Abstract
Ovarian granulosa cell tumors (GCTs) are rare gynecologic tumors in women. Due to the rarity and limited research efforts invested, the etiology of GCTs remains poorly defined. A landmark study has discovered the mutation of forkhead box L2 (FOXL2) as a genetic hallmark of adult GCTs in the human. However, our understanding of the role of cell signaling in GCT development is far from complete. Increasing lines of evidence highlight the importance of TGF-beta (TGFB) superfamily signaling in the pathogenesis of GCTs. This review draws on findings using genetically modified mouse models and human patient specimens and cell lines to reveal SMAD3 activation as a potentially key converging point of dysregulated TGFB superfamily signaling and genetic aberrations in GCT development. It is anticipated that deciphering the role of TGFB superfamily signaling cascades in ovarian tumorigenesis will help develop new therapeutic approaches for GCTs by targeting core signaling elements essential for tumor initiation, growth, and progression.
Keywords: activin, granulosa cell tumor, ovary, SMAD3, TGF-beta
INTRODUCTION
Ovarian sex cord-stromal tumors are rare gynecologic tumors derived from ovarian granulosa, theca, and stromal fibroblast compartments. Ovarian granulosa cell tumors (GCTs), the major type of sex cord-stromal tumors, account for approximately 5% of ovarian tumors and are divided into adult and juvenile subtypes, with the former being more commonly diagnosed [1–3]. Our understanding of the etiology of GCTs is far from complete. Currently, surgical intervention is a standard treatment for early-stage GCT patients. There is no tailored therapeutic option available for this disease.
Two decades ago, the Matzuk laboratory provided striking genetic evidence that transforming growth factor-beta (TGFB) superfamily proteins are involved in sex cord-stromal tumor development in mice of both sexes by demonstrating inhibin alpha (INHA) as a tumor suppressor specific for the gonad using Inha knockout mice [4]. The expression and function of inhibins/activins in ovarian tumors have been reviewed elsewhere [5–7]. Subsequent studies using genetically modified mouse models targeting other TGFB superfamily members and signaling components reinforce the role of this growth factor family in GCT development [8–12]. These studies along with emerging evidence suggest that SMAD3 activation may be one of the key mechanisms underlying the pathogenesis of GCTs.
TGFB SUPERFAMILY SIGNALING
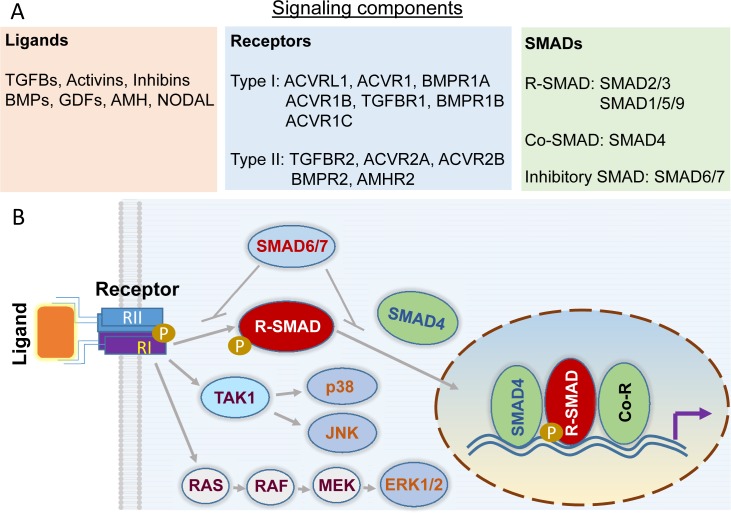
TGFB superfamily signaling regulates fundamental cellular properties, including cell growth and death, differentiation, migration, and invasion. TGFB superfamily ligands (>30 members) contain several subfamily groups such as TGFBs, activins, bone morphogenetic proteins (BMPs), and growth differentiation factors (GDFs), as well as intermediate/distant members including anti-Mullerian hormone (AMH), nodal growth differentiation factor (NODAL), and inhibins [13, 14]. These structurally related proteins signal through transmembrane type II and type I receptor complexes, where type I receptors phosphorylate and activate receptor-regulated SMADs (R-SMADs; SMAD1/2/3/5/9) [14] (Fig. 1A). TGFBs, activins, and NODAL commonly signal via SMAD2/3, while BMPs impinge on SMAD1/5/9 for signal transduction. To gain access to the gene regulatory machinery, R-SMADs form complexes with SMAD4, known as common SMAD (Co-SMAD), and translocate from the cytoplasm to the nucleus, where SMADs regulate gene transcription in the presence of additional coregulators. Besides R-SMAD and Co-SMAD, this pathway is also subject to the negative modulation by inhibitory SMADs (i.e., SMAD6/7) [15] (Fig. 1B). In addition to the aforementioned canonical SMAD-dependent pathway, a noncanonical pathway, also termed SMAD-independent or non-SMAD pathway, can mediate cellular responses to TGFB superfamily signals [16, 17], highlighting the diversity and complexity of the signaling paradigm utilized by TGFB superfamily proteins (Fig. 1B).
FIG. 1.
TGFB signal transduction. A) Core elements of canonical TGFB superfamily signaling. The major ligands, type II and type I receptors, and SMADs are listed. To our knowledge, mRNA and/or protein expression of all listed signaling elements except ACVRL1 and ACVR1C has been reported in human GCT and/or GCT cell lines (i.e., KGN and COV434). B) The TGFB signaling paradigm. TGFB signal transduction is initiated by ligand-receptor binding and propagated through intracellular SMAD proteins. Phosphorylated R-SMADs interact with SMAD4, resulting in nuclear accumulation of the R-SMADs-SMAD4 complex that regulates gene transcription together with coregulators (Co-R) consisting of co-activators and corepressors. SMAD6/7 acts as negative modulators of TGFB signaling activity. Besides the canonical SMAD-dependent signaling, TGFB signaling is also mediated via non-SMAD pathways. SMAD-independent activation of ERK1/2, p38, and JNK is illustrated as examples. This is a simplified illustration of TGFB signaling with the purpose of highlighting major signaling elements.
In this review, we will mainly discuss the involvement of signaling related to TGFBs, activins, inhibins, and BMPs in GCT development. However, the importance of AMH and potential role of NODAL in the pathogenesis of GCTs should not be overlooked. AMH induces male Mullerian duct regression during sex differentiation [18]. AMH has also been found to regulate folliculogenesis, and it is a potential regulator of granulosa cell apoptosis [19]. Moreover, AMH can be used as a circulating marker for GCTs, with prognostic value in clinics [20]. NODAL signaling is critical for multiple events in vertebrate development such as left-right axis specification [21]. In cultured rat granulosa cells, recombinant NODAL induces apoptosis via activin A receptor type 1C (ACVR1C) [22]. Although there are several lines of evidence suggesting a role of NODAL in the pathogenesis of melanoma, breast cancer, and pancreatic ductal adenocarcinoma [23–25], it remains to be determined whether NODAL plays a role in ovarian GCT development.
TGFB SUPERFAMILY SIGNALING IN GCT DEVELOPMENT: LESSONS FROM GENETICALLY MODIFIED MOUSE MODELS
Development of sex cord-stromal tumors has been documented in a number of genetically engineered mouse models [4, 10, 11, 26–32]. INHA is known as a tumor suppressor from the creation of mice lacking INHA [4]. Due to the functional antagonism of activins by inhibins [33], loss of INHA is expected to cause unopposed activin signaling activity. Functional evidence supporting a role of potentiated activin signaling in gonadal tumor development stems from a study demonstrating that activin-induced cachexia-like wasting syndrome was prevented and gonadal tumor formation compromised when an activin antagonist, a chimeric activin receptor type II-murine Fc protein, was administered to Inha−/− mice [34]. Furthermore, genetic deletion of Smad3, but not Smad2, in Inha null background protects/mitigates gonadal tumorigenesis in mice, where a more pronounced effect was observed in the males versus females [8, 35]. This finding suggests that SMAD3 plays a major role in mediating activin signaling during gonadal tumorigenesis. The potential synergy between SMAD2 and SMAD3 and the involvement of non-SMAD signaling in the tumorigenic process resulting from loss of INHA have not been examined. Within the follicular microenvironment, oocytes orchestrate folliculogenesis partially through TGFB superfamily members [36]. Interactions between INHA and oocyte-secreted GDF9 have been revealed during gonadal tumorigenesis using mouse models; and a complex interplay between TGFB superfamily members in regulating the proliferation, differentiation, and malignant transformation of mouse granulosa cells has been proposed [37, 38]. Importantly, these studies indicate a potential role of oocyte paracrine signaling in ovarian tumorigenesis.
Studies using conditional knockout mouse models have shown that signaling mediated by BMP type 1 receptors (i.e., BMPR1A and BMPR1B) and BMP-responsive SMADs (i.e., SMAD1/5) negatively impacts GCT development. Interestingly, ovaries from Smad1/5 and Bmpr1a/Bmpr1b conditional knockout mice express high levels of TGFB downstream target genes such as TGFB induced (Tgfbi) and matrix metallopeptidase 2 (Mmp2) and/or harbor SMAD2/3 activation, in concordance with the antagonism between TGFB and BMP signaling pathways [10, 11, 39, 40]. Generation of Smad1/5/4 triple conditional knockout mice by the Pangas laboratory further indicates that SMAD4-dependent TGFB/activin signaling may be tumorigenic in the mouse ovary [41]. Because canonical BMP signaling activity is attenuated in Smad1/5 conditional knockout mice, disruption of the Smad4 gene in this model is expected to further block SMAD4-dependent TGFB/activin signaling. It is interesting to note that tumors developed in Smad1/5/4 triple conditional knockout mice are not metastatic but are prone to apoptosis [41]. These findings support that TGFB signaling promotes the proliferation of granulosa cells [42–44]. Of note, the role of BMP and TGFB/activin signaling in cell proliferation and differentiation during GCT development is complex, as evidenced by the fact that Smad4 conditional knockout mice show premature granulosa cell luteinization or defective luteal formation [45, 46].
New evidence suggests that GCTs resulting from genetic modifications of some non-TGFB superfamily genes may also harbor TGFB/activin signaling activation [31, 32]. A recent elegant study by the Richards laboratory reveals that triple deletion of forkhead box protein O1 (Foxo1), Foxo3, and phosphatase and tensin homolog (Pten) in the mouse ovary causes GCT formation. This mouse model mimics human GCTs in hormone profiles (e.g., increased levels of estradiol and inhibins). Tumor tissues from these mice are positive for phospho-SMAD2/3, suggesting an activation of TGFB/activin signaling [31]. Another mouse model with constitutive activation of phosphoinositide 3-kinase (termed PIK3CA) in the oocyte demonstrates abnormal follicle growth, ovulatory defects, and formation of bilateral GCTs [32, 47]. Development of GCTs in the PIK3CA mouse model is potentially caused by altered activin signaling, where autocrine activin signaling promotes the growth of tumor cells [32]. As supporting evidence, inhibin beta-A and nuclear SMAD3 are highly expressed in these GCTs [32].
To directly test the effect of TGFB signaling activation in ovarian GCT development, we have recently generated mouse models harboring a constitutively active TGFB receptor 1 (TGFBR1) in the ovary [48]. Constitutive activation of TGFBR1 using anti-Mullerian hormone receptor type 2 (Amhr2)-Cre (TGFBR1-CAAcre) leads to the development of sex cord-stromal tumors, reminiscent of GCTs [48]. These tumors express granulosa cell markers including forkhead box L2 (FOXL2), INHA, and FOXO1. Importantly, serum levels of estradiol, inhibin A, and inhibin B are highly increased and follicle-stimulating hormone (FSH) is dramatically reduced in TGFBR1-CAAcre mice, consistent with the hormone profile of human GCTs. Moreover, constitutive activation of TGFBR1 promotes ovarian cell proliferation, compromises cell differentiation, and disrupts the expression of genes associated with ovarian differentiation and function (e.g., 3β-hydroxysteroid dehydrogenase [Hsd3b], FSH receptor [Fshr], natriuretic peptide type C [Nppc], and wingless-type MMTV integration site family member 4 [Wnt4]) [48]. Further analyses of gene expression using mouse ovaries have indicated potential involvement of increased expression of phospho-AKT and GLI-Kruppel family member GLI1/2 (Gli1/2) and reduced expression of Tgfbr3 in GCT development [48]. This genetic evidence supports the tumorigenic effect of TGFBR1 activation in the ovary. However, the specific contribution of SMAD3 signaling to GCT development in TGFBR1-CAAcre mice has not been determined, although increased expression of phospho-SMAD2/3 has been observed in these mice [48] (Gao and Li, unpublished results). Clarification of the role of SMAD3 requires the generation of mice harboring inactivation of SMAD3 and constitutive activation of TGFBR1 within granulosa cells in the future.
TGFB SUPERFAMILY SIGNALING AND HUMAN GCT DEVELOPMENT
Although limited information on transcriptomic changes of human GCTs is available in publicly available databases including GEO DataSets, Oncomine, Cbioportal, and The Cancer Genome Atlas, partially due to the rarity of this type of tumors in women, emerging evidence indicates that TGFB/activin signaling is active in human GCTs [12, 31, 41]. Currently, there are only two established GCT cell lines available in this research field. KGN and COV434 cells are derived from the respective adult and juvenile GCTs [49, 50]. KGN cells but not COV434 cells bear FOXL2 402C → G mutation [51]. In addition, KGN cells express FOXL2, while COV434 cells lack FOXL2 expression [51], which may be associated with the aggressive property of juvenile GCTs [1, 52]. However, the causes of FOXL2 402C → G mutation in KGN cells and lack of FOXL2 expression in COV434 cells are not known.
Existing literature supports the hypothesis that TGFB superfamily signaling is involved in the pathogenesis of human GCTs. The expression of phospho-SMAD2/3 in human ovarian and testicular juvenile GCTs and extraovarian GCT implants suggests the active status of TGFB/activin signaling in GCTs [12]. Furthermore, SMAD2/3 and nuclear factor kappa B (NFκB) signaling pathways positively influence the survival of KGN cells cultured in vitro [53]. It has been demonstrated that treatment of human COV434 cells with TGFB1, but not activin A, increases cell viability and inhibits apoptosis via TGFBR1-dependent repression of caspase 3/7 activity and poly (ADP-ribose) polymerase 1 (PARP1) cleavage, indicating that activins and TGFBs may play different roles during GCT progression [41]. TGFBR3, also known as betaglycan, promotes the antagonism of activins by inhibins via binding to inhibins [33]. Recent evidence points to a tumor suppressive role of TGFBR3 in GCTs using cultured human GCT cell lines [54, 55]. In support of this concept, transcript levels of TGFBR3 are lower in human GCTs compared with normal ovarian tissues [54]. Immunoreactive signals for TGFBR3 are also weaker in human GCTs versus fibrothecomas [56]. Moreover, stable transfection of TGFBR3 in human GCT cell lines increases cell adhesion and decreases cell invasion [54]. TGFBR3 has also been proposed to inhibit GCT metastasis through interaction with the NFκB pathway based on studies using cultured human GCT cell lines and xenograft mouse model [55].
In addition to TGFB/activin signaling, BMP signaling is also involved in GCT development. Components of the BMP signaling pathway, including BMP ligands, receptors, and downstream SMAD1/5, are present in human GCTs [57]. Human GCT cell line also expresses BMP signaling receptors and SMADs [58]. Treatment of cultured human GCT cell line or immortalized human granulosa cell line (SVOG) with BMPs induces SMAD1/5/9 phosphorylation [59, 60]. Nevertheless, BMP signaling appears to be antitumorigenic, as is corroborated by the aforementioned phenotype of Smad1/5 and Bmpr1a/1b conditional knockout mouse models and the fact that COV434 cells express lower levels of SMAD1/5 versus a nonjuvenile GCT cell line [57]. A study using cultured COV434 cells suggests that the tumor suppressive action of BMP signaling may be mediated through the antagonism of Sp1 transcription factor (SP1) by SMAD1/5 on the promoter region of platelet derived growth factor subunit A (PDGFA) gene, leading to reduced production of PDGFA protein, a multifunctional molecule that has a prominent role in angiogenesis [57]. Of note, TGFB1 stimulates the expression of vascular endothelial growth factor (VEGF) via SP1-dependent transcriptional activation in human cholangiocellular cancer cells, suggesting a potential role of TGFB signaling in tumor angiogenesis [61]. However, TGFB signaling inhibits the expression of VEGFA in the FET cell line that is derived from early-stage colon cancer [62]. These findings suggest that regulation of tumor angiogenesis by TGFB signaling is contextually dependent [63]. However, the role of TGFB signaling-induced angiogenesis in the development of GCTs is not clear. That sustained activation of TGFBR1 promotes ovarian angiogenesis and GCT formation provides circumstantial evidence supporting a link between TGFB signaling-stimulated angiogenesis and GCT development [48].
SMAD3 ACTIVATION AS A POTENTIAL CONVERGING POINT OF DYSREGULATED TGFB SUPERFAMILY SIGNALING AND GENETIC ABERRATIONS IN GCTs
FOXL2 is a transcription factor with a fundamental role in ovarian granulosa cell differentiation and function [64]. A recent study has shown that female adult GCTs (∼97%), but not other types of ovarian tumors, carry a FOXL2 somatic missense mutation (402C → G), resulting in the translation of a mutant FOXL2 (C134W) protein [65]. Although GCTs are rarely found in men, the FOXL2 402C → G mutation has also been reported in adult GCTs from some male patients [66]. The impact of FOXL2 402C → G mutation on FOXL2 function has been highlighted in recent elegant reviews [67, 68]. Current literature supports that FOXL2 402C → G mutation increases cell proliferation, reduces apoptosis, and alters ovarian steroidogenesis to favor the synthesis of estrogen [67, 68]. Studies aimed at identifying FOXL2 partners have uncovered that mutant FOXL2 (C134W) is defective in integrating pro-apoptotic signals in cultured KGN cells, leading the authors to speculate that failure to trigger apoptosis by the mutant FOXL2 may contribute to the oncogenesis of GCTs [69]. The specific role of the mutant FOXL2 (C134W) in granulosa cell tumorigenesis remains to be elucidated. The fact that FOXL2 402C → G mutation occurs nearly universally in adult GCTs but is rare in the juvenile type of GCTs suggests the distinct etiology of the two types of GCTs [65]. Molecular changes associated with FOXL2 mutation have been recently investigated. An interesting question posed is, does FOXL2 mutation link to TGFB/activin signaling activation in human GCTs? New evidence has revealed molecular and functional interactions between FOXL2 and SMAD3 and suggested that SMAD3 activation may serve as a critical converging point of dysregulated TGFB superfamily signaling and genetic aberrations in human GCT development.
First, SMAD3 interacts with FOXL2 and GATA binding protein 4 (GATA4) to regulate the promoter activity of genes associated with GCT cell proliferation and survival (e.g., cyclin D2 [CCND2]) in cultured human GCT cell lines [70]. The balanced regulation of granulosa cell survival and apoptosis by GATA4 and SMAD3 is impaired when FOXL2 mutation occurs [70]. Second, another recent study has shown that GDF9-stimulated follistatin mRNA expression in COV434 cells requires FOXL2 and SMAD3. However, FOXL2 (C134W) mutant protein inhibits the effect of GDF9 on follistatin transcription potentially through impairing the binding of SMAD3 to the SMAD binding site (SBE) [71]. In an independent report using cultured KGN cells, activin A has been shown to promote tumor cell proliferation via an ACVR1-SMAD2/3-SMAD4 circuit [72]. Moreover, overexpression of the wild-type FOXL2 stimulates follistatin expression. However, FOXL2 402C → G mutation renders the FOXL2 (C134W) protein incapable of inducing follistatin expression [72]. Because follistatin antagonizes the effect of activin signaling on cell proliferation, it is conceivable that reduced follistatin may lead to potentiated activin signaling, SMAD2/3 activation, and increased cell proliferation. Last, but not least, a study comparing the transcriptomic profiles of COV434 cells that overexpress wild-type and mutant FOXL2 (C134W) protein has revealed that target genes of FOXL2 (C134W) are enriched for TGFB signaling [73]. Using microarray and Ingenuity Pathway Analysis, the authors have identified the regulation of TGFB superfamily associated genes by mutant FOXL2 (C134W) protein. The candidate genes include, but are not limited to, INHA, SMAD3, ACVR, AP-1, C-JUN, BMPR, SMAD6, INHBA, ID1, ID2, and ID3 [73]. Notably, increased expression of signaling components of activins/TGFBs (ACVR and SMAD3) and reduced expression of INHA and BMP signaling elements (BMPR, SMAD6, ID1, ID2, and ID3) suggest a potential activation of activin/TGFB-SMAD2/3 signaling in human GCTs.
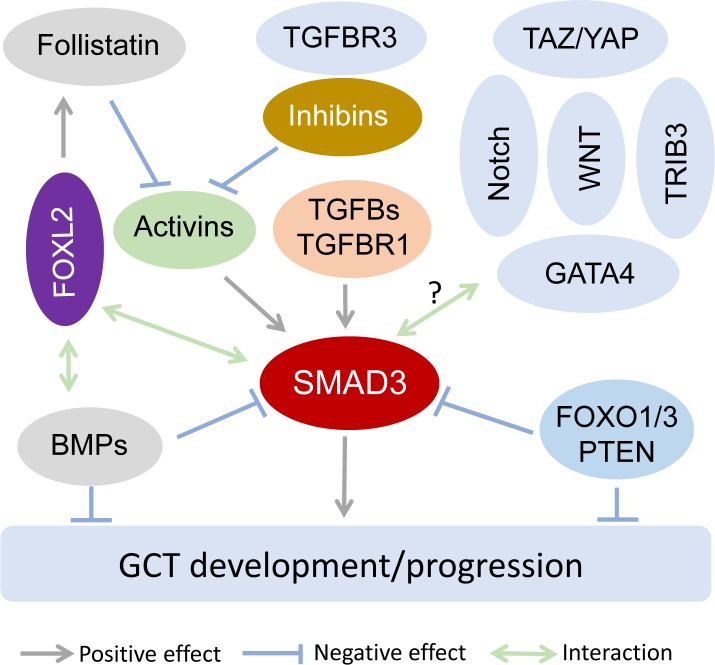
Abnormalities of other transcriptional regulators/pathways that control or influence TGFB signaling activity may also contribute to GCT development. FOXL2 has been shown to interact with SMAD3 to regulate estrogen receptor 2 (Esr2) expression induced by activin in primary mouse follicular cells [74]. Besides SMAD3, FOXL2 interacts with various partners with different target specificities to regulate distinct cellular functions, including apoptosis of KGN cells [69]. Hiemer and colleagues have reported that major transcriptional effectors of the Hippo pathway, transcriptional co-activator with PDZ-binding motif (TAZ) and Yes associated protein 1 (YAP) (TAZ/YAP), interact with SMAD3 in breast cancer cells, directing a TGFB-induced transcriptional program that promotes tumorigenesis [75]. Importantly, YAP regulates KGN cell proliferation, migration, and steroidogenesis [76]. Tribbles homolog 3 (TRIB3) can also interact with SMAD3 to regulate HepG2 tumor cell migration and invasion in vitro [77]. Of note, TGF alpha (TGFA) regulates KGN cell proliferation and migration and activates a variety of signaling molecules including MAPK1/3 and MAPK14 [78], targets that can also be activated by non-SMAD signaling. In addition, activation of WNT and Notch signaling has been known to affect TGFB signaling [79, 80]. Interestingly, mice expressing a dominant stable beta-catenin mutant in granulosa cells develop ovarian GCTs [26]. Therefore, future studies are needed to uncover the contribution of TGFB interactive molecules/pathways to the pathogenesis of GCTs. A hypothetical model is proposed to describe the role of SMAD3 in GCT development (Fig. 2).
FIG. 2.
Hypothetical model depicting a potential role of SMAD3 activation in GCT development. In this model, we hypothesize that SMAD3 activation may serve as a converging point of dysregulated TGFB superfamily signaling and genetic aberrations, and play an important role in GCT development. Activin and TGFB-induced signaling converges on SMAD3 and promotes ovarian tumorigenesis, as is supported by studies using genetically modified mouse models, including Inha−/− [4, 34], Inha−/−; Smad3−/− [8], Smad1/5 conditional knockout [10], Bmpr1a/Bmpr1b conditional knockout [11], and TGFBR1-CAAcre mice [48]. The activity of activins is regulated by follistatin and inhibins (i.e., negative modulators) and TGFBR3, an inhibin-binding protein that promotes the antagonism of activins by inhibins. BMP pathway suppresses GCT development at least partially through influencing SMAD3 activation. FOXL2, a key regulator of granulosa cell differentiation, may inhibit SMAD3 activation through inducing follistatin production and differentially modulating the expression of activin and BMP signaling components [71–73]. FOXO1/3 and PTEN seem to negatively impact SMAD2/3 activation and GCT formation [31]. Additionally, it is postulated that molecules/pathways that influence TGFB signaling activity, particularly those interacting with SMAD3, are potential regulators of GCT development. Such candidates include, but are not limited to, TAZ/YAP, TRIB3, GATA4, WNT, and Notch [70, 75, 77, 79, 80]. Their specific roles and interactions with TGFB signaling in the pathogenesis of GCTs have yet to be experimentally tested. This model does not exclude potential contributions of dysregulated signaling events upstream or downstream of SMAD3, SMAD-independent signaling mechanisms, and other signaling pathways in the pathogenesis of GCTs.
PERSPECTIVES
Although the involvement of inhibins and activins in sex cord-stromal tumors has long been known, the role of TGFB signaling and the converging point of dysregulated TGFB superfamily signaling cascades and genetic aberrations in GCTs have not been recognized until recently. The breakthrough discovery of FOXL2 somatic mutation (FOXL2 402C → G) in adult GCTs and the finding that TGFB/activin signaling is enriched in human GCT cells overexpressing FOXL2 (C134W) protein provide new insights into how genetic aberrations may lead to GCT development via dysregulation of TGFB/activin signaling [65, 73]. The role of SMAD3 in GCT development has been corroborated not only by the aforementioned evidence that deletion of Smad3, but not Smad2, in Inha−/− mice mitigates gonadal tumor development/progression [8, 9, 35], but also by the finding that SMAD3 serves as an important partner of FOXL2 in the regulation of follistatin expression and granulosa cell viability and apoptosis [70, 71]. Furthermore, the consistent detection of phospho-SMAD3 in several genetically modified mouse models targeting genes associated with both TGFB superfamily and non-TGFB superfamily members and the recent demonstration that constitutively active TGFBR1 promotes ovarian tumorigenesis [48] collectively suggest that SMAD3 activation may be a critical converging point of dysregulated TGFB superfamily signaling and genetic aberrations in GCT development. Additional studies are warranted to revisit SMAD3 activation in mouse models where its activation status remains unknown. It needs to be mentioned that although mouse models represent a useful tool to study human GCTs, particularly signaling pathways during tumor development, limitations of these models exist. The differences between mice and humans in developmental biology and physiology represent general limitations for the application of mouse models to GCT research. Moreover, GCT models mimicking FOXL2 402C → G mutation are not available, making it difficult to dissect the initiating cascades of human GCT development. Knocking out Foxl2 in mice does not cause GCT formation, but leads to defects in follicular development and ovarian differentiation [81–83]. Conditional deletion of Foxl2 using inducible Cre in adult mice causes ovary-to-testis transdifferentiation [64]. Thus, introduction of the FOXL2 mutant into mouse granulosa cells may help clarify this question.
In addition to SMAD-mediated signaling, TGFBs can act through SMAD-independent noncanonical pathway. It is well established that mitogen-activated protein kinase 1/3 (MAPK1/3; also known as extracellular signal-regulated kinase 1/2 [ERK1/2]), MAPK14 (also known as p38), and MAPK8 (also known as c-June N-terminal kinase [JNK]) are common mediators of noncanonical TGFB signaling. TGFB signaling can activate MAPK8 and MAPK14 via mitogen-activated protein kinase kinase kinase 7 (MAP3K7 or TAK1). Activation of ERK1/2 is generally associated with RAS-RAF-MEK-mediated signaling [16, 17, 84]. For example, TGFB1 activates AKT, MAPK1/3, and MAPK14 through TGFBR1 in cultured human peripheral blood monocytes [85]. Interestingly, activin A receptor like type 1 (ACVRL1, also known as ALK1) has been involved in SMAD1/5/9 activation by TGFBs in endothelial cells and chondrocytes [86, 87]. It is noteworthy that constitutive activation of MAPK1/3 in KGN cells is associated with cell proliferation [88], suggesting a potential role of noncanonical TGFB signaling in the pathogenesis of GCTs. Future studies are needed to define the contribution of noncanonical TGFB signaling to the development and progression of GCTs.
The evidence that enhanced TGFB/activin signaling promotes GCT development provides a rational basis for testing the role of TGFB and activin receptor modulators in GCT formation and progression. The molecular and functional interactions among TGFB, activin, and BMP signaling during GCT initiation and progression are poorly defined and need to be investigated to achieve a comprehensive roadmap of TGFB superfamily signaling circuitry in GCTs. Further deciphering molecular mechanisms that underpin the tumorigenic function of FOXL2 402C → G mutation, particularly the interaction between mutant FOXL2 and TGFB/activin and other signaling pathways, will help understand the pathogenesis of GCTs and design novel therapeutic strategies.
The role of tumor microenvironment in GCT development is poorly delineated. Tumor-associated macrophages, infiltrating leukocytes recruited to solid tumors, have been shown to promote cancer development [89]. However, the contribution of tumor-associated macrophages to GCT progression is not clear. Notably, chitinase-like 3 (CHIL3/YM1), which is associated with M2 polarization, is detectable in GCTs of mice lacking Foxo1/3 and Pten [31] or harboring a constitutively active TGFBR1 (Gao and Li, unpublished result), suggesting a potential role of altered tumor microenvironment in GCT development.
In summary, understanding the interrelationship among genetic aberrations, signaling circuitry, and tumor microenvironment in the pathogenesis of GCTs will lay a blueprint to guide the discovery of therapeutic targets for GCTs. Significant progress is expected in these areas over the next decade.
ACKNOWLEDGMENT
The authors thank Dr. Robert Burghardt for critical reading of this manuscript. We also wish to thank the anonymous reviewers for the insightful comments during the revision of this manuscript.
Footnotes
Research in the Li laboratory is supported by the Office of the Assistant Secretary of Defense for Health Affairs, through the Ovarian Cancer Research Program (Award No. W81XWH-15-1-0082) and the Eunice Kennedy Shriver National Institute of Child Health & Human Development grant R03HD082416. Opinions are those of the author and are not necessarily endorsed by the funders.
REFERENCES
- Jamieson S, Fuller PJ. Molecular pathogenesis of granulosa cell tumors of the ovary. Endocr Rev. 2012;33:109–144. doi: 10.1210/er.2011-0014. [DOI] [PubMed] [Google Scholar]
- Schumer ST, Cannistra SA. Granulosa cell tumor of the ovary. J Clin Oncol. 2003;21:1180–1189. doi: 10.1200/JCO.2003.10.019. [DOI] [PubMed] [Google Scholar]
- Pectasides D, Pectasides E, Psyrri A. Granulosa cell tumor of the ovary. Cancer Treat Rev. 2008;34:1–12. doi: 10.1016/j.ctrv.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Finegold MJ, Su JG, Hsueh AJ, Bradley A. Alpha-inhibin is a tumour-suppressor gene with gonadal specificity in mice. Nature. 1992;360:313–319. doi: 10.1038/360313a0. [DOI] [PubMed] [Google Scholar]
- Marino FE, Risbridger G, Gold E. The inhibin/activin signalling pathway in human gonadal and adrenal cancers. Mol Hum Reprod. 2014;20:1223–1237. doi: 10.1093/molehr/gau074. [DOI] [PubMed] [Google Scholar]
- Robertson DM, Burger HG, Fuller PJ. Inhibin/activin and ovarian cancer. Endocr Relat Cancer. 2004;11:35–49. doi: 10.1677/erc.0.0110035. [DOI] [PubMed] [Google Scholar]
- Risbridger GP, Schmitt JF, Robertson DM. Activins and inhibins in endocrine and other tumors. Endocr Rev. 2001;22:836–858. doi: 10.1210/edrv.22.6.0450. [DOI] [PubMed] [Google Scholar]
- Li Q, Graff JM, O'Connor AE, Loveland KL, Matzuk MM. SMAD3 regulates gonadal tumorigenesis. Mol Endocrinol. 2007;21:2472–2486. doi: 10.1210/me.2007-0147. [DOI] [PubMed] [Google Scholar]
- Looyenga BD, Hammer GD. Genetic removal of Smad3 from inhibin-null mice attenuates tumor progression by uncoupling extracellular mitogenic signals from the cell cycle machinery. Mol Endocrinol. 2007;21:2440–2457. doi: 10.1210/me.2006-0402. [DOI] [PubMed] [Google Scholar]
- Pangas SA, Li X, Umans L, Zwijsen A, Huylebroeck D, Gutierrez C, Wang D, Martin JF, Jamin SP, Behringer RR, Robertson EJ, Matzuk MM. Conditional deletion of Smad1 and Smad5 in somatic cells of male and female gonads leads to metastatic tumor development in mice. Mol Cell Biol. 2008;28:248–257. doi: 10.1128/MCB.01404-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edson MA, Nalam RL, Clementi C, Franco HL, Demayo FJ, Lyons KM, Pangas SA, Matzuk MM. Granulosa cell-expressed BMPR1A and BMPR1B have unique functions in regulating fertility but act redundantly to suppress ovarian tumor development. Mol Endocrinol. 2010;24:1251–1266. doi: 10.1210/me.2009-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlebrook BS, Eldin K, Li X, Shivasankaran S, Pangas SA. Smad1-Smad5 ovarian conditional knockout mice develop a disease profile similar to the juvenile form of human granulosa cell tumors. Endocrinology. 2009;150:5208–5217. doi: 10.1210/en.2009-0644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmierer B, Hill CS. TGFbeta-SMAD signal transduction: molecular specificity and functional flexibility. Nat Rev Mol Cell Biol. 2007;8:970–982. doi: 10.1038/nrm2297. [DOI] [PubMed] [Google Scholar]
- Massague J. TGF-beta signal transduction. Annu Rev Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- Li Q. Inhibitory SMADs: potential regulators of ovarian function. Biol Reprod. 2015;92:50. doi: 10.1095/biolreprod.114.125203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhurst RJ, Hata A. Targeting the TGF beta signalling pathway in disease. Nat Rev Drug Discov. 2012;11:790–811. doi: 10.1038/nrd3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YE. Non-Smad pathways in TGF-beta signaling. Cell Res. 2009;19:128–139. doi: 10.1038/cr.2008.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behringer RR, Finegold MJ, Cate RL. Mullerian-inhibiting substance function during mammalian sexual development. Cell. 1994;79:415–425. doi: 10.1016/0092-8674(94)90251-8. [DOI] [PubMed] [Google Scholar]
- Seifer DB, Merhi Z. Is AMH a regulator of follicular atresia? J Assist Reprod Genet. 2014;31:1403–1407. doi: 10.1007/s10815-014-0328-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Marca A, Volpe A. The anti-Mullerian hormone and ovarian cancer. Hum Reprod Update. 2007;13:265–273. doi: 10.1093/humupd/dml060. [DOI] [PubMed] [Google Scholar]
- Schier AF, Shen MM. Nodal signalling in vertebrate development. Nature. 2000;403:385–389. doi: 10.1038/35000126. [DOI] [PubMed] [Google Scholar]
- Wang H, Jiang JY, Zhu C, Peng C, Tsang BK. Role and regulation of Nodal/Activin receptor-like kinase 7 signaling pathway in the control of ovarian follicular atresia. Mol Endocrinol. 2006;20:2469–2482. doi: 10.1210/me.2005-0446. [DOI] [PubMed] [Google Scholar]
- Kirsammer G, Strizzi L, Margaryan NV, Gilgur A, Hyser M, Atkinson J, Kirschmann DA, Seftor EA, Hendrix MJC. Nodal signaling promotes a tumorigenic phenotype in human breast cancer. Semin Cancer Biol. 2014;29:40–50. doi: 10.1016/j.semcancer.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topczewska JM, Postovit LM, Margaryan NV, Sam A, Hess AR, Wheaton WW, Nickoloff BJ, Topczewski J, Hendrix MJ. Embryonic and tumorigenic pathways converge via Nodal signaling: role in melanoma aggressiveness. Nat Med. 2006;12:925–932. doi: 10.1038/nm1448. [DOI] [PubMed] [Google Scholar]
- Kong B, Wang WB, Esposito I, Friess H, Michalski CW, Kleeff J. Increased expression of Nodal correlates with reduced patient survival in pancreatic cancer. Pancreatology. 2015;15:156–161. doi: 10.1016/j.pan.2015.02.001. [DOI] [PubMed] [Google Scholar]
- Boerboom D, Paquet M, Hsieh M, Liu J, Jamin SP, Behringer RR, Sirois J, Taketo MM, Richards JS. Misregulated Wnt/beta-Catenin signaling leads to ovarian granulosa cell tumor development. Cancer Res. 2005;65:9206–9215. doi: 10.1158/0008-5472.CAN-05-1024. [DOI] [PubMed] [Google Scholar]
- Boyer A, Paquet M, Lague MN, Hermo L, Boerboom D. Dysregulation of WNT/CTNNB1 and PI3K/AKT signaling in testicular stromal cells causes granulosa cell tumor of the testis. Carcinogenesis. 2009;30:869–878. doi: 10.1093/carcin/bgp051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JS, Fan HY, Liu Z, Tsoi M, Lague MN, Boyer A, Boerboom D. Either Kras activation or Pten loss similarly enhance the dominant-stable CTNNB1-induced genetic program to promote granulosa cell tumor development in the ovary and testis. Oncogene. 2012;31:1504–1520. doi: 10.1038/onc.2011.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilson JH, Abbud RA, Keri RA, Quirk CC. Chronic hypersecretion of luteinizing hormone in transgenic mice disrupts both ovarian and pituitary function, with some effects modified by the genetic background. Recent Prog Horm Res. 2000;55:69–91. [PubMed] [Google Scholar]
- Kananen K, Markkula M, Rainio E, Su JG, Hsueh AJ, Huhtaniemi IT. Gonadal tumorigenesis in transgenic mice bearing the mouse inhibin alpha-subunit promoter/simian virus T-antigen fusion gene: characterization of ovarian tumors and establishment of gonadotropin-responsive granulosa cell lines. Mol Endocrinol. 1995;9:616–627. doi: 10.1210/mend.9.5.7565808. [DOI] [PubMed] [Google Scholar]
- Liu Z, Ren YA, Pangas SA, Adams J, Zhou W, Castrillon DH, Wilhelm D, Richards JS. FOXO1/3 and PTEN depletion in granulosa cells promotes ovarian granulosa cell tumor development. Mol Endocrinol. 2015;29:1006–1024. doi: 10.1210/me.2015-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Ebbert K, Cordeiro MH, Romero MM, Whelan KA, Suarez AA, Woodruff T, Kurita T. Constitutive activation of PI3K in oocytes induces ovarian granulosa cell tumors. Cancer Res. 2016;76:3851–3861. doi: 10.1158/0008-5472.CAN-15-3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis KA, Gray PC, Blount AL, MacConell LA, Wiater E, Bilezikjian LM, Vale W. Betaglycan binds inhibin and can mediate functional antagonism of activin signalling. Nature. 2000;404:411–414. doi: 10.1038/35006129. [DOI] [PubMed] [Google Scholar]
- Li Q, Kumar R, Underwood K, O'Connor AE, Loveland KL, Seehra JS, Matzuk MM. Prevention of cachexia-like syndrome development and reduction of tumor progression in inhibin-deficient mice following administration of a chimeric activin receptor type II-murine Fc protein. Mol Hum Reprod. 2007;13:675–683. doi: 10.1093/molehr/gam055. [DOI] [PubMed] [Google Scholar]
- Rajanahally S, Agno JE, Nalam RL, Weinstein MB, Loveland KL, Matzuk MM, Li Q. Genetic evidence that SMAD2 is not required for gonadal tumor development in inhibin-deficient mice. Reprod Biol Endocrinol. 2010;8:69. doi: 10.1186/1477-7827-8-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppig JJ. Oocyte control of ovarian follicular development and function in mammals. Reproduction. 2001;122:829–838. doi: 10.1530/rep.0.1220829. [DOI] [PubMed] [Google Scholar]
- Wu X, Chen L, Brown CA, Yan C, Matzuk MM. Interrelationship of growth differentiation factor 9 and inhibin in early folliculogenesis and ovarian tumorigenesis in mice. Mol Endocrinol. 2004;18:1509–1519. doi: 10.1210/me.2003-0399. [DOI] [PubMed] [Google Scholar]
- Myers M, Mansouri-Attia N, James R, Peng J, Pangas SA. GDF9 modulates the reproductive and tumor phenotype of female Inha-null mice. Biol Reprod. 2013;88:86. doi: 10.1095/biolreprod.112.104125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Hirschberg R. BMP7 antagonizes TGF-beta -dependent fibrogenesis in mesangial cells. Am J Physiol Renal Physiol. 2003;284:F1006–F1013. doi: 10.1152/ajprenal.00382.2002. [DOI] [PubMed] [Google Scholar]
- Bin S, Li HD, Xu YB, Qi SH, Li TZ, Liu XS, Tang JM, Xie JL. BMP-7 attenuates TGF-beta1-induced fibroblast-like differentiation of rat dermal papilla cells. Wound Repair Regen. 2013;21:275–281. doi: 10.1111/wrr.12015. [DOI] [PubMed] [Google Scholar]
- Mansouri-Attia N, Tripurani SK, Gokul N, Piard H, Anderson ML, Eldin K, Pangas SA. TGFbeta signaling promotes juvenile granulosa cell tumorigenesis by suppressing apoptosis. Mol Endocrinol. 2014;28:1887–1898. doi: 10.1210/me.2014-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao GD, Yin MM, Lian J, Tian H, Liu L, Li X, Sun F. MicroRNA-224 is involved in transforming growth factor-beta-mediated mouse granulosa cell proliferation and granulosa cell function by targeting Smad4. Mol Endocrinol. 2010;24:540–551. doi: 10.1210/me.2009-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saragueta PE, Lanuza GM, Baranao JL. Autocrine role of transforming growth factor beta1 on rat granulosa cell proliferation. Biol Reprod. 2002;66:1862–1868. doi: 10.1095/biolreprod66.6.1862. [DOI] [PubMed] [Google Scholar]
- Dorrington J, Chuma AV, Bendell JJ. Transforming growth factor beta and follicle-stimulating hormone promote rat granulosa cell proliferation. Endocrinology. 1988;123:353–359. doi: 10.1210/endo-123-1-353. [DOI] [PubMed] [Google Scholar]
- Pangas SA, Li X, Robertson EJ, Matzuk MM. Premature luteinization and cumulus cell defects in ovarian-specific Smad4 knockout mice. Mol Endocrinol. 2006;20:1406–1422. doi: 10.1210/me.2005-0462. [DOI] [PubMed] [Google Scholar]
- Yu C, Zhang YL, Fan HY. Selective Smad4 knockout in ovarian preovulatory follicles results in multiple defects in ovulation. Mol Endocrinol. 2013;27:966–978. doi: 10.1210/me.2012-1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Ebbert K, Cordeiro MH, Romero M, Zhu J, Serna VA, Whelan KA, Woodruff TK, Kurita T. Cell autonomous phosphoinositide 3-kinase activation in oocytes disrupts normal ovarian function through promoting survival and overgrowth of ovarian follicles. Endocrinology. 2015;156:1464–1476. doi: 10.1210/en.2014-1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Vincent DF, Davis AJ, Sansom OJ, Bartholin L, Li Q. Constitutively active transforming growth factor beta receptor 1 in the mouse ovary promotes tumorigenesis. Oncotarget. 2016;7:40904–40918. doi: 10.18632/oncotarget.10149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Berg-Bakker CA, Hagemeijer A, Franken-Postma EM, Smit VT, Kuppen PJ, van Ravenswaay Claasen HH, Cornelisse CJ, Schrier PI. Establishment and characterization of 7 ovarian-carcinoma cell-lines and one granulosa tumor-cell line: growth features and cytogenetics. Int J Cancer. 1993;53:613–620. doi: 10.1002/ijc.2910530415. [DOI] [PubMed] [Google Scholar]
- Nishi Y, Yanase T, Mu YM, Oba K, Ichino I, Saito M, Nomura M, Mukasa C, Okabe T, Goto K, Takayanagi R, Kashimura Y, et al. Establishment and characterization of a steroidogenic human granulosa-like tumor cell line, KGN, that expresses functional follicle-stimulating hormone receptor. Endocrinology. 2001;142:437–445. doi: 10.1210/endo.142.1.7862. [DOI] [PubMed] [Google Scholar]
- Jamieson S, Butzow R, Andersson N, Alexiadis M, Unkila-Kallio L, Heikinheimo M, Fuller PJ, Anttonen M. The FOXL2 C134W mutation is characteristic of adult granulosa cell tumors of the ovary. Modern Pathol. 2010;23:1477–1485. doi: 10.1038/modpathol.2010.145. [DOI] [PubMed] [Google Scholar]
- Kalfa N, Philibert P, Patte C, Ecochard A, Duvillard P, Baldet P, Jaubert F, Fellous M, Sultan C. Extinction of FOXL2 expression in aggressive ovarian granulosa cell tumors in children. Fertil Steril. 2007;87:896–901. doi: 10.1016/j.fertnstert.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Bilandzic M, Chu S, Wang Y, Tan HL, Fuller PJ, Findlay JK, Stenvers KL. Betaglycan alters NF kappa B-TGF beta 2 cross talk to reduce survival of human granulosa tumor cells. Mol Endocrinol. 2013;27:466–479. doi: 10.1210/me.2012-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilandzic M, Chu SM, Farnworth PG, Harrison C, Nicholls P, Wang Y, Escalona RM, Fuller PJ, Findlay JK, Stenvers KL. Loss of betaglycan contributes to the malignant properties of human granulosa tumor cells. Mol Endocrinol. 2009;23:539–548. doi: 10.1210/me.2008-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilandzic M, Wang Y, Ahmed N, Luwor RB, Zhu HJ, Findlay JK, Stenvers KL. Betaglycan blocks metastatic behaviors in human granulosa cell tumors by suppressing NF kappa B-mediated induction of MMP2. Cancer Lett. 2014;354:107–114. doi: 10.1016/j.canlet.2014.07.039. [DOI] [PubMed] [Google Scholar]
- Liu J, Kuulasmaa T, Kosma VM, Butzow R, Vanttinen T, Hyden-Granskog C, Voutilainen R. Expression of betaglycan, an inhibin coreceptor, in normal human ovaries and ovarian sex cord-stromal tumors and its regulation in cultured human granulosa-luteal cells. J Clin Endocrinol Metab. 2003;88:5002–5008. doi: 10.1210/jc.2003-030704. [DOI] [PubMed] [Google Scholar]
- Tripurani SK, Cook RW, Eldin KW, Pangas SA. BMP-specific SMADs function as novel repressors of PDGFA and modulate its expression in ovarian granulosa cells and tumors. Oncogene. 2013;32:3877–3885. doi: 10.1038/onc.2012.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi T, Otsuka F, Suzuki J, Takeda M, Inagaki K, Kano Y, Otani H, Mimura Y, Ogura T, Makino H. Mutual regulation of follicle-stimulating hormone signaling and bone morphogenetic protein system in human granulosa cells. Biol Reprod. 2006;74:1073–1082. doi: 10.1095/biolreprod.105.047969. [DOI] [PubMed] [Google Scholar]
- Moore RK, Otsuka F, Shimasaki S. Molecular basis of bone morphogenetic protein-15 signaling in granulosa cells. J Biol Chem. 2003;278:304–310. doi: 10.1074/jbc.M207362200. [DOI] [PubMed] [Google Scholar]
- Chang HM, Cheng JC, Fang L, Qiu X, Klausen C, Taylor EL, Leung PC. Recombinant BMP4 and BMP7 downregulate pentraxin 3 in human granulosa cells. J Clin Endocrinol Metab. 2015;100:E365–E374. doi: 10.1210/jc.2014-2496. [DOI] [PubMed] [Google Scholar]
- Benckert C, Jonas S, Cramer T, Von Marschall Z, Schafer G, Peters M, Wagner K, Radke C, Wiedenmann B, Neuhaus P, Hocker M, Rosewicz S. Transforming growth factor beta 1 stimulates vascular endothelial growth factor gene transcription in human cholangiocellular carcinoma cells. Cancer Res. 2003;63:1083–1092. [PubMed] [Google Scholar]
- Geng L, Chaudhuri A, Talmon G, Wisecarver JL, Wang J. TGF-beta suppresses VEGFA-mediated angiogenesis in colon cancer metastasis. PLoS One. 2013;8:e59918. doi: 10.1371/journal.pone.0059918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardali E, ten Dijke P. Transforming growth factor-beta signaling and tumor angiogenesis. Front Biosci (Landmark Ed) 2009;14:4848–4861. doi: 10.2741/3573. [DOI] [PubMed] [Google Scholar]
- Uhlenhaut NH, Jakob S, Anlag K, Eisenberger T, Sekido R, Kress J, Treier AC, Klugmann C, Klasen C, Holter NI, Riethmacher D, Schutz G, et al. Somatic sex reprogramming of adult ovaries to testes by FOXL2 ablation. Cell. 2009;139:1130–1142. doi: 10.1016/j.cell.2009.11.021. [DOI] [PubMed] [Google Scholar]
- Shah SP, Kobel M, Senz J, Morin RD, Clarke BA, Wiegand KC, Leung G, Zayed A, Mehl E, Kalloger SE, Sun M, Giuliany R, et al. Mutation of FOXL2 in granulosa-cell tumors of the ovary. N Engl J Med. 2009;360:2719–2729. doi: 10.1056/NEJMoa0902542. [DOI] [PubMed] [Google Scholar]
- Lima JF, Jin L, de Araujo AR, Erikson-Johnson MR, Oliveira AM, Sebo TJ, Keeney GL, Medeiros F. FOXL2 mutations in granulosa cell tumors occurring in males. Arch Pathol Lab Med. 2012;136:825–828. doi: 10.5858/arpa.2011-0355-OA. [DOI] [PubMed] [Google Scholar]
- Rosario R, Cohen PA, Shelling AN. The role of FOXL2 in the pathogenesis of adult ovarian granulosa cell tumours. Gynecol Oncol. 2014;133:382–387. doi: 10.1016/j.ygyno.2013.12.012. [DOI] [PubMed] [Google Scholar]
- Leung DT, Fuller PJ, Chu S. Impact of FOXL2 mutations on signaling in ovarian granulosa cell tumors. Int J Biochem Cell Biol. 2016;72:51–54. doi: 10.1016/j.biocel.2016.01.003. [DOI] [PubMed] [Google Scholar]
- L'Hote D, Georges A, Todeschini AL, Kim JH, Benayoun BA, Bae J, Veitia RA. Discovery of novel protein partners of the transcription factor FOXL2 provides insights into its physiopathological roles. Hum Mol Genet. 2012;21:3264–3274. doi: 10.1093/hmg/dds170. [DOI] [PubMed] [Google Scholar]
- Anttonen M, Pihlajoki M, Andersson N, Georges A, L'Hote D, Vattulainen S, Farkkila A, Unkila-Kallio L, Veitia RA, Heikinheimo M. FOXL2, GATA4, and SMAD3 co-operatively modulate gene expression, cell viability and apoptosis in ovarian granulosa cell tumor cells. PLoS One. 2014;9:e85545. doi: 10.1371/journal.pone.0085545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonis D, McTavish KJ, Shimasaki S. Essential but differential role of FOXL2(wt) and FOXL2(C134W) in GDF-9 stimulation of follistatin transcription in co-operation with Smad3 in the human granulosa cell line COV434. Mol Cell Endocrinol. 2013;372:42–48. doi: 10.1016/j.mce.2013.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng JC, Chang HM, Qiu X, Fang L, Leung PC. FOXL2-induced follistatin attenuates activin A-stimulated cell proliferation in human granulosa cell tumors. Biochem Biophys Res Commun. 2014;443:537–542. doi: 10.1016/j.bbrc.2013.12.010. [DOI] [PubMed] [Google Scholar]
- Rosario R, Araki H, Print CG, Shelling AN. The transcriptional targets of mutant FOXL2 in granulosa cell tumours. PLoS One. 2012;7:e46270. doi: 10.1371/journal.pone.0046270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges A, L'Hote D, Todeschini AL, Auguste A, Legois B, Zider A, Veitia RA. The transcription factor FOXL2 mobilizes estrogen signaling to maintain the identity of ovarian granulosa cells Elife 2014. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiemer SE, Szymaniak AD, Varelas X. The transcriptional regulators TAZ and YAP direct transforming growth factor beta-induced tumorigenic phenotypes in breast cancer cells. J Biol Chem. 2014;289:13461–13474. doi: 10.1074/jbc.M113.529115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu D, Lv XM, Hua GH, He CB, Dong JX, Lele SM, Li DWC, Zhai QL, Davis JS, Wang C. YAP regulates cell proliferation, migration, and steroidogenesis in adult granulosa cell tumors. Endocr Relat Cancer. 2014;21:297–310. doi: 10.1530/ERC-13-0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua F, Mu R, Liu JW, Xue JF, Wang ZY, Lin H, Yang HZ, Chen XG, Hu ZW. TRB3 interacts with SMAD3 promoting tumor cell migration and invasion. J Cell Sci. 2011;124:3235–3246. doi: 10.1242/jcs.082875. [DOI] [PubMed] [Google Scholar]
- Wang C, Lv XM, Jiang C, Cordes CM, Fu L, Lele SM, Davis JS. Transforming growth factor alpha (TGF alpha) regulates granulosa cell tumor (GCT) cell proliferation and migration through activation of multiple pathways. PLoS One. 2012;7:e48299. doi: 10.1371/journal.pone.0048299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attisano L, Labbe E. TGFbeta and Wnt pathway cross-talk. Cancer Metastasis Rev. 2004;23:53–61. doi: 10.1023/a:1025811012690. [DOI] [PubMed] [Google Scholar]
- Fu Y, Chang A, Chang L, Niessen K, Eapen S, Setiadi A, Karsan A. Differential regulation of transforming growth factor beta signaling pathways by Notch in human endothelial cells. J Biol Chem. 2009;284:19452–19462. doi: 10.1074/jbc.M109.011833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt D, Ovitt CE, Anlag K, Fehsenfeld S, Gredsted L, Treier AC, Treier M. The murine winged-helix transcription factor Foxl2 is required for granulosa cell differentiation and ovary maintenance. Development. 2004;131:933–942. doi: 10.1242/dev.00969. [DOI] [PubMed] [Google Scholar]
- Uda M, Ottolenghi C, Crisponi L, Garcia JE, Deiana M, Kimber W, Forabosco A, Cao A, Schlessinger D, Pilia G. Foxl2 disruption causes mouse ovarian failure by pervasive blockage of follicle development. Hum Mol Genet. 2004;13:1171–1181. doi: 10.1093/hmg/ddh124. [DOI] [PubMed] [Google Scholar]
- Ottolenghi C, Omari S, Garcia-Ortiz JE, Uda M, Crisponi L, Forabosco A, Pilia G, Schlessinger D. Foxl2 is required for commitment to ovary differentiation. Hum Mol Genet. 2005;14:2053–2062. doi: 10.1093/hmg/ddi210. [DOI] [PubMed] [Google Scholar]
- Li Q. Transforming growth factor beta signaling in uterine development and function. J Anim Sci Biotechnol. 2014;5:52. doi: 10.1186/2049-1891-5-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olieslagers S, Pardali E, Tchaikovski V, ten Dijke P, Waltenberger J. TGF-beta1/ALK5-induced monocyte migration involves PI3K and p38 pathways and is not negatively affected by diabetes mellitus. Cardiovasc Res. 2011;91:510–518. doi: 10.1093/cvr/cvr100. [DOI] [PubMed] [Google Scholar]
- Goumans MJ, Valdimarsdottir G, Itoh S, Rosendahl A, Sideras P, ten Dijke P. Balancing the activation state of the endothelium via two distinct TGF-beta type I receptors. EMBO J. 2002;21:1743–1753. doi: 10.1093/emboj/21.7.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kraan PM. Blaney Davidson EN, Blom A, van den Berg WB. TGF-beta signaling in chondrocyte terminal differentiation and osteoarthritis: modulation and integration of signaling pathways through receptor-Smads. Osteoarthritis Cartilage. 2009;17:1539–1545. doi: 10.1016/j.joca.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Steinmetz R, Wagoner HA, Zeng P, Hammond JR, Hannon TS, Meyers JL, Pescovitz OH. Mechanisms regulating the constitutive activation of the extracellular signal-regulated kinase (ERK) signaling pathway in ovarian cancer and the effect of ribonucleic acid interference for ERK1/2 on cancer cell proliferation. Mol Endocrinol. 2004;18:2570–2582. doi: 10.1210/me.2004-0082. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]