Abstract
Intrauterine growth restriction (IUGR) is an important risk factor for perinatal complications and adult disease. IUGR is associated with down-regulation of placental amino acid transporter expression and activity at birth. It is unknown whether these changes are a cause or a consequence of human IUGR. We hypothesized that placental amino acid transport capacity is reduced prior to onset of reduced fetal growth in baboons with maternal nutrient restriction (MNR). Pregnant baboons were fed either a control (n = 8) or MNR diet (70% of control diet, n = 9) from Gestational Day 30. At Gestational Day 120 (0.65 of gestation), fetuses and placentas were collected. Microvillous (MVM) and basal (BM) plasma membrane vesicles were isolated. System A and system L transport activity was determined in MVM, and leucine transporter activity was assessed in BM using radiolabeled substrates. MVM amino acid transporter isoform expression (SNAT1, SNAT2, and SNAT4 and LAT1 and LAT2) was measured using Western blots. LAT1 and LAT2 expression were also determined in BM. Maternal and fetal plasma amino acids concentrations were determined using mass spectrometry. Fetal and placental weights were unaffected by MNR. MVM system A activity was decreased by 37% in MNR baboon placentas (P = 0.03); however MVM system A amino acid transporter protein expression was unchanged. MVM system L activity and BM leucine transporter activity were not altered by MNR. Fetal plasma concentrations of essential amino acids isoleucine and leucine were reduced, while citrulline increased (P < 0.05) in MNR fetuses compared to controls. In this primate model of IUGR, placental MVM system A amino acid transporter activity is decreased prior to the onset of reduction in the fetal growth trajectory. The reduction in plasma leucine and isoleucine in MNR fetuses may be caused by reduced activity of MVM system A, which is strongly coupled with system L essential amino acid uptake. Our findings indicate that reduced placental amino acid transport may be a cause rather than a consequence of IUGR due to inadequate maternal nutrition.
Keywords: intrauterine growth restriction (IUGR), nutrition, placenta, placental transport, pregnancy
INTRODUCTION
Maternal undernutrition is the most common cause of intrauterine growth restriction (IUGR) in developing countries. In the United States, more than 50 million households have been reported to experience food insecurity or hunger at least sometime during the year [1], indicating that maternal undernutrition remains a significant problem also in developed countries. IUGR is in turn associated with increased perinatal mortality and morbidity [2] as well as increased risk of offspring developing cardiovascular and metabolic disease in childhood and later life [3]. The mechanisms linking maternal undernutrition, IUGR, and developmental programming are poorly understood. IUGR in humans and animal models is associated with specific alterations in the activity and expression of placental nutrient transporters, which have been suggested to contribute to the development of IUGR [4–7]. Because of the lack of noninvasive approaches to assess placental function in women, studies of placental transport prior to term rely on collecting the placenta after delivery and therefore often suffer from being influenced by the effect of preterm birth. The time course of changes in nutrient transport capacity relative to changes in fetal growth during pregnancy therefore remains largely unknown.
The syncytiotrophoblast is the transporting epithelium of the human placenta and is believed to be the primary barrier for transfer of most nutrients from the maternal to the fetal circulation. Specifically, transport across the polarized plasma membranes of the syncytiotrophoblast, the microvillous (MVM) and basal (BM) membranes constitutes the rate-limiting step in transplacental amino acid transfer [8]. The MVM and BM have distinct transport properties, including large differences in surface area (MVM surface area is six times larger than those of the BM) [9] and unequal distribution of placental amino acid transporters [10]. Transplacental amino acid transport is active (energy requiring), resulting in higher amino acid concentrations in the fetal than in maternal blood. The active step in maternal-to-fetal transfer of amino acids is localized in the MVM, in most cases energized by the inwardly directed Na+ gradient, resulting in high intracellular amino acid concentrations in the syncytiotrophoblast. In contrast, transport across the BM occurs via facilitated diffusion down a diffusion gradient mediated by specific transporter systems [8]. Because MVM amino acid uptake is not only dependent on the expression and activity of specific amino acid transporter isoforms, which is subjected to complex regulation, but also modulated by the energy status of the cell, the transport of amino acids across MVM is believed to be the primary rate-limiting step for transplacental amino acid transfer [8].
One of the key amino acid transporters is system A, a Na+-dependent transport system, which mediates the uptake of neutral amino acids. The system A amino acid transporter is encoded by three members of the slc38 family, and all three isoforms (Na+-coupled neutral amino acid transporters SNAT1, 2, and 4) are expressed in the human placenta [11, 12]. The maximal transport capacity of system A is much higher in the MVM than the BM [13]. System A establishes a high intracellular concentration of nonessential amino acids such as glycine, glutamine, and serine, creating a gradient that drives the exchange for essential amino acids via system L [14]. System A activity is therefore necessary for the uptake of both essential and nonessential amino acids. System L, an amino acid exchanger, mediates the uptake of essential amino acids in a Na+-independent manner. System L is a heterodimer made up of a light chain, (large amino acid transporter LAT1 or 2), and a heavy chain (4F2hc/CD98) [14]. In the BM, system L [4, 15, 16] and efflux transporters such as LAT3 [17] are responsible for the transfer of essential amino acids to the fetus.
MVM system A activity is decreased in human IUGR [18] and may be related to the degree of severity of growth restriction [19]. Leucine transport is reduced in both MVM and BM isolated from human term placentas of IUGR pregnancies [4]. Reduced fetal plasma concentrations of several amino acids are also observed in IUGR at term [20]. However, it is not yet established if these changes in amino acid transporter activity occur as a primary event, directly contributing to the pathophysiology of IUGR, or as a secondary consequence of reduced fetal growth and decreased demand. Determining whether changes in placental amino acid transporter expression and activity precede the development of IUGR would provide interesting possible targets for manipulation of these transporters as treatment in order to prevent IUGR. To this end, we have demonstrated that down-regulation of system A activity and expression precedes the development of IUGR in a rat model following a low protein diet [6]. However, rodent placentation is distinct from the human. Studies using nonhuman primate models are more informative and are directly comparable to the human in terms of maternal physiological changes of pregnancy and placentation. Using a baboon model of maternal nutrient restriction (MNR), we have recently shown that protein expression of amino acid transporters SNAT2, LAT1 and 2, and taurine transporter TAUT is reduced in MVM of placentas from MNR baboons near term at Gestational Day (GD) 165, which equates to 0.9 of gestation, compared with controls [7]. These changes are concurrent with reductions in system A and system L transporter activity in MVM in placentas of MNR baboons [21]. At GD 165, IUGR has developed in response to MNR. In the study reported here, we used in vitro approaches to test the hypothesis that placental amino acid transport capacity is reduced prior to the onset of reduced fetal growth in baboons with MNR.
MATERIALS AND METHODS
Animals
All animal procedures were approved by the Texas Biomedical Research Institute Institutional Animal Care and Use Committee and conducted in facilities approved by the Association for the Assessment and Accreditation of Laboratory Animal Care. Baboons (Papio species) were maintained in groups of 10–16 females with one male in outdoor metal and concrete cages at the Southwest National Primate Research Center [22].
System for Controlling and Recording Individual Feeding
Animals were weighed and fed using a feeding system that has been described in detail [22]. Baboons were fed Purina Monkey Diet 5038 (Purina). The basic composition of each biscuit was ≥15% crude protein, ≥5% crude fat, ≤6% crude fiber, 5% ash, ≤3% added minerals, solubilized vitamin C, and other required vitamins. All animals were initially fed 60 biscuits ad libitum in the feeding tray of each individual cage. Baboons were returned to the group cage at the end of the 2-h feeding period, and biscuits that remained in the tray, floor of the cage, and pan beneath the cage were counted. Food consumption, weight, and health status of each animal were recorded daily.
Study Design
Female baboons (8-15 yr old) were selected on the basis of reproductive age, body weight, and absence of extragenital pathological signs, and mated as described previously [22]. Pregnancy was dated according to time of ovulation and changes of sex skin color and confirmed by ultrasonography at GD 30. Subsequently, animals in the MNR group were fed 70% of the total intake of corresponding controls across gestation, calculated per kilogram bodyweight.
Collection of Blood and Tissue Samples
Cesarean sections were performed at GD 120 (0.65 of gestation, term 180–184 days) using general anesthesia and standard sterile techniques as previously described in detail [7, 23]. Animals were tranquilized with ketamine hydrochloride (10 mg/kg), intubated, and anesthetized with isoflurane (starting rate 2% with oxygen, 2 L/min). Heparinized blood samples were taken from the maternal uterine vein and fetal umbilical vein at cesarean section.
Isolation of Trophoblast Plasma MVM and BM Vesicles
Placentas (n = 8 control, n = 9 MNR) were collected, and pieces (1 cm3) of chorionic villous tissue were immediately dissected, washed in saline, and placed in 250 mM sucrose, 10 mM HEPES-Tris, and 1mM ethylenediaminetetraacetic acid, pH 7.4, at 4°C with protease and phosphatase inhibitors. Syncytiotrophoblast plasma MVM and BM were prepared simultaneously from each placenta, according to a well-established protocol with some modifications [24, 25]. Briefly, following initial homogenization and centrifugation steps, BM were separated from MVM by Mg2+ precipitation and purified further on a sucrose gradient. Samples were snap frozen in liquid nitrogen and stored at −80°C. MVM enrichment was assessed using the MVM/homogenate ratio of alkaline phosphatase activity measured by standard assays. MVM vesicle enrichment of alkaline phosphatase activity was not significantly different between the control and MNR groups (control, 3.7 ± 0.5, n = 8 vs. MNR, 5.2 ± 0.6, n = 9; P = 0.12). The protein expression of ferroportin, an iron transporter exclusively localized to the BM [26] was determined using Western blot, and the BM/homogenate-ratio of ferroportin expression was used as a measurement of BM enrichment. BM vesicles were significantly enriched for ferroportin compared to those of MVM (BM, 22 ± 4 vs. MVM, 1.7 ± 0.4; Supplemental Fig. S1, available online at www.biolreprod.org). BM enrichment of ferroportin was not significantly different between control and MNR groups (control, 27 ± 7.5, n = 8 vs. MNR, 17 ± 4.7, n = 9; P = 0.3). Protein content of MVM and BM was assessed using the method of Bradford [27].
Western Blot Analysis
Protein expression of the system A amino acid transporter isoforms SNAT1, 2, and 4, glucose transporter (GLUT1), and taurine transporter (TAUT) was analyzed in MVM as previously described [7, 23]. Protein expression of system L amino acid transporter isoforms LAT1 and LAT2 were analyzed in both MVM and BM. The SNAT1 antibody was received as a generous gift from Dr. Jean Jiang (University of Texas Health Science Center, San Antonio, TX). A polyclonal SNAT2 antibody generated in rabbits [28] was generously provided by Dr. Puttur Prasad (University of Georgia, Athens, GA). Affinity-purified polyclonal anti-SNAT4 antibodies were produced in rabbits using the epitope YGEVEDELLHAYSKV in human SNAT4 (Eurogentec). Antibodies targeting LAT1 and LAT2 were produced in rabbits as described previously [29]. GLUT1 and TAUT antibodies were purchased from Millipore.
Western blot analysis was performed as described previously [7]. Briefly, 20 μg of protein was loaded onto a Bio-Rad Any kD TGX precast polyacrylamide gel, and electrophoresis was performed at 200 V for 40 min. Proteins were transferred onto polyvinylidene fluoride membranes at a constant 35 V. After transfer, membranes were blocked in 5% milk in Tris-buffered saline (w/v) plus 0.1% Tween 80 (v/v) for 1 h at room temperature. Membranes were incubated with primary antibodies overnight at 4°C. Membranes were subsequently incubated with the appropriate peroxidase-labeled secondary antibodies for 1 h at room temperature. After washing, bands were visualized using enhanced chemiluminescence detection reagents (Pierce Biotechnology). Blots were stripped using ReBlot Plus Mild antibody stripping solution (Millipore) and reprobed for β-actin as a loading control. Analysis of the blots was performed by densitometry using Image J software. For each protein target, the mean density of the control sample band was arbitrarily assigned a value of 1. Subsequently, all individual MNR density values were expressed relative to this mean.
Activity of Amino Acid Transporters in MVM and BM
System A transporter activity was assessed in MVM by measuring the uptake of the amino acid analog methylaminoisobutyric acid (MeAIB) using a modification of the protocol of Mahendran et al. [18]. System L activity (MVM) and leucine uptake (BM) was studied by determining the transport of L-leucine as described previously [4, 13] with minor modifications. The rationale to determine system A activity only in MVM and not BM, is that BM system A activity (per mg protein) is only approximately 4% of that in MVM. The quantitative contribution of BM system A to the flux of neutral amino acids across the placental barrier is therefore minimal, in particular when considering the much smaller surface area of the BM [13]. Using 2-aminobicyclo-[2,2,1]-heptane-2-carboxylic acid (BCH), a specific system L inhibitor, we have previously reported that leucine uptake in human MVM is mediated almost exclusively by system L [4], providing the justification for using unlabeled leucine to determine system L activity in MVM.
In the BM, leucine transport is mediated by efflux transporters such as LAT3 [17], in addition to system L. This provides the rationale to designate BM leucine transport inhibitable by unlabeled leucine as mediated leucine transport rather than system L transport.
Vesicles were preloaded and incubated overnight in 300 mmol/L mannitol and 10 mmol/L HEPES-Tris, pH 7.4, at 4°C. Vesicles were subsequently pelleted and resuspended in the same buffer at a protein concentration of approximately 6 mg/ml. Vesicles were kept on ice until immediately prior to transport measurements, when samples were warmed to 37°C using a water bath. Vesicles were mixed rapidly with 30 μl of the appropriate incubation buffer (1:2) including 14C- MeAIB (150 μmol/L) or 3H-L-leucine (0.375 μmol/L). To determine the time course of MeAIB and leucine uptake by MVM (n = 3), uptake of radiolabel was terminated at 8, 12, 20, and 30 sec using ice-cold PBS (pH 7.4) followed by rapid filtration. The uptake of MeAIB was rapid and time dependent, not reaching equilibrium by 20 sec (data not shown). Uptakes at 15 sec were chosen to approximate initial rate in final experiments (n = 8 control, n = 9 MNR). To determine the time course of leucine uptake in BM (n = 3), uptake of radiolabel was terminated at 8, 12, 20, and 30 sec using ice-cold PBS (pH 7.4) followed by rapid filtration. The uptake of leucine in BM was rapid and time dependent, not reaching equilibrium by 10 sec (data not shown). Uptake at 8 sec was chosen to approximate initial rate in final experiments (n = 8 control, n = 9 MNR).
After defined incubation times, vesicles were separated from the substrate medium using rapid filtration over mixed ester filters (0.45 μm pore size; Millipore Corporation) and washed using three times with 2 ml PBS. In studies of MeAIB transport, 150 mmol/L NaCl and 150 mmol/L KCl were used in incubation buffers to measure total and Na+-independent uptake, respectively. In leucine transport experiments, nonmediated flux was studied in the presence of 20 mmol/L unlabeled L-leucine. Each condition was measured in triplicate for each placenta in all uptake experiments.
Filters were dissolved in 2 ml scintillation cocktail and counted. Blanks were subtracted from counts, and uptakes are expressed as picomoles per milligram protein and time. System A activity corresponding to the Na+-dependent uptake of MeAIB was calculated by subtracting Na+-independent uptake from total uptakes. Mediated uptake of leucine was calculated by subtracting nonmediated transport from total uptake. Protein content was measured using the Bradford assay [27].
Amino Acid Analysis
A 20 μl plasma aliquot was combined with stable isotope-labeled internal standards and sodium tetraborate solution prior to derivatization with 5-(dimethylamino) naphthalene-1-sulfonyl chloride. After evaporation to dryness by vacuum centrifugation, the derivatized amino acids were dissolved in mobile phase A (see below) and transferred to autosampler vials for high-performance liquid chromatography (HPLC)-electrospray ionization-mass spectrometry analysis on a Thermo Fisher Q Exactive mass spectrometer used in conjunction with a Thermo Fisher/Dionex Ultimate 3000 HPLC. Analytical conditions were: HPLC column, Kinetex C18 (2.1 × 100 mm, 2.6 μm, 100 Å; Phenomenex); mobile phase A, acetonitrile:water:formic acid (5:95:0.5); mobile phase B, acetonitrile:water:formic acid (95:5:0.5); flow rate, 400 μl/min; gradient, 10% B to 90% B over 5min then held at 90% B for 3 min. Mass spectrometer parameters were: electrospray ionization; scan range, m/z 300–1000; resolution, 70 000 (m/z 300); positive ion detection. Extracted ion chromatograms were generated for the protonated molecule for each derivatized amino acid using a mass window of ± 5 parts per million. Peak areas were determined by processing through QuanBrowser (Thermo Fisher) and compared to calibration curves that were generated by analysis of authentic standards. Linear least squares analysis of the standard 101 curves yielded R2 values of ≥0.99.
Statistical Analysis
The data were found to be normally distributed using the Kolmogorov-Smirnov test; hence a parametric test was applied, and data are represented as mean ± SEM. Amino acid data were transformed to natural logs prior to testing. The significance of the difference between the two groups was calculated using the unpaired Student t-test. Spearman correlation coefficients were calculated for each amino acid to analyze the correlation between maternal and fetal amino acid concentrations in the control and MNR groups. A P-value of less than 0.05 was considered significant.
RESULTS
Fetal and Placental Weights
Fetal and placental weights were not different between the control and MNR groups at GD 120 (n = 8 control, n = 9 MNR, P = 0.73; Table 1). Fetal and placental weights were positively correlated in the MNR group but not in the controls (control, n = 8, r = 0.28, P = 0.5 vs. MNR, n = 9, r = 0.75, P = 0.02; Fig. 1).
TABLE 1.
Fetal and placental weights in control and MNR baboons at GD 120.a
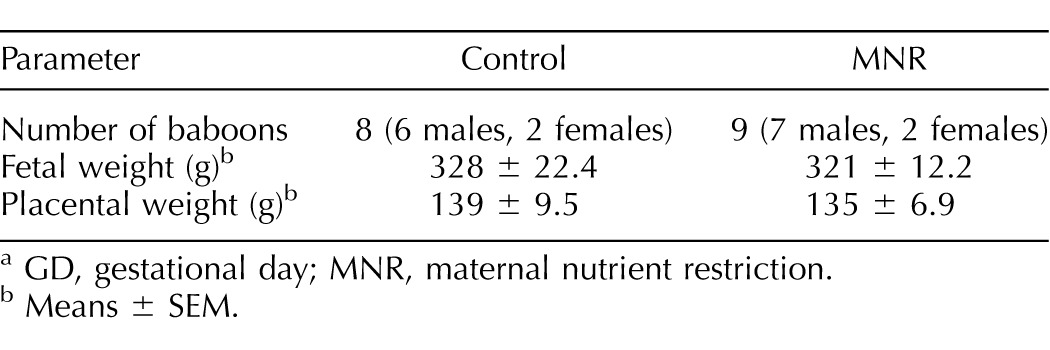
GD, gestational day; MNR, maternal nutrient restriction.
Means ± SEM.
FIG. 1.
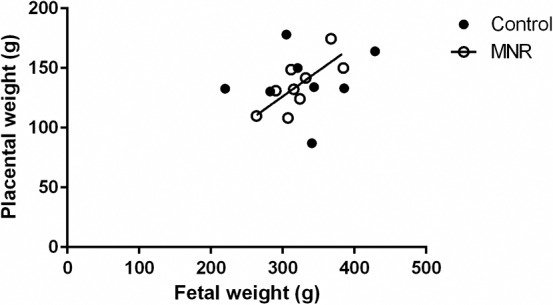
Relationship between placental and fetal weights at GD 120. Fetal weights were positively correlated to placental weights in the MNR group but not the control group. Individual Spearman rank correlation coefficients were calculated for each slope in the control and MNR baboons (control, n = 8, r = 0.28, P = 0.5; MNR, n = 9, r = 0.75, P = 0.02).
Maternal and fetal plasma amino acid concentrations.
Maternal and fetal plasma amino acid concentrations are presented in Table 2. Amino acid concentrations were similar in control and MNR mothers (Table 2). Fetal plasma concentrations of leucine and isoleucine (P < 0.05) were decreased in MNR while fetal citrulline was increased (P < 0.05; Table 3). The maternal and fetal concentrations of alanine were found to be significantly correlated in both the control (r = 0.976, P = 0.004) and MNR (r = 0.733, P = 0.03) groups, albeit without a significant difference in absolute concentrations of alanine between the control and MNR groups in the mother and fetus (P < 0.05). Maternal and fetal concentrations of arginine, citrulline, isoleucine, lysine, threonine, and valine were positively correlated with each other in the control group alone; while maternal and fetal concentrations of cysteine, glutamine, glycine, histidine, phenylalanine, and proline were positively correlated with each other only in the MNR group (Table 4).
TABLE 2.
Maternal plasma amino acid concentrations in control and MNR baboons at GD 120.a
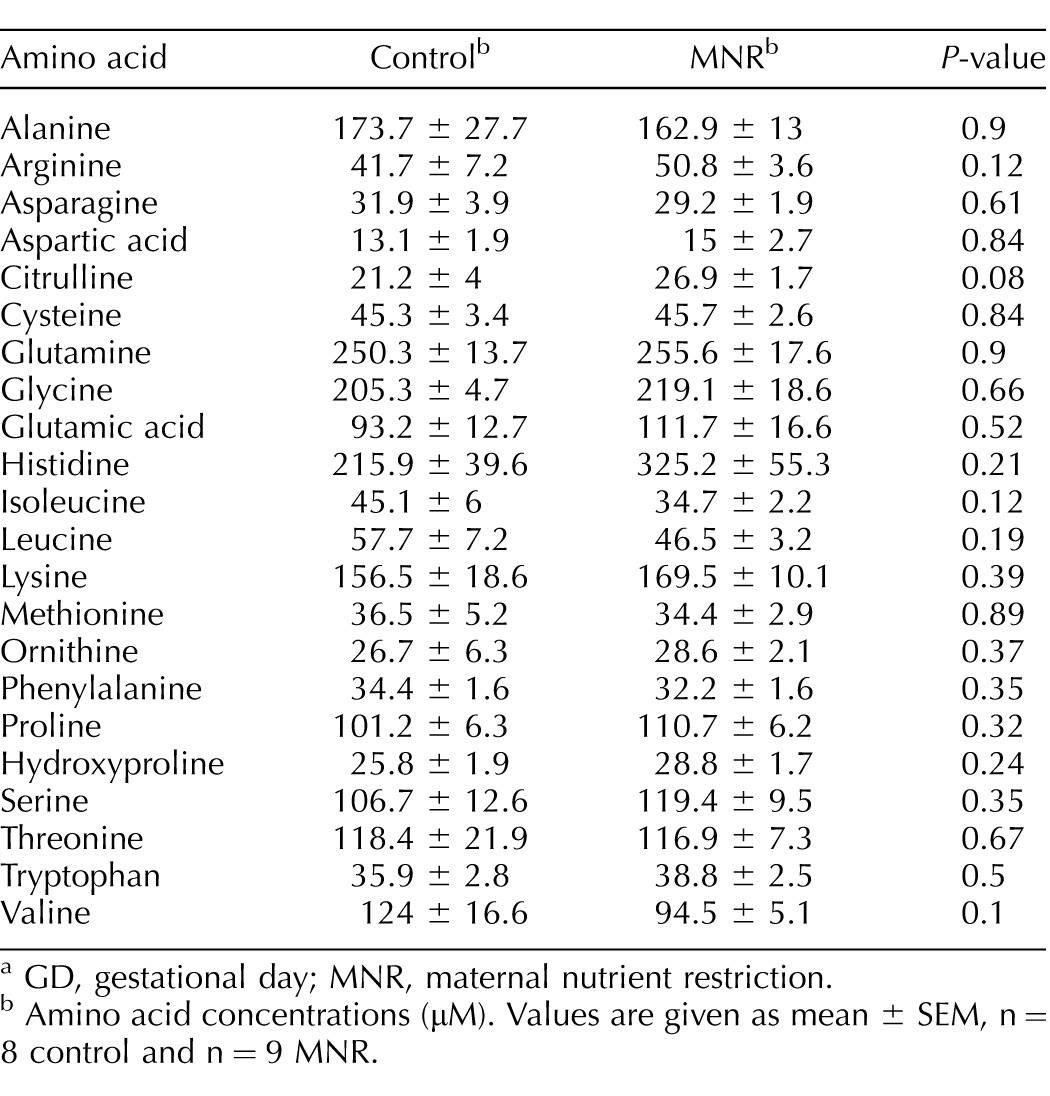
GD, gestational day; MNR, maternal nutrient restriction.
Amino acid concentrations (μM). Values are given as mean ± SEM, n = 8 control and n = 9 MNR.
TABLE 3.
Fetal plasma amino acid concentrations in control and MNR baboons at GD 120.a
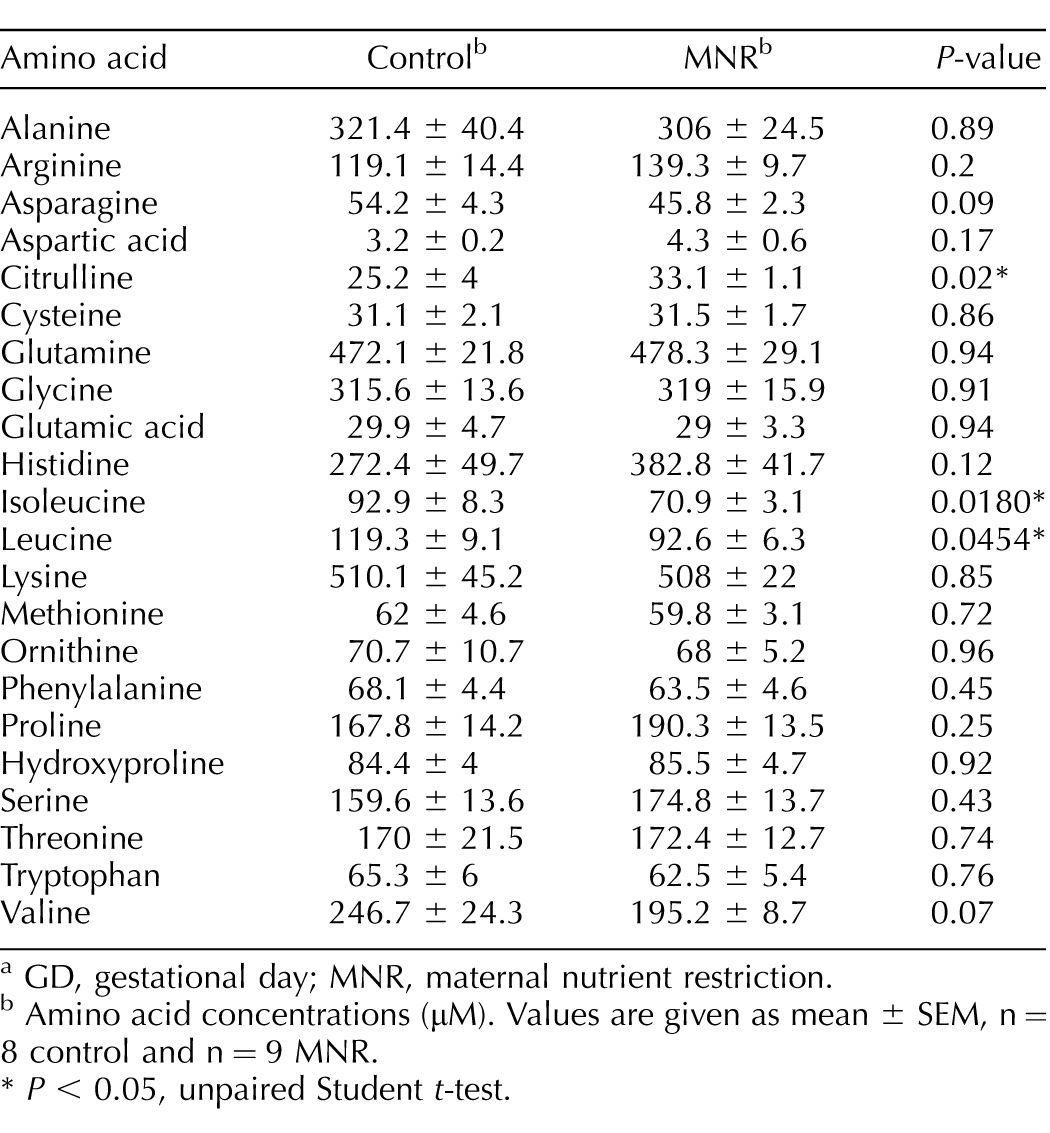
GD, gestational day; MNR, maternal nutrient restriction.
Amino acid concentrations (μM). Values are given as mean ± SEM, n = 8 control and n = 9 MNR.
P < 0.05, unpaired Student t-test.
TABLE 4.
Correlation between maternal and fetal amino acid concentrations in control and MNR baboons at GD 120.a
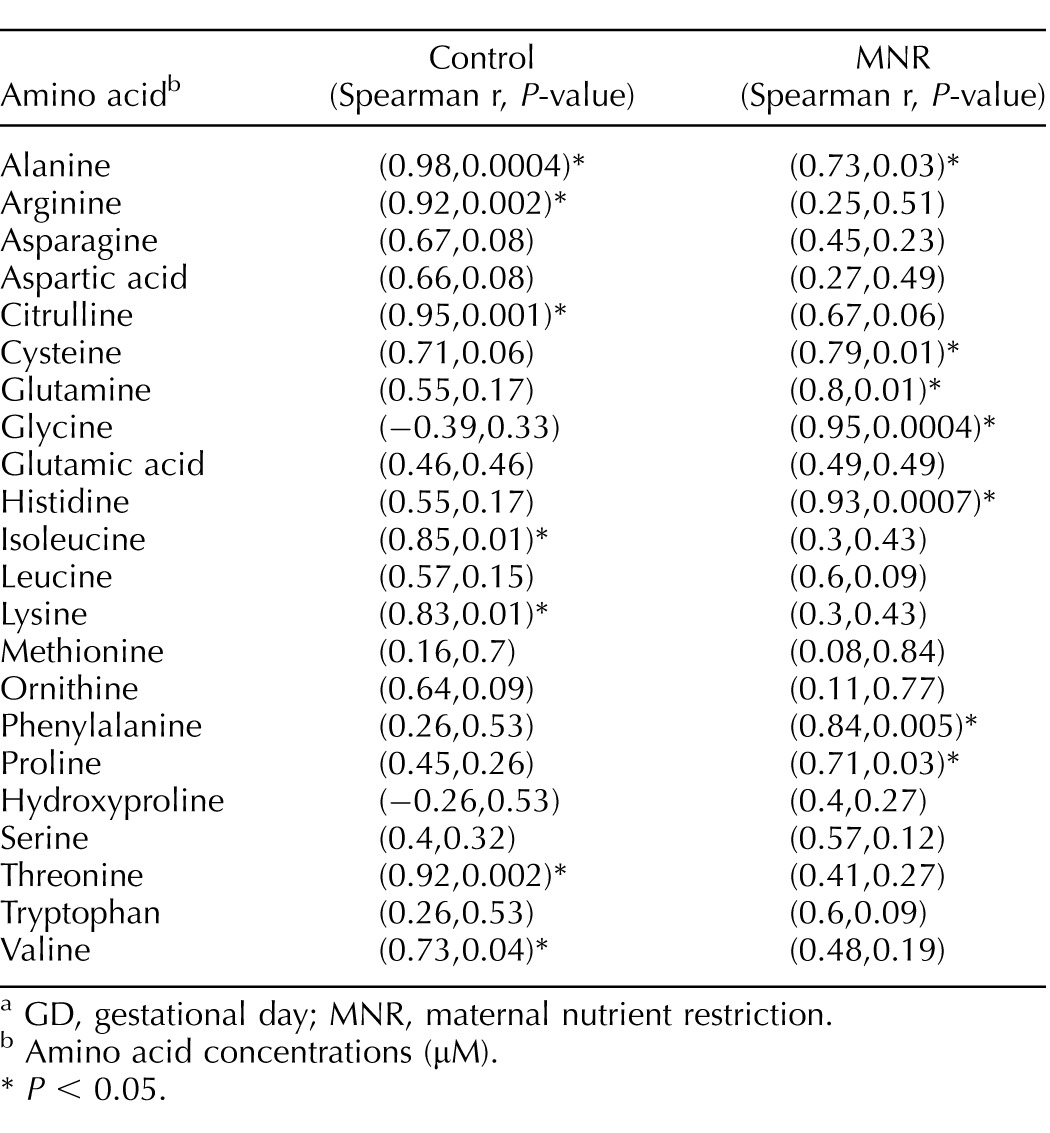
GD, gestational day; MNR, maternal nutrient restriction.
Amino acid concentrations (μM).
P < 0.05.
Glucose and amino acid transporter expression in MVM.
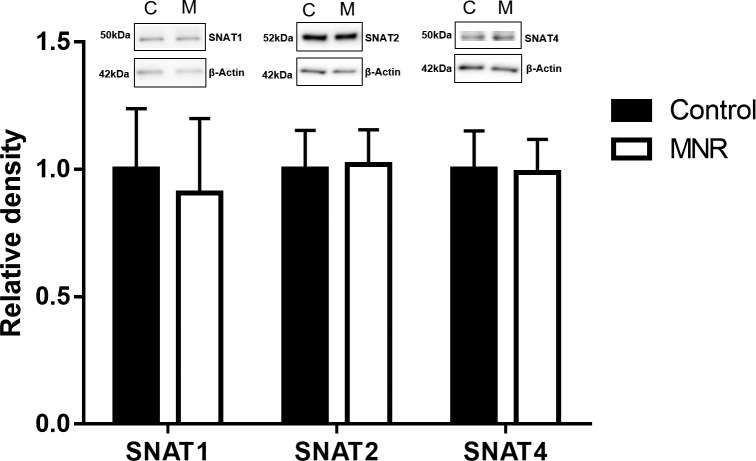
Protein expression of glucose transporter GLUT1 and amino acid transporters TAUT (Fig. 2), SNAT1, 2, and 4 (Fig. 3), and LAT1 and 2 (Fig. 4) were unchanged in MVM isolated from placentas of baboons fed a MNR diet compared to controls (n = 8 control, n = 9 MNR, P > 0.05).
FIG. 2.
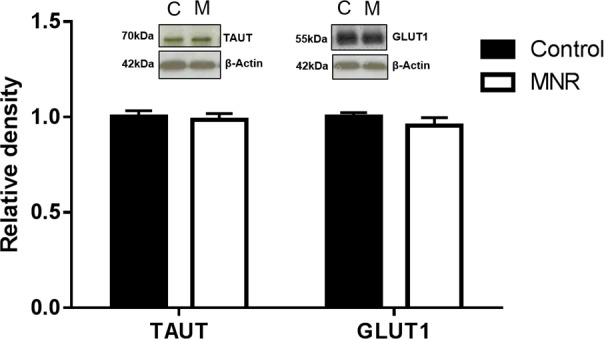
Protein expression of glucose transporter GLUT1 and amino acid transporter isoforms TAUT in MVM isolated from control (C) and MNR (M) baboons at GD 120. Histogram summarizing Western blot analysis data. Equal loading was performed. After normalization to β-actin, the mean density of C samples was assigned an arbitrary value of 1. Values given as mean ± SEM. *P < 0.05, unpaired Student t-test (n = 8 control; n = 9 MNR).
FIG. 3.
Protein expression of amino acid system A transporter isoforms SNAT1, SNAT2, and SNAT4 in MVM isolated from control (C) and MNR (M) baboons at GD 120. Histogram summarizing Western blot analysis data. Equal loading was performed. After normalization to β-actin, the mean density of C samples was assigned an arbitrary value of 1. Values given as mean ± SEM. *P < 0.05, unpaired Student t-test (n = 8 control; n = 9 MNR).
FIG. 4.
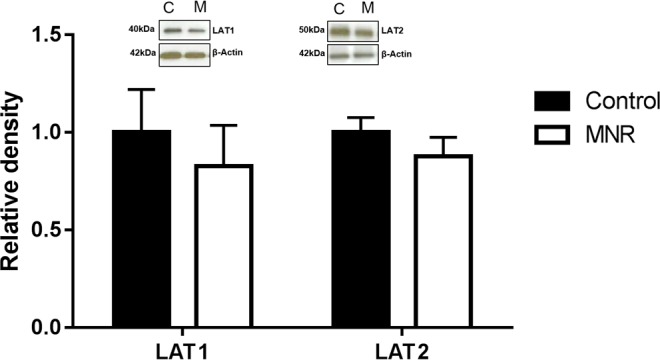
Protein expression of amino acid system L transporter isoforms LAT1 and LAT2 in MVM isolated from control (C) and MNR (M) baboons at GD 120. Histogram summarizing Western blot analysis data. Equal loading was performed. After normalization to β-actin, the mean density of C samples was assigned an arbitrary value of 1. Values given as mean ± SEM. *P < 0.05, unpaired Student t-test (n = 8 control, n = 9 MNR).
System A and System L Activity in MVM
At GD 120, MVM uptake of MeAIB was reduced by 37% in MNR compared to controls (control, n = 8, 26.7 ± 3.2 pmol/mg × 15 sec vs. MNR, n = 9, 16.7 ± 2.4 pmol/mg × 15 sec, *P = 0.035; Fig. 5A). Mediated MVM L-leucine uptake, indicative of system L activity, was unchanged in MNR compared to controls (control, n = 8, 0.18 ± 0.03 pmol/mg × 15 sec vs. MNR, n = 9, 0.12 ± 0.01 pmol/mg × 15 sec, P = 0.35; Fig. 5B) at GD 120.
FIG. 5.
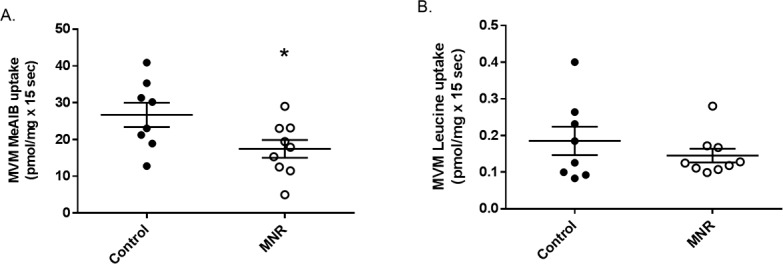
A) Decreased MVM system A activity in MNR. Mediated MeAIB uptake into MVM vesicles isolated from control and MNR placentas of baboons at GD 120. Control and MNR groups were compared using the t-test (n = 8 control, n = 9 MNR, *P < 0.05). B) No change in MVM system L activity in MNR. Mediated L-leucine uptake into MVM vesicles isolated from control and MNR placentas of baboons at GD 120. Control and MNR groups were compared using the t-test (n = 8 control, n = 9 MNR, *P < 0.05).
FIG. 6.
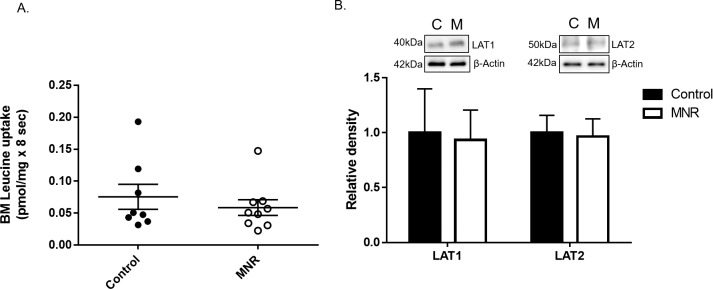
A) BM leucine transport in MNR. Mediated L-leucine uptake into BM vesicles isolated from control and MNR placentas of baboons at GD 120. Control and MNR groups were compared using the unpaired Student t-test (n = 8 control, n = 9 MNR, *P < 0.05, mean ± SEM). B) Protein expression of system L amino acid transporter isoforms LAT1 and LAT2 in BM isolated from control (C) and MNR (M) baboons at GD 120. Histogram summarizing Western blot analysis data. Equal loading was performed. After normalization to β-actin, the mean density of C samples was assigned an arbitrary value of 1. Values given as mean ± SEM. *P < 0.05, unpaired Student t-test (n = 8 control; n = 9 MNR).
Leucine Transport and System L Amino Acid Transporter Expression in BM
BM L-leucine uptake was unchanged in MNR compared to controls (control, n = 8, 0.07 ± 0.01 pmol/mg × 8 sec vs. MNR, n = 9, 0.05 ± 0.01 pmol/mg × 8 sec, P = 0.46; Fig. 6A) at GD 120. BM expression of system L transporters LAT1 and 2 (Fig. 6B) was unaffected by MNR (n = 8 control, n = 9 MNR, P > 0.05).
DISCUSSION
The human placenta is largely inaccessible prior to delivery, and there are currently no approaches to measure placental functions, such as nutrient transport, across gestation in women. As a result, there is no information on placental nutrient transport prior to the development of human IUGR. MNR in the baboon is a well-established model of IUGR with extensive similarities to human IUGR [7, 21, 22, 30–32]. In this report, we used this model to demonstrate for the first time that MNR from GD 30 to GD 120 in the baboon is associated with 1) reduced activity of placental amino acid transport system A in isolated MVM and 2) decreased fetal plasma amino acid concentrations of leucine and isoleucine, and increased concentrations of citrulline, prior to the development of IUGR. Our data support the concept that down-regulation of placental nutrient transport is a primary event, which directly contributes to IUGR. These findings have important implications for our understanding of the pathophysiology of restricted fetal growth and can inform efforts to develop effective intervention strategies in IUGR.
We have previously demonstrated that down-regulation of system A activity precedes the development of IUGR in rats fed a low-protein diet, suggesting that reduced amino acid transport may be a cause rather than a consequence of IUGR [6]. However, rodent and human placentas differ significantly with respect to structure and function. For example, trophoblast invasion is shallow in the rodent placenta and deep in the human [30]. Therefore, in terms of placentation, it is difficult to extrapolate findings in rodents to the human. In contrast, placentation in nonhuman primates, such as the baboon, is strikingly similar to the human, as they have a discoid, hemochorial placenta with a villous structure and trophoblast invasion of the maternal spiral arteries occurs up to the inner third of the myometrium [30]. Maternal undernutrition remains the most common cause of IUGR globally and given that more than 50 million households have been reported to experience food insecurity or hunger at least sometime during the year in the United States [1], our findings have broad applicability for human pregnancy.
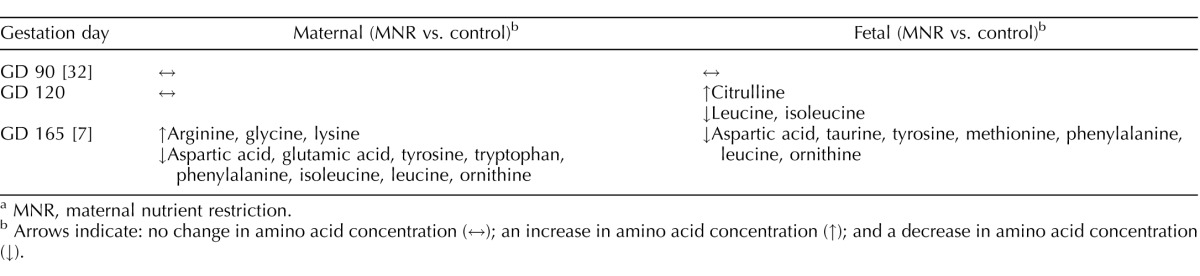
With alanine as the only exception, maternal and fetal plasma concentrations were not significantly correlated for any amino acid in both the control and the MNR groups. Importantly, of the two essential amino acids that were decreased in fetal circulation in MNR, leucine concentrations were not correlated between the maternal and fetal circulation in either the control or MNR group and isoleucine concentrations in maternal and fetal plasma were only significantly correlated in the control group. This may be a consequence of the active nature of placental amino acid transport and the complex metabolism of amino acids in the placenta and suggest that changes in fetal amino acid concentrations in MNR are unlikely to be caused by changes in maternal plasma concentration of amino acids alone. We have previously reported maternal and fetal amino acid plasma concentrations at midgestation (GD 90, 0.5 gestation) [32], and late third trimester (GD 165, 0.9 gestation) [7] in control and MNR animals. Thus, with the data presented in the current study (GD 120, 0.6 of gestation), corresponding to late second trimester, it is possible to compare significant changes in individual amino acids in maternal and fetal plasma across gestation in this baboon model of MNR (Table 5). At GD 90, there were no differences in maternal and fetal plasma amino acid concentrations between control and MNR animals [32]. At GD 120, prior to reduction in fetal and placental weight in baboons with MNR, we found significantly reduced fetal plasma amino acid concentrations of leucine and isoleucine, independent of changes in maternal amino acid concentrations. At GD 165, maternal plasma concentrations of eight amino acids, including leucine and isoleucine, are decreased in MNR [7]. Reduced fetal plasma concentrations of the essential amino acids leucine, phenylalanine, and methionine at GD 165 coincided with down-regulation of placental amino acid transport activity as measured in MVM in vitro, decreased transplacental transport of essential amino acids in vivo, and IUGR at this gestational age [7, 21]. This highlights the importance of decreased fetal availability of essential amino acids, in particular leucine, in the development of IUGR. In support of this idea, decreased fetomaternal enrichment ratios of leucine have been reported to be correlated with the severity of IUGR in the human at term [33, 34]. Furthermore, leucine supplementation has been shown to prevent fetal growth restriction in rats fed a low protein diet [35].
TABLE 5.
Comparison of significant changes in maternal and fetal plasma amino acid concentrations across gestation in MNR baboons.a
MNR, maternal nutrient restriction.
Arrows indicate: no change in amino acid concentration (↔); an increase in amino acid concentration (↑); and a decrease in amino acid concentration (↓).
Leucine transport has been reported to be reduced in MVM (−46%) and BM (−38%) isolated from IUGR term human placentas [4]. While most neutral amino acids are transported by the Na+-dependent transporter system A across the MVM, leucine and isoleucine are primarily transported by system L [4]. Addition of BCH, a specific system L inhibitor, reduces the transport of leucine by MVM to near zero [4]. Conversely, leucine transport across the BM is not entirely suppressed by the addition of BCH, indicating that other mechanisms apart from system L exist to transport leucine across the BM into the fetal circulation, one of which may be substantial non-transporter-mediated transport due to increased membrane fluidity [36] and transporters such as LAT3 [17]. In the present study, we demonstrate that while fetal plasma amino acid concentrations of leucine and isoleucine are decreased, there is no decrease in system L activity in the MVM or leucine transport in the BM. This may be explained by the complex mechanisms of transplacental leucine transport.
The intracellular concentration of leucine in the syncytiotrophoblast is approximately six times higher than in maternal plasma [37]. While the exact mechanisms driving the uphill transport of leucine across the MVM remain to be established, previous work has shown that a high intracellular concentration of other neutral amino acids such as glycine, which is mainly transported across the MVM by system A, may be responsible for creating an uphill gradient driving leucine uptake into the MVM [4]. Glycine is found in high concentrations in the placenta but is limited in fetal circulation, suggesting that it may diffuse back into the maternal circulation [38]. Because glycine is also accepted by system L, an outwardly directed glycine gradient from the MVM back into the maternal circulation may drive the uptake of system L substrates such as leucine and isoleucine across the MVM [4]. In this study, we report decreased activity of system A but not system L in MVM isolated from baboons with MNR at GD 120. It is therefore possible that impaired uptake of nonessential neutral amino acids such as glycine via system A leads to a reduced gradient to drive uphill transport of leucine and isoleucine via system L in vivo, resulting in reduced concentrations of leucine and isoleucine in the fetal circulation.
While we have shown a reduction in the activity of system A transporters in the MVM in response to MNR at GD 120, no changes were observed in corresponding isoform protein expression of SNAT1, 2 or 4. Interestingly, in the previous study investigating placental amino acid transporter protein expression and activity in rats fed a low-protein diet, we also found a discrepancy between system A activity and SNAT isoform expression [6]. In the rodent model, decreased placental and fetal weights as well as reduced system A transporter activity and protein expression was observed in the low protein group at GD 21, which represents close to term. However, at GD 19, placental system A transport activity but not protein expression of SNAT2 was reduced prior to the development of IUGR [6]. The exact mechanisms of reduced system A activity without concurrent reduction in SNAT isoform protein expression remains to be established, but posttranslational modifications of SNAT1, 2, or 4 isoforms may play a role in reducing system A activity in placentas of fetuses with IUGR.
Increased fetal circulating citrulline was also observed in baboons fed a MNR diet at GD 120. Citrulline is formed as an enzymatic by-product of nitric oxide synthesis from arginine by nitric oxide synthase activity [39]. Transplacental transport of citrulline is not well characterized. In the rat small intestine and the apical membrane of renal proximal tubular cells, citrulline transport appears to occur via Na+-dependent systems [40, 41]. IUGR is associated with elevated levels of maternal arginine [42], but intracellular arginine and citrulline are reduced in human umbilical vein cells (HUVECs) [39]. In HUVECs, arginine and citrulline are transported via system y+/CATs (cationic amino acid transporters), and HUVEC y+/CAT activity and expression is decreased in IUGR [39]. In response to MNR in the baboon at GD 165, maternal plasma arginine is elevated, while fetal plasma arginine and citrulline concentrations are increased (albeit these changes were not significant) compared to controls [7]. While increased fetal citrulline was significant, nonsignificant elevations in maternal plasma arginine and citrulline as well as fetal arginine were observed in response to MNR in the baboon at GD 120. It is therefore possible that increased production of citrulline from arginine is responsible for elevated fetal amino acid concentration of citrulline in MNR; however, further work is required to elucidate the mechanisms underlying this increase.
Predominantly based on elegant mouse studies, it has been proposed that placental function is primarily controlled by fetal demand [43–45]. For example, in response to maternal undernutrition or restricted utero-placental blood flow, resulting in decreased placental transfer and limited fetal nutrient availability, the fetal demand model of placental function predicts that the fetus signals to its placenta to up-regulate placental growth and nutrient transport. This model represents a classical homeostatic mechanism by which the fetus compensates for decreased nutrient availability by regulating nutrient supply (i.e., placental transport) in the opposite direction, thereby correcting for deficiencies. However, with few exceptions [44], there are few known fetal demand signals in the human. For example, key placental transporters for amino acids and ions are in general down-regulated in human IUGR, which is inconsistent with a homeostatic or fetal demand model for regulation of placental transport. However, most of these studies were performed at term, and it cannot be excluded that compensatory changes consistent with fetal demand signals are present earlier in pregnancy, as has been shown in mouse models of IUGR [43–45]. In the current study of a well-established IUGR model in baboon, a species with close relationship to the human, we found no evidence of compensatory up-regulation of nutrient transporters at GD 120, corresponding to late second trimester in the human. These observations suggest that the primates lack strong fetal demand signals that regulate placental function.
In this study, we have utilized a nonhuman primate model with extensive similarities to human IUGR, and our studies are therefore relevant to the development of restricted fetal growth in women. Although strongly motivated by the high human relevance of the data obtained, nonhuman primate research is a continuous balancing act between, on one hand, the exhaustive costs for each individual experiment and the ethical requirement to minimize the number of animals used and, on the other hand, the risk for Type I/II errors. With eight and nine animals, respectively, in the two groups, we strongly feel that we have found a reasonable balance between these two opposing aspects. However, the relatively small sample size is a potential limitation of the study.
In this study, we report that reduction in placental nutrient transporter activity occurs at 0.65 of gestation, prior to the development of IUGR, following moderate MNR in a well-established baboon model that has extensive similarities to the human IUGR. We have suggested that the placenta functions as a nutrient sensor, regulating fetal growth rate to match the ability of the maternal supply line to deliver nutrients by altering placental nutrient transport and nutrient delivery to the fetus [6, 46]. The reduction of system A transport in MVM observed following MNR but prior to IUGR is consistent with this hypothesis. The exact mechanism by which system A transporter activity but not isoform protein expression is reduced in MVM in response to MNR remains to be elucidated.
ACKNOWLEDGMENT
We are grateful for the expert contributions of Xiaoli Gao, Ph.D.
Footnotes
Supported by NIH grant P01HD21350. Mass spectrometry analyses were conducted in the metabolomics component of the UTHSCSA Mass Spectrometry Laboratory, supported by UTHSCSA and by an award from the National Institutes of Health, 1S10RR031586-01 (S.T.W.).
REFERENCES
- Nord M, Coleman-Jensen A, Andrews M, Carlson S. Household food security in the United States, 2009. United States Department of Agriculture, Economic Research Services 2010 [Google Scholar]
- Brodsky D, Christou H. Current concepts in intrauterine growth restriction. J Intensive Care Med. 2004;19:307–319. doi: 10.1177/0885066604269663. [DOI] [PubMed] [Google Scholar]
- Barker DJ, Gluckman PD, Godfrey KM, Harding JE, Owens JA, Robinson JS. Fetal nutrition and cardiovascular disease in adult life. Lancet. 1993;341:938–941. doi: 10.1016/0140-6736(93)91224-a. [DOI] [PubMed] [Google Scholar]
- Jansson T, Scholtbach V, Powell TL. Placental transport of leucine and lysine is reduced in intrauterine growth restriction. Pediatr Res. 1998;44:532–537. doi: 10.1203/00006450-199810000-00011. [DOI] [PubMed] [Google Scholar]
- Norberg S, Powell TL, Jansson T. Intrauterine growth restriction is associated with a reduced activity of placental taurine transporters. Pediatr Res. 1998;44:233–238. doi: 10.1203/00006450-199808000-00016. [DOI] [PubMed] [Google Scholar]
- Jansson N, Pettersson J, Haafiz A, Ericsson A, Palmberg I, Tranberg M, Ganapathy V, Powell TL, Jansson T. Down-regulation of placental transport of amino acids precedes the development of intrauterine growth restriction in rats fed a low protein diet. J Physiol. 2006;576:935–946. doi: 10.1113/jphysiol.2006.116509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavitha JV, Rosario FJ, Nijland MJ, McDonald TJ, Wu G, Kanai Y, Powell TL, Nathanielsz PW, Jansson T. Down-regulation of placental mTOR, insulin/IGF-I signaling, and nutrient transporters in response to maternal nutrient restriction in the baboon. FASEB J. 2014;28:1294–1305. doi: 10.1096/fj.13-242271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson T. Amino acid transporters in the human placenta. Pediatr Res. 2001;49:141–147. doi: 10.1203/00006450-200102000-00003. [DOI] [PubMed] [Google Scholar]
- Teasdale F, Jeanjacques G. Intrauterine growth-retardation–morphometry of the microvillous membrane of the human-placenta. Placenta. 1988;9:47–55. doi: 10.1016/0143-4004(88)90072-0. [DOI] [PubMed] [Google Scholar]
- Moe AJ. Placental amino-acid-transport. Am J Physiol. 1995;268:C1321–C1331. doi: 10.1152/ajpcell.1995.268.6.C1321. [DOI] [PubMed] [Google Scholar]
- Desforges M, Lacey HA, Glazier JD, Greenwood SL, Mynett KJ, Speake PF, Sibley CP. SNAT4 isoform of system A amino acid transporter is expressed in human placenta. Am J Physiol Cell Physiol. 2006;290:C305–C312. doi: 10.1152/ajpcell.00258.2005. [DOI] [PubMed] [Google Scholar]
- Cleal JK, Lewis RM. The mechanisms and regulation of placental amino acid transport to the human foetus. J Neuroendocrinol. 2008;20:419–426. doi: 10.1111/j.1365-2826.2008.01662.x. [DOI] [PubMed] [Google Scholar]
- Jansson T, Ekstrand Y, Bjorn C, Wennergren M, Powell TL. Alterations in the activity of placental amino acid transporters in pregnancies complicated by diabetes. Diabetes. 2002;51:2214–2219. doi: 10.2337/diabetes.51.7.2214. [DOI] [PubMed] [Google Scholar]
- Verrey F, Closs EI, Wagner CA, Palacin M, Endou H, Kanai Y. CATs and HATs: the SLC7 family of amino acid transporters. Pflugers Arch. 2004;447:532–542. doi: 10.1007/s00424-003-1086-z. [DOI] [PubMed] [Google Scholar]
- Hoeltzli SD, Smith CH. Alanine transport-systems in isolated basal plasma-membrane of human-placenta. Am J Physiol. 1989;256:C630–C637. doi: 10.1152/ajpcell.1989.256.3.C630. [DOI] [PubMed] [Google Scholar]
- Kudo Y, Boyd CAR. Characterization of amino-acid-transport systems in human placental basal membrane-vesicles. Biochim Biophys Acta. 1990;1021:169–174. doi: 10.1016/0005-2736(90)90030-r. [DOI] [PubMed] [Google Scholar]
- Cleal JK, Glazier JD, Ntani G, Crozier SR, Day PE, Harvey NC, Robinson SM, Cooper C, Godfrey KM, Hanson MA, Lewis RM. Facilitated transporters mediate net efflux of amino acids to the fetus across the basal membrane of the placental syncytiotrophoblast. J Physiol. 2011;589:987–997. doi: 10.1113/jphysiol.2010.198549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahendran D, Donnai P, Glazier JD, D'Souza SW, Boyd RD, Sibley CP. Amino acid (system A) transporter activity in microvillous membrane vesicles from the placentas of appropriate and small for gestational age babies. Pediatr Res. 1993;34:661–665. doi: 10.1203/00006450-199311000-00019. [DOI] [PubMed] [Google Scholar]
- Glazier JD, Cetin I, Perugino G, Ronzoni S, Grey AM, Mahendran D, Marconi AM, Pardi G, Sibley CP. Association between the activity of the system A amino acid transporter in the microvillous plasma membrane of the human placenta and severity of fetal compromise in intrauterine growth restriction. Pediatr Res. 1997;42:514–519. doi: 10.1203/00006450-199710000-00016. [DOI] [PubMed] [Google Scholar]
- Cetin I, Marconi AM, Bozzetti P, Sereni LP, Corbetta C, Pardi G, Battaglia FC. Umbilical amino-acid concentrations in appropriate and small for gestational-age infants–a biochemical difference present in utero. Am J Obstet Gynecol. 1988;158:120–126. doi: 10.1016/0002-9378(88)90792-2. [DOI] [PubMed] [Google Scholar]
- Pantham P, Rosario FJ, Nijland M, Cheung A, Nathanielsz PW, Powell TL, Galan HL, Li C, Jansson T. Reduced placental amino acid transport in response to maternal nutrient restriction in the baboon. Am J Physiol Regul Integr Comp Physiol. 2015 doi: 10.1152/ajpregu.00161.2015. 309:R740–R746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlabritz-Loutsevitch NE, Howell K, Rice K, Glover EJ, Nevill CH, Jenkins SL. Bill Cummins L, Frost PA, McDonald TJ, Nathanielsz PW. Development of a system for individual feeding of baboons maintained in an outdoor group social environment. J Med Primatol. 2004;33:117–126. doi: 10.1111/j.1600-0684.2004.00067.x. [DOI] [PubMed] [Google Scholar]
- Schlabritz-Loutsevitch N, Ballesteros B, Dudley C, Jenkins S, Hubbard G, Burton GJ, Nathanielsz PW. Moderate maternal nutrient restriction, but not glucocorticoid administration, leads to placental morphological changes in the baboon (Papio sp.) Placenta. 2007;28:783–793. doi: 10.1016/j.placenta.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illsley NP, Wang ZQ, Gray A, Sellers MC, Jacobs MM. Simultaneous preparation of paired, syncytial, microvillous and basal membranes from human placenta. Biochim Biophys Acta. 1990;1029:218–226. doi: 10.1016/0005-2736(90)90157-j. [DOI] [PubMed] [Google Scholar]
- Johansson M, Jansson T, Powell TL. Na+-K+-ATPase is distributed to microvillous and basal membrane of the syncytiotrophoblast in human placenta. Am J Physiol-Regul Integr Comp Physiol. 2000;279:R287–R294. doi: 10.1152/ajpregu.2000.279.1.R287. [DOI] [PubMed] [Google Scholar]
- Bastin J, Drakesmith H, Rees M, Sargent I, Townsend A. Localisation of proteins of iron metabolism in the human placenta and liver. Br J Haematol. 2006;134:532–543. doi: 10.1111/j.1365-2141.2006.06216.x. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Ling R, Bridges CC, Sugawara M, Fujita T, Leibach FH, Prasad PD, Ganapathy V. Involvement of transporter recruitment as well as gene expression in the substrate-induced adaptive regulation of amino acid transport system A. Biochim Biophys Acta Biomembr. 2001;1512:15–21. doi: 10.1016/s0005-2736(01)00310-8. [DOI] [PubMed] [Google Scholar]
- Park SY, Kim JK, Kim IJ, Choi BK, Jung KY, Lee S, Park KJ, Chairoungdua A, Kanai Y, Endou H, Kim DK. Reabsorption of neutral amino acids mediated by amino acid transporter LAT2 and TAT1 in the basolateral membrane of proximal tubule. Arch Pharmacal Res. 2005;28:421–432. doi: 10.1007/BF02977671. [DOI] [PubMed] [Google Scholar]
- Carter AM. Animal models of human placentation–a review. Placenta. 2007;28(Suppl A):S41–S47. doi: 10.1016/j.placenta.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Schlabritz-Loutsevitch NE, Hubbard GB, Jenkins SL, Martin HC, Snider CS, Frost PA, Leland MM, Havill LM, McDonald TJ, Nathanielsz PW. Ontogeny of hematological cell and biochemical profiles in maternal and fetal baboons (Papio species) J Med Primatol. 2005;34:193–200. doi: 10.1111/j.1600-0684.2005.00109.x. [DOI] [PubMed] [Google Scholar]
- McDonald TJ, Wu G, Nijland MJ, Jenkins SL, Nathanielsz PW, Jansson T. Effect of 30% nutrient restriction in the first half of gestation on maternal and fetal baboon serum amino acid concentrations. Br J Nutr. 2013;109:1382–1388. doi: 10.1017/S0007114512003261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marconi AM, Paolini CL, Stramare L, Cetin I, Fennessey PV, Pardi G, Battaglia FC. Steady state maternal-fetal leucine enrichments in normal and intrauterine growth-restricted pregnancies. Pediatr Res. 1999;46:114–119. doi: 10.1203/00006450-199907000-00019. [DOI] [PubMed] [Google Scholar]
- Paolini CL, Marconi AM, Ronzoni S, Di Noio M, Fennessey PV, Pardi G, Battaglia FC. Placental transport of leucine, phenylalanine, glycine, and proline in intrauterine growth-restricted pregnancies. J Clin Endocrinol Metab. 2001;86:5427–5432. doi: 10.1210/jcem.86.11.8036. [DOI] [PubMed] [Google Scholar]
- Teodoro GFR, Vianna D, Torres-Leal FL, Pantaleao LC, Matos-Neto EM, Donato J, Tirapegui J. Leucine is essential for attenuating fetal growth restriction caused by a protein-restricted diet in rats. J Nutr. 2012;142:924–930. doi: 10.3945/jn.111.146266. [DOI] [PubMed] [Google Scholar]
- Jansson T, Illsley NP. Osmotic water permeabilities of human placental microvillous and basal membranes. J Membr Biol. 1993;132:147–155. doi: 10.1007/BF00239004. [DOI] [PubMed] [Google Scholar]
- Yudilevich DL, Sweiry JH. Transport of amino-acids in the placenta. Biochim Biophys Acta. 1985;822:169–201. doi: 10.1016/0304-4157(85)90007-3. [DOI] [PubMed] [Google Scholar]
- Cetin I, Marconi AM, Baggiani AM, Buscaglia M, Pardi G, Fennessey PV, Battaglia FC. In-vivo placental transport of glycine and leucine in human pregnancies. Pediatr Res. 1995;37:571–575. doi: 10.1203/00006450-199505000-00002. [DOI] [PubMed] [Google Scholar]
- Casanello P, Sobrevia L. Intrauterine growth retardation is associated with reduced activity and expression of the cationic amino acid transport systems y(+)/hCAT-1 and y(+)/hCAT-2B and lower activity of nitric oxide synthase in human umbilical vein endothelial cells. Circ Res. 2002;91:127–134. doi: 10.1161/01.res.0000027813.55750.e7. [DOI] [PubMed] [Google Scholar]
- Vadgama JV, Evered DF. Characteristics of L-citrulline transport across rat small intestine in vitro. Pediatr Res. 1992;32:472–478. doi: 10.1203/00006450-199210000-00019. [DOI] [PubMed] [Google Scholar]
- Mitsuoka K, Shirasaka Y, Fukushi A, Sato M, Nakamura T, Nakanishi T, Tamai I. Transport characteristics of L-citrulline in renal apical membrane of proximal tubular cells. Biopharm Drug Dispos. 2009;30:126–137. doi: 10.1002/bdd.653. [DOI] [PubMed] [Google Scholar]
- Cetin I, Corbetta C, Sereni LP, Marconi AM, Bozzetti P, Pardi G, Battaglia FC. Umbilical amino-acid-concentrations in normal and growth-retarded fetuses sampled in utero by cordocentesis. Am J Obstet Gynecol. 1990;162:253–261. doi: 10.1016/0002-9378(90)90860-a. [DOI] [PubMed] [Google Scholar]
- Constancia M, Angiolini E, Sandovici I, Smith P, Smith R, Kelsey G, Dean W, Ferguson-Smith A, Sibley CP, Reik W, Fowden A. Adaptation of nutrient supply to fetal demand in the mouse involves interaction between the Igf2 gene and placental transporter systems. Proc Natl Acad Sci U S A. 2005;102:19219–19224. doi: 10.1073/pnas.0504468103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angiolini E, Coan PM, Sandovici I, Iwajomo OH, Peck G, Burton GJ, Sibley CP, Reik W, Fowden AL, Constancia M. Developmental adaptations to increased fetal nutrient demand in mouse genetic models of Igf2-mediated overgrowth. FASEB J. 2011;25:1737–1745. doi: 10.1096/fj.10-175273. [DOI] [PubMed] [Google Scholar]
- Sibley CP, Brownbill P, Dilworth M, Glazier JD. Adaptation in placental nutrient supply to meet fetal growth demand: implications for programming. Placenta. 2010;31:S70–S74. doi: 10.1016/j.placenta.2009.12.020. [DOI] [PubMed] [Google Scholar]
- Jansson T, Powell TL. Role of the placenta in fetal programming: underlying mechanisms and potential interventional approaches. Clin Sci (Lond) 2007;113:1–13. doi: 10.1042/CS20060339. [DOI] [PubMed] [Google Scholar]