysts from the in vitro (left), baicalin-treated (middle), and in vivo (right) groups showing higher degree of apoptosis of nuclei for in vitro group (white arrows) than the baicalin treated or in vivo group (A). Relative mRNA and protein expression levels of Caspase-3 (B), BAX (C) and BCL-2 (D), respectively, in mouse blastocysts (n = 30) from the in vitro, baicalin treated and in vivo groups. * P < 0.05 vs. in vivo control group and ** P < 0.01 vs. in vivo control group; # P < 0.05 vs. IVC group and ## P < 0.01 vs. IVC group.
Discussion
Previous studies have shown that the embryo quality and viability is mainly affected by in vitro culture conditions [4, 22,23,24], and high-quality embryos can be achieved through modifying culture conditions [24, 25]. Baicalin has been reported to promote embryo implantation and maintain pregnancy in mice [13]. This study has demonstrated that baicalin increased 2- and 4-cell embryonic cleavage rates, morula and blastocyst developmental rates, and promoted proliferation of mouse blastocysts cultured in vitro. Consequently, the percentage of mouse embryos that developed to the blastocyst stage increased compared to that reported for the IVC control group. Similarly, Sun et al. demonstrated that baicalin increased mouse blastocyst-hatching rates, and number of hatched blastocysts by inhibiting malondialdehyde (MDA) formation under culture condition [26]. Furthermore, Gao et al. have reported that baicalin increased pregnancy rates and fetal survival rates following transplantation of IVC blastocysts with no side effects on neonatal mice [27].
Apart from morphological criteria, expression levels of developmentally important genes have also been monitored to assess blastocyst quality [4, 20], which may explain how baicalin improves developmental competence of mouse embryos in vitro. The level of Gja1 mRNA, which is one of the gap junction proteins, has been shown to be higher in in vivo derived mouse and bovine blastocysts compared to those produced in vitro [15, 28], which is consistent with the higher quality blastocysts recorded in terms of cryotolerance [29]. Cell adhesion molecule Cdh1 has been reported to mediate the compaction process of morula and regulate subsequent blastocyst formation in mouse [30]. In the present study, baicalin increased Gja1 and Cdh1 gene expression in IVC embryos, which coincided with the higher blastocyst formation rates, similar to the injection of GJA1 or CDH1 double-stranded RNA into bovine zygotes decreased the percentage of zygotes developing to blastocyst by 18.4 and 16.3% in vitro, respectively [31]. It has also been reported that both CDH1 and GJA1 mRNA expression levels are positively correlated with the quality of bovine blastocysts [24]. Our results indicate that baicalin promotes in vitro development of mouse embryos by up-regulating Gja1 and Cdh1 gene expression.
It has been suggested that the in vitro-culture environment increases embryonic cellular stress and apoptosis [32], and embryos adapt to these conditions by adjusting their developmental program [22]. HSP70 is one of the earliest genes that is constitutively expressed in early embryonic development after the activation of the embryo’s transcriptome [33], and it was found that induced thermo-tolerance occurred significantly earlier in in vitro-cultured vs. in vivo-generated murine embryos [34]. Additionally, Hsp70.1 gene expression in IVC 2-cell stage embryos is 15 times higher than that in in vivo-collected 2-cell mouse embryos [33], as well as the blastocyst stage onward [35]. So, it has often been used to assess stress response in IVC embryos [36, 37]. Our observations that baicalin inhibited Hsp70 gene expression in in vitro culture may provide an explanation to how baicalin increases developmental competence of mouse embryos in vitro, because the up-regulated Hsp70 mRNA level, indicates increased embryonic stress and in turn IVC embryos decrease their blastocyst developmental rate [32]. However, it is not clear how baicalin inhibits Hsp70 gene expression in IVC mouse embryos, with a possibility that baicalin optimizes culture environment and reduces embryonic stress.
Apoptosis is an important physiological process for eliminating mutated or damaged cells under stressed condition [38], and the increased incidence of apoptosis in embryonic cells indicates the poor quality of IVC embryos [16]. It has been reported that apoptosis is more frequent in in vitro than in in vivo produced blastocysts [39]. In our study, baicalin reduced Bax and Bcl-2 gene expression in IVC embryos, suggesting an anti-apoptotic effect of baicalin, which protected embryos from apoptosis induced by in vitro culture conditions. Baicalin has also been reported to protect against heat-stress-induced apoptosis [40], and to decrease cellular apoptosis rates through down-regulation of BAX expression in bovine Sertoli cells [41]. Moreover, good-quality bovine blastocysts have lower HSP70 and BAX [42, 43] and higher BCL-2 mRNA levels compared to poor-quality embryos [38]. Our results indicate that baicalin increases developmental competence of mouse embryos in vitro by reducing cellular stress by inhibiting cellular apoptosis and Hsp70 expression.
DNA methylation, a mechanism of epigenetic reprogramming of the genome during embryogenesis [44,45,46], is accomplished through the activities of DNA methyltransferases (DNMTs) [47], which mainly focuses on two different methylation processes: maintenance and de novo, and are catalyzed by DNMT1 and DNMT3a, respectively [48]. The DNMT gene appears to be affected by in vitro culture conditions, which may result in aberrant DNA methylation [48, 49]. Our results in mice and those in bovine [17] and rabbit [49] preimplantation embryos demonstrate that IVC embryos show increased DNMT1 gene expression. Huan et al. also found that DNA methylation inhibitor (5-aza-dC, 5-Aza-2′-deoxycytidine) enhances development of porcine cloned embryos accompanied with lower DNMT1 and higher DNMT3a gene expression [50]. Interestingly, we found decreased Dnmt1 and increased Dnmt3a gene expressions following baicalin treatment of IVC embryos. Although the mechanism behind baicalin-induced up-regulation of Dnmt3a gene expression is unclear, higher Dnmt3a mRNA levels in mouse blastocyst than its early developmental stage to establish a new embryonic methylation pattern have been reported [47]. Moreover, the beneficial effects of melatonin on bovine embryo-quality have also been shown to be due to the increased DNMT3a gene expression [25]. Our study indicates that baicalin enhances developmental competence of mouse embryos in vitro via down-regulating Dnmt1 and up-regulating Dnmt3a gene expressions to improve DNA methylation.
In summary, this study indicates that in vitro culture conditions adversely affect blastocyst quality through modifications in the expression of developmentally important genes. However, baicalin improved the developmental competence of embryos and blastocyst-quality, to a level intermediate between IVC blastocysts and those derived in vivo, by improving the blastocyst developmental rates and DNA methylation, and inhibiting cellular apoptosis and HSP70 expression. This study provides a rudimentary experimental basis for the use of baicalin to optimize embryonic culture medium or to maintain pregnancy in female animals. However, considering the limitations of an in vitro study further investigation is necessary to confirm the protective effect of baicalin in vivo.
Conflict of interest: The authors declare that there are no conflicts of interest.
Acknowledgments
We are grateful to Professor Nazir Ahmad from University of Agriculture, Faisalabad, Pakistan for detailed correction of our manuscript. This work was supported by the National Natural Science Foundation of China (31572590, 31502138) and Shandong province (BS2015NY001), and Higher Educational Science and Technology Program of Shandong Province (J15LF03).
References
- 1.Brison DR, Roberts SA, Kimber SJ. How should we assess the safety of IVF technologies? Reprod Biomed Online 2013; 27: 710–721. [DOI] [PubMed] [Google Scholar]
- 2.Hribal R, Jewgenow K, Braun BC, Comizzoli P. Influence of culture medium composition on relative mRNA abundances in domestic cat embryos. Reprod Domest Anim 2013; 48: 245–251. [DOI] [PubMed] [Google Scholar]
- 3.Jousan FD, de Castro E Paula LA, Brad AM, Roth Z, Hansen PJ. Relationship between group II caspase activity of bovine preimplantation embryos and capacity for hatching. J Reprod Dev 2008; 54: 217–220. [DOI] [PubMed] [Google Scholar]
- 4.Lonergan P, Fair T, Corcoran D, Evans ACO. Effect of culture environment on gene expression and developmental characteristics in IVF-derived embryos. Theriogenology 2006; 65: 137–152. [DOI] [PubMed] [Google Scholar]
- 5.Fernández-González R, de Dios Hourcade J, López-Vidriero I, Benguría A, De Fonseca FR, Gutiérrez-Adán A. Analysis of gene transcription alterations at the blastocyst stage related to the long-term consequences of in vitro culture in mice. Reproduction 2009; 137: 271–283. [DOI] [PubMed] [Google Scholar]
- 6.Xu S, Meng QG. Advance of Research on Antipyretic Effect and Mechanism of Scutellaria. Journal of Chinese Medicine 2008; 06:1179–1181. [Google Scholar]
- 7.Martin J, Dusek J. The Baikal scullcap (Scutellaria baicalensis Georgi)—a potential source of new drugs. Ceska Slov Farm 2002; 51: 277–283.(in Czech) [PubMed] [Google Scholar]
- 8.Zhao ZZ, Xiao PG. Scutellaria baicalensis. In: The contemporary dictionary of medicinal plants. Hong Kong Jockey Club Institute of Chinese Medicine2006; 2: 391–396.
- 9.Huang WH, Lee AR, Yang CH. Antioxidative and anti-inflammatory activities of polyhydroxyflavonoids of Scutellaria baicalensis GEORGI. Biosci Biotechnol Biochem 2006; 70: 2371–2380. [DOI] [PubMed] [Google Scholar]
- 10.Ma AT, Zhong XH, Meng LG. Antiabortive Effect of Baicalin and Its Impact on Cytokines in Mouse. Acta Veterinaria et Zootechnica Sinica 2007; 38: 983–988. [Google Scholar]
- 11.Wang X, Zhao Y, Zhong X. Protective effects of baicalin on decidua cells of LPS-induced mice abortion. J Immunol Res 2014; 2014: 859812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song D, Guo J, Wang Y, Pan G, Li P, Zhang W, Song H. Ingredients of Shuanghuanglian injection powder permeation through placental barrier of rat in pregnancy. Zhongguo Zhong Yao Za Zhi 2010; 35: 1626–1629. (in Chinese) [DOI] [PubMed] [Google Scholar]
- 13.Zhang YM, Zhang YY, Bulbul A, Shan X, Wang XQ, Yan Q. Baicalin promotes embryo adhesion and implantation by upregulating fucosyltransferase IV (FUT4) via Wnt/beta-catenin signaling pathway. FEBS Lett 2015; 589: 1225–1233. [DOI] [PubMed] [Google Scholar]
- 14.Fleming TP, Kwong WY, Porter R, Ursell E, Fesenko I, Wilkins A, Miller DJ, Watkins AJ, Eckert JJ. The embryo and its future. Biol Reprod 2004; 71: 1046–1054. [DOI] [PubMed] [Google Scholar]
- 15.Lonergan P, Rizos D, Gutierrez-Adán A, Moreira PM, Pintado B, de la Fuente J, Boland MP. Temporal divergence in the pattern of messenger RNA expression in bovine embryos cultured from the zygote to blastocyst stage in vitro or in vivo. Biol Reprod 2003; 69: 1424–1431. [DOI] [PubMed] [Google Scholar]
- 16.Fabian D, Koppel J, Maddox-Hyttel P. Apoptotic processes during mammalian preimplantation development. Theriogenology 2005; 64: 221–231. [DOI] [PubMed] [Google Scholar]
- 17.Wrenzycki C, Niemann H. Epigenetic reprogramming in early embryonic development: effects of in-vitro production and somatic nuclear transfer. Reprod Biomed Online 2003; 7: 649–656. [DOI] [PubMed] [Google Scholar]
- 18.Chung YG, Ratnam S, Chaillet JR, Latham KE. Abnormal regulation of DNA methyltransferase expression in cloned mouse embryos. Biol Reprod 2003; 69: 146–153. [DOI] [PubMed] [Google Scholar]
- 19.Filliers M, Goossens K, Van Soom A, Merlo B, Pope CE, de Rooster H, Smits K, Vandaele L, Peelman LJ. Gene expression profiling of pluripotency and differentiation-related markers in cat oocytes and preimplantation embryos. Reprod Fertil Dev 2012; 24: 691–703. [DOI] [PubMed] [Google Scholar]
- 20.Cánepa MJ, Ortega NM, Monteleone MC, Mucci N, Kaiser GG, Brocco M, Mutto A. Expression profile of genes as indicators of developmental competence and quality of in vitro fertilization and somatic cell nuclear transfer bovine embryos. PLoS ONE 2014; 9: e108139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 2008; 3: 1101–1108. [DOI] [PubMed] [Google Scholar]
- 22.Wrenzycki C, Herrmann D, Niemann H. Messenger RNA in oocytes and embryos in relation to embryo viability. Theriogenology 2007; 68(Suppl 1): S77–S83. [DOI] [PubMed] [Google Scholar]
- 23.McElroy SL, Kim JH, Kim S, Jeong YW, Lee EG, Park SM, Hossein MS, Koo OJ, Abul Hashem MD, Jang G, Kang SK, Lee BC, Hwang WS. Effects of culture conditions and nuclear transfer protocols on blastocyst formation and mRNA expression in pre-implantation porcine embryos. Theriogenology 2008; 69: 416–425. [DOI] [PubMed] [Google Scholar]
- 24.Bao ZJ, Zhao S, Haq IU, Zeng SM. Recombinant bovine interferon-τ enhances in vitro development of bovine embryos by upregulating expression of connexin 43 and E-cadherin. J Dairy Sci 2014; 97: 6917–6925. [DOI] [PubMed] [Google Scholar]
- 25.Wang F, Tian X, Zhou Y, Tan D, Zhu S, Dai Y, Liu G. Melatonin improves the quality of in vitro produced (IVP) bovine embryos: implications for blastocyst development, cryotolerance, and modifications of relevant gene expression. PLoS ONE 2014; 9: e93641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun YC, Gao JM, Yu TQ, Chen W, Yang L, Mu X, Zhang JF, Fu WD, Li DD. Effects of three kinds of Chinese medicine effective constituents on mouse early embryos in vitro development. Chin J Vet Sci 2006; 26: 570–573. [Google Scholar]
- 27.Gao JM, Sun YC, Mu X, Chen W, Yu TQ, Yang L, Lu P, Zhang JF, Fan T, Su H. Study on effects of baicalin and ligustrazine on early embryos in vitro culture and the froze embryos transfer in mice. Acta Veterinaria et Zootechnica Sinica 2007; 38: 1120–1125. [Google Scholar]
- 28.Reuss B, Hellmann P, Traub O, Butterweck A, Winterhager E. Expression of connexin31 and connexin43 genes in early rat embryos. Dev Genet 1997; 21: 82–90. [DOI] [PubMed] [Google Scholar]
- 29.Rizos D, Gutiérrez-Adán A, Pérez-Garnelo S, De La Fuente J, Boland MP, Lonergan P. Bovine embryo culture in the presence or absence of serum: implications for blastocyst development, cryotolerance, and messenger RNA expression. Biol Reprod 2003; 68: 236–243. [DOI] [PubMed] [Google Scholar]
- 30.Maître JL, Niwayama R, Turlier H, Nédélec F, Hiiragi T. Pulsatile cell-autonomous contractility drives compaction in the mouse embryo. Nat Cell Biol 2015; 17: 849–855. [DOI] [PubMed] [Google Scholar]
- 31.Tesfaye D, Lonergan P, Hoelker M, Rings F, Nganvongpanit K, Havlicek V, Besenfelder U, Jennen D, Tholen E, Schellander K. Suppression of connexin 43 and E-cadherin transcripts in in vitro derived bovine embryos following culture in vitro or in vivo in the homologous bovine oviduct. Mol Reprod Dev 2007; 74: 978–988. [DOI] [PubMed] [Google Scholar]
- 32.Sananmuang T, Phutikanit N, Nguyen C, Manee-In S, Techakumphu M, Tharasanit T. In vitro culture of feline embryos increases stress-induced heat shock protein 70 and apoptotic related genes. J Reprod Dev 2013; 59: 180–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ealy AD, Hansen PJ. Induced thermotolerance during early development of murine and bovine embryos. J Cell Physiol 1994; 160: 463–468. [DOI] [PubMed] [Google Scholar]
- 34.Christians E, Campion E, Thompson EM, Renard JP. Expression of the HSP 70.1 gene, a landmark of early zygotic activity in the mouse embryo, is restricted to the first burst of transcription. Development 1995; 121: 113–122. [DOI] [PubMed] [Google Scholar]
- 35.Niemann H, Wrenzycki C. Alterations of expression of developmentally important genes in preimplantation bovine embryos by in vitro culture conditions: implications for subsequent development. Theriogenology 2000; 53: 21–34. [DOI] [PubMed] [Google Scholar]
- 36.Edwards JL, Ealy AD, Hansen PJ. Regulation of heat shock protein 70 synthesis by heat shock in the preimplantation murine embryo. Theriogenology 1995; 44: 329–337. [DOI] [PubMed] [Google Scholar]
- 37.Mortensen CJ, Choi YH, Ing NH, Kraemer DC, Vogelsang MM, Hinrichs K. Heat shock protein 70 gene expression in equine blastocysts after exposure of oocytes to high temperatures in vitro or in vivo after exercise of donor mares. Theriogenology 2010; 74: 374–383. [DOI] [PubMed] [Google Scholar]
- 38.Yang MY, Rajamahendran R. Expression of Bcl-2 and Bax proteins in relation to quality of bovine oocytes and embryos produced in vitro. Anim Reprod Sci 2002; 70: 159–169. [DOI] [PubMed] [Google Scholar]
- 39.Yang MY, Rajamahendran R. Involvement of apoptosis in bovine blastocysts produced in vitro. Theriogenology 1999; 51: 336. [Google Scholar]
- 40.Guo X, Chi S, Cong X, Li H, Jiang Z, Cao R, Tian W. Baicalin protects sertoli cells from heat stress-induced apoptosis via activation of the Fas/FasL pathway and Hsp72 expression. Reprod Toxicol 2015; 57: 196–203. [DOI] [PubMed] [Google Scholar]
- 41.Sun CL, Guo XT, Zhao Y, Chen JW, Cong X, Wang X, Jiang ZL, Gao SS, Tian WR. Effects of baicalin on expression of B-cellymphoma-2 (Bcl-2) and Bcl-2Assaciated X protein gene (Bax) and apoptosis rates of pig kidney proximal tubular (LLC-PK1) Cells subjected to heat stress. J Agric Biotechnol 2014; 12: 1553–1560. [Google Scholar]
- 42.Sagirkaya H, Misirlioglu M, Kaya A, First NL, Parrish JJ, Memili E. Developmental and molecular correlates of bovine preimplantation embryos. Reproduction 2006; 131: 895–904. [DOI] [PubMed] [Google Scholar]
- 43.Xiong XR, Wang LJ, Wang YS, Hua S, Zi XD, Zhang Y. Different preferences of IVF and SCNT bovine embryos for culture media. Zygote 2014; 22: 1–9. [DOI] [PubMed] [Google Scholar]
- 44.Young LE, Beaujean N. DNA methylation in the preimplantation embryo: the differing stories of the mouse and sheep. Anim Reprod Sci 2004; 82−83: 61–78. [DOI] [PubMed] [Google Scholar]
- 45.Ehrlich M. Expression of various genes is controlled by DNA methylation during mammalian development. J Cell Biochem 2003; 88: 899–910. [DOI] [PubMed] [Google Scholar]
- 46.Salilew-Wondim D, Fournier E, Hoelker M, Saeed-Zidane M, Tholen E, Looft C, Neuhoff C, Besenfelder U, Havlicek V, Rings F, Gagné D, Sirard MA, Robert C, Shojaei Saadi HA, Gad A, Schellander K, Tesfaye D. Genome-wide DNA methylation patterns of bovine blastocysts developed in vivo from embryos completed different stages of development in vitro. PLoS ONE 2015; 10: e0140467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vassena R, Dee Schramm R, Latham KE. Species-dependent expression patterns of DNA methyltransferase genes in mammalian oocytes and preimplantation embryos. Mol Reprod Dev 2005; 72: 430–436. [DOI] [PubMed] [Google Scholar]
- 48.Uysal F, Akkoyunlu G, Ozturk S. Dynamic expression of DNA methyltransferases (DNMTs) in oocytes and early embryos. Biochimie 2015; 116: 103–113. [DOI] [PubMed] [Google Scholar]
- 49.Reis e Silva AR, Bruno C, Fleurot R, Daniel N, Archilla C, Peynot N, Lucci CM, Beaujean N, Duranthon V. Alteration of DNA demethylation dynamics by in vitro culture conditions in rabbit pre-implantation embryos. Epigenetics 2012; 7: 440–446. [DOI] [PubMed] [Google Scholar]
- 50.Huan Y, Wang H, Wu Z, Zhang J, Liu Z, He H. The expression patterns of DNA methylation reprogramming related genes are associated with the developmental competence of cloned embryos after zygotic genome activation in pigs. Gene Expr Patterns 2015; 18: 1–7. [DOI] [PubMed] [Google Scholar]