Abstract
Background
Amyotrophic lateral sclerosis (ALS) is a rapidly fatal neurodegenerative disease of unknown etiology. We investigated the association between ALS diagnosis and prior cardiovascular disease (CVD), and CVD-specific, hospital admissions in the Danish population.
Methods
We conducted a population-based nested case-control study, including 3,182 Danish residents diagnosed with ALS at age ≥20 years (1982—2009), and 100 randomly selected controls for each case, matched on age, sex and vital status. We estimated odds ratios (OR) associated with CVD, and CVD-specific, hospital admissions, adjusting for socio-economic and marital status, region of residence and past diabetes and obesity diagnoses.
Results
The estimated adjusted OR for any CVD admission at least three years prior to the date of ALS diagnosis was 1.15 (95%CI:1.04–1.27). Our results varied across cause-specific admissions; for atherosclerosis the OR was 1.36 (95%CI:1.02–1.80) and for ischemic heart disease 1.14 (95%CI:0.99–1.31), while we observed no association with hypertensive and cerebrovascular diseases. Adjusting for or stratifying by COPD status, a cigarette-smoking correlate, did not change our results.
Conclusion
In our population-based study we found evidence for a moderately elevated association with CVD that was stronger for specific conditions, such as atherosclerosis. Our findings may have important implications about the ALS pathogenesis.
Keywords: amyotrophic lateral sclerosis (ALS), cardiovascular disease (CVD), ischemic heart disease, atherosclerosis
INTRODUCTION
The etiology of amyotrophic lateral sclerosis (ALS) remains unclear; only 5–10% of patients have familial ALS and thus far there has been no unifying causal hypothesis for the ALS etiology.(1) There is reason to believe that cardiovascular disease and related conditions could play a role in ALS pathogenesis. Defective energy metabolism, hyperlipidemia (2) and cholesterol (3) have been linked to lower risk of ALS, and a lower risk of ALS has been found among subjects with diabetes.(4,5) Statin treatment has also been associated with ALS, although its role in ALS pathogenesis and the direction of the association remain controversial.(2,6)
To our knowledge, only two studies have looked at the association between cardiovascular disease (CVD) and ALS. The first (7) was conducted using hospital-based data and the authors observed a lower ALS rate ratio among patients with coronary heart disease (CHD) than those without CHD. The use of a hospital-based population, however, can potentially introduce Berkson-type selection bias if factors that drive hospitalization are also related to the exposure of interest. The second study was based on self-reported clinical characteristics, and a protective association was also found.(8) However, diabetes was included under the broad CVD definition, and a strong protective association has been found with diabetes specifically.(4,5) To examine the association between CVD, as well as cause-specific CVD hospitalizations, and ALS in a general population-based sample, we used data obtained from the Danish Registers system, with which details of all Danish residents can be linked through the existence of a unique personal identifier.(9)
METHODS
Data Collection
We conducted a case-control study nested within the entire population of Denmark.
ALS Case Ascertainment
ALS cases were identified via the Danish National Patient Register (NPR). NPR, one of the most comprehensive patient registries internationally, was established in 1977 and includes nationwide administrative and clinical records of all non-psychiatric inpatient data.(9) Outpatient data have been included since 1995.(9) Data from this register can be linked to data from other registers via the 10-digit unique personal identifier of each Danish resident.
We defined ALS cases as anyone with an inpatient or outpatient International Classification of Diseases (ICD; World Health Organization)-8 discharge diagnosis of 348.0 (ALS) before 1993 or, starting in 1994 when Denmark adopted the ICD-10, a discharge diagnosis of G12.2 (Motor Neuron Disease, MND), and who was at least 20 years old when first diagnosed. The date of the first relevant code was assigned as the diagnosis date (i.e. index date). We restricted our analyses to cases identified through the NPR between the years 1982–2009, to avoid inclusion of prevalent cases in the early years of the NPR. When compared with medical records, ALS case identification through the NPR was found to have positive predictive value of 93%.(10) In the main analyses, we did not include cases that were identified only in outpatient data because we found a much lower positive predictive value for those.(10)
Control Selection
Through the Danish Civil Registration System (CRS), which includes administrative records on all persons with a residence in Denmark since 1968, for each ALS case we randomly selected 100 controls free of ALS at the index date and individually matched on sex, year of birth and vital status.
CVD-related Hospital Admissions
Cause-specific hospital admissions (inpatient or outpatient records), and the dates of these admissions, were obtained for both cases and controls through the NPR. Using codes from the ICD-8 and ICD-10, we considered any hospital admissions (i.e. either primary or secondary) for all CVD conditions (ICD-8: 390–448, ICD-10: I00–I79). In addition, we separately considered the CVD-specific admissions for ischemic heart disease (ICD-8: 410–414, ICD-10: I20–I25), hypertensive disease (ICD-8: 400–404, ICD-10: I10–I15), cerebrovascular disease (ICD-8: 430–438, ICD-10: I60–I69) and atherosclerosis (ICD-8: 440, ICD-10: I70). We assessed CVD and cause-specific CVD diagnoses as predictors of ALS if they occurred at least three years prior to the index date. To assess the robustness of our findings, avoid potential reverse causality, and occurrence of pseudo-myocardial infarction during the ALS latency period,(11) we repeated analyses using admissions that occurred at least five and ten years prior to the index date.
Covariates
We used the five-category socio-economic status (SES) definitions developed by the Danish Institute of Social Sciences, which are based on job titles and income tax forms. The group with the highest status (group 1) includes corporate managers and people in academics; group 2 includes proprietors, managers of small businesses and teachers; group 3 includes technicians and nurses; group 4 includes skilled workers; and group 5 includes unskilled workers. We also included an additional group for subjects with unknown job titles. If a participant was married, we used the higher of the couple’s individual SES ranks. Further, we included marital status (non-married, married, divorced and widowed), and region of residence at the index date (Copenhagen, Copenhagen suburbs, other large cities, provincial towns, rural areas, Greenland and unknown). Given our recent findings linking diabetes to decreased risk for ALS,(4) we also adjusted for any diabetes-related hospital admission that occurred at least 3 years prior to the index date (ICD-8: 249, 250 and ICD-10: E10, E11). Registry data do not include information on smoking or body mass index (BMI). We, therefore, used diagnoses of chronic obstructive pulmonary disease (COPD; ICD-8: 490–493 and 518, ICD-10: J40–J47) at least one year prior to the ALS diagnosis index date as a proxy indicator for smoking and obesity-specific diagnoses (ICD-8: 277.99, ICD-10: E65–E69) as a surrogate for BMI. As for the CVD diagnoses, diabetes, COPD and obesity diagnoses were obtained using both inpatient and outpatient records and including both primary and secondary diagnoses.
Statistical Analysis
We conducted conditional logistic regression, with strata defined by the 1:100 case-control matched sets to estimate odds ratios (OR) and 95% confidence intervals (CI) for ALS, adjusted for covariates. Since we selected controls using risk set sampling, a form of density sampling, the ORs from our models estimate the rate ratios that would be obtained from a cohort design. We assessed whether associations differed by sex by including a multiplicative term in the model and also repeated the analysis using sex-specific models. Our SES variable has been found to be strongly correlated with smoking in Denmark,(12) but to further reduce residual confounding by smoking we also conducted analyses restricted to those with COPD. For this analysis, we ran unconditional logistic regression, adjusting for the matching factors, to maximize the number of available subjects included.
Sensitivity Analyses
We repeated analyses including ALS cases identified only in outpatient NPR data, i.e. those who never had an inpatient ALS diagnosis and, also, examined potential effect modification by whether first ALS discharge was through the inpatient vs. outpatient data. In addition, in an effort to conduct an analysis closer to the prior report using a hospital-based cohort,(7) we conducted an additional analysis restricting our controls to only those who had been hospitalized for any cause.
Because having CVD could lead to more contact with the medical system, it is possible that the date of ALS diagnosis could be advanced among CVD patients simply because of earlier detection. This could appear as a higher rate of ALS even in the absence of a biological effect of CVD on risk of ALS. Therefore, to examine the impact of this potential bias, we ran additional analyses after artificially delaying the ALS diagnosis date for any ALS case with CVD (and similarly the index date of those cases’ matched controls). We did this using a bootstrap process: for exposed cases, and their corresponding controls, we delayed their date of diagnosis (or index date) by one month on average (SD: 10 days). We then re-assessed exposure based on that date, as any CVD diagnosis at least 3 years prior to the new index date, and repeated our analyses. We repeated this process 100 times for each of multiple delay scenarios, i.e. 1- to 12-month delayed diagnoses, corresponding to CVD hypothetically leading to a 1–12 month earlier ALS diagnosis because of earlier ALS detection.
This study was determined to be exempt by the Harvard School of Public Health Institutional Review Board and was approved by the Danish Data Protection Agency. Participants were not required to provide informed consent by Danish legislation since no biological samples are obtained. All statistical analyses were conducted using the R Statistical Software, version 3.0.3 (Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Between 1982 and 2009, 3,182 ALS cases were identified meeting our inclusion criteria, with a mean age at diagnosis of 65.4 years (SD: 11.6). Table 1 presents the distribution of covariates by CVD status. Among those with CVD at least three years before the index date the average age at first CVD diagnosis in the NPR was 58.7 (SD 10.8) years old.
Table 1.
Distribution of socio-economic status, marital status, region, diabetes, COPD and obesity diagnoses, by cardiovascular disease (CVD) and case status.
| Cases | Controls | |||||||
|---|---|---|---|---|---|---|---|---|
| CVD | No CVD | CVD | No CVD | |||||
| N | (%) | N | (%) | N | (%) | N | (%) | |
| Socio-Economic Status | ||||||||
|
| ||||||||
| Group 1 (high) | 62 | 12.0 | 265 | 9.9 | 4,080 | 8.5 | 26,625 | 9.8 |
| Group 2 | 45 | 8.7 | 313 | 11.7 | 4,891 | 10.2 | 29,882 | 11.1 |
| Group 3 | 110 | 21.3 | 512 | 19.2 | 8,188 | 17.1 | 49,412 | 18.3 |
| Group 4 | 148 | 28.7 | 686 | 25.7 | 13,291 | 27.8 | 72,958 | 27.0 |
| Group 5 (low) | 101 | 19.6 | 498 | 18.7 | 10,939 | 22.9 | 53,583 | 19.8 |
| Unknown job title | 50 | 9.7 | 392 | 14.7 | 6,398 | 13.4 | 37,953 | 14.0 |
|
| ||||||||
| Marital Status | ||||||||
|
| ||||||||
| Non-married | 21 | 4.1 | 203 | 7.6 | 2,674 | 5.6 | 21,779 | 8.1 |
| Married | 316 | 61.2 | 1,628 | 61.1 | 22,168 | 46.4 | 127,578 | 47.2 |
| Divorced | 49 | 9.5 | 260 | 9.8 | 5,261 | 11.0 | 28,940 | 10.7 |
| Widowed | 130 | 25.2 | 575 | 21.86 | 17,684 | 37.0 | 92,116 | 34.1 |
|
| ||||||||
| Region of Residence at Diagnosis/Index Date | ||||||||
|
| ||||||||
| Copenhagen (Capital) | 77 | 14.9 | 365 | 13.7 | 5,357 | 11.2 | 32,502 | 12.0 |
| Copenhagen Suburbs | 109 | 21.1 | 566 | 21.2 | 10,009 | 20.9 | 58,375 | 21.6 |
| Other larger citiesa | 72 | 14.0 | 297 | 11.1 | 5,436 | 11.4 | 29,762 | 11.0 |
| Provincial towns | 187 | 36.2 | 999 | 37.5 | 19,258 | 40.3 | 105,299 | 38.9 |
| Rural areas | 67 | 13.0 | 387 | 14.5 | 7,679 | 16.1 | 42,084 | 15.6 |
| Greenland | 4 | 0.8 | 47 | 1.8 | 11 | 0.0 | 1,028 | 0.4 |
| Unknown | 0 | 0.0 | 5 | 0.2 | 37 | 0.1 | 1,363 | 0.5 |
|
| ||||||||
| Diabetes-Related Admissions | ||||||||
|
| ||||||||
| No Diabetes | 490 | 95.0 | 2,646 | 99.2 | 43,496 | 91.0 | 266,944 | 98.7 |
| Diabetes | 26 | 5.0 | 20 | 0.8 | 4,291 | 9.0 | 3,469 | 1.3 |
|
| ||||||||
| Obesity-Related Admissions | ||||||||
|
| ||||||||
| No Obesity | 497 | 96.3 | 2,656 | 99.6 | 45,483 | 95.2 | 268,846 | 99.4 |
| Obesity | 19 | 3.7 | 10 | 0.4 | 2,304 | 4.8 | 1567 | 0.6 |
|
| ||||||||
| COPDb-Related Admissions | ||||||||
|
| ||||||||
| No COPD | 467 | 90.5 | 2,593 | 97.3 | 42,880 | 89.7 | 262,642 | 97.1 |
| COPD | 49 | 9.5 | 73 | 2.7 | 4,907 | 10.3 | 7,771 | 2.9 |
Aarhus, Odense
COPD: Chronic Obstructive Pulmonary Disease
We found that CVD diagnosis prior to the index date was associated with a higher rate of ALS (Table 2). The estimated adjusted OR for ALS for any CVD admission at least three years prior to the date of ALS diagnosis was 1.15 (95%CI: 1.04–1.27). The associations were similar by sex (Pinteraction = 0.87). The estimated ORs were attenuated when we examined CVD at least 5 and 10 years prior to the index date (Table 2).
Table 2.
Odds ratios (OR) and 95% confidence intervals (CI) for CVD and cause-specific CVD, for 3, 5 and 10 years prior to the index date.
| Cases With Disease: N (%) | Controls With Disease: N (%) | Crudea OR (95%CI) | Adjustedb OR (95%CI) | |
|---|---|---|---|---|
| All Cardiovascular Disease | ||||
|
| ||||
| 3 years | 516 (16.2) | 47,787 (15.0) | 1.10 (1.00–1.22) | 1.15 (1.04–1.27) |
| 5 years | 390 (12.3) | 37,181 (11.7) | 1.06 (0.95–1.18) | 1.10 (0.98–1.23) |
| 10 years | 190 (6.0) | 18,469 (5.8) | 1.03 (0.89–1.20) | 1.07 (0.92–1.25) |
|
| ||||
| Ischemic Heart Disease | ||||
|
| ||||
| 3 years | 231 (7.3) | 21,087 (6.6) | 1.11 (0.97–1.27) | 1.14 (0.99–1.31) |
| 5 years | 180 (5.7) | 16,318 (5.1) | 1.11 (0.96–1.30) | 1.15 (0.98–1.34) |
| 10 years | 85 (2.7) | 7,878 (2.5) | 1.08 (0.87–1.35) | 1.12 (0.90–1.40) |
|
| ||||
| Hypertensive Disease | ||||
|
| ||||
| 3 years | 151 (4.7) | 14,592 (4.6) | 1.04 (0.88–1.23) | 1.10 (0.90–1.31) |
| 5 years | 118 (3.7) | 10,820 (3.4) | 1.10 (0.91–1.32) | 1.17 (0.96–1.41) |
| 10 years | 56 (1.8) | 5,003 (1.6) | 1.12 (0.86–1.47) | 1.20 (0.92–1.58) |
|
| ||||
| Cerebrovascular Disease | ||||
|
| ||||
| 3 years | 98 (3.1) | 10,559 (3.3) | 0.92 (0.75–1.13) | 0.95 (0.77–1.17) |
| 5 years | 64 (2.0) | 7,760 (2.4) | 0.82 (0.64–1.05) | 0.84 (0.66–1.08) |
| 10 years | 39 (1.2) | 3,459 (1.1) | 1.13 (0.82–1.56) | 1.17 (0.85–1.62) |
|
| ||||
| Atherosclerosis | ||||
|
| ||||
| 3 years | 50 (1.6) | 3,911 (1.2) | 1.29 (0.97–1.71) | 1.36 (1.02–1.80) |
| 5 years | 33 (1.04) | 2,823 (0.89) | 1.17 (0.83–1.67) | 1.22 (0.87–1.73) |
| 10 years | 14 (0.35) | 1,126 (0.44) | 1.25 (0.73–2.11) | 1.30 (0.76–2.21) |
The crude analysis from conditional logistic regression includes adjustment for the matching factors
Additionally adjusted for socio-economic status, marital status, region of residence, diabetes, COPD and obesity
To better assess potential confounding by smoking, we stratified our analyses by COPD status. Among subjects with no COPD diagnosis the OR was 1.15 (95%CI: 1.04–1.28), while among subjects with COPD the OR was not materially different (1.18; 95%CI: 0.80–1.73).
Cause-Specific CVD Admissions
In addition to defining the exposure of interest as hospitalization for any form of CVD combined, we also considered each form of CVD as separate exposures of interest. The observed associations varied by admission for different forms of CVD (Table 2). We observed elevated and marginally significant associations for ischemic heart disease, with similar associations for admissions considered at least 3, 5, and 10 years prior to the index date. We also observed significantly elevated associations for atherosclerosis that also remained similar for 3, 5 and 10 years prior to the index date. For 5 and 10 years, however, the confidence intervals were wide and included 1.0. We observed a modestly elevated OR for the association between hypertensive disease at least 3 years prior to the index date and ALS; the associations, however, were stronger when admissions were restricted to those at least 5 and 10 years prior to the index date. Finally, we observed no consistent association with cerebrovascular disease.
We found no evidence of differences in the magnitude of the association between any of the individual forms of CVD and ALS across levels of sex or COPD (Pinteraction ranged between 0.15 – 0.74 for sex and 0.68 – 0.98 for COPD).
Sensitivity Analyses
When we included an additional 468 ALS cases identified only in outpatient records, the estimated associations were only slightly lower, likely the result of increased outcome misclassification. Specifically, for CVD we found OR = 1.10 (95%CI: 1.00–1.21). Results for any CVD type did not differ by which data (inpatient or outpatient) the ALS case was first identified (PInteraction between 0.12 and 0.86).
In contrast to the results from the population-based analysis, when we restricted controls to only those who had been hospitalized at least once for any reason (median: 80, range: 22 – 100 controls per case) the association between CVD and ALS reversed (OR=0.87; 95%CI: 0.79–0.96). Similarly, the ORs for each of the cause-specific CVD admissions moved downwards as well. For example, for ischemic heart disease the estimated OR was 0.89 (95%CI: 0.78–1.02) from this sensitivity analysis (compared to 1.14, 95%CI: 0.99–1.31, in the main analysis).
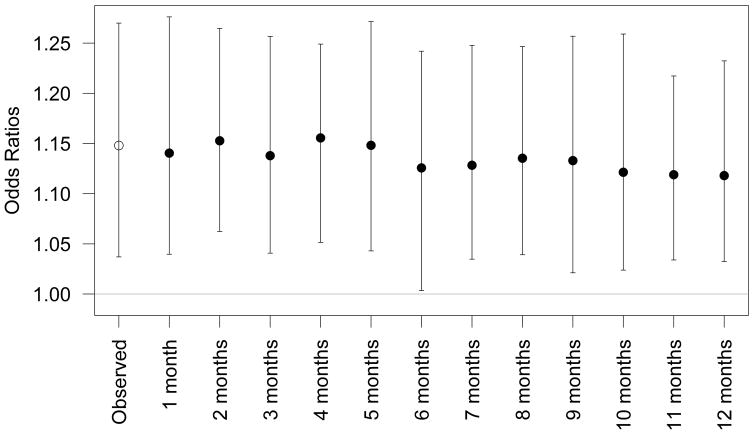
Our bootstrap process showed that if CVD leads to earlier ALS detection, any resulting bias is minimal and does not meaningfully change our results (Figure 1). Even if we assume that CVD advances the ALS diagnosis by a full year, after accounting for this, the OR for CVD was minimally affected (OR=1.12; 95% CI: 1.03–1.23, compared to OR=1.15; 95%CI: 1.04–1.27 in the main analysis). Results for all cause-specific CVD analyses were also all minimally affected. For example, even assuming atherosclerosis could advance ALS diagnosis by a year the OR was 1.39 (95%CI: 1.05–1.75) compared to 1.36 (95%CI: 1.02–1.80) in the main analysis (Supplemental Figure 1).
Figure 1.
Estimated adjusted odds ratios and 95% confidence intervals for the observed association between CVD and ALS (open circle) and after artificially delaying by the number of months indicated the ALS diagnosis date (or index date) among cases with CVD and their corresponding controls using a bootstrap process (closed circle), as described in the Sensitivity Analyses in the Methods section. The presented associations are adjusted for socio-economic status, marital status, region of residence and diabetes, COPD and obesity diagnoses.
DISCUSSION
We conducted a large-scale, population-based study to assess the association between hospital visits for CVD, and cause-specific CVD, and the diagnosis of ALS, using data from the Danish Registers system. We found moderately elevated associations for all forms of CVD combined, and specifically for ischemic heart disease and atherosclerosis, which remained robust to covariate adjustment. On the other hand, we found little evidence for an association with hypertensive disease and no association with cerebrovascular disease. The total CVD results were sensitive to the choice of the number of years prior to ALS index date to exclude CVD admissions; the associations were attenuated with increasing lags. Total CVD, however, is a heterogeneous disease, and the modestly elevated associations we observed for ischemic heart disease and atherosclerosis were robust across lags.
Previous studies have shown risk factors for CVD to be negatively associated with ALS.(1) Specifically, higher body mass index,(13,14) lower levels of physical activity,(14–16) and high vascular risk profile (3) have all been linked with a lower incidence of ALS. Also, a history of diabetes has been found to be associated with a lower risk of ALS.(4,5)
Other hypotheses, however, suggest that CVD risk factors could increase the risk of ALS. For example, neuroinflammation plays an important role in neurodegeneration,(17) and inflammatory processes have been implicated in ALS onset and progression.(18,19) Systemic inflammation is thought to be a shared mechanism of CVD and toxicity of the central nervous system (CNS); circulating cytokines have been shown to exacerbate progression of neurodegeneration,(20) and systemic inflammation has been linked with decreased function in subjects with neurodegenerative diseases(17) and with apoptosis in ALS.(21,22)
To our knowledge, only one other study has linked CHD admissions with ALS onset.(7) In that study, hospital admissions datasets were used to construct two cohorts, a CHD and a non-CHD cohort, and a significantly lower ALS rate was found in the CHD cohort. In our study we found a similar protective association when limiting the analysis to people who had ever appeared (i.e. received any diagnosis) in our hospital registry, but we found the opposite when analyzing the entire population. Creating a cohort based on hospital admissions can lead to Berkson-type selection bias when factors that drive hospitalization are also related to the exposure of interest.(23) In contrast, limiting to a hospital-based population could reduce possible bias from earlier ALS detection among people with CVD because of more frequent contact with the medical system. However, our sensitivity analysis suggested that even if ALS diagnosis was advanced by a full year—an unlikely scenario given the rapid progression of ALS—this would have little impact on the association between CVD, or cause-specific CVD, and ALS.
Our study has some limitations. Potential outcome misclassification cannot be excluded, although we have found this to be minimal in our Danish data.(10) Any resulting bias, however, should be towards the null, as there is no reason to believe that this would be differential by CVD status. This is likely why our sensitivity analysis additionally including ALS cases identified only in the outpatient data (i.e. ALS cases more likely to be misclassified)(10) showed slightly weaker results. Exposure misclassification is also likely, as cardiovascular disease was also assessed using hospital discharge diagnoses. Any resulting bias, however, should also be toward the null, as these binary variables were assessed at least 3 years prior to the index date (with similar results for ischemic heart disease and atherosclerosis when using 5 or 10 years) and, therefore, any misclassification should also be non-differential. Furthermore, validation studies of CVD-related hospital admissions in the Danish NPR have found these diagnoses to be generally highly reliable.(24) Because both ALS cases and controls needed to survive to the index date to be included in our analyses, there could possibly be some bias related to survival after CVD. However, such a bias might be expected to get stronger with longer survival times. Yet the association with ALS did not change with increasing lag time between certain cause-specific CVD admissions, i.e. ischemic heart disease and atherosclerosis, and ALS. Thus, any bias generated through this mechanism is likely to be weak.
We did not have data on behavioral and clinical factors such as weight, smoking and medication use. Adequate adjustment for such variables and assessment of potential effect modification was not possible in our analyses. However, we did use information on obesity-related diagnoses to adjust the estimated ORs. Furthermore, given that weight is associated with increased risk for CVD and reduced risk of ALS,(13,14) any residual confounding should bias our observed associations downwards and, thus, would not explain our observed positive associations with types of CVD, but would rather result in attenuation. Thus, had we been able to more adequately adjust for weight we might have expected the estimated ORs to be even stronger.
There is some evidence suggesting that smoking may increase the risk of ALS, although this remains controversial and some studies suggest that this association may be limited to women.(25) We do not have individual information on smoking, but occupational status has been shown to be correlated with smoking in Denmark;(12) thus, our adjustment for SES based on occupational status would in part also adjust for confounding by smoking status. We also adjusted for COPD status, as a proxy for smoking. Further, in analyses stratified by COPD status we observed no material difference in the associations. Among subjects with COPD, residual confounding by smoking is far less likely because COPD can be attributed to smoking in up to 70% of patients in developed countries.(26) If confounding by smoking were driving our results, we would have expected the estimated ORs from the analysis among subjects with COPD to be much closer to 1.0 compared to the OR from non-COPD subjects.
In conclusion, to our knowledge, this is the first nationwide, population-based study to explore the association between CVD, and cause-specific CVD, and ALS diagnosis. Although our findings should be interpreted in light of our limitations, they suggest that some aspect of CVD—potentially associated with mechanisms involved in ischemic heart disease and atherosclerosis—is related to a higher risk of ALS. Understanding specific biologic mechanisms could help better understand ALS etiology and identify additional, potentially modifiable, risk factors for ALS.
Supplementary Material
Estimated adjusted odds ratios and 95% confidence intervals for the observed association between atherosclerosis and ALS (open circle) and after artificially delaying by the number of months indicated the ALS diagnosis date (or index date) among cases with atherosclerosis and their corresponding controls using a bootstrap process (closed circle), as described in the Sensitivity Analyses in the Methods section. The odd ratios are adjusted for socio-economic status, marital status, region of residence and diabetes, COPD and obesity diagnoses.
Acknowledgments
This work was supported by the National Institute of Environmental Health Sciences [grant numbers R01ES019188 to MW]. MAK and RS were supported in part by an NIH training grant [grant number T32 ES007069]. RS was also supported by the Taplin Fellowship.
Footnotes
Declaration of interests: The authors report no declaration of interest.
References
- 1.Mitchell JD, Borasio GD. Amyotrophic lateral sclerosis. Lancet. 2007;369(9578):2031–2041. doi: 10.1016/S0140-6736(07)60944-1. [DOI] [PubMed] [Google Scholar]
- 2.Dupuis L, Pradat P-F, Ludolph AC, Loeffler J-P. Energy metabolism in amyotrophic lateral sclerosis. Lancet Neurol. 2011;10(1):75–82. doi: 10.1016/S1474-4422(10)70224-6. [DOI] [PubMed] [Google Scholar]
- 3.Sutedja NA, van der Schouw YT, Fischer K, Sizoo EM, Huisman MHB, Veldink JH, et al. Beneficial vascular risk profile is associated with amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2011;82(6):638–642. doi: 10.1136/jnnp.2010.236752. [DOI] [PubMed] [Google Scholar]
- 4.Kioumourtzoglou M-A, Rotem RS, Seals RM, Gredal O, Hansen J, Weisskopf MG. Diabetes Mellitus, Obesity, and Diagnosis of Amyotrophic Lateral Sclerosis. JAMA Neurol. 2015;72(8):905–911. doi: 10.1001/jamaneurol.2015.0910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mariosa D, Kamel F, Bellocco R, Ye W, Fang F. Association between diabetes and amyotrophic lateral sclerosis in Sweden. Eur J Neurol. 2015;22(11):1436–1442. doi: 10.1111/ene.12632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sørensen HT, Lash TL. Statins and amyotrophic lateral sclerosis--the level of evidence for an association. J Intern Med. 2009;266(6):520–526. doi: 10.1111/j.1365-2796.2009.02173.x. [DOI] [PubMed] [Google Scholar]
- 7.Turner MR, Wotton C, Talbot K, Goldacre MJ. Cardiovascular fitness as a risk factor for amyotrophic lateral sclerosis: indirect evidence from record linkage study. J Neurol Neurosurg Psychiatry. 2012;83(4):395–398. doi: 10.1136/jnnp-2011-301161. [DOI] [PubMed] [Google Scholar]
- 8.Seelen M, van Doormaal PTC, Visser AE, Huisman MHB, Roozekrans MHJ, de Jong SW, et al. Prior medical conditions and the risk of amyotrophic lateral sclerosis. J Neurol. 2014;261(10):1949–1956. doi: 10.1007/s00415-014-7445-1. [DOI] [PubMed] [Google Scholar]
- 9.Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scand J Public Health. 2011;39(7 Suppl):30–33. doi: 10.1177/1403494811401482. [DOI] [PubMed] [Google Scholar]
- 10.Kioumourtzoglou M-A, Seals RM, Himmerslev L, Gredal O, Hansen J, Weisskopf MG. Comparison of diagnoses of amyotrophic lateral sclerosis by use of death certificates and hospital discharge data in the Danish population. Amyotroph Lateral Scler Front Degener. 2015;16(3–4):224–229. doi: 10.3109/21678421.2014.988161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodriguez-Castro CE, Elfar A, Gonzalez-Ibarra FP, Siddiqui T, Abbas A. Amyotrophic lateral sclerosis and pseudo-infarct pattern on the electrocardiogram. Am J Med. 2013;126(7):2–4. doi: 10.1016/j.amjmed.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 12.Osler M. Smoking habits in Denmark from 1953 to 1991: a comparative analysis of results from three nationwide health surveys among adult Danes in 1953–1954, 1986–1987 and 1990–1991. Int J Epidemiol. 1992;21(5):862–871. doi: 10.1093/ije/21.5.862. [DOI] [PubMed] [Google Scholar]
- 13.O’Reilly ÉJ, Wang H, Weisskopf MG, Fitzgerald KC, Falcone G, McCullough ML, et al. Premorbid body mass index and risk of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Front Degener. 2013;14(3):205–211. doi: 10.3109/21678421.2012.735240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scarmeas N, Shih T, Stern Y, Ottman R, Rowland LP. Premorbid weight, body mass, and varsity athletics in ALS. Neurology. 2002;59:773–775. doi: 10.1212/wnl.59.5.773. [DOI] [PubMed] [Google Scholar]
- 15.Huisman MHB, Seelen M, de Jong SW, Dorresteijn KRIS, van Doormaal PTC, van der Kooi AJ, et al. Lifetime physical activity and the risk of amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2013;84(9):976–981. doi: 10.1136/jnnp-2012-304724. [DOI] [PubMed] [Google Scholar]
- 16.Pupillo E, Messina P, Giussani G, Logroscino G, Zoccolella S, Chiò A, et al. Physical activity and amyotrophic lateral sclerosis: A European population-based case-control study. Ann Neurol. 2014;75(5):708–716. doi: 10.1002/ana.24150. [DOI] [PubMed] [Google Scholar]
- 17.Perry VH, Nicoll JAR, Holmes C. Microglia in neurodegenerative disease. Nat Rev Neurol. 2010;6(4):193–201. doi: 10.1038/nrneurol.2010.17. [DOI] [PubMed] [Google Scholar]
- 18.Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms Underlying Inflammation in Neurodegeneration. Cell. 2010;140(6):918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evans MC, Couch Y, Sibson N, Turner MR. Inflammation and neurovascular changes in amyotrophic lateral sclerosis. Mol Cell Neurosci. 2013;53:34–41. doi: 10.1016/j.mcn.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 20.Perry VH, Cunningham C, Holmes C. Systemic infections and inflammation affect chronic neurodegeneration. Nat Rev Immunol. 2007;7(2):161–167. doi: 10.1038/nri2015. [DOI] [PubMed] [Google Scholar]
- 21.Dahlke C, Saberi D, Ott B, Brand-Saberi B, Schmitt-John T, Theiss C. Inflammation and neuronal death in the motor cortex of the wobbler mouse, an ALS animal model. J Neuroinflammation. 2015;12(1):215. doi: 10.1186/s12974-015-0435-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hensley K, Floyd RA, Gordon B, Mou S, Pye QN, Stewart C, et al. Temporal patterns of cytokine and apoptosis-related gene expression in spinal cords of the G93A-SOD1 mouse model of amyotrophic lateral sclerosis. J Neurochem. 2002;82(2):365–374. doi: 10.1046/j.1471-4159.2002.00968.x. [DOI] [PubMed] [Google Scholar]
- 23.Roberts RS, Spitzer WO, Delmore T, Sackett DL. An empirical demonstration of Berkson’s bias. J Chronic Dis. 1978;31(2):119–128. doi: 10.1016/0021-9681(78)90097-8. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt M, Schmidt SAJ, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The Danish National patient registry: A review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490. doi: 10.2147/CLEP.S91125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alonso A, Logroscino G, Hernán MA. Smoking and the risk of amyotrophic lateral sclerosis: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2010;81(11):1249–1952. doi: 10.1136/jnnp.2009.180232. [DOI] [PubMed] [Google Scholar]
- 26.Chilvers ER, Lomas Da. Diagnosing COPD in non-smokers: splitting not lumping. Thorax. 2010;65(6):465–466. doi: 10.1136/thx.2009.128421. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Estimated adjusted odds ratios and 95% confidence intervals for the observed association between atherosclerosis and ALS (open circle) and after artificially delaying by the number of months indicated the ALS diagnosis date (or index date) among cases with atherosclerosis and their corresponding controls using a bootstrap process (closed circle), as described in the Sensitivity Analyses in the Methods section. The odd ratios are adjusted for socio-economic status, marital status, region of residence and diabetes, COPD and obesity diagnoses.