Abstract
Objectives: This study examines cardiovascular (CV) effects of guanfacine immediate-release (GUAN-IR), dexmethylphenidate extended-release (DMPH), and their combination (COMB) during acute and long-term treatment of youth with attention-deficit/hyperactivity disorder.
Methods: Two hundred seven participants aged 7–14 years enrolled in an 8-week double-blind randomized trial of GUAN-IR (1–3 milligrams (mg)/day), DMPH (5–20 mg/day), or COMB with fixed–flexible dosing and titrated to optimal behavioral response. Heart rate, systolic blood pressure (BP), diastolic BP, and electrocardiograms were assessed at baseline, end of blinded optimization, and over a 1-year open-label maintenance phase.
Results: During acute titration, GUAN-IR decreased heart rate, systolic BP, and diastolic BP; DMPH increased heart rate, systolic BP, diastolic BP, and corrected QT (QTc) interval; COMB increased diastolic BP, but had no effects on heart rate, systolic BP, or QTc. During maintenance, GUAN-IR-associated decreases in heart rate and DMPH-associated increases in systolic BP returned to baseline values. Other variables across the three groups remained unchanged from the end of blinded titration. There were no discontinuations due to CV adverse events.
Conclusion: GUAN-IR, DMPH, and COMB were well tolerated and safe. Expected changes in CV parameters during acute titration were seen in GUAN-IR and DMPH groups, with COMB values falling intermediately between the two other treatment groups. No serious CV events occurred in any participant. GUAN-IR- and DMPH-associated CV changes generally returned to baseline with sustained therapy. These data suggest that COMB treatment might attenuate long-term CV effects of GUAN-IR and stimulant monotherapy, possibly reducing risk of the small but statistically significant changes associated with either single treatment. Clinicaltrials.gov Identifier: NCT00429273.
Keywords: : ADHD, cardiovascular, stimulant, guanfacine, safety
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is the most commonly diagnosed behavioral disorder of childhood, with an estimated prevalence in the range of 9% of school-age youth (Polanczyk et al. 2007; Wolraich et al. 2012). ADHD is not isolated to childhood and persists in 30%–70% of patients into adulthood. Treating ADHD is compelled by studies showing that symptoms impact nearly all domains of functioning (Barkley et al. 2006; Kessler et al. 2006; Biederman et al. 2012; Miller et al. 2013).
Psychostimulant medications, including various formulations of methylphenidate (MPH) or amphetamine (AMPH), continue to be the mainstay of ADHD pharmacotherapy, with an estimated 3.5 million children receiving prescriptions annually in 2011 (Visser et al. 2014). Psychostimulants as monotherapy produce a robust improvement in symptoms in the short term with responder rates of 65%–75% (Steele et al. 2006; Heal et al. 2012) and approximately full syndrome remission described in roughly half of treated youth (Swanson et al. 2001). Second-tier options for ADHD monotherapy include the norepinephrine reuptake inhibitor atomoxetine as well as the alpha-2 agonists, guanfacine (GUAN) and clonidine. FDA has granted approval for GUAN extended-release (GUAN-XR) and clonidine extended-release formulations as ADHD therapies. Controlled trials of GUAN-XR suggest response rates of 50%–65% during acute treatment (Biederman et al. 2008; Sallee et al. 2009).
Recognition of the negative impact of untreated ADHD symptoms (Biederman et al. 1998) and concomitant wishes to maximize treatment response and higher rates of remission have led investigators to examine possible benefits of adjunctive alpha-2 agonist therapy in patients exhibiting partial stimulant responses (Spencer et al. 2009; Wilens et al. 2012). Efficacy and safety data from these studies are largely limited to short-term trials conducted with extended-release formulations in stimulant-refractory samples.
Despite their long history of frequent use, there have been ongoing concerns raised about the cardiovascular (CV) safety of ADHD medications, particularly for psychostimulants (Martinez-Raga et al. 2013). Specifically, the possible association of stimulant medication use and increased risk for sudden cardiac death, potential links to corrected QT (QTc) interval prolongation on electrocardiogram (EKG), and risk of arrhythmia have prompted U.S. Food and Drug Administration (FDA) reviews and several independent reports. A black box warning on potential cardiac risks was debated, but ultimately not implemented by the FDA due to lack of support for an association.
One study described a significant association between cases of unexplained sudden death and stimulant medications, although the report did not establish causality and had certain methodological weaknesses that might have influenced findings (Gould et al. 2009). A second study conducted in a large cohort did not find statistically significant differences between children taking stimulants and nonusers in rates of sudden death, ventricular arrhythmia, or all causes of death (Schelleman et al. 2011). Another large matched-cohort longitudinal study of 1,200,438 children and young adults similarly found no association between MPH use and serious cardiac events (Cooper et al. 2011).
A separate prospective longitudinal cohort study of 714,258 Danish children containing 8300 diagnosed with having ADHD and followed for a mean of 9.5 years reported an increased overall rate of any CV event, that is, CV disease NOS, arrhythmias, and hypertension, in stimulant users vs. nonusers (Dalsgaard et al. 2014). However, all observations of serious CV events (e.g., cardiac arrest, ischemic heart disease) or death due to any cause occurred in the nonstimulant users. While patients with underlying heart disease might be at greater risk for sudden death, there is no explicit recommendation against psychostimulant usage in such patients. In all patients, reports of sudden death directly related to stimulants are rare (Olfson et al. 2012). A review of studies of ∼40,000 person-years of stimulant use found no reports of sudden cardiac death (Winterstein et al. 2007).
The impact of ADHD medications on CV functioning remains another area of active concern, especially given higher obesity rates seen with ADHD as well as other data suggesting that adolescent ADHD itself is a risk factor for adult hypertension (Fuemmeler et al. 2011). Numerous reports have demonstrated statistically significant increases in heart rate and blood pressure (BP) with stimulants (Samuels et al. 2006; Stiefel and Besag 2010; Hammerness et al. 2011), including studies showing increases from 3 to 10 beats per minute (bpm), 1–8 millimeters of mercury (mmHg), and 1–15 mmHg for heart rate, systolic BP, and diastolic BP, respectively. The long-term effects of stimulant medication on CV health have not been fully described, although one study suggests that increases in heart rate and BP persist over time (Hammerness et al. 2011).
The majority of reports fail to find statistically significant CV effects or effects that were determined to be clinically meaningful (Silva et al. 2004; Stiefel and Besag 2010; Hammerness et al. 2011). The determination of the clinical significance of these known CV changes is complicated by the fact that few reports include the specific identification of subjects with CV values falling outside of accepted clinical cutoff values for age, sex, and height, with some exceptions (Stowe et al. 2002; Donner et al. 2007; Grisaru et al. 2013).
The alpha-2 agonist GUAN has been found to affect CV parameters in directions opposite that of MPH. A study of 240 children receiving GUAN-XR showed small decreases in BP and heart rate (Biederman et al. 2008). These reductions were not found to be dangerous or clinically significant and there were no differences in positional BP or orthostasis compared with placebo (Scahill et al. 2001). Additionally, GUAN-XR is thought to prevent major fluctuations in peak-to-trough drug concentrations, compared with immediate release formulations, which could minimize the degree of CV effects and improve tolerability (Sallee et al. 2009).
Studies of EKG changes similarly fail to find consistent clinically meaningful effects of ADHD pharmacotherapies. The sum of the literature does not find clinically or statistically significant changes in EKG intervals (Hammerness et al. 2011). No clinically significant EKG changes have been reported in studies of GUAN-XR (Sallee et al. 2009) or combination GUAN-XR plus stimulant (Wilens et al. 2012).
There are sparse long-term data on the safety of combined stimulant and alpha agonist treatment in children with ADHD, despite earlier concerns (Cantwell et al. 1997) and the increasing frequency of the combination in community settings. Since MPH tends to increase (while GUAN tends to decrease) heart rate and BP, there is speculation that combination treatment might in fact attenuate the CV effects of each drug and prevent marked changes from patients’ baseline hemodynamic profiles (Roesch et al. 2013). Preliminary studies suggest that combination MPH and GUAN-XR treatment is safe and well tolerated, with small decreases in BP and heart rate noted when GUAN-XR is added to ongoing MPH therapy (Spencer et al. 2009).
A similar safety profile was found when GUAN-XR was added to partial stimulant responders (Wilens et al. 2012). Overall, no serious safety issue has been found in patients taking combination psychostimulant and GUAN-XR (Spencer et al. 2009; Wilens et al. 2012). Notably, however, the majority of monotherapy and combination treatment studies on CV effects are limited to acute trials of a few months’ duration. There is a paucity of research on the longer-term CV impact of combination regimens with ongoing medication maintenance (Spencer et al. 2009; Wilens et al. 2012).
In this investigation, we compare potential changes in CV parameters among groups of ADHD-affected youth randomly assigned to treatment with dexmethylphenidate extended-release (DMPH), immediate-release GUAN (GUAN-IR) monotherapy, or these two medications in combination (COMB) both during acute titration and after 14 months of continued treatment.
Methods
Participants
Youth 7–14 years of age with a primary diagnosis of having ADHD, any subtype, as defined by the Diagnostic and Statistical Manual of Mental Disorders, 4th edition, Text Revision (DSM-IV-TR) (American Psychiatric Association 2000), assessed using the Kiddie Schedule for Affective Disorders and Schizophrenia–Present and Lifetime version (K-SADS-PL) (Kaufman et al. 1997) and clinical interview, and with Clinical Global Impressions (CGI)-severity ≥4 (Guy 1976) were enrolled.
Participants were excluded for (1) autistic disorder, chronic tic disorder, psychosis, bipolar disorder, or structural heart defects; (2) current major depression or panic disorder; (3) systolic or diastolic BP 95th or <5th percentile for age and body–mass index (Robertson and Shilkofski 2005); (4) past history of unexplained syncopal episodes, family history of sudden cardiac death before age 30 years, known clinically significant structural cardiac defects, or other medical conditions that in the physician investigators’ judgment contraindicated treatment with stimulants or alpha agonists; and (5) need for chronic use of other central nervous system medications similarly assessed by a study physician. Before initiation of any study procedures, participants and parents received thorough oral and written explanations of all study requirements and provided written informed permission and assent under procedures approved by the university's Institutional Review Board.
Study design
The current investigation was conducted as part of a larger project designed to test the hypothesis that clinical and cognitive responses in ADHD to combination pharmacotherapies that robustly enhance both dopamine and norepinephrine signaling would be superior to monotherapies (McCracken et al. 2016). The study comprised two phases. Phase I was an 8-week randomized, comparative, parallel-group, fixed–flexible dosing study of three treatments. Participants were randomized equally to (1) twice-daily GUAN-IR; (2) once daily DMPH; or (3) COMB, randomized 1:1:1 and stratified by younger (7–10) and older (11–14) age ranges. Treatments were applied sequentially. During the first 4 weeks participants received GUAN-IR or placebo. Beginning in week 5, participants continued week 4 treatments and additionally received DMPH or placebo, creating the three groups.
Initial GUAN-IR dosing was 0.5 mg twice daily for week 1; in week 2, dose increased to 1 mg twice daily as tolerated; and week 3 doses advanced to 1.5 mg twice daily as tolerated. For DMPH, participants weighing less than 25 kg received 5 mg DMPH once daily for week 5; week 6 was advanced to 10 mg DMPH daily, and week 7 moved to 15 mg daily. For participants weighing 25 kg or more, DMPH began in week 5 with 10 mg DMPH once daily; week 6 advanced to 15 mg daily; and week 7 moved to 20 mg daily. Although attempts were made to complete titration over the full range of specified doses, study physicians were permitted to deviate from prescribed dosage increase if problems with tolerability emerged.
Optimal dosing of GUAN-IR and DMPH was established by blinded clinical assessment of symptoms, CGI-Improvement (CGI-I) ratings (Guy 1976), and side effect rating scales by consensus agreement by two independent study clinicians at week 3 and 7 visits. Double-blind outcomes were assessed at week 8.
In Phase II, participants remained within assigned treatment arms and received open-label therapy assessed at monthly intervals for an additional year. Dose adjustments were permitted to maintain efficacy or reduce side effects. CV outcomes, including heart rate, BP, and EKGs, were obtained at each visit. Heart rate and BP were measured while participants were seated, using an Omron Digital Blood Pressure Monitor Model HEM907XL. EKG variables examined included corrected QTc and QRS intervals. QTc was calculated using the Bazett formula (Phan et al. 2015)
Data analyses
Descriptive statistics for participant characteristics at baseline were derived and groups compared to assess potential differences. CV measures were assessed for effects of assigned treatment group and time with the general linear mixed model using PROC MIXED (SAS Version 9.2), which automatically handles missing observations. Separate analyses were conducted for Phase I and Phase II data. All tests were two-tailed, with an a priori significance level of p < 0.05.
Results
Participant characteristics
There were 207 participants randomized to the three treatment groups: GUAN-IR (N = 68), DMPH (N = 69), and COMB (N = 70). The sample was 69% male, 69% white, and 21% Hispanic. Mean (SD) age was 10.2 (2.1) years and there were no statistically significant baseline differences for age, weight, height, sex distribution, heart rate, BP, or EKG measures between groups. Mean (SD) final week 8 daily doses of DMPH were 16.0 (3.9) mg for DMPH-Only and 15.1 (4.8) mg for COMB. Mean final daily doses of GUAN-IR were 2.2 (0.7) mg for GUAN-IR-Only and 2.4 (0.6) mg for COMB. Mean mg/kg daily doses of GUAN-IR were 0.06 (0.03) mg/(kg·d) for both GUAN groups.
Phase I results
Phase I changes in CV variables, including heart rate, BP, and QTc, are summarized in Table 1. Within-group variables largely remained unchanged over the 8-week titration, although some group × visit interaction effects were evidenced over time. QTc prolongation occurred only with DMPH, with no significant changes in QTc in the other two groups. No significant changes were noted in QRS intervals.
Table 1.
Changes in Cardiovascular Variables During Blinded Titration
| Baseline | Week 8 | Visit effect | ||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Group | Mean (SD) | Mean (SD) | F df | P | All group effects | F df | P |
| Heart rate | GUAN-IR | 79.4 (10.9) | 73.8 (14.0) | 15.8 1/54 | 0.0002 | Visit | 0.2 1/170 | 0.6 |
| DMPH | 79.8 (11.0) | 81.4 (13.6) | 1.1 1/59 | 0.3 | Group | 2.8 2/208 | 0.07 | |
| COMB | 78.5 (10.9) | 81.2 (13.9) | 2.6 1/57 | 0.1 | Visit × Group | 8.3 2/170 | 0.0004 | |
| BP systolic | GUAN-IR | 109.1 (8.6) | 105.2 (11.6) | 7.0 1/54 | 0.01 | Visit | 2.5 1/170 | 0.1 |
| DMPH | 109.6 (8.7) | 115.4 (11.2) | 22.5 1/59 | <0.0001 | Group | 7.6 2/208 | 0.0006 | |
| COMB | 107.8 (8.6) | 109.8 (11.5) | 1.8 1/57 | 0.2 | Visit × Group | 12.4 2/170 | <0.0001 | |
| BP diastolic | GUAN-IR | 65.5 (6.7) | 61.2 (9.3) | 12.9 1/54 | 0.0007 | Visit | 4.5 1/170 | 0.04 |
| DMPH | 64.3 (6.8) | 69.6 (9.0) | 25.5 1/59 | <0.0001 | Group | 5.6 2/208 | 0.004 | |
| COMB | 64.4 (6.7) | 67.5 (9.2) | 7.7 1/57 | 0.008 | Visit × Group | 19.0 2/170 | <0.0001 | |
| EKG QTc | GUAN-IR | 417.3 (20.0) | 415.3 (21.7) | 0.8 1/50 | 0.4 | Visit | 7.4 1/158 | 0.007 |
| DMPH | 416.3 (20.1) | 426.5 (21.1) | 11.9 1/53 | 0.001 | Group | 1.5 2/206 | 0.2 | |
| COMB | 417.4 (19.8) | 421.7 (21.6) | 2.6 1/55 | 0.1 | Visit × Group | 5.1 2/158 | 0.007 |
BP, blood pressure; COMB, combination; DMPH, dexmethylphenidate extended-release; EKG, electrocardiogram; GUAN, guanfacine immediate-release.
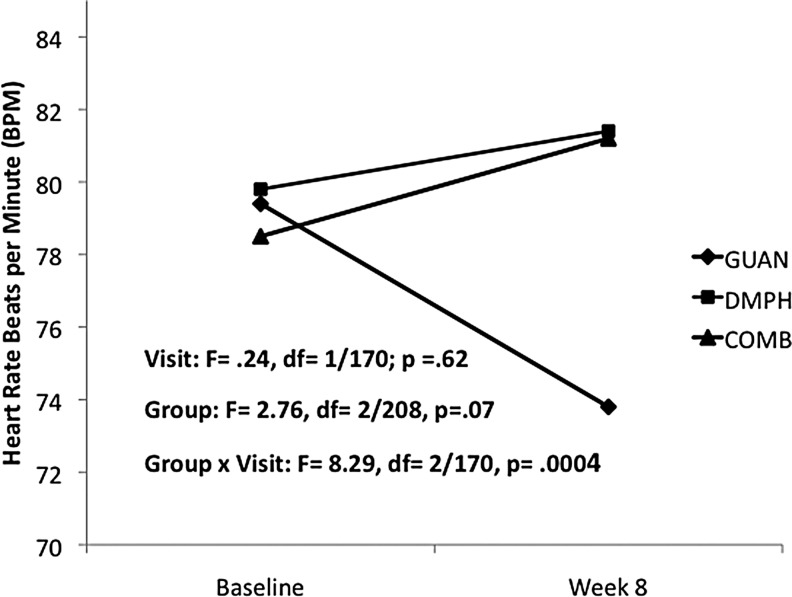
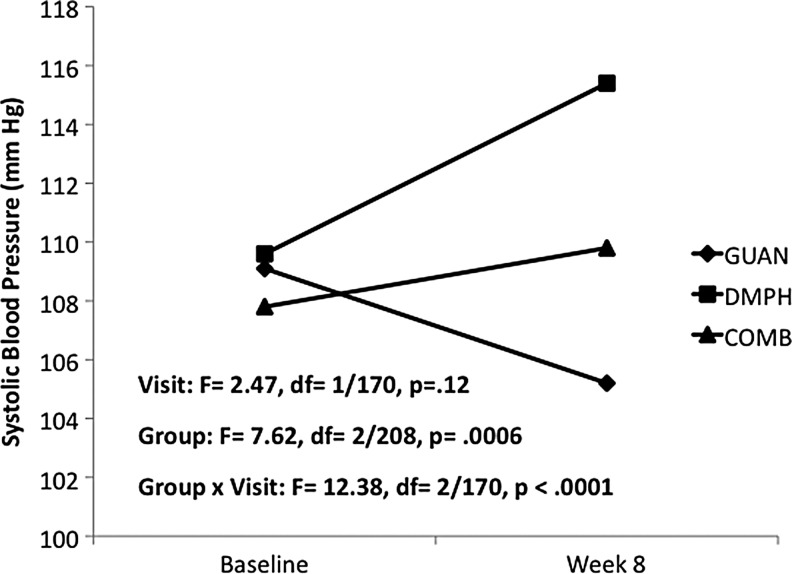
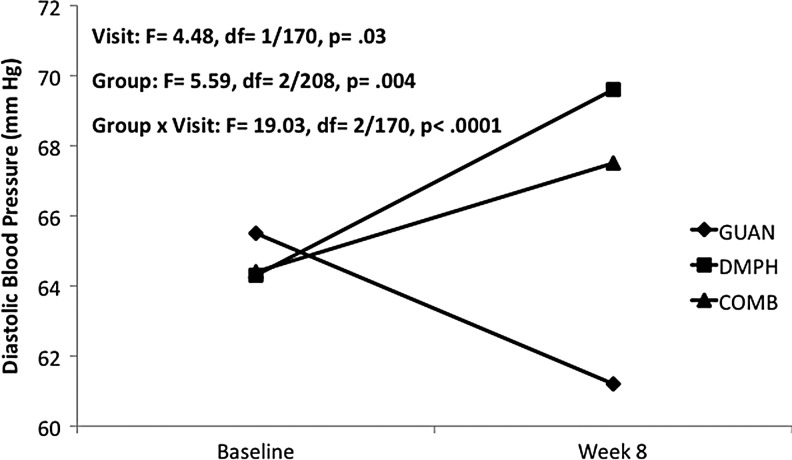
For heart rate, a significant decrease was seen with GUAN-IR, while DMPH and COMB remained unchanged from baseline (Fig. 1). Systolic BP decreased with GUAN-IR, increased with DMPH, and remained unchanged with COMB (Fig. 2). Diastolic BP decreased with GUAN-IR and increased with both DMPH and COMB, with COMB-associated changes intermediate compared with the monotherapies (Fig. 3). All treatments were well tolerated. Of the early terminations from Phase I, only 1 of 68 (GUAN-IR), 1 of 60 (DMPH), and 2 of 70 (COMB) discontinued for problems related to tolerability. None of these were due to CV-related adverse events.
FIG. 1.
Heart rate changes during acute titration. COMB, combination; DMPH, dexmethylphenidate extended-release; GUAN, guanfacine immediate-release.
FIG. 2.
Systolic blood pressure during acute titration. COMB, combination; DMPH, dexmethylphenidate extended-release; GUAN, guanfacine immediate-release.
FIG. 3.
Diastolic blood pressure during acute titration. COMB, combination; DMPH, dexmethylphenidate extended-release; GUAN, guanfacine immediate-release.
Phase II results
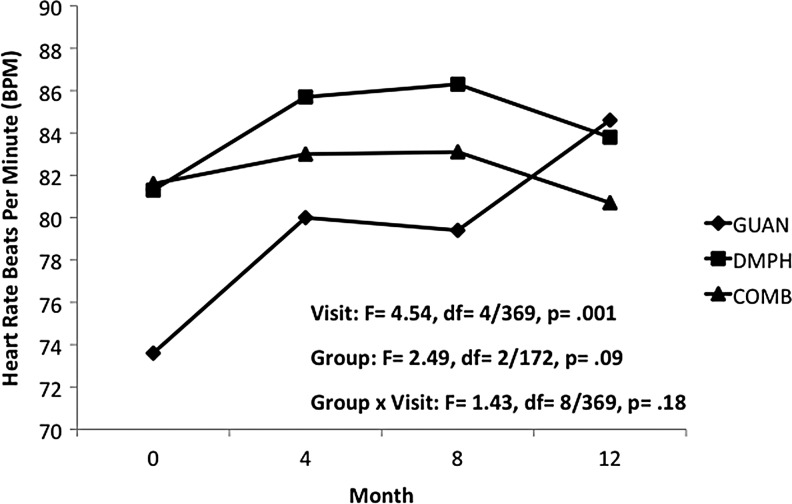
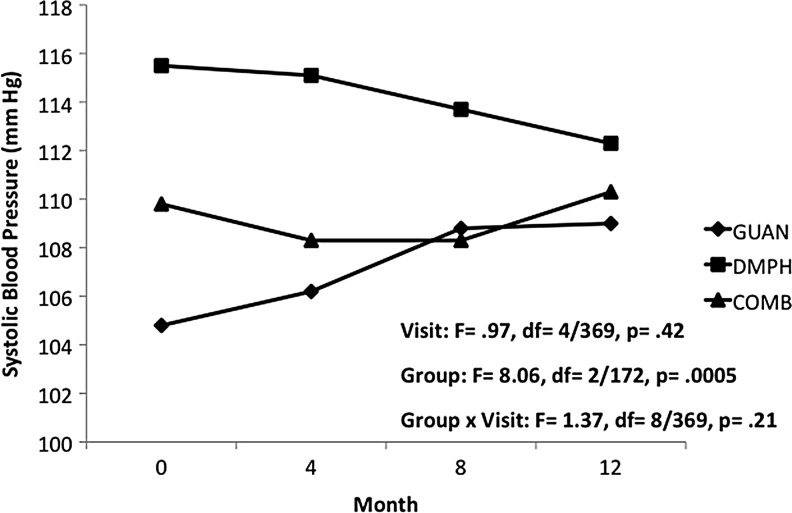
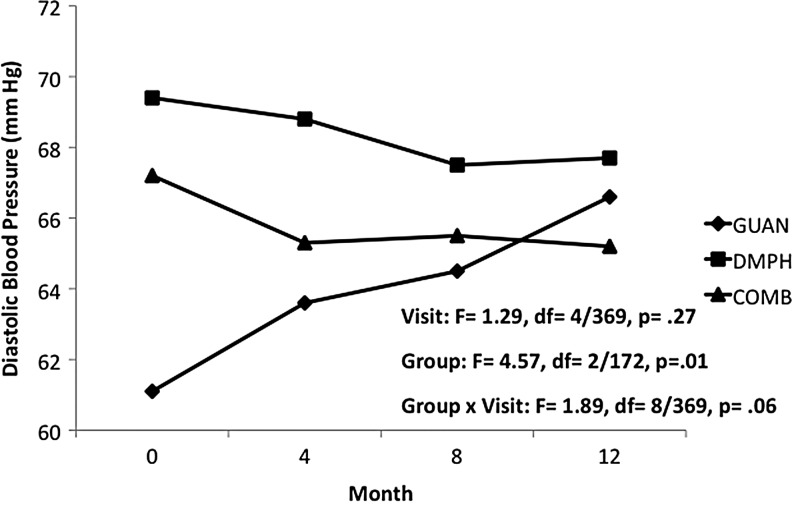
In Phase II, reductions in heart rate seen with GUAN-IR during acute titration returned to baseline values, with a mean (SD) increase of 11.6 (23.4) bpm over the 12 months of open treatment. For DMPH and COMB, heart rate remained unchanged from both baseline and end of acute titration. Differences in heart rate between groups trended toward statistical significance, likely due to the relatively lower heart rates since during initial months of GUAN-IR (Fig. 4). Group differences for both systolic BP (Fig. 5) and diastolic BP (Fig. 6) persisted over the 12-month maintenance phase. There was a trend for differences in diastolic BP changes over time within groups, likely due to increases seen with GUAN-IR. No differences over time were evidenced in diastolic BP with DMPH or COMB. For both heart rate and BP, changes seen with COMB were intermediate to those seen with GUAN-IR and DMPH. There were no changes in QTc or QRS in any group.
FIG. 4.
Heart rate changes during 12 months of maintenance treatment. COMB, combination; DMPH, dexmethylphenidate extended-release; GUAN, guanfacine immediate-release.
FIG. 5.
Systolic blood pressure during 12 months of maintenance treatment. COMB, combination; DMPH, dexmethylphenidate extended-release; GUAN, guanfacine immediate-release.
FIG. 6.
Diastolic blood pressure during 12 months of maintenance treatment. COMB, combination; DMPH, dexmethylphenidate extended-release; GUAN, guanfacine immediate-release.
Discussion
This study examined the acute and long-term CV effects of GUAN-IR, DMPH, and COMB treatment for up to 14 months of exposure in youth with ADHD. Participants in this study had no clinically meaningful CV changes in either the acute blinded titration or long-term open-maintenance phases. These findings add to several previous studies that suggest that ADHD medications are generally safe and without meaningful CV risk in otherwise healthy children (Cooper et al. 2011). Importantly, the COMB treatment was not associated with any evidence of CV risk or unexpected safety signals.
During acute treatment, GUAN-IR and DMPH had opposing effects on heart rate, BP, and QTc, consistent with prior findings (Sallee et al. 2009; Hammerness et al. 2011). In addition, similar to previous research, trends for acute decreases in heart rate and BP were noted with GUAN (Biederman et al. 2008; Connor et al. 2010; Hirota et al. 2014). Although prior reports of stimulant-associated increases in BP and QTc intervals have lacked clinical significance (Findling et al. 2001, 2005; Donner et al. 2007), the relationship between QTc changes and GUAN-IR is less clear. While our study found a small (2 ms) decrease in QTC, another reported a small (5.3 ms) increase (Hirota et al. 2014), while a third found no meaningful change (Kollins et al. 2011). These discrepancies merit further investigation.
CV parameters in the GUAN-IR group remained unchanged during 1-year follow-up, with the exception of heart rate increases and diastolic BP decreases that returned to baseline values. Reversal of acute decreases in heart rate with sustained GUAN-IR treatment was also found in a 2-year open-label follow-up study (Sallee et al. 2009). Together, these data suggest that minor changes in CV parameters seen during acute ADHD therapy return to pretreatment values over time.
CV parameters for the COMB group fell between the values of DMPH and GUAN-IR. A similar phenomenon of GUAN-XR mitigating heart rate increases from stimulants has been found in other studies (Wilens et al. 2012). This demonstrates the possibility that combination treatment might not only improve ADHD symptoms and enhance cognition (McCracken et al. 2016) but might also attenuate CV effects of either monotherapy. Our study's safety profile, with a lack of clinically significant CV implications, is in line with combination studies in which GUAN-XR was added onto existing psychostimulant treatment (Spencer et al. 2009; Wilens et al. 2012).
Study limitations
While the exclusion criteria used in this study allowed for a specific focus on children and adolescents with ADHD alone, many patients in the clinical setting have comorbid psychiatric diagnosis (Scahill and Schwab-Stone 2000). Along with this, polypharmacy to treat other comorbid diagnoses may impact the CV profiles of patients on dual ADHD treatment and should be further investigated.
In addition, although the CV changes observed are deemed minor and are consistent with those reported in the literature, more safety data using longitudinal designs over longer treatment periods and in larger samples are needed to further bolster our conclusions regarding the safety for these treatments. Likewise, more intense efforts to study the day-to-day variability of CV parameters in real-world settings using methods such as ambulatory monitoring might add further appreciation of the functional impact, if any, of these treatment approaches. Finally, additional efforts should be made to identify those individuals who might be at highest risk for the rare but serious CV events sometimes observed to be associated with ADHD therapies.
Conclusions
For children and adolescents with ADHD to achieve the acknowledged goal of symptom remission, consideration of combining stimulant and alpha-2 agonist monotherapies is becoming more commonplace. This study failed to demonstrate any clinically significant adverse CV outcomes with such pairing. Indeed, if confirmed in additional studies, the moderation of usual stimulant- and guanfacine-induced CV changes by their combination might further increase the long-term safety of these two established ADHD treatments.
Clinical Significance
This study provides additional data suggesting that combination psychostimulant and alpha-2 agonist therapy creates no additional adverse impact on CV health and might in fact attenuate CV changes seen with either monotherapy
Disclosures
Dr. J.J.M. has received research support from NeuroSigma, Inc., Purdue Pharma, and Shire Pharmaceuticals, as well as consulting honoraria from Akili Interactive, Inc., Merck, and Neurovance, and as a DSMB member for Sunovion Pharmaceuticals. Dr. J.T.M. has received consultant income from BioMarin, Roche, and Think Now, Inc., as well as research contract support from Roche, Seaside Therapeutics, and donated study medication from AstraZeneca and Shire Pharmaceuticals. Drs. G.R.S., J.L., and J.C., Ms. A.S., and Mr. E.C. report no conflicts of interest.
References
- American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th ed., Text Revision. Washington (District of Columbia), American Psychiatric Association, 2000 [Google Scholar]
- Barkley RA, Fischer M, Smallish L, Fletcher K: Young adult outcomes of hyperactive children: Adaptive functioning in major life activities. J Am Acad Child Adolesc Psychiatry 45:192–202, 2006 [DOI] [PubMed] [Google Scholar]
- Biederman J, Petty CR, Woodworth KY, Lomedico A, Hyder LL, Faraone SV: Adult outcomes of attention-deficit/hyperactivity disorder. J Clin Psychiatry 73:941–950, 2012 [DOI] [PubMed] [Google Scholar]
- Biederman J, Melmed RD, Patel A, McBurnett K, Donahue J, Lyne A: Long-term, open-label extension study of guanfacine extended release in children and adolescents with ADHD. CNS Spectr 13:1047–1055, 2008 [DOI] [PubMed] [Google Scholar]
- Biederman J, Mick E, Faraone SV: Normalized functioning in youths with persistent attention-deficit/hyperactivity disorder. J Pediatrics 133:544–551, 1998 [DOI] [PubMed] [Google Scholar]
- Cantwell DP, Swanson J, Connor DF: Case study: Adverse response to clonidine. J Am Acad Child Adolesc Psychiatry 36:539–544, 1997 [DOI] [PubMed] [Google Scholar]
- Connor DF, Findling RL, Kollins SH, Sallee F, Lopez FA, Lyne A, Tremblay G: Effects of guanfacine extended release on oppositional symptoms in children aged 6–12 years with attention-deficit hyperactivity disorder and oppositional symptoms. CNS Drugs 24:755–768, 2010 [DOI] [PubMed] [Google Scholar]
- Cooper WO, Habel LA, Sox CM, Chan A, Arbogast PG, Cheetham TC, Murray KT, Quinn VP, Stein M, Callahan ST, Fireman BH, Fish FA, Kirshner HS, O'Duffy A, Connell FA, Ray WA: ADHD drugs and serious cardiovascular events in children and young adults. N Eng J Med 365:1896–1904, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalsgaard S, Kvist AP, Leckman JF, Nielsen HS, Simonsen M: Cardiovascular safety of stimulants in children with attention-deficit/hyperactivity disorder: A nationwide prospective cohort study. J Child Adolesc Psychopharmacol 24:302–310, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner R, Michaels MA, Ambrosini PJ: Cardiovascular effects of mixed amphetamine salts extended release in the treatment of school-aged children with attention-deficit hyperactivity disorder. Biol Psychiatry 61:706–712, 2007 [DOI] [PubMed] [Google Scholar]
- Findling RL, Biederman J, Wilens TE, Spencer TJ, McGough JJ, Lopez FA, Tulloch SJ: Short- and long-term cardiovascular effects of mixed amphetamine salts extended release in children. J Pediatr 147:348–353, 2005 [DOI] [PubMed] [Google Scholar]
- Findling RL, Short EJ, Manos M: Short-term cardiovascular effects of methylphenidate and adderall. J Am Acad Child Adolesc Psychiatry 40:525–529, 2001 [DOI] [PubMed] [Google Scholar]
- Fuemmeler BF, Ostbye T, Yang C, McClernon FJ, Kollins SH: Association between attention-deficit/hyperactivity disorder symptoms and obesity and hypertension in early adulthood: A population-based study. Int J Obes 35:852–862, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould MS, Walsh T, Munfakh JL, Kleinman M, Duan N, Olfson M, Greenhill L, Cooper T: Sudden death and use of stimulant medications in youths. Am J Psychiatry 166:992–1001, 2009 [DOI] [PubMed] [Google Scholar]
- Grisaru S, Yue MW, Mah JC, Hamiwka LA: Ambulatory blood pressure monitoring in a cohort of children referred with suspected hypertension: Characteristics of children with and without Attention Deficit Hyperactivity Disorder. Int J Hypertens 2013:419208, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy W: ECDEU Assessment Manual for Psychopharmacology. Rockville (Maryland), US Department of Heath, Education, and Welfare Public Health Service Alcohol, Drug Abuse, and Mental Health Administration, 1976 [Google Scholar]
- Hammerness PG, Perrin JM, Shelley-Abrahamson R, Wilens TE: Cardiovascular risk of stimulant treatment in pediatric attention-deficit/hyperactivity disorder: Update and clinical recommendations. J Am Acad Child Adolesc Psychiatry 50:978–990, 2011 [DOI] [PubMed] [Google Scholar]
- Heal DJ, Smith SL, Findling RL: ADHD: Current and future therapeutics. Curr Top Behav Neurosci 9:361–390, 2012 [DOI] [PubMed] [Google Scholar]
- Hirota T, Schwartz S, Correll CU: Alpha-2 agonists for attention-deficit/hyperactivity disorder in youth: A systematic review and meta-analysis of monotherapy and add-on trials to stimulant therapy. J Am Acad Child Adolesc Psychiatry 53:153–173, 2014 [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N: Schedule for affective disorders and schizophrenia for school-age children-present and lifetime ersion (K-SADS-PL): Initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 36:980–988, 1997 [DOI] [PubMed] [Google Scholar]
- Kessler RC, Adler L, Barkley R, Biederman J, Conners CK, Demler O, Faraone SV, Greenhill LL, Howes MJ, Secnik K, Spencer T, Ustun TB, Walters EE, Zaslavsky AM: The prevalence and correlates of adult ADHD in the United States: Results from the National Comorbidity Survey Replication. Am J Psychiatry 163:716–723, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollins SH, Lopez FA, Vince BD, Turnbow JM, Farrand K, Lyne A, Wigal SB, Roth T: Psychomotor functioning and alertness with guanfacine extended release in participants with attention-deficit/hyperactivity disorder. J Child and Adolesc Psychopharmacology 21:111–120, 2011 [DOI] [PubMed] [Google Scholar]
- Martinez-Raga J, Knecht C, Szerman N, Martinez MI: Risk of serious cardiovascular problems with medications for attention-deficit hyperactivity disorder. CNS Drugs 27:15–30, 2013 [DOI] [PubMed] [Google Scholar]
- McCracken JT, McGough JJ, Loo S, Levitt J, Del'Homme M, Cowen J, Sturm A, Whelan F, Hellenmann G, Sugar C, Bilder R: Combined stimulant and guanfacine administration in attention—deficit/hyperactivity disorder (ADHD): A controlled, comparative study. J Am Acad Child Adolesc Psychiatry 55:657–666, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M, Sheridan M, Cardoos SL, Hinshaw SP: Impaired decision-making as a young adult outcome of girls diagnosed with attention-defiict/hyperactivity disorder in childhood. J Int Neuropsychol Soc 19:110–114, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olfson M, Huang C, Gerhard T, Winterstein AG, Crystal S, Allison PD, Marcus SC: Stimulants and cardiovascular events in youth with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 51:147–156, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan DQ, Silka MJ, Lan YT, Chang RK: Comparison of formulas for calculation of the corrected QT interval in infants and young children. J Pediatr 166:960–964, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanczyk G, Silva de Lima M, Horta BL, Biederman J, Rohde LA: The worldwide prevalence of ADHD: A systematic review and metaregression analysis. Am J Psychiatry 164:942–948, 2007 [DOI] [PubMed] [Google Scholar]
- Robertson J, Shilkofski N. (eds): Harriet Lane Handbook: A Manual for Pediatric House Officers: 17th Edition. Maryland Heights (Missouri), Mosby Press, 2005 [Google Scholar]
- Roesch B, Corcoran ME, Fetterolf J, Haffey M, Martin P, Preston P, Purkayastha J, Wang P, Ermer J: Pharmacokinetics of coadministered guanfacine extended release and lisdexamfetamine dimesylate. Drugs R D12:119–129, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallee FR, Lyne A, Wigal T, McGough JJ: Long-term safety and efficacy of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol 19:215–226, 2009 [DOI] [PubMed] [Google Scholar]
- Samuels JA, Franco K, Wan F, Sorof JM: Effects of stimulants on 24-h ambulatory blood pressure in children with ADHD: A double-blind, randomized, cross-over trial. Pediatr Nephrol 21:92–95, 2006 [DOI] [PubMed] [Google Scholar]
- Scahill L, Chappell PB, Kim YS, Schultz RT, Katsovich L, Shepherd E, Arnsten AF, Cohen DJ, Leckman JF: A placebo-controlled study of guanfacine in the treatment of children with tic disorders and attention deficit hyperactivity disorder. Am J Psychiatry 158:1067–1074, 2001 [DOI] [PubMed] [Google Scholar]
- Scahill L, Schwab-Stone M: Epidemiology of ADHD in school-age children. Child Adolesc Psychiatr Clin N Am 9:541–555, 2000 [PubMed] [Google Scholar]
- Schelleman H, Bilker WB, Strom BL, Kimmel SE, Newcomb C, Guevara JP, Daniel GW, Cziraky MJ, Hennessy S: Cardiovascular events and death in children exposed and unexposed to ADHD agents. Pediatrics 127:1102–1110, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva R, Tilker HA, Cecil JT, Kowalik S, Khetani V, Faleck H, Patin J: Open-label study of dexmethylphenidate hydrochloride in children and adolescents with attention deficit hyperactivity disorder. J Child Adolesc Psychopharmacol 14:555–563, 2004 [DOI] [PubMed] [Google Scholar]
- Spencer TJ, Greenbaum M, Ginsberg LD, Murphy WR: Safety and effectiveness of coadministration of guanfacine extended release and psychostimulants in children and adolescents with attention-deficit/hyperactivity disorder. J Child and Adolesc Psychopharmacology 19:501–510, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele M, Jensen PS, Quinn DM: Remission versus response as the goal of therapy in ADHD: A new standard for the field? Clin Ther 28:1892–1908, 2006 [DOI] [PubMed] [Google Scholar]
- Stiefel G, Besag FMC: Cardiovascular effects of methylphenidate, amphetamines and atomoxetine in the treatment of attention-deficit hyperactivity disorder. Drug Saf 33:821–842, 2010 [DOI] [PubMed] [Google Scholar]
- Stowe CD, Gardner SF, Gist CC, Schultz EG, Wells TG: 24-hour ambulatory blood pressure monitoring in male children receiving stimulant therapy. Ann Pharmacother 36:1142–1149, 2002 [DOI] [PubMed] [Google Scholar]
- Swanson JM, Kraemer HC, Hinshaw SP, Arnold LE, Conners CK AB.ikoff HB, Clevenger W, Davies M, Elliott GR, Greenhill LL, Hechtman L, Hoza B, Jensen PS, March JS, Newcorn JH, Owens EB, Pelham WE, Schiller E, Severe JB, Simpson S, Bitiello B, Wells K, Wigal T, Wu M: Clinical relevance of the primary findings of the MTA: Success rates based on severity of ADHD and ODD symptoms at the end of treatment. J Am Acad Child Adolesc Psychiatry 40:168–179, 2001 [DOI] [PubMed] [Google Scholar]
- Visser SN, Danielson ML, Bitsko RH, Holbrook JR, Kogan MD, Ghandour RM, Perou R, Blumberg SJ: Trends in the parent-report of health care provider-diagnosed and medication attention-deficit/hyperactivity disorder: United States, 2003–2011. J Am Acad Child Adolesc Psychiatry 53:34–46, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilens TE, Bukstein O, Brams M, Cutler AJ, Childress A, Rugino T, Lyne A, Grannis K, Youcha S: A controlled trial of extended-release guanfacine and psychostimulants for attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 51:74–85, 2012 [DOI] [PubMed] [Google Scholar]
- Winterstein AG, Gerhard T, Shuster J, Johnson M, Zito JM, Saidi A: Cardiac safety of central nervous system stimulants in children and adolescents with attention-deficit/hyperactivity disorder. Pediatrics 120:1494–1501, 2007 [DOI] [PubMed] [Google Scholar]
- Wolraich ML, McKeown RE, Bard D, Cuffe S, Neas B, Geryk LL, Doffing M, Bottai M, Abramowitz AJ, Beck L, Holbrook JR, Danielson M: The prevalence of ADHD: Its diagnosis and treatment in four school districts across two states. J Atten Disord 18:563–575, 2012 [DOI] [PubMed] [Google Scholar]