Abstract
Background
Acute kidney injury (AKI) is a well-documented complication of pediatric hematopoietic stem cell transplantation (HSCT). Dialysis after HSCT is associated with a lower overall survival (OS); however, the association between less severe AKI and OS is unclear.
Method
We retrospectively studied 205 consecutive pediatric HSCT patients to determine the incidence and impact of all stages of AKI on OS in pediatric HSCT recipients. We used the peak pRIFLE grade during the first 100 days to classify AKI (R=risk, I= injury, F= failure, L= loss of function, E= End-stage renal disease) and used the modified Schwartz formula to estimate glomerular filtration rate.
Results
AKI was observed in 173 of the 205 patients (84%). The 1-year OS decreased significantly with an increasing severity of pRIFLE grades (p < 0.01). There was no difference in the OS between patients without AKI and the R/I group. Regardless of the dialysis status, stages F/L/E had significantly lower OS compared with patients without AKI or R/I (p < 0.01). There was no difference in OS among patients with dialysis and F/L/E without dialysis (p 0.65). Stages F/L/E predicted mortality independent of acute graft versus host disease, gender, and malignancy.
Conclusion
The OS of children after HSCT decreases significantly with an increasing severity of AKI within the first 100 days posttransplant. While our data did not show an increased risk of mortality with stages R/I, stages F/L/E predicted mortality regardless of dialysis. Prevention and minimization of AKI may improve survival after pediatric HSCT.
Introduction
Hematopoietic stem cell transplantation (HSCT) is the treatment of choice for a wide array of hematologic, neoplastic, metabolic and immunologic conditions 1. With advances in HLA typing, less toxic conditioning regimens, and improved detection and treatment of fungal and viral infections, overall survival (OS) has markedly improved in recent years 2. For children undergoing unrelated bone marrow transplant for acute leukemia, 2-year OS improved from 35% in 1987–1995 to 58% in 2003–2006 3. Despite these improvements, mortality following HSCT remains substantial. It is, therefore, important to examine HSCT complications that contribute to mortality.
Since the first report by Zager et al. in 1989 4, several adult and pediatric studies have documented the incidence of acute kidney injury (AKI) after HSCT 5–11. In critically ill pediatric patients, all stages of AKI are associated with an increased risk of mortality 12. It is therefore reasonable to suspect that all stages of AKI contribute to mortality in HSCT recipients. Zager et al. 4 reported a mortality rate of 84% in adult HSCT recipients requiring dialysis compared to 17% in patients without AKI. Lane et al. 13 documented a mortality rate of 77% in pediatric HSCT recipients who required dialysis. In a recent retrospective study, Rajpal et al. 14 not only demonstrated a higher mortality in patients requiring dialysis but found an unchanged incidence of dialysis in pediatric HSCT recipients over the last two decades. The AKI data on adult and pediatric HSCT recipients are quite heterogeneous due to a lack of utilization of standardized definitions of AKI. While the association between dialysis and a higher mortality is explicit, uncertainties exist regarding the understudied earlier stages of AKI and the risk of mortality. The current body of evidence is inadequate to support aggressive interventions to minimize early AKI in HSCT recipients.
In this study, we aimed to investigate the association between various stages of AKI and the OS in pediatric HSCT recipients. We used pRIFLE criteria to define the stages of AKI (Table 1). As shown in the table, pRIFLE criteria define AKI based on its severity and outcome. The pRIFLE criteria were first developed by Akcan-Arikan et al. using prospective data on 150 critically ill children 15. The sensitivity and specificity of pRIFLE were subsequently validated by Plotz et al. in 2008 16. We are the first group to use the pRIFLE criteria to assess the incidence of AKI in pediatric HSCT recipients. We hypothesized that all stages of AKI decreased OS following HSCT in children. We also assessed the prevalence of chronic kidney disease (CKD) among 1-year survivors of pediatric HSCT. To our knowledge, this is the largest single-center study of pediatric HSCT recipients examining the outcomes of AKI.
Table 1.
pRIFLE Staging
| pRIFLE stage | Estimated glomerular filtration rate(eGFR) |
|---|---|
| R = Risk for renal dysfunction | eGFR decreased by 25% |
| I = Injury to the kidney | eGFRL decreased by 50% |
| F = Failure of kidney function | eGFR decreased by 75% or eGFR < 35 ml/min per 1.73 m2 |
| L = Loss of kidney function | Persistent failure > 4 weeks |
| E = End-stage renal disease | Persistent failure > 3 months |
Patients and Methods
Patient population
This is a retrospective cohort study of 205 consecutive pediatric patients, aged 21 years or less, who received HSCT at the University of Minnesota between 1/20/11 and 10/23/13. We retrieved data from a prospectively maintained HSCT database at the University of Minnesota. The database included information on the date of transplant, age at transplant, race, gender, indication for HSCT, conditioning regimen, source of stem cells, acute graft versus host disease (aGVHD), veno-occlusive disease (VOD), number of donors, type of donors, total body irradiation (TBI), and dialysis. The pretransplant measured glomerular filtration rate (GFR), serum creatinine values, height and body mass index (BMI) were unavailable in the database and were extracted from the electronic medical records. The institutional review board at the University of Minnesota approved this study.
Study Endpoints
The primary endpoint of this study was 1-year OS. We assigned a pRIFLE stage to each recipient using their peak serum creatinine within the first 100 days posttransplant and determining the percentage decrease in the estimated GFR (eGFR) compared to baseline (table 1). The eGFR was estimated using serum creatinine, height, and a proportionality constant according to the modified Schwartz equation 17. The baseline eGFR was calculated using serum creatinine that was drawn immediately prior to the initiation of the pretransplant conditioning regimen. We did not use urine output data to define AKI as it was not easily available.
Because a number of patients (63%) underwent a nuclear medicine study for GFR determination prior to the initiation of pretransplant conditioning to allow accurate dosing of chemotherapy we were able to validate our estimations of GFR using the Schwartz method by comparing the estimated GFR with the measured GFR in this subset of patients. For GFR measurement, 1.2 to 1.4 mCi of Tc-99m diethylene triamine pentaacetic acid (DTPA) were injected intravenously and blood was sampled. We used the serum creatinine that was drawn immediately before or after the nuclear medicine study for calculation of the GFR for comparison. Our results revealed Pearson correlation coefficient of 0.6 (p < 0.001) for patients with BMI of < 25 and 25 −80 percentiles and 0.3 (p < 0.02) for patients with BMI > 80 percentile. The stratification of BMI was arbitrary.
The secondary endpoint of this study was the prevalence of chronic kidney disease (CKD) among the HSCT recipients who were alive at 1 year posttransplant. According to the National Kidney Foundation-Kidney Disease Outcomes Quality Initiative (NKF-KDOQI) guideline, CKD was defined as GFR < 90 ml/min/1.73m2 with structural or functional kidney abnormality (abnormal composition of blood or urine and/or abnormal imaging studies) for ≥ 3 months or GFR < 60ml/min/1.73m2 with or without kidney damage for ≥ 3 months 18. We did not examine structural or functional kidney damage in individuals with GFR > 90 ml/min/1.73m2.
Statistical analysis
Data on transplantation patient characteristics, post-transplantation complications and outcomes were prospectively collected by the Biostatistical Support Group at the University of Minnesota using standardized procedures. We stratified the patients into four groups based on their pRIFLE stages and whether or not they received dialysis. These groups were no AKI, risk/injury (R/I), failure/loss of kidney function/end-stage renal disease (F/L/E) without dialysis and F/L/E with dialysis. The categories R/I and F/L/E were collapsed to enhance the power of the study. Patient and disease characteristics were summarized using descriptive statistics. Statistical comparisons of these variables between AKI and no AKIgroups were completed by the nonparametric Wilcoxon test for continuous factors and Pearson chi-square test for categorical factors. Mantel test for trend (1 degree of freedom chi square test) was used to assess a linear trend of mortality with the increasing severity of AKI. The one year overall survival (OS) for each group was estimated using Kaplan-Meier methods and compared using log-rank tests. Univariate analyses were conducted to identify variables that were significantly associated with both AKI and OS. A multivariate Cox proportional hazards model was created using variables that significantly predicted OS in the univariate analysis. VOD and donor source were not included in the model however due to their high correlation with AKI. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated adjusting for gender, malignant indication for HSCT and grade 3–4 acute graft versus host disease (GVHD). A p value of < 0.05 was considered statistically significant. All statistical analyses were performed with Statistical Analysis System software version 9.3 (SAS Institute, Inc., Cary, NC).
Results
The demographic and clinical characteristics are presented in table 2. The median age at the time of transplant was 8.0 years (range: 0.3 – 21). Our cohort included 61% male and 75% white patients. AKI was detected in 173 of the 205 (84%) recipients within the first 100 days posttransplant. The distribution of various stages of AKI is shown in table 3. Thirty-five percent of all recipients developed stage R and 33% developed stage I of AKI according to the pRIFLE criteria. Patients with F/L/E but without dialysis comprised 10% whereas the patients with F/L/E and dialysis constituted 7% of all patients.
Table 2.
Demographic and clinical characteristics
| Variables | All patients (n 205) |
AKI (n 173) |
No AKI (n 32) |
P value |
|---|---|---|---|---|
| Age at transplant in years | 8.0(0.3–21.0) | 8.0(0.3–21.0) | 8.4(0.7–20.6) | 0.81 |
| Median (range) | ||||
| Gender | 0.03 | |||
| Male | 125(61%) | 100(80%) | 25(20%) | |
| Female | 80(39%) | 73(91%) | 7(9%) | |
| Race | < 0.01 | |||
| Caucasian | 154(75%) | 132(86%) | 22(14%) | |
| African American | 15(7%) | 15(100%) | 0 | |
| Native American | 3(2%) | 1(33%) | 2(67%) | |
| Asian | 11(5%) | 7(64%) | 4(36%) | |
| Other | 10(5%) | 7(70%) | 3(30%) | |
| Unknown | 12(6%) | 11(92%) | 1(8%) | |
| Indication for HSCT | 0.60 | |||
| Malignant | 113(55%) | 94(83%) | 19(17%) | |
| Nonmalignant | 92(45%) | 79(86%) | 13(14%) | |
| Conditioning Intensity | 0.91 | |||
| Myeloablative | 149(73%) | 126(85%) | 23(15%) | |
| Reduced intensity | 56(27%) | 47(84%) | 9(16%) | |
| Conditioning regimen | ||||
| Allogeneic recipients | 0.81 | |||
| Cyclophos/TBI based | 91(53%) | 79 (87%) | 12 (13%) | |
| Busulfan/Cyclophos based | 43 (25%) | 39 (91%) | 4 (9%) | |
| Melphalan based | 10 (6%) | 9 (90%) | 1 (10%) | |
| Cyclophos based | 8 (5%) | 7 (88%) | 1 (12%) | |
| Carboplatin/etoposide | 0 (0%) | 0 (0%) | 0 (0%) | |
| Busulfan/Melphalan based | 7 (4%) | 7 (100%) | 0 (0%) | |
| Others | 15 (9%) | 12 (80%) | 3 (20%) | |
| Autologous recipients | 0.04 | |||
| Cyclophos/TBI based | 2 (6%) | 2 (100%) | 0 (0%) | |
| Busulfan/Cyclophos based | 0 (0%) | 0 (0%) | 0 (0%) | |
| Melphalan based | 7 (2%) | 2 (29%) | 5 (71%) | |
| Cyclophos based | 6 (2%) | 6(100%) | 0 (0%) | |
| Carboplatin/etoposide | 4 (1%) | 4(100%) | 0 (0%) | |
| Busulfan/Melphalan based | 8 (3%) | 4(50%) | 4(50%) | |
| Others | 4 (1%) | 2(50%) | 2(50%) | |
| Use of TBI | 0.25 | |||
| Yes | 96(47%) | 84(88%) | 12(12%) | |
| No | 109(53%) | 89(82%) | 20(18%) | |
| Stem Cell Source | <0.01 | |||
| Bone Marrow | 79(39%) | 68(86%) | 11(14%) | |
| Peripheral blood | 34(17%) | 22(65%) | 12(35%) | |
| Umbilical cord | 92(45%) | 83(90%) | 9(10%) | |
| Donor Source | 0.02 | |||
| Autologous | ||||
| MRD | 31(15%) | 20(65%) | 11(35%) | |
| MMRD | 35(17%) | 31(89%) | 4(11%) | |
| MURD | 6(3%) | 5(83%) | 1(17%) | |
| MMURD | 82(40%) | 74(90%) | 8(10%) | |
| 51(25%) | 43(84%) | 8(16%) | ||
| aGVHD (grades 3–4) | 0.98 | |||
| Yes | 13(6%) | 11(85%) | 2(15%) | |
| No | 192(94%) | 162(84%) | 30(16%) | |
| VOD | 0.04 | |||
| Yes | 21 (10%) | 21(100%) | 0 | |
| No | 184 (90%) | 152(83%) | 32(17%) |
Abbreviation: AKI – acute kidney injury; TBI- total body irradiation; MRD – matched related donor; MMRD – mismatched related donor; MURD – matched unrelated donor; MMURD – mismatched unrelated donor; aGVHD – acute graft versus host disease; VOD – veno - occlusive disease; HSCT – hematopoietic stem cell transplant; cyclophos - cyclophosphamide
Table 3.
Distribution of pRIFLE stages of AKI
| Stages of AKI | N (205) |
|---|---|
| No AKI | 32 (16%) |
| R | 71 (35%) |
| I | 67 (33%) |
| F/L/E without dialysis | 21 (10%) |
| F/L/E with dialysis | 14 (7%) |
Abbreviation: AKI – acute kidney injury; R – risk; I – injury; F/L/E – failure, loss and end-stage renal disease
Females were more likely to develop AKI compared to males (91% vs. 80% p = 0.03). AKI was observed in 100% of African American recipients (n= 15). Fifty-five percent of recipients underwent transplant for a malignant indication. Those receiving autologous HSCT had the lowest incidence of AKI (65%; p=0.02). The incidence of AKI was 86%, 65% and 90% in the bone marrow, peripheral blood and umbilical cord recipients respectively (p < 0.01). However when subgroup analysis was performed for autologous and allogeneic HSCT recipients, no association was found between the source of stem cell and AKI (p 0.37). There was no difference among patients with and without AKI in terms of age, indication for HSCT, conditioning intensity, the use of TBI and the presence of grade 3–4 aGVHD.
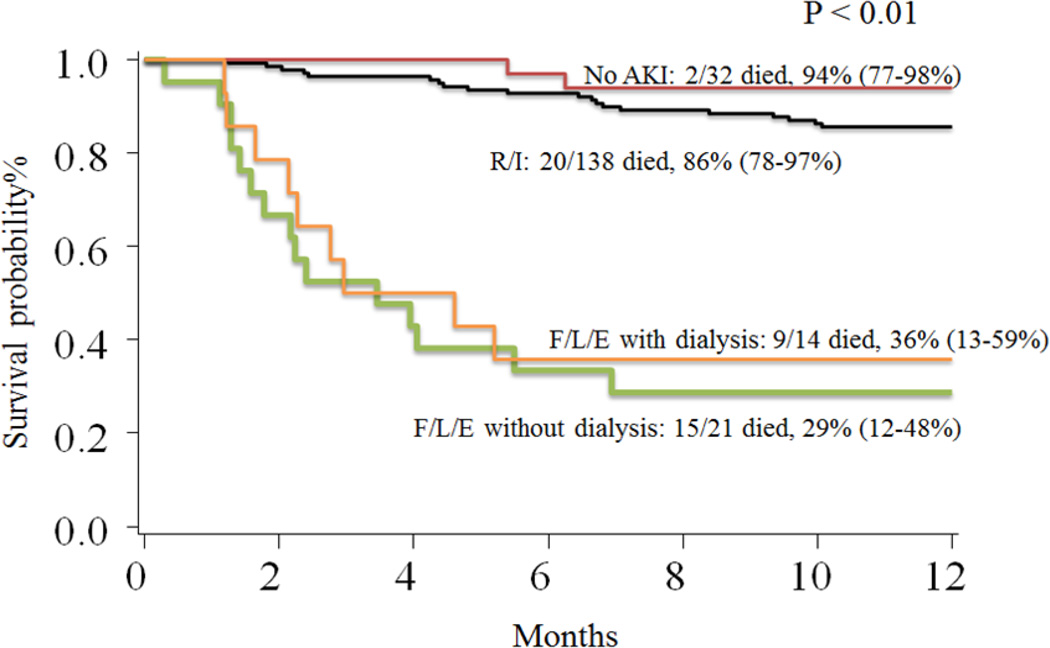
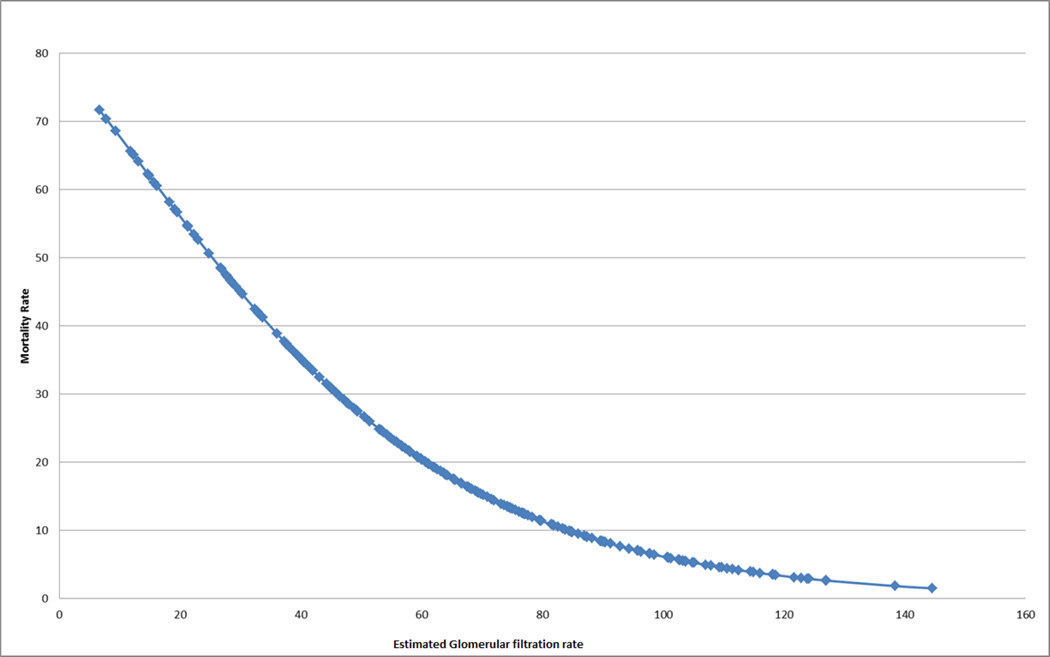
The univariate survival analysis is presented in table 4. OS was significantly lower among patients with AKI compared to those without AKI (75% vs. 94%; p = 0.02). The OS was 86% (95%CI: 78–90%) for patients with stages R and I, 36% (95%CI: 13 – 59%) for stages F/L/E with dialysis and 29 %( 95%CI: 12–48%) for stages FLE without dialysis (p < 0.01) (Figure 1). There was no difference in the OS among patients without AKI and those with stages R/I (94% vs. 86%; p = 0.22). Stages F/L/E had a significantly lower OS compared to no AKI and R/I (31% vs. 87%; p < 0.01). Stages F/L/E had similar OS for groups with and without dialysis. Although our results did not show a difference in the OS between the groups no AKI and R/I, Mantel’s test for trend had a significant p value of < 0.01 indicating an increasing trend of mortality with the increasing severity of AKI (degree of freedom = 1). The univariate survival analysis using the lowest eGFR within the first 100 days as a continuous variable showed that an increase of 1 ml/min/1.73m2 in the eGFR was associated with a 3% reduction in the risk of overall mortality (HR 0.97; 95% CI 0.96–0.98. p < 0.01) (Figure 2). Other factors associated with OS in the univariate analysis included malignant indication for HSCT, donor source, the presence of grades 3–4 aGVHD and the presence of VOD after HSCT (table 4).
Table 4.
Univariate survival analysis
| Variable | Survival estimate % (95% Confidence interval) |
P value (log rank test) |
|---|---|---|
| AKI | 0.02 | |
| Yes | 75 (67–80) | |
| No | 94 (77–98) | |
| AKI Categories | < 0.01 | |
| R/I | 86 (78–90) | |
| F/L/E with dialysis | 36 (13–59) | |
| F/L/E without dialysis | 29 (12–48) | |
| AKI categories | 0.22 | |
| No AKI | 94 (77–98) | |
| R/I | 86 (78–90) | |
| AKI categories | <0.01 | |
| No AKI and R/I | 87 (81–91) | |
| F/L/E | 31 (17–47) | |
| AKI categories | 0.65 | |
| F/L/E with dialysis | 29 (12–48) | |
| F/L/E without dialysis | 36 (13–59) | |
| Gender | ||
| Male | 78 (69–84) | 0.98 |
| Female | 78 (67–85) | |
| Malignant indication for HSCT | 0.05 | |
| Yes | 82 (74–88) | |
| No | 72 (61–80) | |
| Stem Cell Source | ||
| Bone Marrow | 78 (68–86) | 0.07 |
| Peripheral blood | 91 (75–97) | |
| Umbilical cord | 72 (61–80) | |
| Donor Source | ||
| Autologous | 94 (77–98) | 0.04 |
| MRD | 80 (63–90) | |
| MMRD | 50 (11–80) | |
| MURD | 74 (63–82) | |
| MMURD | 75 (60–84) | |
| aGVHD (grade 3–4) | ||
| Yes | 46 (19–70) | <0.01 |
| No | 80 (73–85) | |
| VOD | ||
| Yes | 48 (26–67) | <0.01 |
| No | 81 (75–86) | |
| TBI | ||
| Yes | 75 (65–82) | 0.42 |
| No | 80 (71–86) |
Abbreviations: AKI - acute kidney injury; R/I - risk and injury; F/L/E - failure, loss and end-stage renal disease; HSCT - hematopoietic stem cell transplant; aGVHD - acute graft versus host disease; VOD - veno-occlusive disease; TBI - total body irradiation
Figure 1.
1-year OS by severity of acute kidney injury
Abbreviations: AKI – acute kidney injury; R/I – risk and injury; F/L/E – failure, loss and end-stage renal disease
P value of < 0.01 indicates that at least one of the groups is statistically different from others
Pairwise comparison:
No AKI versus R/I – p value 0.22
F/L/E with dialysis versus F/L/E without dialysis – p value 0.65
No AKI plus R/I versus F/L/E with and without dialysis – p value < 0.01
Figure 2.
1-year mortality rate by the lowest estimated glomerular filtration rate within the first 100 days posttransplant
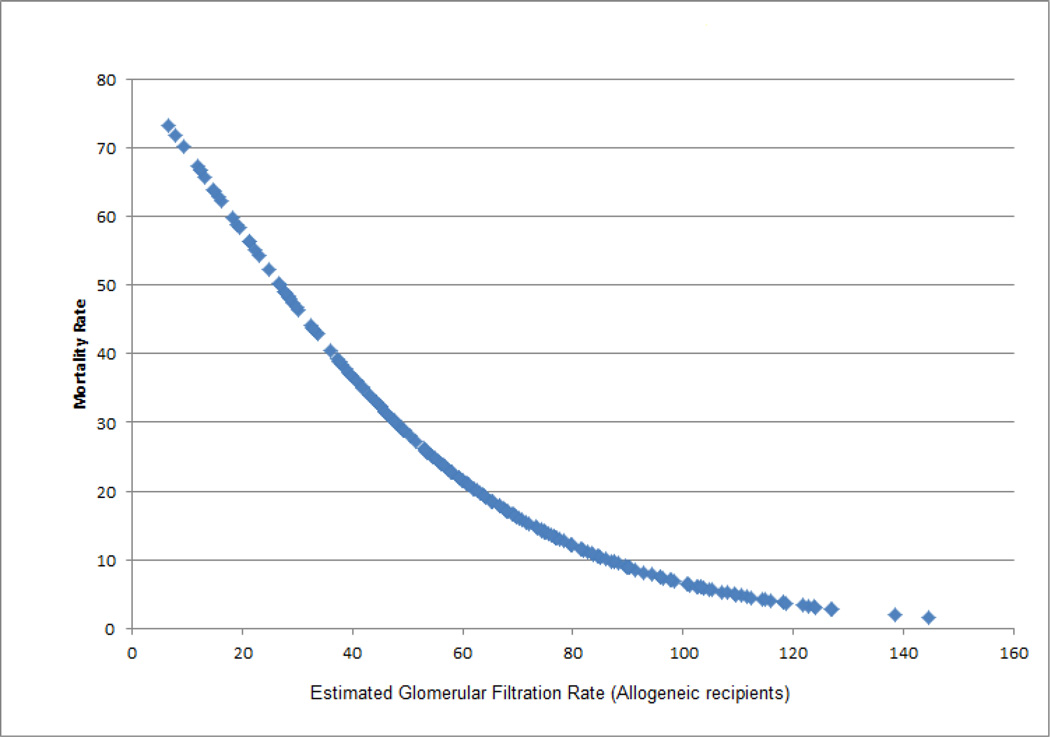
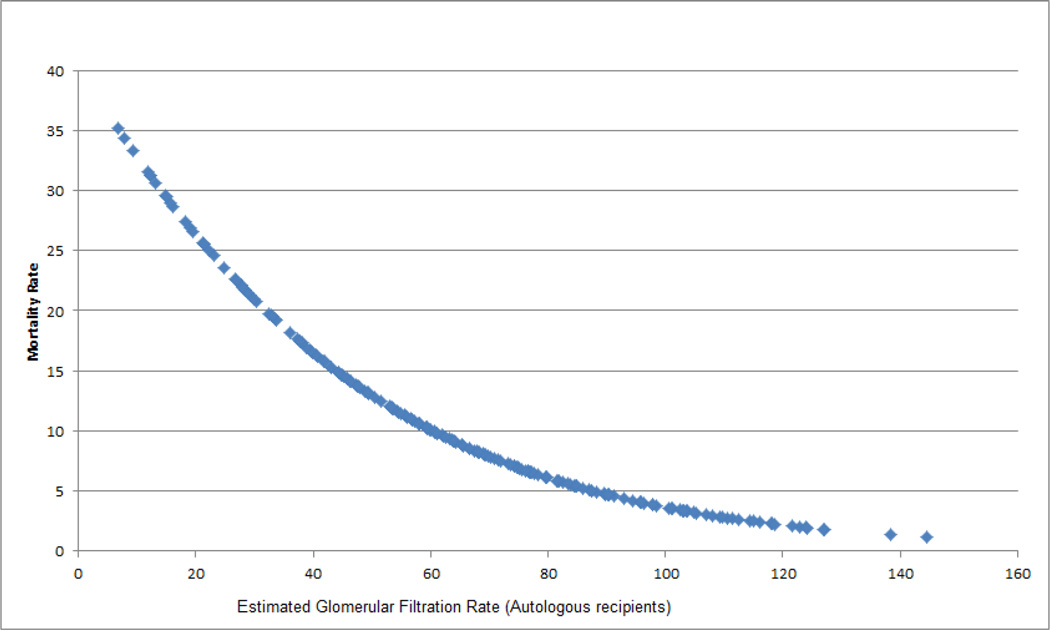
The results of our multivariate Cox proportional hazards regression model are shown in table 5. Stages F/L/E predicted mortality in pediatric HSCT recipients independent of gender, indication for HSCT and presence of grades 3–4 aGVHD with an adjusted HR of 8.4 (95% CI: 4.55–15.53). Although the absence of AKI appeared to be protective against mortality compared to stages R/I, the difference was not statistically significant (adjusted HR 0.4; 95% CI: 0.09–1.72). Gender, malignant indication for HSCT and grades 3–4 aGVHD were not significant predictors of survival in the multivariate model. Subgroup analysis by the type of transplantation (autologous versus allogeneic) showed that the stages F/L/E with and without dialysis were significant predictors of the 1-year OS in the allogeneic recipients (adjusted HR 7.3, 95% CI: 3.92–13.71). Similar to the overall analysis, the difference between stages R/I and no AKI for the allogeneic recipients was not statistically significant. The analysis of autologous recipients did not show a significant impact of AKI on mortality. This was likely due to a substantially smaller number of patients in the autologous group (n=31).
Table 5.
Multivariate analysis
| Variable | Hazard ratio (95% CI) | P value |
|---|---|---|
| AKI Category | ||
| R/I | 1.00 | < 0.01 |
| F/L/E | 8.41 (4.55–15.53) | |
| NO AKI | 0.40 (0.09–1.72) | |
| Gender | ||
| Male | 1.00 | 0.39 |
| Female | 0.77 (0.42–1.40) | |
| Malignant Indication for HSCT | 0.11 | |
| Yes | 1.00 | |
| No | 1.62 (0.90–2.91) | |
| aGVHD (grades 3–4) | ||
| Yes | 1.00 | 0.11 |
| No | 1.96 (0.87–4.46) |
Abbreviations: AKI – acute kidney injury; R/I – risk and injury; F/L/E – failure, loss and end-stage renal disease; HSCT – hematopoietic stem cell transplant; aGVHD – acute graft versus host disease
One hundred and fifty nine patients (76%) survived to 1-year post HSCT. Twenty-seven patients had missing serum creatinine data at 1 year. Of the remaining 132 patients, 10 patients (7.5%) had evidence of CKD at 1 year. Three patients had stage 2 CKD characterized by a combination of abnormal urine (hematuria, proteinuria) and metabolic acidosis. Stage 3 CKD was present in 3 patients and stage 4 was present in one. One patient developed end-stage renal disease and commenced chronic hemodialysis. All patients with CKD had developed AKI during the first 100 days; 5 patients had stages F/L/E of AKI while 5 had stages R/I.
Discussion
This is the first pediatric study to assess the incidence of AKI among pediatric HSCT recipients and to investigate the impact of various stages of AKI on OS according to the validated pRIFLE criteria. We found AKI to be very common in pediatric HSCT recipients within the first 100 days posttransplant. The OS was significantly lower among patients with stages F/L/E of AKI, regardless of their dialysis status. Although the OS for stages R/I was not significantly lower than no AKI, we noticed a significant reduction in the risk of mortality with every ml/min/1.73m2 increase in the lowest eGFR within the first 100 days posttransplant. Additionally, we noticed a worsening trend of mortality with the increasing severity of AKI (Mantel test for trend: p value < 0.01).
AKI is a well-documented complication of HSCT. The estimated incidence of AKI in adult HSCT recipients ranges from 36 to 73% depending on the population studied and the criteria used to define AKI 8. The pediatric data on the incidence of AKI in HSCT recipients is very limited. In a recent prospective study of 34 pediatric allogenic HSCT recipients, Hazer et al. 19 reported AKI (defined as doubling of serum creatinine) in 26.4% of recipients within the first 3 months posttransplant. Previously, Kistvan et al. 20 had reported the incidence of AKI to be 21% in their prospective study of 66 pediatric HSCT recipients. Recently, Ileri et al. 21 published a prospective study of 61 pediatric myeloablative and allogenic HSCT recipients and reported the incidence of AKI to be 42% within the first 100 days posttransplant. A recent systemic review of AKI in pediatric HSCT recipients included five observational studies and concluded that one-third of pediatric HSCT recipients developed AKI 10. The cited studies used doubling of serum creatinine as their criterion for diagnosis of AKI except for Ileri et al. The incidence of AKI in these studies is substantially lower than in the present study. This is partly due to a difference in the definitions used for AKI. Four of the five studies did not count patients with 25% reduction in their eGFR towards AKI while in the present study these patients (stage R) constituted 41% of all cases.
One of the strengths of the present study is the utilization of a standardized definition of AKI. Due to the heterogeneity in AKI data, the Acute Dialysis Quality Initiative group proposed the RIFLE criteria in 2004 to standardize the definition of AKI 22. Later, the Acute Kidney Injury Network proposed AKIN criteria for definition of AKI 23. The RIFLE criteria include three grades of severity (R, I and F) and two measures of outcome (L and E). RIFLE uses a change in serum creatinine or a change in GFR to determine the severity. AKIN criteria, on the other hand, only include the three grades of severity that are determined based on a percentage increase (>50%) in serum creatinine or an absolute increase of 0.3 mg/dl within a span of 48 hours. The pRIFLE criteria were derived from the RIFLE criteria using data on critically ill children. The performance of RIFLE, AKIN and conventional graded criteria 24 was compared in a recent retrospective cohort study of 249 adult Japanese HSCT recipients; AKI was detected in 52.6% of patients using RIFLE and 46.5% of patients using AKIN criteria. The authors concluded that RIFLE was more sensitive than AKIN in the HSCT population. Similarly, Sutherland et al. 12 applied pRIFLE, AKIN and KDIGO criteria to hospitalized pediatric patients and showed a higher sensitivity of pRIFLE criteria (KDIGO criteria are similar to AKIN except for a 7-day timeframe for an increase in serum creatinine 25). Ileri et al. used the AKIN criteria to determine the incidence of AKI. The difference between the present study and that of Ileri et al. may partly be due to the higher sensitivity of pRIFLE criteria compared to AKIN. Additionally, Ileri et al. used changes in serum creatinine to define AKI while we used changes in eGFR. Zapitelli et al. 26, in their study of critically and noncritically ill children, noted that changes in eGFR led to a higher prevalence of AKI compared to changes in serum creatinine.
Previous studies have delineated several risk factors for AKI in HSCT recipients. Pediatric data have identified sepsis, VOD, TBI, non-HLA-identical related or matched unrelated donors and nephrotoxicity associated with cyclosporine, amphotericin B and forscarnet as risk factors 20, 21, 27. Although adult studies have elucidated myeloablative conditioning 28, female sex, high-risk disease and aGVHD29 as risk factors for AKI, pediatric data have not replicated these findings 10. This may be because pediatric studies are smaller and underpowered. We identified gender, race, donor type and VOD as risk factors for AKI. However, gender was not associated with AKI when the analysis was conducted separately for allogeneic and autologous transplantation. The higher incidence of AKI in females was likely because a higher number of females compared to males underwent allogeneic transplantation. Racial differences in the incidence of AKI are well known 30. While the reasons for these differences are not completely understood, the higher susceptibility of African American individuals to AKI may partly be attributed to certain high-risk alleles of apolipoprotein L1 which are associated with a rapid decline in GFR among the individuals of African ancestry 31, 32. As mentioned above, donor type was associated with AKI in our study. Studies have shown that unrelated donor transplants are at a significantly higher risk of severe graft versus host disease, organ toxicity and severe infections 33, 34. These complications may serve as mediators of AKI after the unrelated donor transplantation. We also found an association between AKI and VOD. While it remains unclear how VOD leads to multi-organ failure, one proposed mechanism is decreased hepatic clearance of toxins that mediate organ injuries 35. We showed a lower incidence of AKI with peripheral blood transplant compared to bone marrow or umbilical cord. However, stem cell source also lost statistical significance when the analysis was performed separately for allogeneic and autologous HSCT recipients. Unlike adult studies, we did not find an association between AKI and grades 3–4 aGVHD. A possible explanation may be that only a small number of patients in this cohort (6.3%) developed aGVHD. We also did not find an association between AKI and TBI. Although Van Why et al. 9 identified TBI as a risk factor for AKI in their retrospective study of 92 pediatric patients, subsequent pediatric studies have had conflicting results.
A meta-analysis of 1211 adult HSCT recipients (6 studies) 36 demonstrated that the risk of mortality increased by two-fold in patients with doubling of serum creatinine and exceeded 80% for patients requiring dialysis. Earlier stages of AKI were not included in this analysis. In a pediatric study 11, doubling of serum creatinine increased the mortality to 55% within the first 30 days posttransplant as opposed to an overall mortality of 19%. In a recent retrospective study of 1427 pediatric HSCT recipients, our institution demonstrated a 1-year OS of 15% with dialysis compared to 71% without dialysis 14. As is evident from these studies, the risk of mortality is alarmingly high with severe renal failure in both adult and pediatric patients. In the present study, we showed that renal injury, even in the absence of dialysis, was an independent predictor of mortality. Stages F/L/E had significantly lower OS compared to no AKI, regardless of dialysis status. Stages R and I also had a lower OS than no AKI, however, the difference did not achieve statistical significance. These results are compatible with an adult study by Lopes et al. 37 that showed lower 5-year OS with every RIFLE stage of AKI. Importantly, we found that increasing the lowest eGFR by 1 ml/min/1.73m2 within the first 100 days was associated with a 3% reduction in the risk of mortality (p < 0.01). This is consistent with adult studies showing increased mortality with minimal increases in serum creatinine among patients with cardiovascular disease 38.
We found CKD in 8% of recipients who were alive at 1-year. This is similar to other pediatric studies reporting a prevalence of 10–11% at 1-year 20, 21. Adult studies report a higher prevalence compared to pediatric studies 39. Some of the variation is due to a difference in the definition of CKD and some due to a difference in the duration of follow up. Additionally, adult patients have a different set of comorbid conditions that may predispose them to a higher incidence of CKD. AKI has been identified as a risk factor for CKD 40. In the present study, all patients with CKD had AKI posttransplant.
This study has several potential limitations. First, the study was likely underpowered to detect a statistically significant difference in the OS between early AKI (R/I) and no AKI. Second, since this was a retrospective study we could not adjust the results for confounders that were not captured in the original dataset. Third, we estimated GFR using serum creatinine, which may not be an accurate marker of renal function. However, serum creatinine was used in the original pRIFLE study by Akcan-Arikan et al 15. Additionally, we found a strong correlation between eGFR using serum creatinine and measured GFR at baseline. Fourth, we used the modified Schwartz equation for eGFR determination, whereas the pRIFLE criteria were developed using the original Schwartz equation. Lastly, we could not adequately capture the urine output data and only used eGFR for AKI determination. Although a limitation of this study, adult literature does not provide any evidence that combination of urine output and eGFR data improves the performance of RIFLE criteria in predicting mortality 41.
This is the first pediatric study to evaluate the incidence of AKI in pediatric HSCT recipients using pRIFLE criteria. More than three-fourths of our patients had evidence of some degree of AKI and severe AKI was an independent predictor of mortality. Although our study does not establish causality, when taken together with the existing pediatric and adult literature, it is likely that prevention of AKI would improve OS in HSCT recipients. We recommend that pediatric HSCT recipients be closely monitored for AKI. We encourage the use of a sensitive measure of AKI such as pRIFLE rather than relying on serum creatinine alone. Medication lists should frequently be reviewed with pharmacists and drug levels should closely be monitored to minimize nephrotoxicity. A non-nephrotoxic alternative should be used when possible, especially in the presence of other risk factors for AKI. Nephrology consultation should be considered for AKI to optimize care. Early nephrology involvement is important because even mild AKI is associated with residual kidney damage 42. Interventions for minimization of AKI and their impact on mortality should further be evaluated in large prospective studies.
Figure 3.
1-year mortality rate by the lowest estimated glomerular filtration rate within the first 100 days posttransplant (Allogeneic recipients)
Figure 4.
1-year mortality rate by the lowest estimated glomerular filtration rate within the first 100 days posttransplant (Autologous recipients)
Acknowledgments
Sarah J. Kizilbash was supported by National Institute of Diabetes and Digestive and Kidney Diseases grant 5T32DK007784-15
Contributor Information
Sarah J. Kizilbash, Pediatric Nephrology Fellow, University of Minnesota.
Clifford E. Kashtan, Pediatric Nephrology, University of Minnesota.
Blanche M. Chavers, Pediatric Nephrology, University of Minnesota.
Qing Cao, Biostatistics and Bioinformatics Core, Masonic Cancer Center University of Minnesota.
Angela R. Smith, Pediatric Blood and Marrow Transplant, University of Minnesota.
References
- 1.Ljungman P, Urbano-Ispizua A, Cavazzana-Calvo M, et al. Allogeneic and autologous transplantation for haematological diseases, solid tumours and immune disorders: definitions and current practice in Europe. Bone marrow transplantation. 2006;37:439–449. doi: 10.1038/sj.bmt.1705265. [DOI] [PubMed] [Google Scholar]
- 2.Gooley TA, Chien JW, Pergam SA, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. The New England journal of medicine. 2010;363:2091–2101. doi: 10.1056/NEJMoa1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MacMillan ML, Davies SM, Nelson GO, et al. Twenty years of unrelated donor bone marrow transplantation for pediatric acute leukemia facilitated by the National Marrow Donor Program. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2008;14:16–22. doi: 10.1016/j.bbmt.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 4.Zager RA, O'Quigley J, Zager BK, et al. Acute renal failure following bone marrow transplantation: a retrospective study of 272 patients. Am J Kidney Dis. 1989;13:210–216. doi: 10.1016/s0272-6386(89)80054-x. [DOI] [PubMed] [Google Scholar]
- 5.Gruss E, Bernis C, Tomas JF, et al. Acute renal failure in patients following bone marrow transplantation: prevalence, risk factors and outcome. Am J Nephrol. 1995;15:473–479. doi: 10.1159/000168889. [DOI] [PubMed] [Google Scholar]
- 6.Hingorani SR, Guthrie K, Batchelder A, et al. Acute renal failure after myeloablative hematopoietic cell transplant: incidence and risk factors. Kidney international. 2005;67:272–277. doi: 10.1111/j.1523-1755.2005.00078.x. [DOI] [PubMed] [Google Scholar]
- 7.Kersting S, Koomans HA, Hene RJ, Verdonck LF. Acute renal failure after allogeneic myeloablative stem cell transplantation: retrospective analysis of incidence, risk factors and survival. Bone marrow transplantation. 2007;39:359–365. doi: 10.1038/sj.bmt.1705599. [DOI] [PubMed] [Google Scholar]
- 8.Lopes JA, Jorge S. Acute kidney injury following HCT: incidence, risk factors and outcome. Bone marrow transplantation. 2011;46:1399–1408. doi: 10.1038/bmt.2011.46. [DOI] [PubMed] [Google Scholar]
- 9.Van Why SK, Friedman AL, Wei LJ, Hong R. Renal insufficiency after bone marrow transplantation in children. Bone marrow transplantation. 1991;7:383–388. [PubMed] [Google Scholar]
- 10.Didsbury MS, Mackie FE, Kennedy SE. A systematic review of acute kidney injury in pediatric allogeneic hematopoietic stem cell recipients. Pediatric transplantation. 2015;19:460–470. doi: 10.1111/petr.12483. [DOI] [PubMed] [Google Scholar]
- 11.Patzer L, Kentouche K, Ringelmann F, Misselwitz J. Renal function following hematological stem cell transplantation in childhood. Pediatric nephrology (Berlin, Germany) 2003;18:623–635. doi: 10.1007/s00467-003-1146-9. [DOI] [PubMed] [Google Scholar]
- 12.Sutherland SM, Byrnes JJ, Kothari M, et al. AKI in hospitalized children: comparing the pRIFLE, AKIN, and KDIGO definitions. Clinical journal of the American Society of Nephrology : CJASN. 2015;10:554–561. doi: 10.2215/CJN.01900214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lane PH, Mauer SM, Blazar BR, Ramsay NK, Kashtan CE. Outcome of dialysis for acute renal failure in pediatric bone marrow transplant patients. Bone marrow transplantation. 1994;13:613–617. [PubMed] [Google Scholar]
- 14.Rajpal JS, Patel N, Vogel RI, Kashtan CE, Smith AR. Improved survival over the last decade in pediatric patients requiring dialysis after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2013;19:661–665. doi: 10.1016/j.bbmt.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 15.Akcan-Arikan A, Zappitelli M, Loftis LL, Washburn KK, Jefferson LS, Goldstein SL. Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney international. 2007;71:1028–1035. doi: 10.1038/sj.ki.5002231. [DOI] [PubMed] [Google Scholar]
- 16.Plotz FB, Bouma AB, van Wijk JA, Kneyber MC, Bokenkamp A. Pediatric acute kidney injury in the ICU: an independent evaluation of pRIFLE criteria. Intensive Care Med. 2008;34:1713–1717. doi: 10.1007/s00134-008-1176-7. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz GJ, Munoz A, Schneider MF, et al. New equations to estimate GFR in children with CKD. Journal of the American Society of Nephrology : JASN. 2009;20:629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inker LA, Astor BC, Fox CH, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis. 2014;63:713–735. doi: 10.1053/j.ajkd.2014.01.416. [DOI] [PubMed] [Google Scholar]
- 19.Hazar V, Gungor O, Guven AG, et al. Renal function after hematopoietic stem cell transplantation in children. Pediatric blood & cancer. 2009;53:197–202. doi: 10.1002/pbc.22030. [DOI] [PubMed] [Google Scholar]
- 20.Kist-van Holthe JE, Goedvolk CA, Brand R, et al. Prospective study of renal insufficiency after bone marrow transplantation. Pediatric nephrology (Berlin, Germany) 2002;17:1032–1037. doi: 10.1007/s00467-002-0989-9. [DOI] [PubMed] [Google Scholar]
- 21.Ileri T, Ertem M, Ozcakar ZB, et al. Prospective evaluation of acute and chronic renal function in children following matched related donor hematopoietic stem cell transplantation. Pediatric transplantation. 2010;14:138–144. doi: 10.1111/j.1399-3046.2009.01182.x. [DOI] [PubMed] [Google Scholar]
- 22.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Critical care (London, England) 2004;8:R204–R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Critical care (London, England) 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ando M, Mori J, Ohashi K, et al. A comparative assessment of the RIFLE, AKIN and conventional criteria for acute kidney injury after hematopoietic SCT. Bone marrow transplantation. 2010;45:1427–1434. doi: 10.1038/bmt.2009.377. [DOI] [PubMed] [Google Scholar]
- 25.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron. Clinical practice. 2012;120:c179–c184. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- 26.Zappitelli M, Parikh CR, Akcan-Arikan A, Washburn KK, Moffett BS, Goldstein SL. Ascertainment and epidemiology of acute kidney injury varies with definition interpretation. Clinical journal of the American Society of Nephrology : CJASN. 2008;3:948–954. doi: 10.2215/CJN.05431207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kist-van Holthe JE, van Zwet JM, Brand R, van Weel MH, Vossen JM, van der Heijden AJ. Bone marrow transplantation in children: consequences for renal function shortly after and 1 year post-BMT. Bone marrow transplantation. 1998;22:559–564. doi: 10.1038/sj.bmt.1701388. [DOI] [PubMed] [Google Scholar]
- 28.Parikh CR, Schrier RW, Storer B, et al. Comparison of ARF after myeloablative and nonmyeloablative hematopoietic cell transplantation. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2005;45:502–509. doi: 10.1053/j.ajkd.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 29.Hahn T, Rondeau C, Shaukat A, et al. Acute renal failure requiring dialysis after allogeneic blood and marrow transplantation identifies very poor prognosis patients. Bone marrow transplantation. 2003;32:405–410. doi: 10.1038/sj.bmt.1704144. [DOI] [PubMed] [Google Scholar]
- 30.Grams ME, Matsushita K, Sang Y, et al. Explaining the racial difference in AKI incidence. Journal of the American Society of Nephrology : JASN. 2014;25:1834–1841. doi: 10.1681/ASN.2013080867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Limou S, Nelson GW, Kopp JB, Winkler CA. APOL1 kidney risk alleles: population genetics and disease associations. Advances in chronic kidney disease. 2014;21:426–433. doi: 10.1053/j.ackd.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lipkowitz MS, Freedman BI, Langefeld CD, et al. Apolipoprotein L1 gene variants associate with hypertension-attributed nephropathy and the rate of kidney function decline in African Americans. Kidney international. 2013;83:114–120. doi: 10.1038/ki.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mogul MJ. Unrelated cord blood transplantation vs matched unrelated donor bone marrow transplantation: the risks and benefits of each choice. Bone Marrow Transplant. 2000;(25 Suppl 2):S58–S60. doi: 10.1038/sj.bmt.1702372. [DOI] [PubMed] [Google Scholar]
- 34.Parody R, Martino R, Rovira M, et al. Severe infections after unrelated donor allogeneic hematopoietic stem cell transplantation in adults: comparison of cord blood transplantation with peripheral blood and bone marrow transplantation. Biol Blood Marrow Transplant. 2006;12:734–748. doi: 10.1016/j.bbmt.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 35.McDonald GB, Hinds MS, Fisher LD, et al. Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation: a cohort study of 355 patients. Annals of internal medicine. 1993;118:255–267. doi: 10.7326/0003-4819-118-4-199302150-00003. [DOI] [PubMed] [Google Scholar]
- 36.Parikh CR, McSweeney P, Schrier RW. Acute renal failure independently predicts mortality after myeloablative allogeneic hematopoietic cell transplant. Kidney international. 2005;67:1999–2005. doi: 10.1111/j.1523-1755.2005.00301.x. [DOI] [PubMed] [Google Scholar]
- 37.Lopes JA, Goncalves S, Jorge S, et al. Contemporary analysis of the influence of acute kidney injury after reduced intensity conditioning haematopoietic cell transplantation on long-term survival. Bone marrow transplantation. 2008;42:619–626. doi: 10.1038/bmt.2008.207. [DOI] [PubMed] [Google Scholar]
- 38.Lassnigg A, Schmidlin D, Mouhieddine M, et al. Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: a prospective cohort study. Journal of the American Society of Nephrology : JASN. 2004;15:1597–1605. doi: 10.1097/01.asn.0000130340.93930.dd. [DOI] [PubMed] [Google Scholar]
- 39.Hingorani S. Chronic kidney disease in long-term survivors of hematopoietic cell transplantation: epidemiology, pathogenesis, and treatment. Journal of the American Society of Nephrology : JASN. 2006;17:1995–2005. doi: 10.1681/ASN.2006020118. [DOI] [PubMed] [Google Scholar]
- 40.Greenberg JH, Coca S, Parikh CR. Long-term risk of chronic kidney disease and mortality in children after acute kidney injury: a systematic review. BMC nephrology. 2014;15:184. doi: 10.1186/1471-2369-15-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lopes JA, Fernandes P, Jorge S, et al. Acute kidney injury in intensive care unit patients: a comparison between the RIFLE and the Acute Kidney Injury Network classifications. Critical care (London, England) 2008;12:R110. doi: 10.1186/cc6997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Menon S, Kirkendall ES, Nguyen H, Goldstein SL. Acute kidney injury associated with high nephrotoxic medication exposure leads to chronic kidney disease after 6 months. The Journal of pediatrics. 2014;165:522–527. doi: 10.1016/j.jpeds.2014.04.058. e522. [DOI] [PubMed] [Google Scholar]