Abstract
The incidence of food allergy, a disease characterized by adverse immune responses that can render common foods life-threatening, is rising. Yet our current standard of care is simply avoidance of allergenic foods and administration of emergency medications upon accidental exposure. Significant advances have been made in food allergy oral immunotherapy, which is emerging as a potential preventive and curative treatment for this disease. The fundamental strategy of oral immunotherapy is to mitigate adverse immune responses to allergenic food proteins through repeated exposure; reduced reactivity to food allergens (desensitization) often results, but the establishment of sustained immune unresponsiveness or of permanent resolution (tolerance) is not certain. This review examines exciting recent developments in oral immunotherapy for food allergy.
Introduction
The worldwide prevalence of food allergy (FA) is 1–10% [1] and rising [2]. In the U.S. alone the prevalence of FA is estimated at 8% among children under 18, and 30% of these food allergic children suffer from multiple food allergies [3]. The burden of FA on patients, their families, and healthcare systems is significant and growing. An estimated 203,000 food-allergy related emergency department visits occur in the U.S. each year, including about 90,000 for food-induced anaphylaxis [4]. The ever-present threat of accidental exposure to a food allergen that could trigger a potentially fatal allergic reaction produces a significant negative impact on the psychosocial well being of FA patients and their families [5,6]. Although FA is recognized as a major public health issue, FA patients have no effective treatment options: the accepted standard of care is simply the strict avoidance of allergenic foods, with rapid administration of emergency medication upon accidental exposure.
Novel therapeutic approaches are being developed to surmount the challenges of FA. The fundamental strategy of FA oral immunotherapy (OIT) is to deliver gradually increasing doses of specific food allergens with the goal of establishing desensitization and ‘immune tolerance’ [7]. A preliminary double-blind, placebo-controlled oral food challenge (DBPCFC) is used for screening to ascertain the food allergic status of a patient; the DBPCFC is the only definitive diagnostic test for FA, as other diagnostic tools (e.g., skin prick tests and measurements of food-antigen-specific IgE levels (sIgE) in the plasma) are susceptible to false positives. OIT then begins with an initial day dose escalation, in which the patient is exposed to increasing amounts of food allergen under clinical supervision to determine the highest tolerated dose. The resulting dose is then used to initiate the subsequent dose-escalation phase, in which the dose is incremented weekly or biweekly until a targeted maintenance dose is reached. The patient consumes the maintenance dose of the food allergen daily throughout the maintenance phase, which ranges from months to years. When the treatment period is complete, a final oral food challenge (OFC) is used to evaluate the efficacy of the OIT protocol: success is defined by a statistically significant increase in the tolerated food allergen dose to a level that protects the patient upon accidental exposure. Desensitization, or reduced reactivity to food allergens that is maintained through regular exposure, and is often achieved. Sustained unresponsiveness (SU) to food allergens is reduced reactivity that persists indefinitely, even in the absence of regular food allergen exposure. Tolerance is the resolution of FA, the ideal outcome of OIT that would be measured as SU at an OFC at any point in the patient’s life after OIT, regardless of the frequency of exposure; because an OFC does not provide information about a patient’s future allergic status, the achievement of actual tolerance is not certain even when SU is found.
This review highlights recent results from clinical studies of OIT. To ensure their focus on true FA, we include only studies requiring a screening DBPCFC (sDBPCFC). We preferred clinical studies having a placebo arm, clear and defined dosing, associated mechanistic studies, and long-term follow up to evaluate sustained unresponsiveness, as well as peer reviewed publication in a journal having an impact factor >4 (Table 1). We focus on the contributions of these studies to addressing current challenges in FA OIT, including its safety, efficacy, need for mechanistic understanding (Figure 1), and the complexity arising from the variety of FA and the presence of multiple FAs in 30% of FA patients. The need to address the heterogeneity among OIT trials has been excellently reviewed elsewhere [8]; and standard guidelines for FA immunotherapy [9] and FA prevalence, prevention and management are emerging [10,11,12].
Table 1.
Selected Food Allergy Oral Immunotherapy Trials.
| Trial | Therapy | Allergen | Patients in Test Group | Allergen-Specific IgE Range (kUA/L) | sDBPCFC Range (mg) | Final OFC Range (mg) | % Desensitized |
|---|---|---|---|---|---|---|---|
| Syed (2014); Ryan (2016) | OIT | Peanut | 23 | 19–317 | 6–100 | 6–2000 | 87% (24mo = end of OIT), 30% (27mo), 13% (30 mo) |
| Vazquez-Ortiz (2014) | OIT | Egg | 50 | 1.84–24 | 120–1900 | 800–3800 | 80% complete and 2% partial (initial phase 16wks): 54% complete and 8% partial (maintainence phase 12mo) |
| Yanagida (2015) | OIT | Cow’s milk | 12 | 1.7–278 | 750–3000 | 750–3000 | 58.3% |
| Sato (2015) | OIT | Wheat | 18 | 2.92–100 | 20–1,300 | 5,200 | 61.1% |
| Anagnostou (2014) | OIT | Peanut | 39 | 20–3971 | 5–400 | 100–1400 | 62%; 91% of placebo group who crossed over into OIT |
| Goldberg (2015) | OIT | Baked milk | 15 | - | 6–30 | 90–1800 | 21% |
| Begin (2014); Begin (2015) | Omalizumab + multi-OIT | Multiple (2 to 5) | 25 | 67–1829 | 0.1–100 | 4.000 per allergen (maintenance dose reached) | 100% |
| Wood (2016) | Omalizumab + OIT | Milk | 28 | 9.4–83 | 0–100 | 5.500–10,000 (28mo), 0–10,000 (32mo) | 88.9% (23mo); 71.4% (32mo) |
| Schneider (2013); Burton (2014) | Omalizumab + OIT | Peanut | 13 | 21–617 | 1–100 | 4000–8000 | 92% |
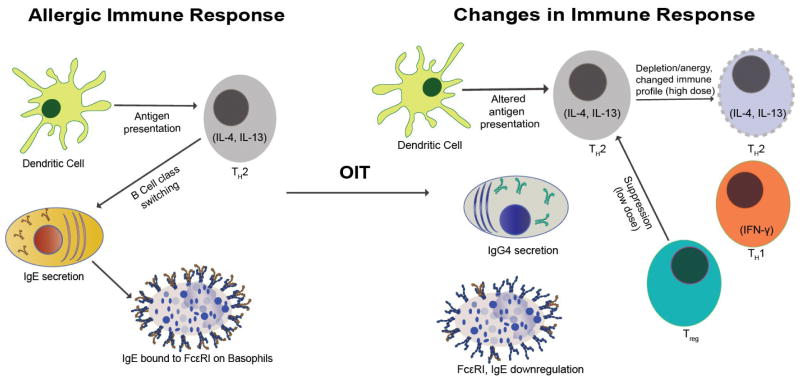
Figure 1.
The Immune System in Sickness and in Health. FA OIT induces changes in T cells, B cell responses (IgE and IgG4), and basophil activation.
OIT Alone
Towards Predicting ‘Immune Tolerance’
A phase 1, single-site trial targeted the identification of specific immune mechanisms associated with a tolerant clinical phenotype, where clinical ‘immune tolerance’ is defined as having no detectable allergic reaction to an OFC after the withdrawal of therapy and resumption of allergen avoidance [13••]. 23 patients with peanut allergy established by sDBPCFC underwent OIT for 24 months, and were followed at 3 and 6 months thereafter. Peripheral blood was collected longitudinally and analyzed for levels of specific immunoglobulins E (sIgE) and G4 (sIgG4), basophil activation, immunophenotype profiles, and methylation sites. 20 of 23 patients achieved desensitization at 24 months; 7 of these 20 achieved clinical ‘immune tolerance’ at 27 months, and 3 of these 7 retained clinical ‘immune tolerance’ at 30 months. While sIgE, sIgG4, and basophil activation did not statistically differentiate between clinical ‘immune tolerance’ and its absence, reduced methylation of forkhead box protein 3 (FOXP3) CpG sites in antigen-induced regulatory T cells (aiTreg) were associated with clinical ‘immune tolerance.’ In tolerant participants who regained sensitivity, FOXP3 CpG methylation in aiTregs increased. Thus, in peanut OIT, epigenetic changes in aiTregs were shown to be predictive of clinical ‘immune tolerance.’ An additional mechanistic study of 10 subjects from this trial examined changes associated with clinical ‘immune tolerance’ in peanut-specific CD4+ T cells [14]. Using single-cell sorting and transcriptional profiling of individual T cells collected throughout OIT, this study discovered the induction of anergic memory and nonallergic populations of peanut-specific CD4+ T lymphocytes, which occurred only in clinical ‘immune tolerance.’
Safety
A study focused on predicting which FA patients are likely to avoid severe allergic reactions during OIT retrospectively correlated reaction severity and frequency of patients having sDBPCFC-confirmed FA with their initial sIgE levels, finding that sIgE levels below 8.85 kU/L were associated with a 77% probability of having reduced reactivity during OIT, whereas having an sIgE level above this value indicated a 95% probability of persistent reaction, some of which were severe enough to require discontinuation of OIT [15]. This study demonstrated a correlation between baseline specific IgE and the safety of egg OIT.
A common target dose of cow’s milk OIT is 200 mL, although this dose often induces severe reactions. To test the efficacy and safety of low-dose-induction OIT with cow’s milk, a study enrolled children with positive screening OFC to 3 mL cow’s milk [16]. After 1 year, 58.3% of the OIT group and 13.8% of the control group tolerated 3 mL milk, and 33.3% of the OIT group also tolerated 25 mL milk, whereas none of the control group did; the OIT group also showed a significant decrease in casein sIgE levels. Adverse reactions were rare, and most were mild. This study demonstrated that the use of a small dose of milk was as effective as and safer than using a larger dose of milk to induce desensitization, even in a high-risk group that had initially reacted to the smaller dose.
Although wheat is the third most common allergen to cause anaphylaxis in Japan, very few studies have focused on wheat OIT. A study designed to determine the efficacy of wheat OIT followed 16 subjects with wheat anaphylaxis confirmed by sDBPCFC with heated wheat flour containing 1.3 g wheat protein [17]. After 2 years of wheat OIT with a target dose of 200 g boiled udon containing 5.2 g wheat protein, OIT was terminated, and 2 weeks later subjects underwent a final DBPCFC. 60% of the subjects passed the final DBPCFC and were therefore deemed to have achieved ‘immune tolerance,’ with a 10% reported adverse reaction rate, suggesting that wheat OIT can be effective and safer than conventional milk OIT.
Efficacy
A phase 2 randomized controlled crossover trial was designed to determine the efficacy of peanut OIT [18]. Patients who had passed a sDBPCFC were assigned either to an active OIT group or to a control (peanut avoidance) group: 24 of 39 active group subjects achieved desensitization to 1,400 mg peanut protein after 6 months of OIT, whereas none of the control group did. Control group subjects then underwent OIT, with 54% tolerating a 1,400 mg peanut OFC after 6 months. Overall quality of life improved after OIT and the side effects were mild, suggesting that peanut OIT can be safe and effective in inducing desensitization.
To investigate whether baked milk oral immunotherapy could desensitize allergic patients to unheated milk, children who had failed to complete a milk oral immunotherapy protocol and were reactive to baked milk were studied [19]. Only 21% of the subjects were able to reach the target dose of 1.3 g baked milk in 12 months, and these 3 experienced only a limited increase in threshold dose of unheated milk to induce a reaction upon OFC. These results suggest that OIT alone may not be sufficiently safe and effective to treat highly sensitive individuals such as these.
OIT in Combination with Omalizumab
The possibility of desensitizing patients to multiple food allergens simultaneously and rapidly was tested in a Phase I single site trial of OIT in combination with omalizumab, a monoclonal antibody that binds free IgE [20••]. 25 children with multiple food allergies (each confirmed by sDBPCFC) received OIT for up to 5 food allergens simultaneously, with omalizumab administered as of 8 weeks before the initiation of OIT and continuing for 16 weeks. Participants reached the 4 g maintenance dose at a median of 18 weeks, whereas the median time to reach this dose with OIT alone had been 85 weeks [21]. The median reaction rate was 3.2 per 100 doses, with 94% of the reactions mild. This was the first study to use OIT in combination with omalizumab for multiple food allergens simultaneously; demonstrated that this “rush OIT” method could achieve desensitization to doses of 4 g per food allergen as safely as could multi-allergen OIT alone, but 67 weeks earlier. A Phase II Peanut Reactivity Reduced by Oral Tolerance in an Anti-IgE Clinical Trial (PRROTECT) is underway [22], as is a trial of early-intervention (at 9–36 months of age) peanut OIT [23].
A mechanistic study of a subset of these subjects (3 from the OIT group and 3 controls) investigated T cell clonotype changes associated with rush OIT [24]. Next-generation sequencing of peanut-proliferative TCR β from peripheral blood mononuclear cells collected at 0, 9, and 18 months revealed an extremely diversified set of peanut-responsive T cell clones, only 6 % of which persisted over time, regardless of treatment group. However, the relative frequency of each clone in this persistent population did not change in the control group, while it did change over the course of OIT, suggesting that T cell replacement may contribute to the establishment of desensitization.
The safety and efficacy of OIT was compared with that of OIT + omalizumab in a double-blind, placebo-controlled trial in which omalizumab treatment began 4 months before the initiation of cow’s milk OIT [25]. At 28 months, omalizumab was discontinued and patients who passed OFC were considered desensitized and continued OIT for 2 more months. Those who then passed OFC at 32 months were deemed to have achieved sustained unresponsiveness (SU). While the percentage of patients achieving desensitization and SU did not differ significantly between groups, adverse reaction rate and severity, and the number of doses required to reach maintenance were significantly reduced with the addition of omalizumab.
A pilot study targeting peanut-allergic patients at high risk of allergic reaction even to trace amounts of peanut tested the safety and efficacy of desensitization via peanut OIT in combination with omalizumab [26•]. All 13 patients had high sIgE levels and had failed an initial DBPCFC of ≤100 mg peanut flour. OIT was initiated after 12 weeks of treatment with omalizumab: all subjects tolerated a cumulative dose of 992 mg peanut flour on the first day, and 12 reached the maximum maintenance dose of 4 g in a median 8 weeks, when omalizumab was discontinued. After 12 more weeks of OIT, all of these 12 tolerated an OFC of 8 g peanut flour. This study indicates that the addition of omalizumab can make OIT more quickly effective and safer for even high-risk peanut allergic patients.
To test whether sIgG4 could be useful in mitigating FA, sera collected from these patients at week 0 and week 52 of OIT were compared in their ability to enhance or suppress peanut-induced basophil activation [27••]. The ratio of IgG4 to IgE was found to have increased significantly after OIT, and pre-OIT sera sensitized basophils for activation, whereas post-OIT sera suppressed basophil activation by pre-OIT sera; antibodies against FcγRII block this inhibition. Similar results were obtained from experiments run in parallel in a mouse model of FA. These encouraging results suggest that sIgGs induced during OIT inhibit IgE-mediated FA reactions, and may be useful in FA treatment.
Conclusion
Mechanistic understanding of the immune system processes that give rise to desensitization and SU are pointing the way towards the development of FA biomarkers and therapies, and contributing to the correlation of in FA endotypes with clinical phenotypes. New findings include the discovery that epigenetic changes in antigen-induced regulatory T cells can be predictive of ‘immune tolerance’ in peanut OIT, the discovery of the induction of anergic memory and nonallergic populations of peanut-specific CD4+ T lymphocytes in ‘immune tolerance,’ and the discovery that sIgGs induced during OIT inhibit IgE-mediated FA reactions and may be useful in FA treatment. The safety of FA OIT may be enhanced without compromising effectiveness through the use of lower doses, and through combination with omalizumab, which enables maintenance to be reached more rapidly than in OIT alone, without compromising safety. Combination with omalizumab is shown to put FA OIT within reach of even high-risk peanut allergic populations. In total, recent developments in food allergy oral immunotherapy represent significant advances towards tolerance.
HIghlights.
Clinical and mechanistic food allergy OIT studies point the way to novel biomarkers and therapies.
OIT with omalizumab can be more rapidly effective and safe even for high-risk patients.
First study of multiple food allergen OIT with omalizumab achieved very rapid desensitization.
Epigenetic changes in antigen-induced regulatory T cells may predict ‘immune tolerance.’
Specific IgGs induced during OIT inhibit IgE-mediated reactions and may be useful in treatment.
Acknowledgments
The authors would like to thank Vanitha Sampath for creating the figure. This work was supported by the Sean N. Parker Center for Allergy and Asthma Research, Food Allergy Research and Education Center of Excellence, Myra Reinhard Foundation, and Trip Advisor, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Prescott SL, Pawankar R, Allen KJ, Campbell DE, Sinn JKh, Fiocchi A, Ebisawa M, Sampson HA, Beyer K, Lee BW. A global survey of changing patterns of food allergy burden in children. World Allergy Organ J. 2013;6:21. doi: 10.1186/1939-4551-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koplin JJ, Mills EN, Allen KJ. Epidemiology of food allergy and food-induced anaphylaxis: is there really a Western world epidemic? Curr Opin Allergy Clin Immunol. 2015;15:409–416. doi: 10.1097/ACI.0000000000000196. [DOI] [PubMed] [Google Scholar]
- 3.Gupta RS, Springston EE, Warrier MR, Smith B, Kumar R, Pongracic J, Holl JL. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics. 2011;128:e9–17. doi: 10.1542/peds.2011-0204. [DOI] [PubMed] [Google Scholar]
- 4.Clark S, Espinola J, Rudders SA, Banerji A, Camargo CA., Jr Frequency of U.S. emergency department visits for food-related acute allergic reactions. J Allergy Clin Immunol. 2011;127:682–683. doi: 10.1016/j.jaci.2010.10.040. [DOI] [PubMed] [Google Scholar]
- 5.Henson M, Burks AW. The future of food allergy therapeutics. Semin Immunopathol. 2012;34:703–714. doi: 10.1007/s00281-012-0319-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta R, Holdford D, Bilaver L, Dyer A, Holl JL, Meltzer D. The economic impact of childhood food allergy in the United States. JAMA Pediatr. 2013;167:1026–1031. doi: 10.1001/jamapediatrics.2013.2376. [DOI] [PubMed] [Google Scholar]
- 7.Nadeau KC, Kohli A, Iyengar S, DeKruyff RH, Umetsu DT. Oral Immunotherapy and Anti-IgE Antibody-Adjunctive Treatment for Food Allergy. Immunol Allergy Clin North Am. 2012;32:111–133. doi: 10.1016/j.iac.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 8.Yee CS, Rachid R. The Heterogeneity of Oral Immunotherapy Clinical Trials: Implications and Future Directions. Curr Allergy Asthma Rep. 2016;16:25. doi: 10.1007/s11882-016-0602-0. [DOI] [PubMed] [Google Scholar]
- 9.Sampson HA, Gerth van Wijk R, Bindslev-Jensen C, Sicherer S, Teuber SS, Burks AW, Dubois AE, Beyer K, Eigenmann PA, Spergel JM, et al. Standardizing double-blind, placebo-controlled oral food challenges American Academy of Allergy, Asthma, & Immunology-European Academy of Allergy and Clinical Immunology PRACTALL consensus report. J Allergy Clin Immunol. 2012;130:1260–1274. doi: 10.1016/j.jaci.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 10.Nwaru BI, Hickstein L, Panesar SS, Roberts G, Muraro A, Sheikh A an EAACI Food Allergy & Anaphylaxis Guidelines Group. Prevalence of common food allergies in Europe: a systematic review and meta-analysis. Allergy. 2014;69:992–1007. doi: 10.1111/all.12423. [DOI] [PubMed] [Google Scholar]
- 11.ASCIA [Australian Society of Clinical Immunology and Allergy] Guidelines - Infant feeding and allergy prevention. 2016 available at http://www.allergy.org.au/healthprofessionals/papers/ascia-guidelines-for-infant-feeding-and-allergy-prevention?highlight=WyJndWlkZWxpbmVzIiwiZm9vZCIsIidmb29kIiwiYWxsZXJneSIsImFsbGVyZ3knLiIsIidhbGxlcmd5IiwiJ2FsbGVyZ3knIiwiJ2FsbGVyZ3knLiIsImZvb2QgYWxsZXJneSJd.
- 12.Vale S, Smith J, Said M, Mullins RJ, Loh R. ASCIA guidelines for prevention of anaphylaxis in schools, pre-schools and childcare: 2015 update. J Paediatr Child Health. 2015;51:949–954. doi: 10.1111/jpc.12962. [DOI] [PubMed] [Google Scholar]
- ••13.Syed A, Garcia MA, Lyu SC, Bucayu R, Kohli A, Ishida S, Berglund JP, Tsai M, Maecker H, O’Riordan G, et al. Peanut oral immunotherapy results in increased antigen induced Treg function and hypomethylation of FOXP3. J Allergy Clin Immunol. 2014;133:500–510. doi: 10.1016/j.jaci.2013.12.1037. This study demonstrated that epigenetic changes in antigen-induced regulatory T cells were predictive of clinical ‘immune tolerance’ in peanut OIT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ryan JF, Hovde R, Glanville J, Lyu SC, Ji X, Gupta S, Tibshirani RJ, Jay DC, Boyd SD, Chinthrajah RS, et al. Successful immunotherapy induces previously unidentified allergen-specific CD4+ T-cell subsets. Proc Natl Acad Sci U S A. 2016;113:e1286–1295. doi: 10.1073/pnas.1520180113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vazquez-Ortiz M, Alvaro M, Piquer M, Dominguez O, Machinena A, Martín-Mateos MA, Plaza AM. Baseline specific IgE levels are useful to predict safety of oral immunotherapy in egg-allergic children. Clin Exp Allergy. 2014;44:130–141. doi: 10.1111/cea.12233. [DOI] [PubMed] [Google Scholar]
- 16.Yanagida N, Sato S, Asaumi T, Okada Y, Ogura K, Ebisawa M. A Single-Center, Case-Control Study of Low-Dose-Induction Oral Immunotherapy with Cow’s Milk. Int Arch Allergy Immunol. 2015;168:131–137. doi: 10.1159/000442157. [DOI] [PubMed] [Google Scholar]
- 17.Sato S, Utsunomiya T, Imai T, Yanagida N, Asaumi T, Ogura K, Koike Y, Hayashi N, Okada Y, Shukuya A, et al. Wheat oral immunotherapy for wheat-induced anaphylaxis. J Allergy Clin Immunol. 2015;136:1131–1133. doi: 10.1016/j.jaci.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 18.Anagnostou K, Islam S, King Y, Foley L, Pasea L, Bond S, Palmer C, Deighton J, Ewan P, Clark A. Assessing the efficacy of oral immunotherapy for the desensitisation of peanut allergy in children (STOP II): a phase 2 randomised controlled trial. Lancet. 2014;383:1297–1304. doi: 10.1016/S0140-6736(13)62301-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldberg MR, Nachshon L, Appel MY, Elizur A, Levy MB, Eisenberg E, Sampson HA, Katz Y. Efficacy of baked milk oral immunotherapy in baked milk–reactive allergic patients. J Allergy Clin Immunol. 2015;136:1601–1606. doi: 10.1016/j.jaci.2015.05.040. [DOI] [PubMed] [Google Scholar]
- •20.Bégin P, Dominguez T, Wilson SP, Bacal L, Mehrotra A, Kausch B, Trela A, Tavassoli M, Hoyte E, O’Riordan G, et al. Phase 1 results of safety and tolerability in a rush oral immunotherapy protocol to multiple foods using Omalizumab. Allergy Asthma Clin Immunol. 2014;10:7. doi: 10.1186/1710-1492-10-7. This was the first study to use OIT in combination with omalizumab for multiple food allergens simultaneously. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bégin P, Winterroth LC, Dominguez T, Wilson SP, Bacal L, Mehrotra A, Kausch B, Trela A, Hoyte E, O’Riordan G, et al. Safety and feasibility of oral immunotherapy to multiple allergens for food allergy. Allergy Asthma Clin Immunol. 2014;10:1. doi: 10.1186/1710-1492-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peanut Reactivity Reduced by Oral Tolerance in an Anti-IgE Clinical Trial. https://clinicaltrials.gov/ct2/show/study/NCT01781637.
- 23.Johnson Kate. Early Oral Peanut Immunotherapy Successful in Preschoolers. 2015 Feb 24; http://www.medscape.com/viewarticle/840273_print.
- 24.Bégin Philippe, Nadeau Kari C. Changes in peanut-specific T-cell clonotype with oral immunotherapy. J Allergy Clin Immunol. 2015;135:1636–1638. doi: 10.1016/j.jaci.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 25.Wood RA, Kim JS, Lindblad R, Nadeau K, Henning AK, Dawson P, Plaut M, Sampson HA. A randomized, double-blind, placebo-controlled study of omalizumab combined with oral immunotherapy for the treatment of cow’s milk allergy. J Allergy Clin Immunol. 2016;137:1103–1110. doi: 10.1016/j.jaci.2015.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •26.Schneider LC, Rachid R, LeBovidge J, Blood E, Mittal M, Umetsu DT. A pilot study of omalizumab to facilitate rapid oral desensitization in high-risk peanut-allergic patients. J Allergy Clin Immunol. 2013;132:1368–1374. doi: 10.1016/j.jaci.2013.09.046. This pilot study shows that OIT in combination with omalizumab can make OIT more quickly effective and safer even for high-risk peanut allergic patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••27.Burton OT, Logsdon SL, Zhou JS, Medina-Tamayo J, Abdel-Gadir A, Noval Rivas M, Koleoglou KJ, Chatila TA, Schneider LC, Rachid R, et al. Oral immunotherapy induces IgG antibodies that act through FcγRIIb to suppress IgE-mediated hypersensitivity. J Allergy Clin Immunol. 2014;134:1310–1317. doi: 10.1016/j.jaci.2014.05.042. This study suggests that sIgGs induced during OIT inhibit IgE-mediated FA reactions, and may be useful in FA treatment. [DOI] [PMC free article] [PubMed] [Google Scholar]