Abstract
Density gradient centrifugation (DGC) and swim-up techniques have been reported for semen preparation in assisted reproductive techniques in humans. We investigated whether semen preparation using a combination of DGC and swim-up techniques could effectively decrease morphologically abnormal human sperms at the ultrastructural level. Semen samples were obtained from 16 infertile males and fractionated by swim-up following DGC. Ultrastructural abnormalities of sperms obtained from original semen, lower layer of swim-up following DGC, and upper layer of swim-up following DGC were analyzed by transmission electron microscopy. The correlation among ultrastructural head abnormality in sperms from the upper layer of swim-up, fertilization in in vitro fertilization, and pregnancy after embryo transfer was also investigated. Furthermore, sperms with DNA fragmentation in the samples processed via a combination of DGC and swim-up was assessed in a sperm chromatin structure assay. Ultrastructural abnormalities in sperm heads and tails in the upper layer after swim-up following DGC was the lowest among the three groups. Sperms with nuclear vacuoles were the most difficult to eliminate using a combination of DGC and swim-up in all types of head abnormalities. A negative correlation was confirmed between the fertilization rates of intracytoplasmic sperm injection and head abnormality of sperms obtained from the upper layer of the swim-up following DGC. Sperms with DNA fragmentation were effectively decreased using the combination of two techniques. In conclusion, the combination of DGC and swim-up effectively decreased the number of sperms with ultrastructural abnormalities both in the head and in the tail. However, sperms with ultrastructural abnormalities that cannot be completely decreased using a combination of DGC and swim-up may impair fertilization in some cases of intracytoplasmic sperm injection.
Keywords: Density gradient centrifugation, Sperm morphology, Sperm selection, Swim-up
Suitable spermatozoa are physiologically selected from the female reproductive tract during natural mating [1]. Spermatozoa with good quality should be selected to increase the probability of successful fertilization, viable embryos and healthy offspring. Therefore, several in vitro techniques have been developed to process spermatozoa for use in assisted reproductive technology (ART) [2, 3]. Sperms with an abnormal morphology and/or a low motility exhibit a reduced potential to attach to the zona pellucida of oocytes, and such sperm properties have been postulated to underlie the pathogenesis of low fertilization ability [4, 5]. Dead sperms and white blood cells secrete hazardous reactive oxygen species (ROS) [6,7,8,9] and often impair the viability of normal sperms. Thus, the separation of hazardous leucocytes, dead cells, and sperms with abnormal morphology from sperms with good quality in semen is critically important for in vitro fertilization (IVF) [10].
In most ART centers, semen preparation is performed by density gradient centrifugation (DGC) or the swim-up method to obtain normal sperm [10,11,12,13]. However, these methods cannot exclude all abnormal and dead sperms. Recent reports regarding motile sperm organelle morphology examination indicated that sperm with fine structural abnormalities remained in the sample after the DGC or swim-up method [13, 14]. In addition, abnormal sperms that were not eliminated impaired IVF outcomes [13,14,15]. This indicates that semen processing by the DGC or swim-up method alone was insufficient for reducing IVF failure.
The swim-up and DGC methods are based on different underlying principles. The swim-up method was designed to isolate highly motile sperms and the DGC method was designed to separate sperms depending on chromosome packaging [3, 12, 16,17,18]. Thus, combining both methods may improve sample quality. Ng et al. [19] revealed that sperms with good motility were effectively enriched when the methods were combined compared to the swim-up method alone; however, they did not report the elimination of sperms with ultrastructural abnormal morphologies. Ultrastructural analysis of morphology using a transmission electron microscope (TEM) can detect sperm abnormalities more precisely than a light microscope (LM), and IVF outcomes can be predicted based on the rate of ultrastructural sperm abnormalities [20, 21]. Therefore, analysis of the fine structure of sperms using TEM may lead to the establishment of a sperm washing protocol for sorting good-quality sperms. Moreover, the nuclear properties of sperms, including DNA fragmentation and maturity, have been shown to affect IVF outcomes [22, 23]. Although the DGC or swim-up method was shown to effectively decrease sperms with abnormal nuclear structures [24, 25], the efficacy of the combination of these methods remains unknown.
The objective of this study was to determine the efficacy of combining these two methods to decrease sperms with an abnormal nuclear structure at the microscopic level. Next, we investigated whether the number of sperms with abnormal morphology, identified at the ultrastructural level using TEM, could be reduced. The relationship between IVF outcomes and sperm morphology was also assessed.
Materials and Methods
This study was approved by the local ethics Institutional Review Board of IVF Namba Clinic and the Japan Society of Obstetrics and Gynecology (Registry No. 147). Semen samples used in the study were donated from volunteers and patients who provided informed consent.
Sperm preparation for morphology analysis using a LM and nucleus integrity
Semen samples from two fertile volunteers (40 and 30 years old, respectively) were evaluated in this study. Ejaculated semen samples were liquefied at 37°C for 30 min. Total sperm concentration, motile sperm concentration, and motile rate were analyzed using a Macklar chamber® (Sefi Medical Instruments, Haifa, Israel) under × 200 magnification. The sperm samples were prepared via four different methods as described below.
Washing: The semen was washed with culture medium (human tubal fluid-buffered with 21 mM HEPES containing 2.7 mM glucose and 0.33 mM pyruvate (Modified HTF Medium; Irvine Scientific, Irvine, CA, USA) containing 5% (v/v) synthetic serum substitute (SSS; Irvine Scientific) and centrifuged at 370 × g for 10 min. The supernatant was discarded and sperms were collected from the pellet.
Combination of washing and swim-up method (washing + swim-up): After washing, the supernatant was discarded and swim-up was performed by overlaying 1 ml of the culture medium on the washed sample at 37°C for 30 min. Sperms were collected from the upper fraction.
DGC: Semen was layered on 80% Percoll (Sigma Aldrich, St. Louis, MO, USA). Semen and the upper Percoll layer were then stirred and centrifuged at 600 × g for 30 min, and then the supernatant was discarded. Sperms were collected from the pellet.
Combination of DGC and swim-up methods: DGC was performed, the supernatant was discarded, and swim-up was similarly performed. Sperms were collected from the upper fraction.
The motility, nuclear integrity, and morphology of the sperm samples were analyzed and compared in five groups: 1) original, 2) washing, 3) washing + swim-up, 4) DGC, and 5) DGC + swim-up. Sperm morphology was evaluated according to Kruger’s strict criteria [26] as described below following fixation and Diff-Quick staining.
Type 1 (normal): the shape of the sperm head was a smooth oval configuration with a well-defined acrosome involving 40–70% of the sperm head, as well as the absence of neck, midpiece, or tail defects. No cytoplasmic droplets in the sperm head should be present. The length of a normal sperm head is 5–6 μm and the diameter is 2.5–3.5 μm.
● Type 2 (abnormal): sperm has slightly amorphous head, slightly elongated, loss of oval shape, acrosome 40–70%, and diameter of 2–2.5 μm.
● Type 3 (abnormal): sperm has thick neck but normal-shaped head.
● Type 4 (abnormal): sperm has an acrosome involving > 70% of the sperm head.
● Type 5 (abnormal): sperm has an acrosome involving < 30% of the sperm head.
● Type 6 (abnormal): sperm has round, small, large, tapered, or double head, double or coiled tail, or cytoplasmic droplets in the head.
Analysis of DNA fragmentation and immature nucleus structure
Semen samples from two fertile volunteers (40 and 30 years old, respectively) were used in this analysis, and nuclear integrity was compared among the five groups (original, washing, washing + swim-up, DGC, DGC + swim-up) prepared as described above. A sperm chromatin structure assay was performed according to Evenson’s method [27]. Briefly, 400 μl acid detergent solution (0.08 N HCl, 0.15 M NaCl, 0.1% Triton X-100) was added to 2 × 106 cells/ml of sperm sample. After a 30-sec incubation, 1.2 ml acridine orange staining solution was added to the sample and 10,000 events were analyzed using a FACSCaliburTM (BD Biosciences, Franklin Lakes, NJ, USA). The DNA fragmentation index (DFI) was obtained by analyzing the rate of sperm with DNA fragmentation in each sample. Nucleus immaturity (high DNA stainability; HDS) was determined by the uptake rate of an intercalator (acridine orange, Sigma Aldrich) in each sample.
Sperm preparation for ultrastructural morphological analysis
Specimens obtained from 16 infertile couples were subjected to IVF from April to May 2007 in the IVF Namba Clinic. The average ages of the female and male patients were 38.5 ± 4.3 (mean ± SD) and 39.0 ± 5.6 years old, respectively. The average parameters of conventional semen samples were as follows: total sperm concentration (87.9 ± 66.9) × 106 cells/ml; motile sperm rate 48.2 ± 15.7%; abnormal morphology 37.1 ± 12.7%. Samples obtained from the upper fraction after swim-up following DGC were used for IVF. Surplus samples from the upper layer of swim-up following DGC, lower layer of swim-up, and original semen were used for morphological analysis.
Morphological analysis using TEM
The ultrastructures of sperms from the original semen, upper fraction of swim-up following DGC, and lower fraction of swim-up following DGC were compared. TEM samples were prepared as described previously [28]. Briefly, collected sperm samples were fixed with 1% glutaraldehyde in a phosphate buffer (pH 7.4) at 4°C for 24 h. Samples were washed in the same buffer overnight, post-fixed in 1% osmium tetroxide at 4°C for 2 h, dehydrated in an increasing concentration of ethanol, and then immersed in n-butyl glycidyl ether at 25°C for 20 min. After washing in a mixture of 50% n-butyl glycidyl ether and 50% Epon 812 resin overnight, sperm samples were embedded in Epon 812 resin (TAAB Laboratories, Berkshire, UK). Ultra-thin sections (thickness: 90 nm) were stained with uranyl acetate for 10 min and Reynold’s lead citrate for 10 min, and then examined using a TEM (JEM-1011; JEOL, Tokyo, Japan).
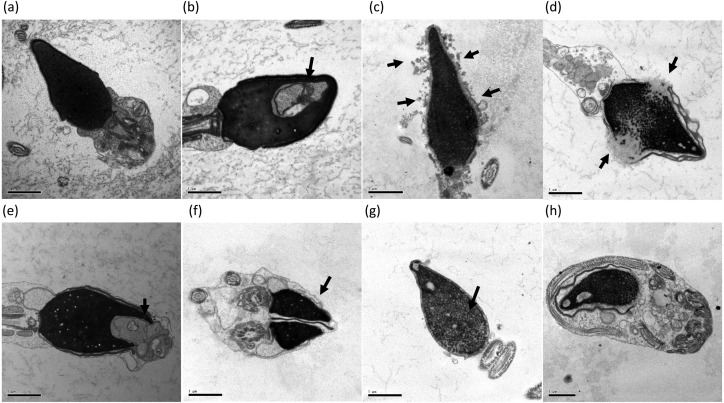
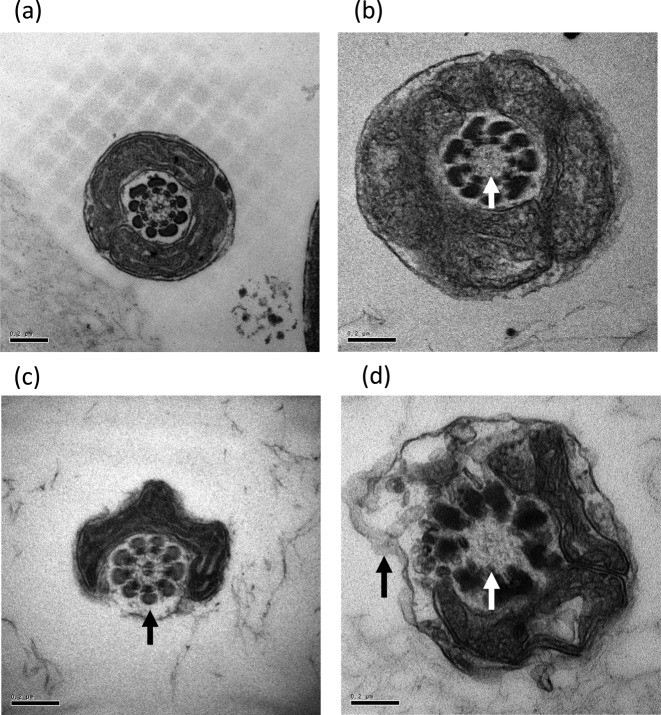
Head and tail structures of sperms were analyzed and any morphological abnormality was evaluated by TEM as described previously [29,30,31,32]. The head structure of sperms with nuclear vacuoles, low condensation of the nucleus, acrosomal alteration, disrupted membranes, deformity, immature structure, and double heads were all categorized as abnormal (Fig. 1). Sperms with tails lacking mitochondria and/or a pair of centriole microtubules were also considered abnormal (Fig. 2).
Fig. 1.
Classification of head compartment morphology. (a) Normal, (b) nuclear vacuole, (c) acrosomal alteration, (d) disrupted membrane, (e) head deformity, (f) double head, (g) condensation failure, (h) immature. Arrows indicate abnormal area. Bars: 1 μm.
Fig. 2.
Classification of tail compartment morphology. (a) Normal, (b) lack of centriole microtubule pair alone, (c) lack of mitochondria alone, (d) lack of both mitochondria and centriole microtubule pair. Arrows indicate abnormal area. Bars: 0.2 μm.
The heads and tails of 100 sperm specimens (a total of 4800 sections in each head and tail) were analyzed for the original semen, lower fraction, and upper fraction samples. The numbers of sperms with abnormal morphology were compared among samples. Further, the rate of sperms with head abnormalities decreased when a combination of DGC and swim-up methods was employed. The decreasing rate of sperms with abnormal morphology was calculated as follows: difference in the rate of abnormal sperm between original semen and after swim-up following DGC divided by the rate of abnormal sperm in original semen.
Relationship between abnormal sperm structure and IVF outcome
The correlation between the fertilization rate and rate of morphologically abnormal sperms in the upper fractions of swim-up following DGC was analyzed both in intra-cytoplasmic sperm injection (ICSI) and conventional IVF cycles (10 ICSI cycles and 6 conventional IVF cycles). Fertilization was determined by the confirmation of two pronuclei on day 1 after insemination. Embryo transfer (ET) was carried out in 14 patients (five cases of fresh ET and nine vitrified and warmed ET). Embryos were vitrified and warmed as previously described [33]. Eight patients received cleavage-stage ET and 4 received blastocyst transfer. Two patients received two-step ET [34]. The relationship between the pregnancy rate and rate of abnormal head morphology in the upper fraction after swim-up was also analyzed. Two weeks after ET, the plasma levels of human chorionic gonadotropin were measured using the ECLusys reagent HCGII STAT (Roche Diagnostics, Basel, Switzerland). A human chorionic gonadotropin level of more than 100 mIU/ml was diagnosed as pregnancy. To assess the influence of sperm head morphology on pregnancy, patients were divided into three groups, highly abnormal (> 20%), moderate (10–20%), and low (< 10%) according to the rate of sperms with head abnormality. Pregnancy rates were compared among the three groups.
Statistical analysis
The differences among groups were analyzed using the Tukey-Kramer test following ANOVA. Correlations were calculated by single regression analysis. Statcel 2, version 2 (OMS Ltd., Tokyo, Japan) was used for statistical analysis and a P value of < 0.05 was considered statistically significant.
Results
Analysis by LM
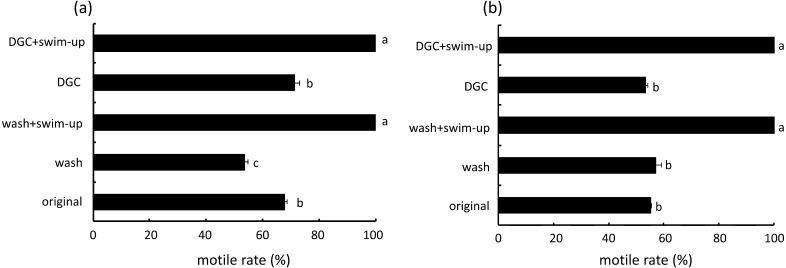
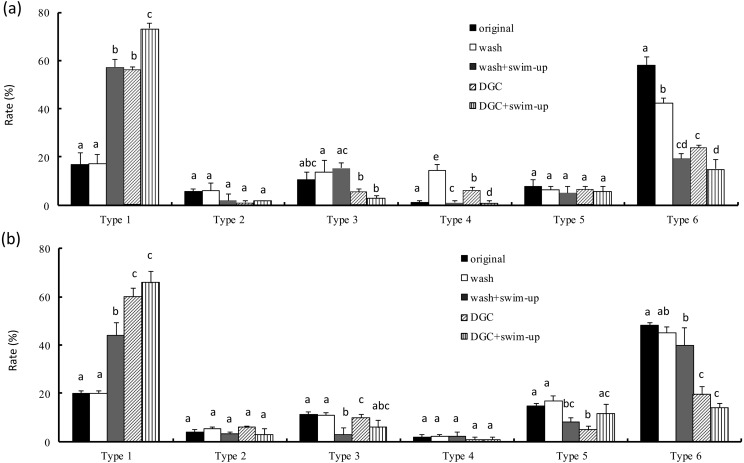
The total sperm concentrations in original semen of volunteer A and B were 49.1 × 106 and 76.7 × 106 cells/ml, respectively. The rates of motile sperms in the original semen of volunteer A and B were 67.8 and 55.3%, respectively. The rate of motile sperms in samples obtained by DGC alone was similar to that in the original semen (Fig. 3). The motility of motile sperms in samples obtained by a combination of DGC and swim-up was higher than that in the original semen and DGC alone. Morphological assessment of the samples from volunteer A revealed that type 1 sperms (normal) were significantly increased by a combination of DGC and swim-up (Fig. 4). Type 6 abnormal (severe abnormality) sperm in the original semen was higher than that in other types. The rate of type 6 sperms obtained after swim-up following DGC and was the lowest in all groups. A similar pattern was observed in samples from volunteer B.
Fig. 3.
Rate of motile sperm after four different treatments. (a) and (b) indicate the semen data from different fertile men: (a): volunteer A; (b): volunteer B. DGC = Percoll density gradient centrifugation. Wash = original semen washed by HTF. The conventional semen parameters were: (a): total sperm concentration 49.1 × 106 cells/ml, motile sperm rate 55.3%, (b): total sperm concentration 76.7 × 106 cells/ml, motile sperm rate 67.8%. a–d Different letters indicate statistically significant differences (P < 0.05) by the Tukey-Kramer method following ANOVA.
Fig. 4.
Rate of abnormal morphology at the LM level after different four treatments. (a) and (b) indicate the semen data from different fertile men: (a): volunteer A; (b): volunteer B. DGC = Percoll density gradient centrifugation. Wash = original semen washed by HTF. Vertical axis indicates rate of each type of morphology. Type 1 indicates sperm with normal morphology. Type 6 indicates sperm with severe abnormal morphology. a-dDifferent letters in each abnormal type indicate statistically significant differences (P < 0.05) by the Tukey-Kramer method following ANOVA.
Elimination of sperms with DNA fragmentation and an immature nuclear structure
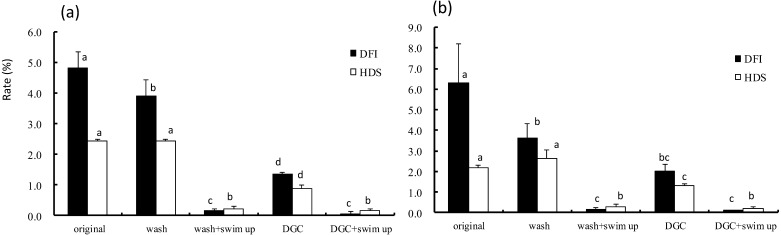
DFI of the sperm from volunteer A decreased by washing or DGC (Fig. 5). Nearly all sperms showing DNA fragmentation or an immature nucleus (HDS) were eliminated by a combination of DGC and swim-up or by a combination of washing and swim-up. A similar pattern was observed in samples from volunteer B.
Fig. 5.
DFI and HDS values after four different treatments. (a) and (b) indicate the semen data from different fertile men: (a): volunteer A; (b): volunteer B. DGC = Percoll density gradient centrifugation. Wash = original semen washed by HTF. Vertical axis indicates the value of DFI and HDS. The values of DFI and HDS among four different treatments were compared. a–d Different letters in DFI or HDS indicate statistically significant differences (P < 0.05) by the Tukey-Kramer method following ANOVA.
Sperm ultrastructural analysis using a TEM
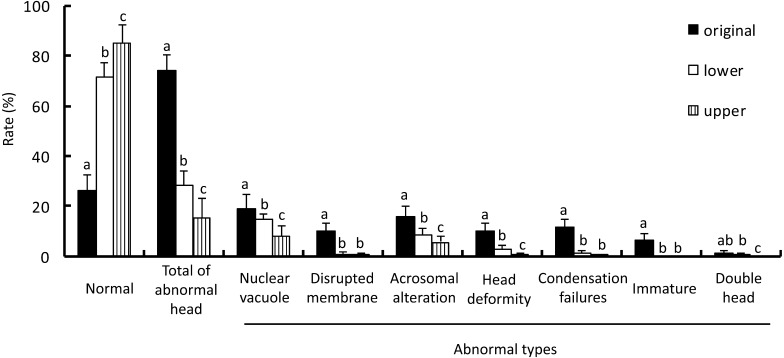
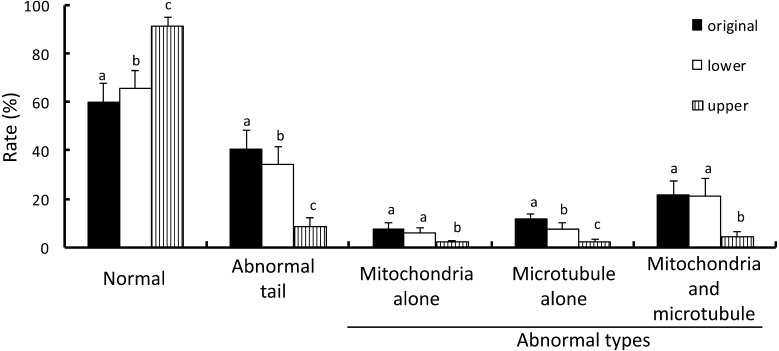
The rate of sperm with ultrastructural head abnormality obtained from the upper fractions of swim-up following DGC was the lowest among the 3 groups (Fig. 6). Samples from the upper fractions of swim-up following DGC contained a significantly lower number of morphologically abnormal sperms including nuclear vacuoles, disrupted membrane, acrosomal alteration, head deformity, condensation failures, immaturity, and double head than the original semen. The rates of sperms showing nuclear vacuoles, disrupted membranes, acrosomal alteration, head deformity, condensation failure, and immaturity from the lower fractions of swim-up following DGC were lower than in the original samples. Sperms with nuclear vacuoles, acrosomal alteration, head deformity, and double heads were observed more frequently in the lower fractions of swim-up following DGC than in the upper fractions. The proportion of sperm with tail abnormality (abnormal mitochondria, abnormal microtubules, and abnormality in both mitochondria and microtubules) in the samples obtained from the upper fractions of swim-up following DGC was the lowest among the 3 groups (Fig. 7). The lower fractions of swim-up following DGC contained significantly fewer sperms with microtubule abnormality than the original semen. In contrast, the number of sperms with abnormal mitochondria and abnormalities in both mitochondria and microtubules obtained from the lower fractions of swim-up following DGC was similar to that in the original semen.
Fig. 6.
Rate of sperm with normal and abnormal head morphology at the ultrastructural level in each fraction. The specimens were obtained from original semen and the lower and the upper fractions after DGC and swim-up. The head morphology of 100 sperms from each fraction was observed. Vertical axis indicates the rate of normal morphology and each type of head abnormality. The rates of normal and each type of abnormal morphology among the three fractions were compared. a–c Different letters in each morphology indicate statistically significant differences (P < 0.05) by the Tukey-Kramer method following ANOVA.
Fig. 7.
Rate of sperm with normal and abnormal tail morphology at the ultrastructural level in each fraction. The specimens were obtained from original semen and the lower and the upper fractions after DGC and swim-up. The tail morphology of 100 sperms in each fraction was observed. Vertical axis indicates the rate of normal morphology and each type of tail abnormality. The rates of normal and each type of abnormal morphology among the three fractions were compared. a–c Different letters in each morphology indicate statistically significant differences (P < 0.05) by the Tukey-Kramer method following ANOVA.
Elimination of sperms with abnormal head morphology by a combination of DGC and swim-up
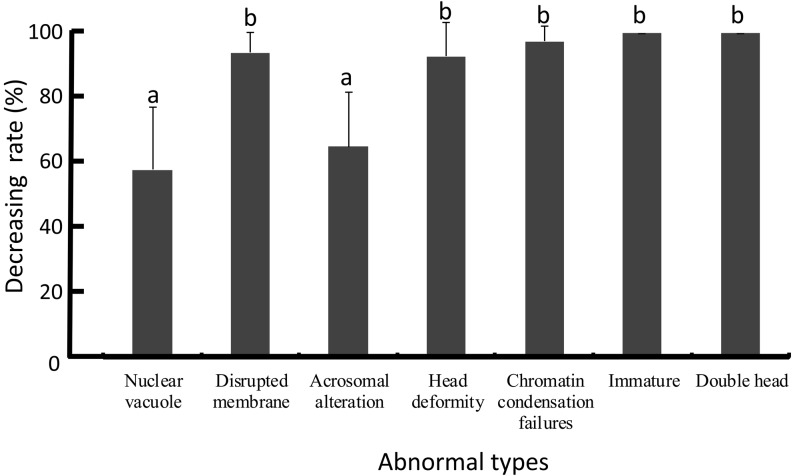
The lowering rates of abnormal sperms with nuclear vacuoles and acrosomal alteration using a combination of DGC and swim-up was poorer than for other abnormality types including disrupted membrane, head deformity, chromatin condensation failure, immaturity, and double head (Fig. 8).
Fig. 8.
Decreasing rate of each type of abnormal head morphology after combination treatment. a,b Different letters indicate statistically significant differences (P < 0.05) by the Tukey-Kramer method following ANOVA.
Relationship between ultrastructural abnormality of sperms and IVF outcomes
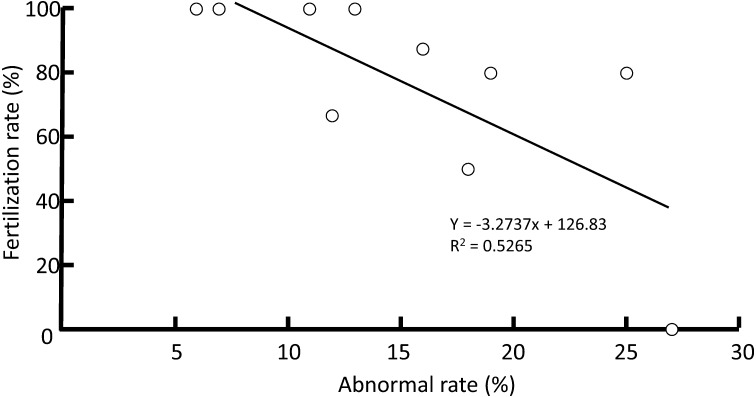
A negative correlation was observed between the rate of head abnormality in the upper fractions following DGC and fertilization in ICSI cycles (R2 = 0.53, P < 0.05, n = 10) (Fig. 9). However, there was no relationship between these parameters in conventional IVF cycles (R2 = 0.04, n = 6) and in all cycles (R2 = 0.16, n = 16). The pregnancy rate of patients with sperms categorized as low was 60% (n = 5) and the rate of patients categorized as moderate was 42.9% (n = 7), respectively. In contrast, pregnancy was never achieved in patients with their sperms categorized as highly abnormal (n = 4).
Fig. 9.
Correlation between the rate of total abnormal head morphology in upper fractions and fertilization rates in ICSI cycles. A significant correlation (R2 = 0.53, P < 0.05, n = 10) was found by simple regression analysis.
Discussion
The present study revealed that a combination of DGC and swim-up effectively enriched sperms showing normal morphology, even at the ultrastructural level, compared to DGC alone. There was a significant negative correlation between the rate of total abnormal head morphology in the upper fractions of swim-up and fertilization rate of ICSI.
The combination of DGC and swim-up was also more effective in eliminating sperms with DNA fragmentation than DGC alone. Although Xue et al. [35] indicated that sperms with DNA fragmentation were excluded by either swim-up or DGC alone, our data showed that swim-up was more effective in eliminating such sperms than DGC. Previous studies demonstrated that sperms with intact DNA were incubated for a longer period of time with cells that produced ROS in the process of swim-up than in DGC, resulting in a higher level of DNA damage to sperms in the samples processed by swim-up compared to DGC [25, 26]. Sperms with intact DNA can be efficiently separated from cells producing ROS by using DGC [10]. In addition, Liu et al. [36] reported that DNA fragmentation of sperms with lower motility was more severe than for highly motile sperms. The motility of sperms in the samples processed by the combination of DGC and swim-up was higher than for DGC alone in the present study. For the combination technique, swim-up was performed after the elimination of harmful components by DGC. Therefore, sperms with not only high motility but also integrity of DNA were obtained using both techniques as compared to DGC alone. Recently, a Korean group reported no difference in the clinical outcome between the combination technique and swim-up only [37]. Although we did not assess the clinical outcomes using sperm obtained after only swim-up, conventional observation under a microscope (× 400) may allow morphologically abnormal sperm that were not reduced without DGC to be removed from sperms for ICSI.
Head abnormalities at ultrastructural level decreased with the number of washing steps. Sperms with normal mature morphology were screened during DGC, which separates cells based on chromosome packaging [10, 11]. As a result, sperms with a disrupted membrane, chromatin condensation failure, and immaturity were excluded during DGC. Sperm head abnormality at the ultrastructural level also decreased during swim-up, which is consistent with a previous report showing that swim-up can eliminate sperms with ultrastructural morphologies exhibiting signs of apoptosis and necrosis [38]. Accordingly, our data showed that a combination of DGC and swim-up more effectively decreased sperms with ultrastructural head abnormality.
Abnormal sperm tails such as mitochondrial and microtubular abnormalities decreased during swim-up, although DGC was not effective for reducing abnormal sperm tails. Sperms with low motility have been shown to have severe ultrastructural tail abnormalities as compared to highly motile sperms [20]. Thus, sperms with high motility and normal tail morphology can be enriched using the combination technique. Taken together, sperms with head abnormality can be primarily reduced by DGC, and sperms with tail and/or head abnormality can be further reduced by swim-up. Our data shows that the combination technique is effective for enriching sperms with normal head and tail morphologies.
Nuclear vacuoles and acrosomal alteration were not effectively reduced by DGC and swim-up as compared to other head abnormalities. It has been shown that 70–90% of sperms processed by swim-up displayed nuclear vacuoles [39] and that 90% of sperms processed by DGC had vacuoles [40], suggesting that most sperms with vacuoles cannot be excluded by either swim-up or DGC alone. Thus, the decreasing rate of sperms with nuclear vacuoles after the combination technique may be poor compared to other head abnormalities. For IVF outcomes, we found a significant negative correlation between fertilization rate after ICSI and ultrastructural head abnormality, although no correlation between fertilization rate of conventional IVF and head abnormality was detected. It has been shown that sperms with the competence to adhere to the zona pellucida had intact DNA and normal morphology at the LM level compared to non-adherent sperms [5, 41]. Normal sperms can be selected in conventional IVF through granulosa cells and zona pellucida surrounding oocytes, but not in ICSI; therefore, there may be no correlation with conventional IVF. It has been shown that ICSI of morphologically abnormal sperm resulted in poor fertilization compared to morphologically normal sperms [3, 42]. Thus, it may be difficult in ICSI to sort normal sperms at the ultrastructural level compared to conventional IVF, resulting in poor fertilization.
Sperm selection techniques, such as DGC and swim-up methods, were found to be effective for IVF or artificial insemination in cattle [43, 44], pigs [45], and horses [46]. A combination of DGC and swim-up has been also suggested to remove bovine viral diarrhea virus in artificially contaminated semen in cattle [47]. Thus, DGC treatment followed by swim-up may be an effective measure for obtaining high-quality sperm for successful fertilization, viable embryos, and healthy offspring in mammals.
In the present study, sperms with morphologically abnormal heads and tails at the ultrastructural level were effectively reduced using a combination of DGC and swim-up compared to using DGC alone. Furthermore, sperms with abnormal nuclear structures were also reduced. However, not all types of abnormal sperm heads were effectively decreased. A few sperms with abnormal morphology were observed, such as nuclear vacuoles at the ultrastructural level even after swim-up following DGC. The results of the present study suggest that a more precise sperm selection technique that can efficiently reduce sperms with ultrastructural abnormalities is required to improve ICSI outcomes.
Acknowledgments
This work was supported in part by a grant from the Japan Agency for Medical Research and Development to SH and YM.
References
- 1.Suarez SS. Interactions of spermatozoa with the female reproductive tract: inspiration for assisted reproduction. Reprod Fertil Dev 2007; 19: 103–110. [DOI] [PubMed] [Google Scholar]
- 2.Morrell J, Rodríguez-Martínez H. Biomimetic techniques for improving sperm quality in animal breeding: a review. The Open Andrology J. 2009; 1: 1–9. [Google Scholar]
- 3.Henkel RR, Schill WB. Sperm preparation for ART. Reprod Biol Endocrinol 2003; 1: 108–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mullins KJ, Saacke RG. Study of the functional anatomy of bovine cervical mucosa with special reference to mucus secretion and sperm transport. Anat Rec 1989; 225: 106–117. [DOI] [PubMed] [Google Scholar]
- 5.Liu DY, Baker HW. Morphology of spermatozoa bound to the zona pellucida of human oocytes that failed to fertilize in vitro. J Reprod Fertil 1992; 94: 71–84. [DOI] [PubMed] [Google Scholar]
- 6.Lo Monte G, Murisier F, Piva I, Germond M, Marci R. Focus on intracytoplasmic morphologically selected sperm injection (IMSI): a mini-review. Asian J Androl 2013; 15: 608–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saleh RA, Agarwal A, Kandirali E, Sharma RK, Thomas AJ, Nada EA, Evenson DP, Alvarez JG. Leukocytospermia is associated with increased reactive oxygen species production by human spermatozoa. Fertil Steril 2002; 78: 1215–1224. [DOI] [PubMed] [Google Scholar]
- 8.Aitken RJ, Bronson R, Smith TB, De Iuliis GN. The source and significance of DNA damage in human spermatozoa; a commentary on diagnostic strategies and straw man fallacies. Mol Hum Reprod 2013; 19: 475–485. [DOI] [PubMed] [Google Scholar]
- 9.Aitken RJ, Baker MA. Causes and consequences of apoptosis in spermatozoa; contributions to infertility and impacts on development. Int J Dev Biol 2013; 57: 265–272. [DOI] [PubMed] [Google Scholar]
- 10.Morrell JM, Rodriquez-M H. Practical applications of sperm selection techniques as a tool for improving reproductive efficiency. Vet Med Int 2011; 2011: 1−9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. Sperm preparation techniques. In: WHO laboratory manual for the examination of human semen and sperm-cervical mucus interaction. 4th ed. Cambridge: Cambrige University Press; 2000: 34–35.
- 12.Mortimer D. Sperm preparation methods. J Androl 2000; 21: 357–366. [PubMed] [Google Scholar]
- 13.Bartoov B, Berkovitz A, Eltes F, Kogosowski A, Menezo Y, Barak Y. Real-time fine morphology of motile human sperm cells is associated with IVF-ICSI outcome. J Androl 2002; 23: 1–8. [DOI] [PubMed] [Google Scholar]
- 14.Monqaut AL, Zavaleta C, López G, Lafuente R, Brassesco M. Use of high-magnification microscopy for the assessment of sperm recovered after two different sperm processing methods. Fertil Steril 2011; 95: 277–280. [DOI] [PubMed] [Google Scholar]
- 15.Obara H, Shibahara H, Tsunoda H, Taneichi A, Fujiwara H, Takamizawa S, Idei S, Sato I. Prediction of unexpectedly poor fertilization and pregnancy outcome using the strict criteria for sperm morphology before and after sperm separation in IVF-ET. Int J Androl 2001; 24: 102–108. [DOI] [PubMed] [Google Scholar]
- 16.Englert Y, Van den Bergh M, Rodesch C, Bertrand E, Biramane J, Legreve A. Comparative auto-controlled study between swim-up and Percoll preparation of fresh semen samples for in-vitro fertilization. Hum Reprod 1992; 7: 399–402. [DOI] [PubMed] [Google Scholar]
- 17.Chen SU, Ho HN, Chen HF, Chao KH, Lin HR, Huang SC, Lee TY, Yang YS. Comparison between a two-layer discontinuous Percoll gradient and swim-up for sperm preparation on normal and abnormal semen samples. J Assist Reprod Genet 1995; 12: 698–703. [DOI] [PubMed] [Google Scholar]
- 18.Prakash P, Leykin L, Chen Z, Toth T, Sayegh R, Schiff I, Isaacson K. Preparation by differential gradient centrifugation is better than swim-up in selecting sperm with normal morphology (strict criteria). Fertil Steril 1998; 69: 722–726. [DOI] [PubMed] [Google Scholar]
- 19.Ng FL, Liu DY, Baker HW. Comparison of Percoll, mini-Percoll and swim-up methods for sperm preparation from abnormal semen samples. Hum Reprod 1992; 7: 261–266. [DOI] [PubMed] [Google Scholar]
- 20.Visco V, Raffa S, Elia J, Delfino M, Imbrogno N, Torrisi MR, Mazzilli F. Morphological sperm defects analyzed by light microscopy and transmission electron microscopy and their correlation with sperm motility. Int J Urol 2010; 17: 259–266. [DOI] [PubMed] [Google Scholar]
- 21.Małgorzata K, Depa-Martynów M, Butowska W, Filipiak K, Pawelczyk L, Jedrzejczak P. Human spermatozoa ultrastructure assessment in the infertility treatment by assisted reproduction technique. Arch Androl 2007; 53: 297–302. [DOI] [PubMed] [Google Scholar]
- 22.Virro MR, Larson-Cook KL, Evenson DP. Sperm chromatin structure assay (SCSA) parameters are related to fertilization, blastocyst development, and ongoing pregnancy in in vitro fertilization and intracytoplasmic sperm injection cycles. Fertil Steril 2004; 81: 1289–1295. [DOI] [PubMed] [Google Scholar]
- 23.Boe-Hansen GB, Fedder J, Ersbøll AK, Christensen P. The sperm chromatin structure assay as a diagnostic tool in the human fertility clinic. Hum Reprod 2006; 21: 1576–1582. [DOI] [PubMed] [Google Scholar]
- 24.Amiri I, Ghorbani M, Heshmati S. Comparison of the DNA fragmentation and the sperm parameters after processing by the density gradient and the swim up methods. J Clin Diagn Res 2012; 6: 1451–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakkas D, Manicardi GC, Tomlinson M, Mandrioli M, Bizzaro D, Bianchi PG, Bianchi U. The use of two density gradient centrifugation techniques and the swim-up method to separate spermatozoa with chromatin and nuclear DNA anomalies. Hum Reprod 2000; 15: 1112–1116. [DOI] [PubMed] [Google Scholar]
- 26.Kruger TF, Acosta AA, Simmons KF, Swanson RJ, Matta JF, Oehninger S. Predictive value of abnormal sperm morphology in in vitro fertilization. Fertil Steril 1988; 49: 112–117. [DOI] [PubMed] [Google Scholar]
- 27.Evenson DP, Jost LK, Marshall D, Zinaman MJ, Clegg E, Purvis K, de Angelis P, Claussen OP. Utility of the sperm chromatin structure assay as a diagnostic and prognostic tool in the human fertility clinic. Hum Reprod 1999; 14: 1039–1049. [DOI] [PubMed] [Google Scholar]
- 28.Hashimoto S, Suzuki N, Yamanaka M, Hosoi Y, Ishizuka B, Morimoto Y. Effects of vitrification solutions and equilibration times on the morphology of cynomolgus ovarian tissues. Reprod Biomed Online 2010; 21: 501–509. [DOI] [PubMed] [Google Scholar]
- 29.Collodel G, Moretti E, Capitani S, Estenoz M, Manca D, Piomboni P, Baccetti B. Ultrastructural sperm study in infertile males with microdeletions of Y chromosome. J Submicrosc Cytol Pathol 2006; 38: 45–50. [PubMed] [Google Scholar]
- 30.Baccetti B, Burrini AG, Collodel G, Piomboni P, Renieri T. A “miniacrosome” sperm defect causing infertility in two brothers. J Androl 1991; 12: 104–111. [PubMed] [Google Scholar]
- 31.Bourgain C, Nagy ZP, De Zutter H, Van Ranst H, Nogueira D, Van Steirteghem AC. Ultrastructure of gametes after intracytoplasmic sperm injection. Hum Reprod 1998; 13(Suppl 1): 107–116. [DOI] [PubMed] [Google Scholar]
- 32.Chemes HE, Alvarez Sedo C. Tales of the tail and sperm head aches: changing concepts on the prognostic significance of sperm pathologies affecting the head, neck and tail. Asian J Androl 2012; 14: 14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuwayama M, Vajta G, Ieda S, Kato O. Comparison of open and closed methods for vitrification of human embryos and the elimination of potential contamination. Reprod Biomed Online 2005; 11: 608–614. [DOI] [PubMed] [Google Scholar]
- 34.Goto S, Shiotani M, Kitagawa M, Kadowaki T, Noda Y. Effectiveness of two-step (consecutive) embryo transfer in patients who have two embryos on day 2: comparison with cleavage-stage embryo transfer. Fertil Steril 2005; 83: 721–723. [DOI] [PubMed] [Google Scholar]
- 35.Xue X, Wang WS, Shi JZ, Zhang SL, Zhao WQ, Shi WH, Guo BZ, Qin Z. Efficacy of swim-up versus density gradient centrifugation in improving sperm deformity rate and DNA fragmentation index in semen samples from teratozoospermic patients. J Assist Reprod Genet 2014; 31: 1161–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu DY, Liu ML. Clinical value of sperm DNA damage should be assessed in motile sperm fraction rather than whole ejaculated sperm. Fertil Steril 2013; 99: 367–371. [DOI] [PubMed] [Google Scholar]
- 37.Kim EK, Kim EH, Kim EA, Lee KA, Shin JE, Kwon H. Comparison of the effect of different media on the clinical outcomes of the density-gradient centrifugation/swim-up and swim-up methods. Clin Exp Reprod Med 2015; 42: 22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Piomboni P, Bruni E, Capitani S, Gambera L, Moretti E, La Marca A, De Leo V, Baccetti B. Ultrastructural and DNA fragmentation analyses in swim-up selected human sperm. Arch Androl 2006; 52: 51–59. [DOI] [PubMed] [Google Scholar]
- 39.Fekonja N, Štrus J, Tušek Žnidarič M, Knez K, Vrtacnik Bokal E, Verdenik I, Virant-Klun I. Clinical and structural features of sperm head vacuoles in men included in the in vitro fertilization programme. Biomed Res Int 2014; 2014: 927841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oliveira JB, Cavagna M, Petersen CG, Mauri AL, Massaro FC, Silva LF, Baruffi RL, Franco JG., Jr Pregnancy outcomes in women with repeated implantation failures after intracytoplasmic morphologically selected sperm injection (IMSI). Reprod Biol Endocrinol 2011; 9: 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu DY, Baker HW. Human sperm bound to the zona pellucida have normal nuclear chromatin as assessed by acridine orange fluorescence. Hum Reprod 2007; 22: 1597–1602. [DOI] [PubMed] [Google Scholar]
- 42.De Vos A, Van De Velde H, Joris H, Verheyen G, Devroey P, Van Steirteghem A. Influence of individual sperm morphology on fertilization, embryo morphology, and pregnancy outcome of intracytoplasmic sperm injection. Fertil Steril 2003; 79: 42–48. [DOI] [PubMed] [Google Scholar]
- 43.Parrish JJ, Krogenaes A, Susko-Parrish JL. Effect of bovine sperm separation by either swim-up or Percoll method on success of in vitro fertilization and early embryonic development. Theriogenology 1995; 44: 859–869. [DOI] [PubMed] [Google Scholar]
- 44.Samardzija M, Karadjole M, Getz I, Makek Z, Cergolj M, Dobranic T. Effects of bovine spermatozoa preparation on embryonic development in vitro. Reprod Biol Endocrinol 2006; 4: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sjunnesson YC, Morrell JM, González R. Single layer centrifugation-selected boar spermatozoa are capable of fertilization in vitro. Acta Vet Scand 2013; 55: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lindahl J, Dalin AM, Stuhtmann G, Morrell JM. Stallion spermatozoa selected by single layer centrifugation are capable of fertilization after storage for up to 96 h at 6°C prior to artificial insemination. Acta Vet Scand 2012; 54: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Galuppo AG, Junior NB, Arruda NS, Corbellini AO, Chiappetta CM, Pavão DL, D’Angelo M, Canal CW, Rodrigues JL. Evaluation of the effectiveness of semen processing techniques to remove bovine viral diarrhea virus from experimentally contaminated semen samples. J Virol Methods 2013; 187: 443–448. [DOI] [PubMed] [Google Scholar]