Abstract
Objective:
Use of generic medicines is important to reduce rising health-care costs. Proper knowledge and perception of medical students and doctors toward generic medicines are important. Xavier University School of Medicine in Aruba admits students from the United States, Canada, and other countries to the undergraduate medical (MD) program. The present study was conducted to study the knowledge and perception about generic medicines among basic science MD students.
Materials and Methods:
The cross-sectional study was conducted among first to fifth semester students during February 2015. A previously developed instrument was used. Basic demographic information was collected. Respondent’s agreement with a set of statements was noted using a Likert-type scale. The calculated total score was compared among subgroups of respondents. One sample Kolmogorov–Smirnov test was used to study the normality of distribution, Independent samples t-test to compare the total score for dichotomous variables, and analysis of variance for others were used for statistical analysis.
Results:
Fifty-six of the 85 students (65.8%) participated. Around 55% of respondents were between 20 and 25 years of age and of American nationality. Only three respondents (5.3%) provided the correct value of the regulatory bioequivalence limits. The mean total score was 43.41 (maximum 60). There was no significant difference in scores among subgroups.
Conclusions:
There was a significant knowledge gap with regard to the regulatory bioequivalence limits for generic medicines. Respondents’ level of knowledge about other aspects of generic medicines was good but could be improved. Studies among clinical students in the institution and in other Caribbean medical schools are required. Deficiencies were noted and we have strengthened learning about generic medicines during the basic science years.
Key words: Caribbean, generic medicines, knowledge, medical students, perception
Key message: Use of generic medicines reduces the cost of treatment and promotes rational use of medicines. Generic medicines are widely used in developed countries. Medical students as future doctors have an important role in promoting use of generic medicines. Knowledge gaps with regard to use of these medicines exist and must be corrected through appropriate education.
A generic medicine is a multisource pharmaceutical product which is intended to be interchangeable with the originator product and is marketed after patents and other exclusivity rights of the innovator pharmaceutical company have expired.[1,2] Globally, health-care costs have been increasing rapidly and governments have adopted cost containment measures in an attempt to ensure efficient utilization of scarce resources. One of the important mechanisms to reduce health-care costs is to promote the use of cheaper generic medicines instead of more expensive branded equivalents.[3]
The usage of generic medicines is high in industrialized countries where price levels for pharmaceuticals are usually high. In the United States (US), over the period from 2003 to 2012, generic medicines have resulted in savings of US$ 1.2 trillion to the health-care system.[4] In the US, generic medicines account for approximately 75% of prescriptions but incur only 13% of the cost of newer, innovative, and more expensive medications.[5] During the year 2006, generic medicines accounted for 42% of dispensed packs among 27 European countries, but the cost was only 18% of the total pharmaceutical expenditure.[6] A study published in 2004 concluded that the combined effect of federal price regulations, provincial price freezes, and generic substitution policies have been effective in controlling price-related determinants of drug spending in Canada.[7]
Xavier University School of Medicine (XUSOM) is a private offshore medical school in Aruba, Kingdom of the Netherlands admitting students from the US, Canada, and other countries to the undergraduate medical (MD) course. From January 2013, the school has shifted to an integrated organ system-based curriculum with early clinical exposure.[8] Students complete the first six semesters (2 years) of the course in Aruba, and then, do their clinical rotations in the US and Canada. At the present, the pharmacology curricula have been tailored to enhance rational use of medicines by the future practitioners. Students learn to select a personal or P-drug for a disease condition and verify the suitability of their selected P-drug for a particular patient. They are also briefly introduced to generic medicines and also learned that to reduce the cost associated with medicines, promoting the use of generic medicines is important.
However, generic prescribing is still a contentious issue among prescribers and the issues of bioequivalence, quality, and safety remain problem areas.[9] Previous studies have shown that changing prescribing behavior among doctors may be difficult.[10] A national survey conducted in Australia, among senior medical students found that medical students need to be better taught about generic medicines and issues related to generic prescribing.[11] Another study comparing the knowledge and perceptions of senior medical students and pharmacy preregistrants in Australia found that though there were few differences in response, both groups had knowledge deficiencies about the quality, safety, and effectiveness of generic medicines which may need to be addressed by educational curricula.[12]
Students’ perception about generic medicines has not been previously studied in the institution. Hence, the present study was conducted to study the knowledge and perception about generic medicines among basic science undergraduate medical students in the institution.
Materials and Methods
The study was conducted among basic science undergraduate medical (MD) students (first to fifth semester) at XUSOM during the month of February 2015. A previously developed and validated survey[11,12] was used to study the student knowledge and perception about generic medicines. The first author of the previous two surveys was also an author of the present study and permission to use the instrument was obtained from him. Students were explained the aims and objectives of the study and invited to participate. Written informed consent was obtained from all respondents. The questionnaire used is shown in the appendix and consisted of three parts. The first part collected demographic information about the respondent including gender, age range in years, semester of study, and nationality. Part B of the questionnaire focused on the respondent’s knowledge toward generic medicines. There was a statement about the allowable regulatory bioequivalence limits when comparing generic and brand-name products of the same drug molecule, and six questions framed for responses on a five-point Likert-type scale. The scale was 5 = strongly agree with the statement, 4 = agree, 3 = neutral, 2 = disagree, and 1 = strongly disagree with the statement. Statements 4, 5, and 6 were negatively worded and the scores were reversed while calculating the total score.
Part C of the questionnaire studied respondents’ perception toward issues pertaining to generic medicines utilization. Statement 4 was negatively worded and was reverse scored. Information was collected using printed versions of the questionnaire. The data were entered into and analyzed using IBM Corp. Released 2011. IBM SPSS Statistics for Windows, Version 20.0. (Armonk, NY: IBM Corp.). The percentage of correct responses to the question about the regulatory bioequivalence limits was noted. The respondents’ agreement with the statements scored using a Likert-type scale was added together to compute the total score. The frequency and percentage of different demographic characteristics of respondents were noted.
The normality of the distribution of the scores of individual statements and of the total score was tested using the one-sample Kolmogorov–Smirnov test. Mean and standard deviation (SD) were calculated for scores following a normal distribution while median and interquartile ranges were calculated for nonparametric variables. The total scores were compared among different subgroups of respondents using independent sample t-test for dichotomous variables and one-way analysis of variance for others. A P < 0.05 was taken as statistically significant. The study was approved by the Institutional Review Board of the XUSOM vide notification number XUSOM/IRB/2015/01 dated January 27, 2015.
Results
Fifty-six of the eighty-five students (65.9%) participated in the study. Table 1 shows the demographic characteristics of the respondents [Table 1]. The questionnaires returned by two respondents were incomplete and were not included in the study. Around 55% of respondents were male and most were between 20 and 25 years of age. Around 55% of respondents were of American nationality.
Table 1.
Demographic characteristics of student respondents
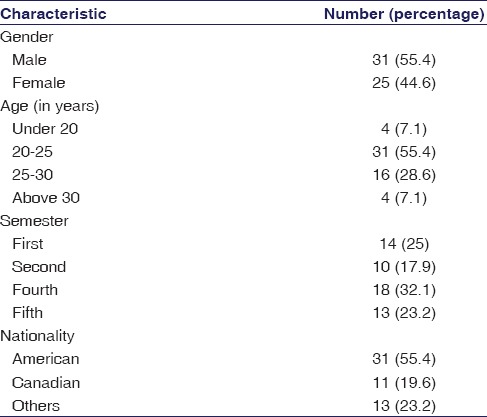
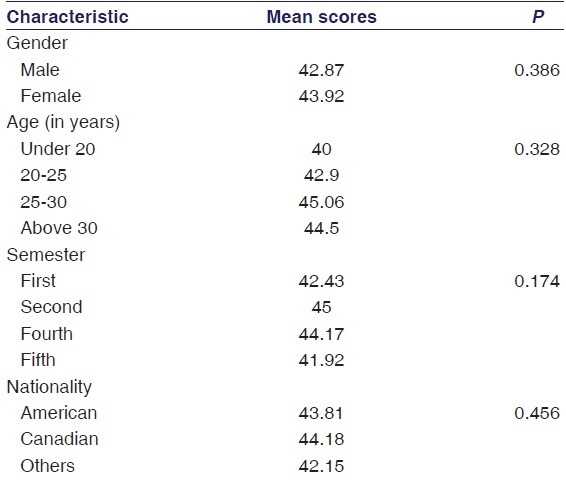
Only three of the fifty-six respondents (5.3%) gave the correct answer to the question about the regulatory bioequivalence limits. The mean ± SD total score was 43.41 ± 4.53 (maximum possible score was 60). As described in the methods section, the distribution of the total score followed a normal distribution while the scores of individual statements were not doing so. Table 2 shows the mean total scores among different subgroups of respondents. There was no significant difference in scores according to the respondents’ personal characteristics [Table 2].
Table 2.
Mean total scores among different subgroups of respondents
There were agreement-type six questions relating to knowledge. The mean agreement score for the statement regarding bioequivalence of generic and brand-name medicines was 3.89. The reversed scores of statements about generic medicines being less effective, producing more side effects, and brand-name medicines requiring to meet higher safety standards compared to generic medicines were all >3.5 denoting a low level of agreement with the original statements. There were six statements dealing with respondents’ perceptions about generic medicines. The mean score for the statement regarding the need for a standard guideline for health-care providers regarding the brand substitution process was 3.89. Respondents strongly agreed that quality use of generic medicines can be achieved if health-care providers work together (mean score of 4.05). The mean score for the statement about patients should be provided adequate information about generic medicines to ensure that they really understand the medicine they are taking was 4.32. The respondents’ agreement with the statements regarding requiring more information about the safety and efficacy of generic medicines was high (mean score 3.83), but the agreement with the statement regarding hospital procurement budgets affecting their choice of medicines was lower (mean score 3.34).
The median scores of all individual statements were 4. In Part B, statements 4, 5, and 6 were negatively worded. While in Part C, statement 4 was negatively worded. These statements were reverse scored.
Discussion
Medical educators have an important responsibility to teach the future doctors about the cost-effective use of medicines.[13] Increasing use of generic medicines has been strongly supported by policymakers to reduce the cost of pharmaceutical expenditure to both the health-care system and to individual consumers.[14] The authors of a previous paper had mentioned that to increase the rate of generic medicines use medical doctors must have a sound understanding of the issues surrounding their use and should be able to work in cooperation with other health-care practitioners.[12] In a previous study, majority of medical and pharmacy students were unable to identify the correct bioequivalence limits allowed for approval of generic medicines.[12] In our study, only about 5% of respondents were aware of the correct limits.
In our study, 45 of the 56 respondents (80.36%) either strongly agreed or agreed with the statement that a generic medicine is bioequivalent to a brand-name medicine while 42 respondents (75%) either strongly disagreed or disagreed with the statement that generic medicines are less effective compared to brand-name medicines. In the Australian study, about 85% of respondents in both the senior medical student group and the pharmacy preregistrant group agreed that a medicine rated as a generic equivalent should be therapeutically equivalent to the comparator brand-name product.[12] More than 80% of respondents agreed that a generic medicine was bioequivalent to the corresponding brand-name medicine.
A qualitative study conducted in Pakistan, among dispensing doctors had shown mixed views of the practitioners toward generic medicines.[15] The authors concluded that knowledge of dispensing doctors about generic medicines was sparse. Many doctors were confused about the term “generic medicines” and the international nonproprietary name of a drug.
In the study conducted in Australia, it was noted that respondents perceived generic medicines to be inferior in quality, less effective, produced more side effects, and were less safer compared to brand-name medicines.[12] More than 90% of respondents agreed that generic medicines cost much less than their brand-name counterparts. Deficiencies in knowledge and problems with perception toward generic medicines were noted in a study conducted among general practitioners (GPs) in Ireland.[16] GPs overall had a positive attitude toward generic medicines but voiced concerns over patient experience, clinical effectiveness, and manufacturing quality. A similar finding was noted among GPs in a northern state of Malaysia.[17] The authors concluded that GPs seem to have largely accepted the use of generic medicines but still had concerns about the quality and reliability of such products.
At XUSOM, Aruba, under the new curriculum, students learn pharmacology in an integrated organ system-based manner with other basic science subjects. Practical sessions in P-drug selection, verifying the suitability of a selected P-drug for a particular patient, and writing a prescription are conducted. Students are introduced to generic medicines during a small group session on social issues in use of medicines during the first semester. We broadly follow a similar scheme for the session as described in a Nepalese medical school.[18] The issue of cost of medicines is repeatedly addressed during sessions on P-drug selection during which medicine cost is an important selection criteria. Although the number of respondents who participated in the study is small compared to other studies of a similar nature elsewhere, the findings do suggest that lack of knowledge to issues surrounding to generic medicines needs to be addressed among the future practitioners. During the first semester, an activity-based session on social issues in use of medicines is conducted and during this session, we have strengthened student learning about cost of medicines, patents, generics, and the quality standards which generic medicines should meet. During the interactive lecture session on pharmacokinetics, the bioavailability requirements for generics and the concept of bioequivalence are also discussed. Cost of medicines, patents, generics, and innovator brands is again addressed during the second semester during the small group session on understanding and responding to pharmaceutical promotion. Issues of quality of generic medicines and regulatory requirements for generic medicines and the process for approving generic medicines are discussed. We also address the issue of cost of medicines during the personal drug selection sessions and emphasize that though most Canadians and many Americans have health insurance, the high cost of medicines strains the budgets of health systems and cost containment strategies are being increasingly emphasized. Increasing use of generic medicine is an important strategy towards cost containment.
Conclusions
There was a significant knowledge gap with regard to the regulatory bioequivalence limits for generic medicines. The median scores for positively worded and the reversed scores for negatively worded statements were 4 indicating a reasonable level of knowledge about generic medicines but which could nevertheless be improved. Social issues related to the use of medicines and cost of medicines are addressed during the basic science years of the MD course and based on the results of the present study education about generic medicines have been strengthened during the basic science years. Studies among clinical students in the institution and in other Caribbean medical schools are required.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
References
- 1.World Health Organization. Multisource (Generic) Pharmaceutical Products: Guidelines on Registration Requirements to Establish Interchangeability. Working Document QAS/04.093/Rev. 4. 2005. [Last accessed on 2016 Oct 02]. Available from: http://www.who.int/medicines/services/expertcommittees/pharmprep/QAS04_093Rev4_final.pdf .
- 2.Al-Gedadi NA, Hassali MA, Shafie AA. A pilot survey on perceptions and knowledge of generic medicines among consumers in Penang, Malaysia. Pharm Pract (Granada) 2008;6:93–7. doi: 10.4321/s1886-36552008000200006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kanavos P. Do generics offer significant savings to the UK National Health Service? Curr Med Res Opin. 2007;23:105–16. doi: 10.1185/030079907X159506. [DOI] [PubMed] [Google Scholar]
- 4.Generic Pharmaceutical Association. Generic Drug Savings in the US. Fifth Annual Edition. [Last accessed on 2016 Oct 02]. Available from: http://www.gphaonline.org/media/cms/2013_Savings_Study_12.19.2013_FINAL.pdf .
- 5.Kohl H, Shrank WH. Increasing generic drug use in medicare part D: The role of government. J Am Geriatr Soc. 2007;55:1106–9. doi: 10.1111/j.1532-5415.2007.01286.x. [DOI] [PubMed] [Google Scholar]
- 6.Simoens S. Trends in generic prescribing and dispensing in Europe. Expert Rev Clin Pharmacol. 2008;1:497–503. doi: 10.1586/17512433.1.4.497. [DOI] [PubMed] [Google Scholar]
- 7.Morgan S. Drug spending in Canada: Recent trends and causes. Med Care. 2004;42:635–42. doi: 10.1097/01.mlr.0000129494.36245.4b. [DOI] [PubMed] [Google Scholar]
- 8.Shankar PR, Bharti R, Ramireddy R, Balasubramanium R, Nuguri V. Students’ perception of the learning environment at Xavier University School of Medicine, Aruba: A follow-up study. J Educ Eval Health Prof. 2014;11:9. doi: 10.3352/jeehp.2014.11.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Birkett DJ. Generics-equal or not? Aust Prescr. 2003;26:85–7. [Google Scholar]
- 10.Helin-Salmivaara A, Huupponen R, Klaukka T, Hoppu K. Steering Group of the ROHTO Programme. Focusing on changing clinical practice to enhance rational prescribing – Collaboration and networking enable comprehensive approaches. Health Policy. 2003;66:1–10. doi: 10.1016/s0168-8510(03)00020-4. [DOI] [PubMed] [Google Scholar]
- 11.Hassali MA, Stewart K, Kong DC. A national survey on knowledge and perceptions of senior medical students in Australia about generic medicines. Med J Aust. 2008;188:123–4. doi: 10.5694/j.1326-5377.2008.tb01543.x. [DOI] [PubMed] [Google Scholar]
- 12.Hassali MA, Kong DC, Stewart K. A comparison between senior medical students’ and pharmacy pre-registrants’ knowledge and perceptions of generic medicines. Med Educ. 2007;41:703–10. doi: 10.1111/j.1365-2923.2007.02791.x. [DOI] [PubMed] [Google Scholar]
- 13.Gafa M, Bilbija S, Martinova A, Bates I. Pharmacoeconomics. A view of EPSA member countries on issues related to awareness of the topic and the undergraduate curriculum. Pharm Educ. 2002;2:171–5. [Google Scholar]
- 14.Lofgren H. Generic drugs: International trends and policy developments in Australia. Aust Health Rev. 2004;27:39–48. doi: 10.1071/ah042710039. [DOI] [PubMed] [Google Scholar]
- 15.Jamshed SQ, Hassali MA, Ibrahim MI, Babar ZU. Knowledge attitude and perception of dispensing doctors regarding generic medicines in Karachi, Pakistan: A qualitative study. J Pak Med Assoc. 2011;61:80–3. [PubMed] [Google Scholar]
- 16.Dunne SS, Shannon B, Cullen W, Dunne CP. Beliefs, perceptions and behaviours of GPs towards generic medicines. Fam Pract. 2014;31:467–74. doi: 10.1093/fampra/cmu024. [DOI] [PubMed] [Google Scholar]
- 17.Chua GN, Hassali MA, Shafie AA, Awaisu A. A survey exploring knowledge and perceptions of general practitioners towards the use of generic medicines in the Northern state of Malaysia. Health Policy. 2010;95:229–35. doi: 10.1016/j.healthpol.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 18.Shankar P. Introducing Medical Students to Social Issues in Use of Medicines. MedEdPORTAL. 2012. [Last accessed on 2016 Oct 02]. Available from: http://www.mededportal.org/publication/9183 .


