Abstract
We previously reported that active sensitization of rats resulted in the appearance of a unique system for rapid and specific antigen uptake across intestinal epithelial cells. The current studies used rats sensitized to horseradish peroxidase (HRP) to define the essential components of this antigen transport system. Sensitization of rats to HRP stimulated increased HRP uptake into enterocytes (significantly larger area of HRP-containing endosomes) and more rapid transcellular transport compared with rats sensitized to an irrelevant protein or naive control rats. Whole serum but not IgE-depleted serum from sensitized rats was able to transfer the enhanced antigen transport phenomenon. Immunohistochemistry demonstrated that sensitization induced expression of CD23, the low-affinity IgE receptor (FcεRII), on epithelial cells. The number of immunogold-labeled CD23 receptors on the enterocyte microvillous membrane was significantly increased in sensitized rats and was subsequently reduced after antigen challenge when CD23 and HRP were localized within the same endosomes. Finally, pretreatment of tissues with luminally added anti-CD23 antibody significantly inhibited both antigen transport and the hypersensitivity reaction. Our results provide evidence that IgE antibodies bound to low-affinity receptors on epithelial cells are responsible for the specific and rapid nature of this novel antigen transport system.
Introduction
Allergic diseases are the most common of all immunologically mediated conditions, affecting 20–30% of the U.S. population, and are increasing in prevalence in most countries of the developed world (1). Food allergy is one type of allergic disorder that affects both adults and children. After ingestion of a specific food antigen, a sensitized individual may experience local gastrointestinal symptoms such as nausea, vomiting, and diarrhea; extraintestinal symptoms can occur in the skin and airways (2, 3). In severe reactions, such as those to peanut antigen, systemic anaphylaxis can occur and be life threatening. Treatment of food allergies usually consists of avoidance of suspected foods. In some cases, particularly in children, elimination diets can become so restrictive that nutrition may be compromised. Therefore, it is important to have a clear understanding of the mechanisms involved in food allergic/intestinal hypersensitivity reactions in order to develop effective therapeutic strategies.
The accepted sequence of events in allergy is that symptoms are triggered when antigen cross-links IgE antibodies bound to the surface of mast cells. Released bioactive mediators then act on receptors on other cell types to alter function. Studies in humans and animal models of food allergy have shown that intestinal reactions occur very quickly and result in dramatic physiological changes. Within minutes, epithelial secretion of ions, water and mucin begins, vasodilation and vascular permeability increase, and contraction of smooth muscle occurs (4–6). The rapid nature of the response has resulted in allergic reactions being termed “immediate” hypersensitivity. The exact mechanism responsible for the rapidity of the allergic symptom production has never been fully explained, as the epithelial lining of the gastrointestinal tract should theoretically restrict access of macromolecular antigens to effector cells such as mast cells, located in the subepithelial lamina propria.
Normally, macromolecules penetrate the epithelium in very limited quantities. M cells, specialized cells in the epithelium covering Peyer’s patches (located mainly in the distal small and large intestine), transport antigens from the lumen to immune cells in the patch (7). This process is thought to be important in the generation of oral tolerance, a mechanism to actively suppress immune responses (8). However, the number of M cells is relatively small compared with the number of columnar epithelial cells (enterocytes) that line the entire intestinal tract. Enzymes anchored in the enterocyte microvillus membrane degrade most ingested proteins into nonantigenic amino acids and peptides. Enterocytes do take up some intact protein into endosomes that are transported across the cells, but the majority of endocytosed protein is hydrolyzed by lysosomal enzymes after fusion of endosomes with lysosomes (9). Thus, the amount of immunologically intact protein that arrives in the circulation comprises less than 0.01% of that ingested. Transepithelial protein transport has been shown to be relatively slow, requiring 20–30 minutes (10).
In contrast to this normal process, our previous results indicated a much faster event in sensitized rats. In early studies of rats sensitized to ovalbumin (OVA), in vivo perfusion of the small intestine with antigen-containing buffer resulted in significantly altered transport of ions and water within 20 minutes, associated with mast cell activation (11). Subsequently, we examined segments of small and large intestine (rodents and humans) in Ussing chambers and demonstrated that challenge with antigen or anti-IgE resulted in active Cl– ion secretion (the driving force for water secretion leading to diarrhea), indicated on-line by an increase in the short-circuit current (Isc) (12–15). Surprisingly, when antigen was added to the luminal surface of intestinal tissues from sensitized rats, the change in Isc began in only 3 minutes (16). In addition, intestinal responsiveness in this animal model was retained for at least 8 months (17).
Recently, we conducted studies designed to determine the effect of sensitization on the rate and route of transepithelial antigen transport. We sensitized rats to horseradish peroxidase (HRP) and subsequently challenged tissues in Ussing chambers with this protein. HRP was used as a model antigen because it is similar in size to typical food antigens and its reaction product can be visualized in cells and tissues by electron microscopy. We found that endosomal uptake of HRP by enterocytes was enhanced in rats sensitized to this protein, but not in those sensitized to OVA (18). Both the amount of HRP antigen endocytosed and its rate of transport across the cells were significantly increased by specific sensitization, such that at 2 minutes, HRP was already present in the lamina propria in tissues from sensitized rats, whereas HRP-containing endosomes remained confined to the apical region of enterocytes in control tissues. The specificity of the antigen uptake phenomenon in sensitized rats suggested that it was mediated by an immunoglobulin recognition mechanism.
The present study was designed to investigate the essential components of this enhanced specific antigen transport system in sensitized rats. We found that rapid transepithelial transport could be passively transferred to naive rats by injection of serum from actively sensitized rats, but that depletion of IgE from the serum eliminated the effect. Expression of the low-affinity IgE receptor, FcεRII/CD23, on the enterocyte microvillus membrane was stimulated by sensitization. Immunogold receptor localization showed that the number of receptors on the apical membrane was increased by sensitization and then reduced by antigen challenge. Subsequently, we localized CD23 and HRP antigen within the same endosomes. Finally, pretreatment of tissues with anti-CD23 mAb before HRP challenge concentration dependently inhibited both transepithelial transport of antigen and the hypersensitivity reaction. Our results provide evidence that an IgE-CD23 mechanism accounts for enhanced transepithelial antigen transport in sensitized rats.
Methods
Animals.
All studies were approved by the McMaster University Animal Care Committee. Pathogen-free male Sprague-Dawley rats (mean weight 300 g; Charles River, St. Constant, Quebec, Canada) were actively sensitized to HRP (type II) or OVA (type VI) (both from Sigma Chemical Co., St. Louis, Missouri, USA) by injection of 1 mg protein in alum (subcutaneously) plus pertussis vaccine or toxin (intraperitoneal) adjuvants to induce IgE production, as described previously (16–18). Controls were rats sham-sensitized by injection of saline. Experiments were conducted 14 days after active/sham sensitization.
Rats were passively sensitized to HRP by injection (intraperitoneal) of 1.5 ml of high IgE–containing serum (anti-HRP IgE titer = 1:1,024) generated in HRP-sensitized and boosted Brown-Norway rats (Harlan Sprague-Dawley Inc., Indianapolis, Indiana, USA), a high responder strain. In pilot studies, we determined that heat treatment (56°C for 2 hours) of this serum abolished its ability to transfer enhanced antigen uptake passively, suggesting the involvement of heat-labile IgE antibodies. Therefore, we specifically depleted IgE by immunoprecipitation using monoclonal mouse anti-rat IgE (MARE-1; Serotec, Raleigh, North Carolina, USA) coupled to Sepharose 4B beads. Serum and beads were incubated overnight at 4°C and then centrifuged to remove the IgE-bead complexes. Controls were rats sham-sensitized by injection of serum from naive rats. Experiments were conducted 3 days after passive/sham sensitization.
Ussing chambers.
A 15-cm segment of jejunum (beginning 10 cm distal to the ligament of Treitz) was removed from anesthetized rats. The external muscle layer was stripped off and mucosal sheets (usually four per rat) were mounted in Ussing chambers (surface area = 0.6 cm2) (16). Care was taken to avoid segments with Peyer’s patches. The tissues were bathed in 10 ml of oxygenated Krebs buffer (115.0 mM NaCl, 8.0 mM KCl, 1.25 mM CaCl2, 1.2 mM MgCl2, 2.0 mM KH2PO4, and 25.0 mM NaHCO3 (pH 7.35 ± 0.02) at 37°C). The buffer in the serosal compartment contained 10 mM glucose osmotically balanced by 10 mM mannitol in the mucosal (luminal) compartment of the chamber. Tissues were short-circuited at zero volts by injection of Isc (in μA/cm2) using an automated voltage clamp (WPI Instruments; Narco Scientific, Mississauga, Ontario, Canada). At intervals, the Isc was turned off, and the spontaneous potential difference was recorded. Tissue conductance (in mS/cm2) was calculated according to Ohm’s Law. After an equilibration period of 20 minutes, 5 × 10–5 M HRP (or in some cases OVA as a nonspecific antigen control) was added to the mucosal buffer (luminal side of the tissue).
Epithelial transport of antigen.
The increase in Isc, indicating the ion secretory response, began approximately 3 minutes after the addition of HRP to the mucosal compartment of chambers containing jejunum from HRP-sensitized rats (18). Therefore, to examine epithelial uptake of HRP before the hypersensitivity reaction, tissues were removed from the chambers at 2 minutes and fixed for electron microscopy. (We previously showed that mast cells were not activated 2 minutes after addition of antigen to the mucosal buffer of chambers containing tissues from sensitized rats [ref. 18]). Tissues were fixed in 2% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.4), washed, incubated in 3,3′-diaminobenzadine tetrahydrochlorine (Sigma Chemical Co.) and H2O2, and subsequently processed for transmission electron microscopy. Ultrathin sections of midvillus epithelium (cut in the longitudinal plane) were stained with uranyl acetate and lead citrate. Photomicrographs of epithelial cells were taken at a magnification of 8,000. To quantify epithelial HRP uptake, the total area of HRP-containing endosomes in fixed size windows (206 μm2) in the apical region of enterocytes was measured using a computerized image processing system. Measurements were made by one investigator (P.-C. Yang, who was unaware of the rat treatment) on 12–24 coded photomicrographs per group (obtained from 4–6 rats per treatment group).
To examine transport of HRP protein across the tissues, HRP was added to the mucosal buffer and samples of serosal buffer (1 ml) were collected at 30 minutes intervals for 90 minutes. The concentration of HRP was determined by a kinetic enzymatic assay (18). Briefly, 150 μl of sample was added to 800 μl of phosphate buffer containing 0.003% H2O2 and 80 μg/ml o-dianisidine (Sigma Chemical Co.), and the enzyme activity was determined from the rate of increase in optical density at 460 nm. The mucosal-to-serosal flux was calculated using a standard formula and expressed as pmol/cm2/h.
Epithelial expression of CD23.
In rodents, the high-affinity receptor for IgE (FcεRI) is restricted to mast cells and basophils due to the necessity of the β chain for receptor stability and transport to the cell surface, whereas in humans, expression requires only the α and γ chains and distribution is more extensive (19). However, expression of FcεRI on epithelial cells has not been reported in either humans or rodents. In contrast, the low-affinity receptor (FcεRII/CD23) has been identified on epithelial cells in the intestine (20) and airways (21). Therefore, we determined the effect of sensitization of rats on epithelial expression of CD23. The hybridoma cell line, B3B4, producing a well-described anti-CD23 mAb (IgG2a anti-mouse CD23, cross-reactive with rat) (22), was grown in DMEM with 5% FCS. The antibody was isolated from culture supernatant by ammonium sulfate precipitation followed by affinity purification in a protein G-Sepharose column (Pharmacia Biotech Inc., Uppsala, Sweden). In preliminary experiments, we confirmed that this antibody similarly identified CD23 expression on rat as well as mouse splenic B cells. The B3B4 antibody was used for immunohistochemical detection of CD23 on intestinal epithelial cells. For light microscopy, segments of jejunum were immersed in OCT, snap frozen in liquid nitrogen and stored at –70°C. Cryosections were dried at room temperature overnight and fixed with acetone for 15 minutes. Endogenous peroxidase was extinguished by treating sections with 0.01% H2O2 for 10 minutes, and with 1% BSA for 30 minutes. CD23 was identified by the biotin-streptavidin method (B3B4 primary antibody, secondary biotinylated antibody from DAKO Corp., Mississauga, Ontario, Canada). Controls included sections where the B3B4 primary antibody was omitted or replaced with an irrelevant isotype-matched antibody (Rockland Immunochemicals, Gilbertsville, Pennsylvania, USA). No immunoreactive cells were observed in the control sections.
For immunoelectron microscopy, tissues were fixed in 2% paraformaldehyde/0.75% glutaraldehyde and dehydrated with a series of graded ethanols. The specimens were saturated in 50%, 75%, and 100% LR White at 4°C and embedded with LR White, and then polymerized in a freezer under ultraviolet radiation. Ultrathin sections were treated with 1% BSA mixed with 5% rabbit serum for 30 minutes, incubated with primary antibody for 1 hour at room temperature, then incubated with gold-conjugated rabbit anti-rat IgG antibody for 1 hour. Sections were post-fixed in osmium and stained with uranyl acetate and lead citrate. Sections were observed with the electron microscope and coded photomicrographs prepared. Immunogold labels were counted on the microvillus membrane of epithelial cells and expressed per 100 μm. Controls for electron microscopy included sections where the B3B4 primary antibody was omitted or replaced with the isotype-control antibody. No labels were detected in these control sections.
Effect of blocking CD23 on antigen uptake and transport.
B3B4 antibody has been shown to block/displace binding of IgE to its receptor (22). Therefore, we examined the effect of excess antibody on antigen uptake. Thirty minutes before HRP challenge, B3B4 antibody (10–40 μg/ml) or isotype control antibody was added to the mucosal buffer bathing tissues from rats actively sensitized to HRP. To examine the effect of anti-CD23 on transepithelial antigen transport before the hypersensitivity reaction, tissues were removed 2 minutes after challenge and processed to identify HRP in tissues. The area of HRP-containing endosomes in enterocytes was measured in 12 windows per rat group. To examine the effect of anti-CD23 on the transport of HRP protein across the tissues, samples of serosal buffer were collected for three 30-minute periods. The concentration of HRP was determined, and the mucosal-to-serosal flux was calculated.
Effect of blocking CD23 on the hypersensitivity reaction.
To examine the effect of anti-CD23 on the hypersensitivity reaction itself, we determined the following responses to luminal HRP challenge of sensitized tissues after preincubation with various concentrations (10–40 μg/ml) of B3B4 or isotype control antibody: (a) the increase in Isc (maximum change within 15 minutes after HRP challenge), indicating ion secretion; and (b) the increase in conductance (change at 30 minutes after HRP challenge), indicating enhanced permeability mainly of the paracellular pathway.
Statistics.
Differences between groups were tested by ANOVA, with post hoc analysis using Newman Keuls test or Student’s t test when appropriate. The data were expressed as mean ± SEM. A value of P < 0.05 was considered to be significant.
Results
Sensitization enhances epithelial uptake of specific antigen.
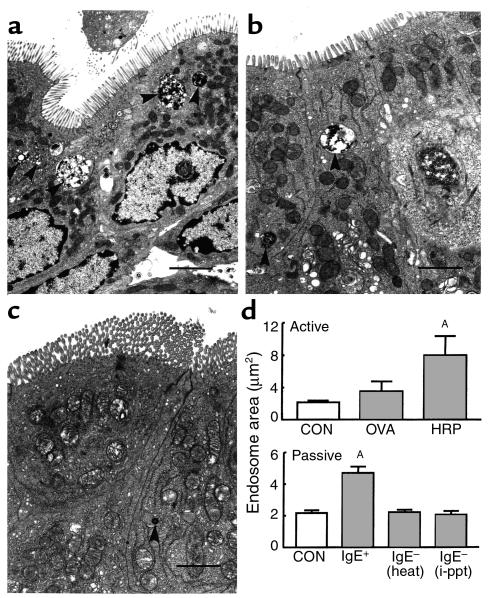
To examine the effect of sensitization on the uptake of specific versus bystander antigen, tissues from control, HRP-sensitized, and OVA-sensitized rats were fixed for electron microscopy 2 minutes after HRP addition to the mucosal compartment of Ussing chambers. In electron photomicrographs from rats actively sensitized to HRP, many large HRP-containing endosomes were present in enterocytes (example shown in Figure 1a). Such endosomes were observed in all regions of the cell, and some HRP was also identified in the lamina propria. However, in photomicrographs from control rats (Figure 1c) and those sensitized to OVA, only a few HRP-containing endosomes were identified in the apical region of enterocytes. Image analysis revealed that the total area of epithelial HRP endosomes in fixed-size apical windows was increased severalfold in HRP-sensitized rats compared with naive rats (P < 0.01) or rats sensitized to OVA (Figure 1d, upper panel). This result (similar to our previous finding [ref. 18]) indicates the specific nature of the enhanced antigen uptake phenomenon and implicates an immunoglobulin recognition mechanism.
Figure 1.
HRP uptake into enterocyte endosomes. HRP was added to the mucosal buffer of chambers containing jejunal tissues obtained from naive control rats or rats actively sensitized to OVA or HRP. Tissues were fixed for electron microscopy 2 minutes after HRP challenge and processed to visualize HRP reaction product. Representative photomicrographs are shown for tissues obtained from: (a) a rat actively sensitized to HRP, (b) a rat passively sensitized to HRP, or (c) a naive control rat. HRP-containing endosomes are indicated by arrowheads (bars indicate 1 μm). These photomicrographs are representative of those used to obtain the quantitative measurements of endosomal area shown in (d). Upper panel: active sensitization. Rats, actively sensitized to OVA or HRP, were compared with naive controls (CON); jejunal tissues from all rats were challenged with HRP. The total area of HRP endosomes was measured in fixed-size windows in the apical region of enterocytes. Lower panel: passive sensitization. For passive sensitization, experimental rats were injected intraperitoneally with serum from actively sensitized rats, either untreated [IgE+], heat-treated [IgE– (heat)], or IgE-depleted [IgE– (i-ppt)]. Values represent means ± SEM. AP < 0.01 compared with control; n = 12–24 views analyzed for each group (from four rats per group).
IgE is required for the enhanced epithelial uptake of antigen.
Rats passively sensitized to HRP by injection of high IgE-containing serum also demonstrated large HRP-containing endosomes in enterocytes (example shown in Figure 1b). Image analysis confirmed that the area of endosomes was significantly greater (P < 0.01) than those in controls (Figure 1d, lower panel), although the magnitude of the increase was less than in actively sensitized rats. Both heat- treatment of the serum to destroy heat-labile IgE, and immunoprecipitation to remove antibodies of the IgE isotype specifically, completely abolished the enhanced HRP uptake restoring the area of HRP-containing endosomes to the control value. This result indicates that IgE was mediating the enhanced epithelial uptake of HRP antigen in sensitized rats, most likely by binding to an IgE receptor expressed on the microvillus membrane of enterocytes.
Sensitization stimulates the expression of CD23 on intestinal epithelial cells.
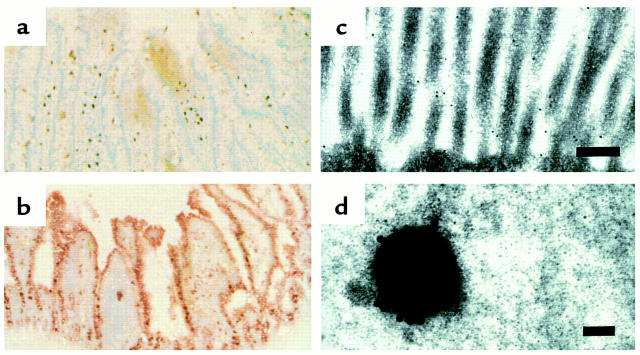
Immunohistochemical staining to examine expression of FcεRII/CD23 on epithelial cells was performed using anti-CD23 mAb. In sections from naive control rats, minimal immunoreactivity was evident on enterocytes, although some cells (appeared to be mononuclear cells) in the lamina propria were positive (Figure 2a). However, in sections from sensitized rats, immunoreactivity was clearly observed on epithelial cells (Figure 2b). The most prominent staining was located on the apical membrane, with lighter staining in the apical portion of the cells.
Figure 2.
Light and electron photomicrographs showing expression of CD23 on intestinal epithelial cells. Jejunal tissues for light microscopy were obtained from (a) a naive control rat or (b) an actively sensitized rat. Sections were cryofixed and stained with anti-CD23 antibody. Strong immunoreactivity for CD23 is demonstrated on epithelial cells in the section from the sensitized rat but not in the section from the control rat. ×40. (c) Immunoelectron photomicrograph of enterocyte microvilli from a HRP sensitized rat before HRP challenge demonstrating gold-labeled CD23 receptors on the microvillus membrane (bar = 200 nm). (d) After HRP challenge, endosomes containing HRP with several CD23 labels on the endosome membrane are visualized (bar = 100 nm). These photomicrographs are representative of those prepared from four rats per group.
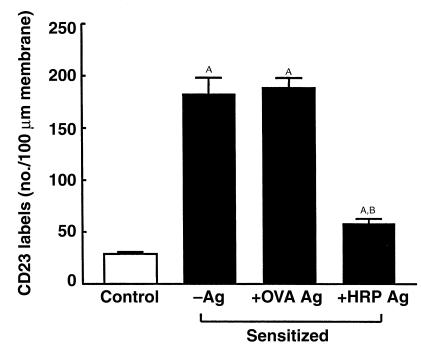
At the ultrastructural level, gold labeling indicated CD23 immunoreactivity along the microvilli of enterocytes with many labels present in sensitized rats (Figure 2c) and relatively few in control rats. The number of labels per 100 μm of microvillus membrane was significantly greater (P < 0.01) in actively sensitized rats compared with controls (Figure 3). Specific antigen challenge with HRP, but not OVA, reduced (P < 0.01) the presence of CD23 on the surface of enterocyte microvilli; however, the number of labels was still greater (P < 0.05) than the control value. Similar results were obtained for passively sensitized rats when compared with controls the number of CD23 labels was increased (P < 0.01), and then reduced after HRP challenge (16 ± 1,132 ± 9 and 52 ± 1 labels per 100 μm microvillus membrane, respectively; n = 20 views analyzed in each group (from four rats per group). These results suggest that antigen challenge caused a redistribution of CD23, perhaps by internalization into the epithelial cells.
Figure 3.
Effect of sensitization and antigen challenge on CD23 expression on the enterocyte microvillus membrane. Rats were actively sensitized to HRP (black bars) and either unchallenged (–Ag), or challenged with OVA (+OVA Ag) or HRP (+HRP Ag); antigens were added to the mucosal buffer 30 minutes before tissues were removed and processed for immunogold labeling using anti-CD23 antibody. The open bar indicates the value for naive control rats. Values represent means ± SEM. AP < 0.01 compared with control; BP < 0.01 compared with –Ag. n = 12 views analyzed for each group (from four rats per group).
CD23 and antigen are colocalized in endocytic vesicles after antigen challenge.
To examine if CD23 was internalized into endocytic vesicles after HRP antigen challenge, tissue sections were processed to visualize both HRP and CD23. In HRP-sensitized and challenged rats, numerous vesicles were identified that contained both HRP and CD23 (example shown in Figure 2d). No such vesicles were observed in tissues from control or OVA-sensitized rats.
Anti-CD23 antibodies inhibit epithelial antigen uptake and transport.
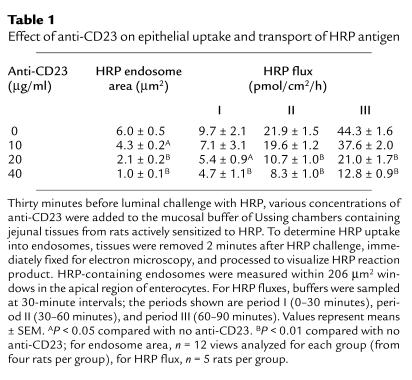
To confirm the role of the low-affinity IgE receptor, FcεRII/CD23, in the enhanced epithelial uptake and transport of antigen, tissues from HRP sensitized rats were incubated with increasing concentrations of anti-CD23 or control antibody added to the mucosal buffer before HRP challenge. Anti-CD23, in a concentration-dependent manner, reduced the total area of HRP containing endosomes within enterocytes (Table 1). At the highest concentration of antibody used (40 μg/ml), the inhibition of antigen uptake was 83%, restoring the value for the area of HRP-containing endosomes in sensitized rats to that in unsensitized rats (Figure 1d). In contrast, the isotype control antibody had no significant effect on endosomal area (6.0 ± 0.5 vs. 5.7 ± 0.6 μm2 at 40 μg/ml; n = 12). In addition, the transmucosal flux of HRP protein was inhibited by anti-CD23 as indicated by the significantly reduced flux values (Table 1). For example, with 40 μg/ml in period III, the flux value was inhibited 71% from 44.3 ± 1.6 to 12.8 ± 0.9 pmol/cm2/h (n = 5 rats in each group). This value in sensitized rats was then similar to that in unsensitized rats (11.9 ± 1.6 pmol/cm2/h). This data provides evidence that the mechanism responsible for enhanced sampling and transport of antigen by epithelial cells in sensitized rats involves CD23.
Table 1.
Effect of anti-CD23 on epithelial uptake and transport of HRP antigen
Anti-CD23 antibodies inhibit the intestinal hypersensitivity reaction.
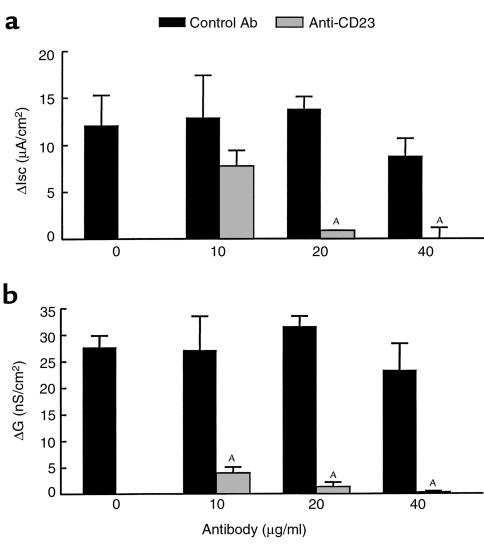
Anti-CD23 antibody added to the luminal surface of epithelial cells before antigen challenge also inhibited functional changes characteristic of the intestinal hypersensitivity reaction (Figure 4). The Isc response to HRP challenge of tissues from sensitized rats was reduced, and the magnitude of the inhibition depended on the concentration of antibody used (Figure 4a). In fact, at the highest concentration (40 μg/ml), there was no secretory response at all to luminal antigen. Figure 4b shows that the rise in conductance was similarly inhibited by the anti-CD23 antibody. In contrast, the isotype control antibody had no significant effect on either of the two measures of antigen-induced intestinal pathophysiology. These results suggest that CD23 on epithelial cells plays an important functional role in the intestinal hypersensitivity reaction to luminal antigen.
Figure 4.
Effect of anti-CD23 on Isc and conductance values after antigen challenge. Thirty minutes before luminal challenge with HRP, various concentrations of anti-CD23 (gray bars) or isotype control antibody (black bars) were added to the mucosal buffer of Ussing chambers containing jejunal tissues from rats actively sensitized to HRP. (a) The increase in Isc (ΔIsc) is the difference between the peak value (within 15 minutes) after challenge and the base-line value. This antigen-induced secretory response was significantly reduced by anti-CD23 in a dose-dependent manner. (b) The increase in conductance (ΔG) is the difference between the value at 30 minutes after challenge and the base-line value. This antigen-induced permeability increase was significantly reduced by anti-CD23 in a dose-dependent manner. Values represent means ± SEM. AP < 0.01 compared with control values; n = 5 rats per group.
Discussion
Our studies in a rat model of food allergy provide evidence that enhanced specific uptake of antigen by intestinal epithelial cells is mediated by IgE antibodies binding to the low-affinity IgE receptor CD23/FcεRII. Although CD23 expression has been noted previously on epithelial cells, as far as we are aware this study is the first to define a role for CD23 in facilitated antigen transport by enterocytes. In addition, we showed that anti-CD23 interferes both with antigen uptake and the subsequent allergic reaction in intestinal tissues. This information may be important in understanding and treating food allergic conditions.
One of the main roles of the intestinal epithelium is to act as a barrier to limit the influx into the body of antigens and other potentially noxious material in the gut lumen. Under normal conditions, delivery of luminal antigens to the gut mucosal immune system results in oral tolerance (8). However, in food-allergic humans and animals, antigen transported into the mucosa results in a local hypersensitivity reaction. Studies from our group and others have documented that luminal antigen challenge stimulates secretion of ions and water and increases motility associated with the development of diarrhea (reviewed in ref. 5). These changes occur faster than would be expected based on the normal physiology of endosomal protein transport across epithelial cells to reach and activate lamina propria mast cells, shown to be the key effector cells in the intestinal hypersensitivity reaction (13, 23). In fact, Ussing chamber studies of jejunal tissues from sensitized rats demonstrated that the Isc response to luminal antigen begins in only approximately 3 minutes (16, 18). In addition, we found that in vivo intestinal transport of antigen from lumen to blood was increased in sensitized rats when compared with naive animals (24), although the mechanism was not elucidated.
Recently, we designed a series of studies to investigate whether sensitization alters transepithelial antigen transport in a qualitative or quantitative manner. Using rats sensitized to HRP, we visualized HRP within intestinal cells/tissues by electron microscopy and determined the route and rate of antigen transport (18). Compared with rats sensitized to an irrelevant protein (OVA), rats sensitized to HRP preferentially endocytosed this antigen and rapidly delivered significantly greater amounts of this protein across the epithelium. This finding suggested a mechanism for recognition of antigen at the level of the epithelium. However, because mast cells bear IgE receptors and reside in close proximity to epithelial cells in intestinal tissues, it was possible that mast cells were responsible for the identification of specific antigen. Therefore, we conducted similar studies in mast cell-deficient Ws/Ws rats (23). We confirmed that these animals had no mast cells in the intestinal tract, but produced normal levels of IgE antibodies. Jejunal tissues from sensitized Ws/Ws rats also demonstrated enhanced epithelial uptake of HRP, but did not display any hypersensitivity reaction based on the complete lack of Isc or conductance response after luminal antigen challenge. In addition, after antigen challenge, tissues from mast cell-replete rats demonstrated HRP in the paracellular spaces between epithelial cells and the overall flux of HRP across tissues was significantly increased; however, such findings were never observed in tissues from Ws/Ws rats.
Taken together, our previous studies suggested two phases of enhanced antigen transport: an early phase (phase I) before the hypersensitivity reaction, and a later phase (phase II) subsequent to activation of mast cells (25). In phase I, antigen is transported via the transcellular route in endosomes that rapidly traverse epithelial cells; this phase is induced by sensitization and is specific for the sensitizing antigen but is mast cell independent. In contrast, phase II begins after mast cell activation and involves recruitment of the paracellular route that amplifies the barrier defect, resulting in nonspecific uptake of antigens and other luminal molecules. Here, we designed experiments to investigate the mechanism responsible for the first phase of specific antigen uptake across the intestinal epithelium. In addition, because the first phase may be the most crucial in delivering antigen into the body, we determined the effect of inhibiting phase I antigen transport on the hypersensitivity reaction.
IgE is present in human intestinal secretions of individuals with food allergies (26). Impressively, in nematode parasitized rats, the concentration of IgE was shown to be greater in intestinal fluid than in serum or mesenteric lymph (27). In addition, a luminally directed transepithelial transport pathway for labeled myeloma IgE (but not IgG1) was induced in rats by nematode infection or intravenous infusion of IL-4 (28). These results suggest a receptor-mediated mechanism for transepithelial transport of IgE into the lumen. In this study, we documented enhanced epithelial uptake from the lumen of a model antigen, HRP, in rats sensitized to the specific antigen compared with those sensitized to an irrelevant protein, OVA, or naive rats. This finding also implicates an immunoglobulin recognition system at the level of the epithelium. In addition, passively sensitized rats demonstrated enhanced uptake of HRP antigen into enterocyte endosomes with an absolute requirement for IgE. Based on this background, we postulated that a functional epithelial receptor for IgE may be present on the apical membrane of gut epithelial cells in sensitized rats.
Intestinal hypersensitivity reactions are mediated by antigen cross-linking of IgE antibodies bound to its high-affinity receptor (FcεRI) on mast cells. However, this receptor has not been demonstrated on epithelial cells. The low-affinity IgE receptor (CD23/FcεRII) has been identified on the apical membrane of enterocytes in biopsies from humans, with increased expression in individuals with food allergy and inflammatory bowel disease (20). CD23 has also been demonstrated on airway epithelial cells in asthmatics (21). Therefore, we determined the effect of sensitization of rats on epithelial expression of CD23. The B3B4 anti-CD23 antibody we used in the expression studies has been well-characterized as identifying CD23 on mouse B cells and blocking binding or displacing IgE from its receptor (22). There is a high degree of homology between mouse and rat (and even human) CD23 (29). We confirmed that the B3B4 antibody similarly identifies CD23 on splenic B cells from rats and mice. Immunohistochemistry of light microscopic sections showed minimal CD23 expression on epithelial cells in control animals, although there was some positive staining in the lamina propria. In sensitized rats, CD23 immunoreactivity on enterocytes was dramatically enhanced; the expression appeared to be mainly on and immediately below the apical membrane. A clearer picture of expression of the IgE low-affinity receptor emerged in the electron microscope, where immunogold labeling showed a small number of CD23 receptors on the microvillus membrane of enterocytes in control rats relative to the number in sensitized rats. In addition, the number of such labels was decreased in sensitized rats after antigen challenge. Receptor internalization was confirmed by the finding of numerous endosomes containing both CD23 and HRP antigen. The increased expression of CD23 induced by sensitization is most likely due to activation of the IL-4 enhancer element on the CD23 gene (30). IL-4 is produced in excess by intestinal mucosal T lymphocytes after sensitization (31) and is also present in serum of atopic individuals (32). Therefore, IL-4 (and/or possibly other unidentified factors) in the serum used to passively sensitize rats may have stimulated expression of epithelial CD23. Studies in progress in IL-4–deficient mice confirm the requirement for IL-4 for both elevated CD23 expression and enhanced epithelial antigen uptake. CD23 has been described previously as mediating endocytosis of antigen in human B cells (33); therefore, it is not unreasonable that it might play a similar role in enterocytes.
Our results suggest that the initial phase of enhanced transepithelial antigen transport in sensitized rats is mediated by HRP binding to anti-HRP IgE attached to low-affinity receptors on the apical membrane of enterocytes. In confirmation of the role of CD23 in transepithelial antigen transport, anti-CD23 mAb inhibited enterocyte uptake and transport of HRP antigen in a concentration-dependent manner. Taken together, these findings and those from our previous studies (18, 23) suggest that binding to IgE/CD23 protects antigen from degradation during transepithelial transport, resulting in large quantities of immunogenic protein gaining access to the lamina propria in a short period. This antigen then is available to activate mast cells and initiate the hypersensitivity reaction. In confirmation of this hypothesis, luminal treatment of tissues with anti-CD23 inhibited the intestinal responses characteristic of gut hypersensitivity. The increase in Isc after luminal HRP challenge of tissues from sensitized rats was reduced, indicating inhibition of the secretory response. In addition, the elevated conductance was decreased by anti-CD23, indicating inhibition of nonspecific epithelial permeability. Thus inhibition of phase I of transepithelial antigen transport interfered with the intestinal anaphylactic reaction, including the secretory response and the increase in conductance/permeability.
Although several aspects of the specific antigen transport system remain to be defined, our results provide convincing evidence that enhanced uptake of antigen by enterocytes in sensitized rats is mediated by IgE and CD23. The specificity of the uptake can be explained by the involvement of IgE antibodies. The increased quantity of antigen delivered across the epithelium into the body can be explained by a receptor-mediated process that protects antigen from degradation. Our study also demonstrates that this system for uptake of specific antigen is important in the allergy, as blocking the binding of IgE to its receptor inhibits both transepithelial antigen transport and the hypersensitivity reaction. Preliminary experiments in sensitized mice suggest the existence of a similar mechanism for antigen uptake. If this process also occurs in humans, it may be a novel target for treatment of food allergic conditions.
Acknowledgments
We thank R. Bell for helpful discussions during the early phase of these experiments and M. Benjamin for technical assistance. This research was supported by the Medical Research Council of Canada.
References
- 1.de Vries JE. Atopic allergy and other hypersensitivities. Curr Opin Immunol. 1994;6:835–837. doi: 10.1016/0952-7915(94)90001-9. [DOI] [PubMed] [Google Scholar]
- 2.Sampson HA. Food allergy. Part 1: immunopathogenesis and clinical disorders. J Allergy Clin Immunol. 1999;103:717–728. doi: 10.1016/s0091-6749(99)70411-2. [DOI] [PubMed] [Google Scholar]
- 3.Lessof MH, Wraith DG, Merrett TG, Merrett J, Buisseret PD. Food allergy and intolerance in 100 patients: local and systemic effects. Q J Med. 1980;49:259–271. [PubMed] [Google Scholar]
- 4.Knutson TW, et al. Intestinal anaphylaxis in man. Rapid increase in mucosal release of macromolecules and inflammatory mediators. Gastroenterology. 1992;102:A646. (Abstr.) [Google Scholar]
- 5.Crowe SE, Perdue MH. Gastrointestinal food hypersensitivity: basic mechanisms of pathophysiology. Gastroenterology. 1992;103:1075–1095. doi: 10.1016/0016-5085(92)90047-3. [DOI] [PubMed] [Google Scholar]
- 6.Scott RB, Diamant SC, Gall DG. Motility effects of intestinal anaphylaxis in the rat. Am J Physiol. 1988;255:G505–G511. doi: 10.1152/ajpgi.1988.255.4.G505. [DOI] [PubMed] [Google Scholar]
- 7.Neutra MR. Role of M cells in transepithelial transport of antigens and pathogens to the mucosal immune system. Am J Physiol. 1998;274:G785–G791. doi: 10.1152/ajpgi.1998.274.5.G785. [DOI] [PubMed] [Google Scholar]
- 8.Strobel S, Mowat AM. Immune responses to dietary antigens: oral tolerance. Immunol Today. 1998;19:173–181. doi: 10.1016/s0167-5699(97)01239-5. [DOI] [PubMed] [Google Scholar]
- 9.Sanderson IR, Walker WA. Uptake and transport of macromolecules by the intestine: possible role in clinical disorders (an update) Gastroenterology. 1993;104:622–639. doi: 10.1016/0016-5085(93)90436-g. [DOI] [PubMed] [Google Scholar]
- 10.Bomsell M, Prydz K, Parton RG, Gruenberg J, Simons K. Endocytosis in filter-grown Madin-Darby canine kidney cells. J Cell Biol. 1989;109:3243–3258. doi: 10.1083/jcb.109.6.3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perdue MH, Chung M, Gall DG. The effect of intestinal anaphylaxis on gut function in the rat. Gastroenterology. 1984;86:391–397. [PubMed] [Google Scholar]
- 12.Perdue MH, Gall DG. Intestinal anaphylaxis in the rat: jejunal response to in vitro antigen exposure. Am J Physiol. 1986;250:G427–G431. doi: 10.1152/ajpgi.1986.250.4.G427. [DOI] [PubMed] [Google Scholar]
- 13.Forbes D, Patrick M, Perdue M, Buret A, Gall DG. Intestinal anaphylaxis: in vivo and in vitro studies in the rat proximal colon. Am J Physiol. 1988;255:G201–G205. doi: 10.1152/ajpgi.1988.255.2.G201. [DOI] [PubMed] [Google Scholar]
- 14.Perdue MH, Masson S, Wershil BK, Galli SJ. Role of mast cells in ion transport abnormalities associated with intestinal anaphylaxis. Correction of the diminished secretory response in genetically mast cell-deficient W/Wv mice by bone marrow transplantation. J Clin Invest. 1991;87:687–693. doi: 10.1172/JCI115047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crowe SE, Perdue MH. Anti-immunoglobulin E-stimulated ion transport in human large and small intestine. Gastroenterology. 1993;105:764–772. doi: 10.1016/0016-5085(93)90894-i. [DOI] [PubMed] [Google Scholar]
- 16.Crowe SE, Sestini P, Perdue MH. Allergic reactions of rat jejunal mucosa. Ion transport responses to luminal antigen and inflammatory mediators. Gastroenterology. 1990;99:74–82. doi: 10.1016/0016-5085(90)91232-u. [DOI] [PubMed] [Google Scholar]
- 17.Kosecka U, Marshall JM, Crowe SE, Bienenstock J, Perdue MH. Pertussis toxin stimulates hypersensitivity and enhances nerve-mediated antigen uptake in rat intestine. Am J Physiol. 1994;267:G745–G753. doi: 10.1152/ajpgi.1994.267.5.G745. [DOI] [PubMed] [Google Scholar]
- 18.Berin MC, et al. Rapid transepithelial antigen transport in rat jejunum: impact of sensitization and the hypersensitivity reaction. Gastroenterology. 1997;113:856–864. doi: 10.1016/s0016-5085(97)70180-x. [DOI] [PubMed] [Google Scholar]
- 19.Kinet JP. The high-affinity IgE receptor (FcεRI): from physiology to pathology. Annu Rev Immunol. 1999;17:931–972. doi: 10.1146/annurev.immunol.17.1.931. [DOI] [PubMed] [Google Scholar]
- 20.Kaiserlian D, Lachaux A, Grosjean I, Graber P, Bonnefoy JY. Intestinal epithelial cells express the CD23/Fc-epsilon-RII molecule: enhanced expression in enteropathies. Immunology. 1993;80:90–95. [PMC free article] [PubMed] [Google Scholar]
- 21.Campbell AM, Vignola AM, Chanez P, Godard P, Bousquat J. Low-affinity receptor for IgE on human bronchial epithelial cells in asthma. Immunology. 1994;82:506–508. [PMC free article] [PubMed] [Google Scholar]
- 22.Rao M, Lee WT, Conrad DH. Characterization of a monoclonal antibody directed against the murine B lymphocyte receptor for IgE. J Immunol. 1999;138:1845–1851. [PubMed] [Google Scholar]
- 23.Berin MC, et al. The influence of mast cells on pathways of antigen transport in rat intestine. J Immunol. 1998;161:2561–2566. [PubMed] [Google Scholar]
- 24.Crowe SE, Perdue MH. Intestinal permeability in allergic rats: nerve involvement in antigen-induced changes. Am J Physiol. 1993;264:G617–G623. doi: 10.1152/ajpgi.1993.264.4.G617. [DOI] [PubMed] [Google Scholar]
- 25.Perdue MH. The mucosal antigen barrier: crosstalk with mucosal cytokines. Am J Physiol. 1999;277:G1–G5. doi: 10.1152/ajpgi.1999.277.1.G1. [DOI] [PubMed] [Google Scholar]
- 26.Belut D, Moneret-Vautrin DA, Nicholas JP, Grilliat JP. IgE levels in intestinal juice. Dig Dis Sci. 1980;25:323–332. doi: 10.1007/BF01308055. [DOI] [PubMed] [Google Scholar]
- 27.Negrao-Correa D, Adams LS, Bell RG. Intestinal transport and catabolism of IgE: a major blood-independent pathway of IgE dissemination during a Trichinella spiralis infection of rats. J Immunol. 1996;157:4037–4046. [PubMed] [Google Scholar]
- 28.Ramaswamy K, Hakimi J, Bell RG. Evidence for an interleukin-4-inducible immunoglobulin E uptake and transport mechanism in the intestine. J Exp Med. 1994;180:1793–1803. doi: 10.1084/jem.180.5.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Conrad DH, Campbell KA, Bartlett WC, Squire CM, Dierks SE. Structure and function of the low-affinity IgE receptor. Adv Exp Med Biol. 1994;347:17–30. doi: 10.1007/978-1-4615-2427-4_3. [DOI] [PubMed] [Google Scholar]
- 30.Richards ML, Katz DH. Regulation of the murine FcεRII (CD23) gene. Functional characterization of an IL-4 enhancer element. J Immunol. 1994;152:3453–3466. [PubMed] [Google Scholar]
- 31.Hauer AC, Breese EJ, Walker-Smith JA, MacDonald TT. The frequency of cells secreting interferon-γ and interleukin-4, -5, and -10 in the blood and duodenal mucosa of children with cow’s milk hypersensitivity. Pediatr Res. 1997;42:629–638. doi: 10.1203/00006450-199711000-00014. [DOI] [PubMed] [Google Scholar]
- 32.Hashimoto S, et al. Elevation of soluble IL-2 receptor and IL-4, and non-elevation of IFNγ in sera from patients with allergic asthma. Ann Allergy. 1993;71:455–458. [PubMed] [Google Scholar]
- 33.Yokata A, et al. Two forms of the low affinity Fc receptor for IgE differentially mediate endocytosis and phagocytosis: identification of the critical cytoplasmic domains. Proc Natl Acad Sci USA. 1992;89:5030–5034. doi: 10.1073/pnas.89.11.5030. [DOI] [PMC free article] [PubMed] [Google Scholar]