Abstract
Antithrombin is a plasma protease inhibitor that inhibits thrombin and contributes to the maintenance of blood fluidity. Using targeted gene disruption, we investigated the role of antithrombin in embryogenesis. Mating mice heterozygous for antithrombin gene (ATIII) disruption, ATIII+/–, yielded the expected Mendelian distribution of genotypes until 14.5 gestational days (gd). However, approximately 70% of the ATIII–/– embryos at 15.5 gd and 100% at 16.5 gd had died and showed extensive subcutaneous hemorrhage. Histological examination of those embryos revealed extensive fibrin(ogen) deposition in the myocardium and liver, but not in the brain or lung. Furthermore, no apparent fibrin(ogen) deposition was detected in the extensive hemorrhagic region, suggesting that fibrinogen might be decreased due to consumptive coagulopathy and/or liver dysfunction. These findings suggest that antithrombin is essential for embryonic survival and that it plays an important role in regulation of blood coagulation in the myocardium and liver.
Introduction
Antithrombin is a 58-kDa plasma serine protease inhibitor (serpin) that, through the inhibition of thrombin and other activated serine proteases, plays important roles in the regulation of coagulation (1). A recent report has demonstrated that the cleaved conformation of antithrombin has potent antiangiogenic activity and that antithrombin may allow for the precise regulation of angiogenesis (2).
The inhibition of thrombin by antithrombin is relatively slow, but is dramatically enhanced in the presence of heparin (1, 3). Heparin-like molecules, heparan sulfate proteoglycans (HSPGs), are closely associated with endothelial cells and enhance the action of circulating antithrombin (4, 5). The syndecan and glypican core protein families constitute the major membrane-associated HSPGs generated within the cardiovascular system (6). Among them, syndecan-4 (ryudocan) was originally cloned from endothelial cells as an antithrombin-binding molecule (7, 8), and we recently generated syndecan-4–null mice by targeted gene disruption (9).
Individuals with functionally defective antithrombin are susceptible to venous thromboembolic diseases (10–12). Congenital antithrombin deficiency is usually heterozygous and classified into two types, type I (quantitative deficiency) and type II (qualitative deficiency), which includes a reactive defect, heparin-binding site defect, and pleiotrophic effect (12–14). Although some cases of homozygous qualitative antithrombin deficiency have been reported (15–17), homozygous quantitative antithrombin deficiency has not. Thus, complete deficiency of antithrombin is speculated to cause embryonic lethality similar to that of other anticoagulant proteins (18, 19). It is not known, however, when or in what way antithrombin is necessary in normal embryogenesis. To further investigate the physiological roles of antithrombin, especially those in embryogenesis, we generated antithrombin-deficient mice by gene targeting.
Methods
Isolation of the mouse antithrombin gene.
Oligonucleotide primers mAT3S (5′-ATGATGTACCAGGAAGGCAA, cDNA positions 865–884) and mAT3A (5′-GGAATGCGTCGGAGACATAG, 1219–1200), based on the published murine antithrombin cDNA sequence (20), were used to obtain a partial cDNA of mouse antithrombin from liver mRNA of C57BL/6J mice (CLEA, Tokyo, Japan) using RT-PCR. The resulting 355-bp PCR product was used as a probe to isolate a genomic clone containing a segment of the antithrombin gene from a 129SVJ lambda FIX II genomic library (Stratagene, La Jolla, California, USA), as described previously (21), and a 16-kb segment of DNA (λmATG1) containing the antithrombin initiation codon to the sixth intron was obtained. The sequence data have been deposited in DNA Data Bank of Japan (DDBJ)/European Molecular Biology Laboratory (EMBL)/GenBank (accession number AB043785). The detailed features of the gene structure and its comparison with the human antithrombin gene will be discussed elsewhere.
Construction of a targeting vector for homologous recombination in embryonic stem cells.
The targeting vector was constructed from a basic vector with MC1neo (polyoma virus thymidine kinase gene promoter and neomycin resistance gene) and DTA (diphtheria toxin fragment A gene) (22) and antithrombin gene fragments. We used a 2.1-kb SspI/PvuII fragment as the 5′ arm and a 7.3-kb HindIII/SmaI fragment as the 3′ arm to replace 1,781 bases, including exon 2 of the antithrombin gene with MC1neo by homologous recombination (Figure 1a). The generation of targeted D3 embryonic stem (ES) cells and blastocyst injection were performed as described previously (9, 23).
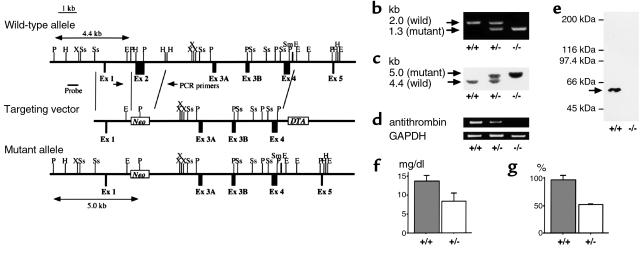
Figure 1.
Antithrombin gene-disruption in mice. (a) Antithrombin replacement construct and partial restriction map. P, PvuII; H, HindIII; X, XbaI; Ss, SspI; E, EcoRI; Sm, SmaI; Ex, exon; Neo, neomycin resistance gene. (b) PCR. External primers (PCR primers in a) were used. The mutant allele gave a 1.3-kb band, whereas the wild-type allele gave a 2.0-kb band. +/+, ATIII+/+; +/–, ATIII+/–; –/–, ATIII–/– embryos. (c) Southern blot analysis after PvuII digestion. An external probe (probe in a) was used. The mutant allele gave a 5.0-kb band, whereas the wild-type allele gave a 4.4-kb band. (d) RT-PCR demonstrating that mRNA expression of antithrombin was detected in total RNA derived from the whole of an ATIII+/+ or ATIII+/– embryo, whereas it was not detected in total RNA derived from the whole ATIII–/– embryo at 14.5 gd. (e) Western blot analysis demonstrating that protein expression of antithrombin was detected in homogenate derived from the whole liver of an ATIII+/+ embryo, whereas it was not detected in homogenate derived from that of an ATIII–/– embryo at 14.5 gd. The arrow indicates the position where antithrombin migrates. (f) The amount of antithrombin antigen in plasma was significantly reduced in ATIII+/– mice compared with ATIII+/+ mice (n = 8, P < 0.001). The values are means ± SD. The P value was calculated with Student’s t test. (g) The functional activity of antithrombin in plasma was significantly reduced in ATIII+/– mice compared with ATIII+/+ mice (n = 8, P < 0.001). The values are means ± SD. The P value was calculated with Student’s t test.
Generation of antithrombin-deficient mice.
Male chimeric mice were mated with C57BL/6J female mice, and F1 mice heterozygous for antithrombin gene (ATIII) disruption, ATIII+/–, were mated with each other to yield null mutant mice. Noon on the day of vaginal plug detection was defined as 0.5 gestational days (gd). Females were sacrificed and the embryos harvested at various times during gestation. We defined embryonic death by the absence of a heartbeat at first inspection. The lower limbs and tail of the embryos were used for Southern blot and PCR analyses. The remaining embryos were used for RT-PCR, Western blot, or histological analyses. For histological analysis, the embryos were fixed overnight in Carnoy’s solution (methanol/chloroform/acetic acid = 6:3:1). The fixed embryos were then dehydrated, embedded in paraffin, and sectioned (6 μm thick).
Preparation of anti-mouse antithrombin antisera.
The antisera against mouse antithrombin were raised in rabbits by direct introduction of the encoding cDNA in pCI vector (Promega, Madison, Wisconsin, USA) (24). From the antisera, nonspecific Ab’s were absorbed with dry powder derived from ATIII–/– embryos at 14.5 gd. Serum titers were determined by Western blot analysis using recombinant mouse antithrombin fused to glutathione-S-transferase protein (Amersham Pharmacia Biotech, Tokyo, Japan) and rat antithrombin (Sigma-Aldrich, St. Louis, Missouri, USA).
Detection of antithrombin-deficient mice
DNA analysis.
PCR was performed with a forward primer (5′-CCTTCCAGACCGAACTGTCC) and a reverse primer (5′-GTAATCCCAGCCTTCTCCTG) to detect the expected deletion (Figure 1a). The PCR conditions were 35 cycles of denaturation for 30 seconds at 94°C, annealing for 1 minute at 68°C, and extension for 3 minutes at 72°C. The wild-type allele gave a 2.0 kb band, whereas the mutant allele gave a 1.3-kb band (Figure 1b).
We also performed Southern blot analysis. After digestion with PvuII and hybridization with an external probe (a 658-base HindIII/SspI fragment; Figure 1a), the wild-type allele gave a 4.4-kb band, whereas the mutant allele gave a 5-kb band (Figure 1c).
RNA analysis.
Total RNA was isolated from the single embryo of each genotype at 14.5 gd by the guanidinium thiocyanate-phenol-chloroform method (25). RT-PCR was performed with primers mAT3S and mAT3AS, described above, yielding a 355-bp fragment. The PCR conditions were 25 cycles of denaturation for 30 seconds at 94°C, annealing for 1 minute at 55°C, and extension for 1 minute at 72°C.
Protein analysis.
The whole liver of a single ATIII+/+ or ATIII–/– embryo at 14.5 gd was homogenized in PBS (500 μl). After centrifugation, the supernatant was rotated with protein G-Sepharose (40 μl of 50% slurry) (Amersham Pharmacia Biotech) for 1 hour at 4°C. After centrifugation, the supernatant was rotated with goat anti-human antithrombin Ab (8 μg) (Cedarlane Laboratories, Hornby, Ontario, Canada) and protein G-Sepharose (40 μl of 50% slurry) for 1 hour. The bound antithrombin was isolated together with IgG protein–G-Sepharose complex by centrifugation, washed with PBS, and eluted by incubation at 100°C for 5 minutes with 4% SDS (30 μl). Samples were applied to 8% SDS-polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane for blotting with rabbit anti-mouse antithrombin antisera (1:10 dilution). Anti-rabbit IgG Ab conjugated with horseradish peroxidase was used for detection. The samples were visualized with enhanced chemiluminescence (ECL; Amersham Pharmacia Biotech).
Measurement of plasma antithrombin.
The levels of antithrombin antigen and activity in plasma were determined using N-assay TIA AT-III, which is a turbidimetric immunoassay for antigen-Ab complex, and N-test ATIII-S, which determines its enzymatic activity using a chromogenic substrate (Nittohbo, Tokyo, Japan), according to the manufacturer’s instructions.
Statistical analysis.
Stat View 4.5 (SAS Institute Inc., Cary, North Carolina, USA) was used for statistical analyses. P values were calculated with Student’s t test.
Antifibrin(ogen) immunohistochemical staining.
Slides were deparaffinized in xylene, transferred to 100% ethanol, and then incubated for 30 minutes in 0.3% H2O2/methanol. After rinsing with PBS, the slides were incubated successively with 5% normal goat serum/PBS (20 minutes, room temperature), rabbit anti-mouse fibrin(ogen) Ab (26) (1:200 dilution, overnight, 4°C), anti-rabbit IgG Ab conjugated with biotin (1:500 dilution, 1 hour, room temperature), and avidin-biotin complex conjugated with horseradish peroxidase (Vector Laboratories, Burlingame, California, USA) (30 minutes, room temperature). The staining was visualized with diaminobenzidine tetrahydrochloride-Ni3+, Co2+ (Amersham Pharmacia Biotech).
Results
Generation of antithrombin-deficient mice.
To inactivate the murine ATIII, a targeting vector (Figure 1a) was introduced into D3 ES cells by electroporation. Among 314 ES clones, we found four clones lacking ATIII from a chromosome by homologous recombination and confirmed, using Southern blot analysis with a probe to neomycin resistance gene, that three of them did not have any random integrations. Blastocyst injection of two independent ES clones yielded chimeric mice. The chimeric mice derived from one ES clone transmitted the mutant allele into the germ line. ATIII+/– mice were produced by mating the chimeric mice with female C57BL/6J mice and then crossed to yield ATIII–/– embryos. PCR, Southern blot, RT-PCR, and Western blot analyses confirmed that ATIII–/– embryos lacked the antithrombin gene, mRNA, and protein (Figure 1, b–e). The plasma levels of antithrombin antigen and activity were significantly reduced in ATIII+/– mice, compared with ATIII+/+ mice (P < 0.001) (Figure 1, f and g). However, the appearance and body weight of ATIII+/– mice were not different from those of ATIII+/+ mice until 4 months of age. None of the 54 ATIII+/– mice died or showed apparent thrombosis during the observation period.
Characterization of the ATIII–/– mice.
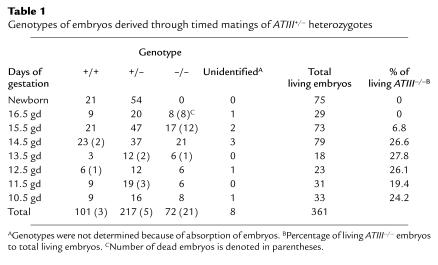
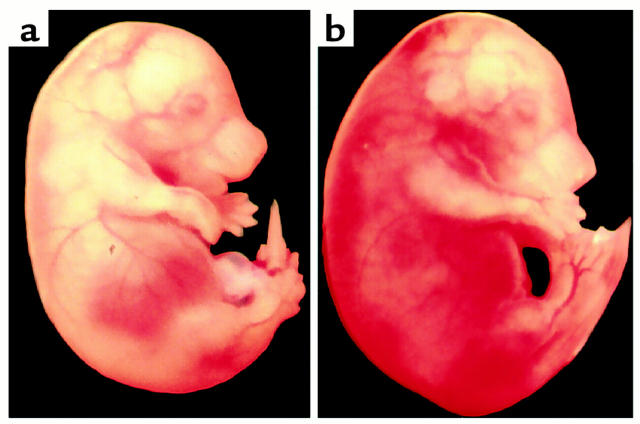
Until 14.5 gd, the frequency of living ATIII–/– embryos matched the Mendelian rate (Table 1). Their appearance was indistinguishable from that of ATIII+/– or ATIII+/+ embryos (data not shown). However, approximately 70% of the ATII–/– embryos (12 of 17 embryos) at 15.5 gd and 100% (eight of eight embryos) at 16.5 gd had died and showed extensive subcutaneous hemorrhage (Figure 2). No ATIII–/– mice were observed among the 75 F2 newborn mice (Table 1). Thirteen dead ATII–/– embryos (five embryos at 15.5 gd and eight at 16.5 gd) were examined histologically. In all of them, fibrin(ogen) deposition was detected in the degenerated myocardium and liver (Figure 3, c–f). In the liver, fibrin(ogen) deposition was observed in the sinusoids, but not in the central veins (Figure 3f). Even in all of five living ATIII–/– embryos at 15.5 gd and one of four living ATIII–/– embryos examined at 14.5 gd, the myocardium was partially degenerated where fibrin(ogen) deposition was detected (Figure 4). In three of five living ATIII–/– embryos at 15.5 gd, the liver was also partially degenerated where fibrin(ogen) was detected in the sinusoids (data not shown). These observations suggest that the degeneration of the myocardium and liver of dead ATIII–/– embryos is due to intravascular coagulation rather than the natural progression of the absorption of dead embryos. It seems likely that fibrin deposition among degenerating myocardial fibers is secondary, perhaps from hemorrhage into the necrotic tissue. In other tissues, including the brain, lung, and kidney, no fibrin(ogen) deposition was observed by immunohistochemical staining, even though there was apparent tissue degeneration. Extensive hemorrhage was observed subcutaneously and intracranially (Figure 5a), but no apparent fibrin(ogen) deposition was detected by immunohistochemical staining (Figure 5b), suggesting that fibrinogen in plasma may have been decreased by consumptive coagulopathy and/or liver dysfunction in the dead ATIII–/– embryos.
Table 1.
Genotypes of embryos derived through timed matings of ATIII+/– heterozygotes
Figure 2.
Macroscopic observation of ATIII+/+ (a) and dead ATIII–/– (b) embryos at 15.5 gd. A dead ATIII–/– embryo at 15.5 gd showed extensive subcutaneous hemorrhage.
Figure 3.
Microscopic observation of ATIII+/+ (a and b) and dead ATIII–/– (c–f) embryos at 15.5 gd. (a and c) Hematoxylin-eosin and (b and d) immunochemical staining with antifibrin(ogen) Ab. (e and f) Magnification of the areas indicated in d. (e) Myocardium. (f) Liver. A dead ATIII–/– embryo at 15.5 gd showed degeneration of the myocardium and liver (c). Fibrin(ogen) deposition was observed in the myocardium and sinusoids of the liver (d, e, and f). Size bars, 100 μm.
Figure 4.
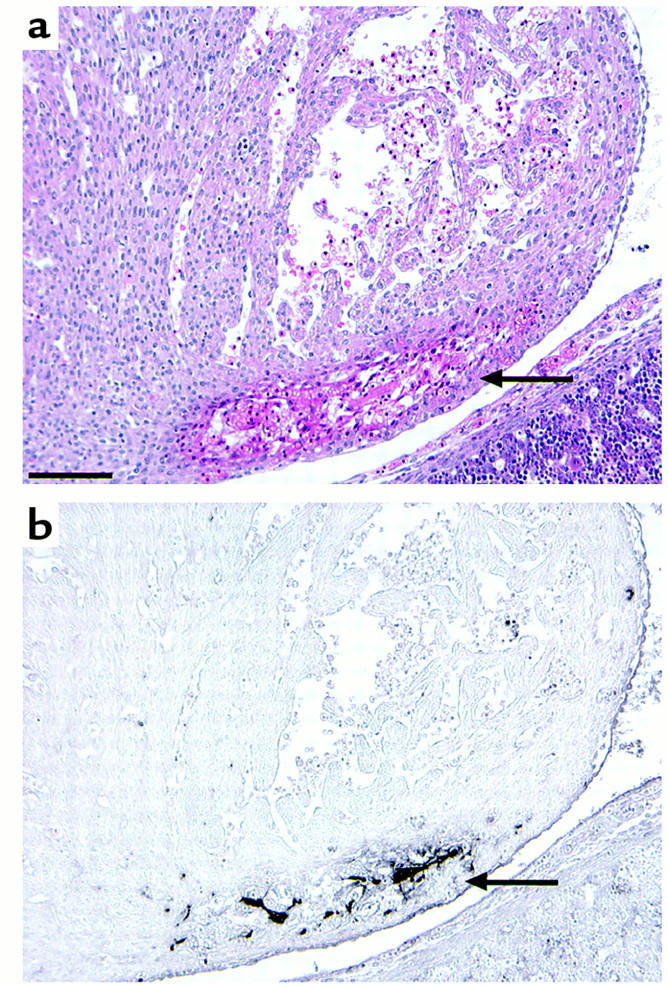
Hematoxylin-eosin (a) and immunohistochemical staining with anti-fibrin(ogen) Ab (b) of the myocardium of a living ATIII–/– embryo at 14.5 gd. Arrows indicate partial degeneration (a) and fibrin(ogen) deposition (b) in the myocardium.
Figure 5.
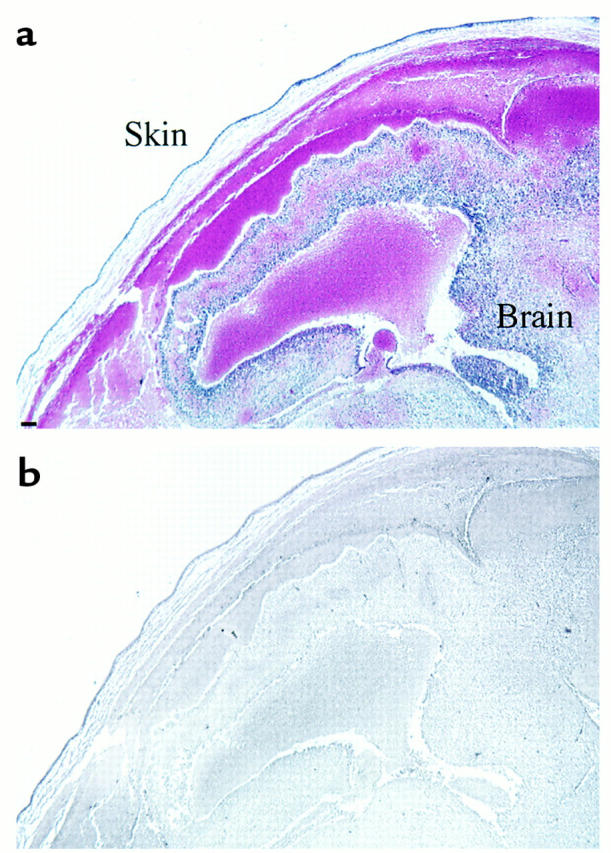
Hematoxylin-eosin (a) and immunohistochemical staining with anti-fibrin(ogen) Ab (b) of the head of a dead ATIII–/– embryo at 16.5 gd. Extensive hemorrhage was observed subcutaneously and intracranially (a), but there was no apparent fibrin(ogen) deposition (b). Size bar, 100 μm.
Discussion
It has been suggested that the contribution of a given anticoagulant mechanism to the hemostatic balance varies from one vascular bed to another, and thrombosis occurs in an organ-specific fashion through the critical interplay of genetic and environmental factors (27). Whereas individuals with heterozygous antithrombin deficiency are susceptible to venous thromboembolic diseases (10–12), a homozygous variant of antithrombin that lacks affinity for heparin causes thrombosis not only in veins but also in arteries (15–17). In this study, we demonstrated that complete antithrombin deficiency was lethal to embryos. The reason for embryonic death during mid-late embryogenesis appears to be fibrin deposition in the myocardium and liver, since antithrombin deficiency caused fibrin(ogen) deposition around 15.5 gd in the degenerated myocardium and liver, but apparently did not in other regions. Although a small amount of fibrin(ogen) deposition undetectable by immunohistochemical staining might be present in several other organs, the myocardium and liver seemed to be more susceptible than other tissues to fibrin(ogen) deposition in antithrombin deficiency.
Previous reports have demonstrated that disruption of the tissue-factor pathway inhibitor (TFPI) or protein C (PC) gene also causes fibrin(ogen) deposition in the liver (18, 28), although its location was not discussed in detail. In ATIII–/– embryos, fibrin(ogen) deposition was detected in the sinusoids of the liver, but not in the central veins. It has been reported that expression of thrombomodulin and TFPI is not detected in the sinusoids of the liver (29, 30), suggesting that anticoagulation in the sinusoid of the liver might be dependent on antithrombin. In contrast, such disruption of the TFPI or PC gene does not cause severe fibrin(ogen) deposition in the embryonic myocardium (18, 28); this is peculiar to ATIII–/– embryos. Although the mechanism leading to the organ-specific fibrin(ogen) deposition in ATIII–/– embryos remains to be elucidated, these observations suggest that antithrombin may be an important anticoagulant protein in the myocardium and sinusoids of the liver during embryogenesis.
Beyond 15.5 gd, dead ATIII–/– embryos had extensive hemorrhage, with no fibrin(ogen) deposition detectable by immunohistochemical staining, suggesting that fibrinogen may be decreased by consumptive coagulopathy and/or liver dysfunction. Mice with fibrinogen deficiency suffer hemorrhagic symptoms postnatally but are born in normal numbers (31). On the other hand, embryos with TFPI (18) or antithrombin deficiency have a severe hemorrhagic phenotype. Severe hemorrhage of these anticoagulant-deficient embryos may be attributed to the combined defects of plasma and cellular hemostatic components with extensive intravascular coagulation.
The plasma levels of antithrombin antigen and activity were significantly reduced in ATIII+/– mice compared with ATIII+/+ mice. However, ATIII+/– mice appear to be indistinguishable from ATIII+/+ mice and did not experience any apparent thromboembolic events during observation through 4 months of age. Most subjects with heterozygous antithrombin deficiency are asymptomatic (32, 33), although antithrombin deficiency is obviously a risk factor for thromboembolic diseases, especially deep venous thrombosis of the lower limb and pulmonary embolism (10–12). The ATIII+/– mouse will be a useful animal model for studying the mechanism of thromboembolic events observed in human heterozygous antithrombin deficiency.
Acknowledgments
We thank Eriko Yamafuji and Chika Wakamatsu for their excellent technical support. This work is supported by Grants-in-Aid for Scientific Research (10557090, 10670942, 10178102, and 10CE2006) from the Ministry of Education, Science, Sports and Culture, and for Research on Specific Diseases from the Ministry of Health and Welfare.
References
- 1.Lane DA, Caso R. Antithrombin: structure, genomic organization, function and inherited deficiency. Baillieres Clin Haematol. 1989;2:961–998. doi: 10.1016/s0950-3536(89)80054-x. [DOI] [PubMed] [Google Scholar]
- 2.O’Reilly MS, Pirie-Shepherd S, Lane WS, Folkman J. Antiangiogenic activity of the cleaved conformation of the serpin antithrombin. Science. 1999;285:1926–1928. doi: 10.1126/science.285.5435.1926. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg RD, Damus PS. The purification and mechanism of action of human antithrombin-heparin cofactor. J Biol Chem. 1973;248:6490–6505. [PubMed] [Google Scholar]
- 4.Marcum JA, Fritze L, Galli SJ, Karp G, Rosenberg RD. Microvascular heparin-like species with anticoagulant activity. Am J Physiol. 1983;245:H725–H733. doi: 10.1152/ajpheart.1983.245.5.H725. [DOI] [PubMed] [Google Scholar]
- 5.Marcum JA, Rosenberg RD. Anticoagulantly active heparin-like species molecules from vascular tissue. Biochemistry. 1984;23:1730–1737. doi: 10.1021/bi00303a023. [DOI] [PubMed] [Google Scholar]
- 6.Rosenberg RD, Shworak NW, Liu J, Schwartz JJ, Zhang L. Heparan sulfate proteoglycans of the cardiovascular system: specific structures emerge but how is synthesis regulated? J Clin Invest. 1997;99:2062–2070. doi: 10.1172/JCI119377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kojima T, Leone CW, Marchildon GA, Marcum JA, Rosenberg RD. Isolation and characterization of heparan sulfate proteoglycans produced by cloned rat microvascular endothelial cells. J Biol Chem. 1992;267:4859–4869. [PubMed] [Google Scholar]
- 8.Kojima T, Shworak NW, Rosenberg RD. Molecular cloning and expression of two distinct cDNA-encoding heparan sulfate proteoglycan core proteins from a rat endothelial cell line. J Biol Chem. 1992;267:4870–4877. [PubMed] [Google Scholar]
- 9.Ishiguro K, et al. Syndecan-4 deficiency impairs focal adhesion formation only under restricted conditions. J Biol Chem. 2000;275:5249–5252. doi: 10.1074/jbc.275.8.5249. [DOI] [PubMed] [Google Scholar]
- 10.Egeberg O. Inherited antithrombin III deficiency causing thrombophilia. Thromb Diath Haemorrh. 1965;13:516–530. [PubMed] [Google Scholar]
- 11.Demers C, Ginsberg JS, Hirsh J. Thrombosis in antithrombin-III-deficient persons. Report of a large kindred and literature review. Ann Intern Med. 1992;116:754–761. doi: 10.7326/0003-4819-116-9-754. [DOI] [PubMed] [Google Scholar]
- 12.Lane DA, et al. Antithrombin mutation database: 2nd update. Thromb Haemost. 1997;77:197–211. [PubMed] [Google Scholar]
- 13.Lane DA, Kunz G, Olds RJ, Thein SL. Molecular genetics of antithrombin deficiency. Blood Rev. 1996;10:59–74. doi: 10.1016/s0268-960x(96)90034-x. [DOI] [PubMed] [Google Scholar]
- 14.Bayston TA, Lane DA. Antithrombin: molecular basis of deficiency. Thromb Haemost. 1997;78:339–343. [PubMed] [Google Scholar]
- 15.Chowdhury V, et al. Homozygous antithrombin deficiency: report of two new cases (99 Leu to Phe) associated with arterial and venous thrombosis. Thromb Haemost. 1994;72:198–202. [PubMed] [Google Scholar]
- 16.Okajima K, et al. Homozygous variant of antithrombin III that lacks affinity for heparin, AT III Kumamoto. Thromb Haemost. 1989;61:20–24. [PubMed] [Google Scholar]
- 17.Boyer C, Wolf M, Vedrenne J, Meyer D, Larrieu MJ. Homozygous variant of antithrombin III: ATIII Fontainebleau. Thromb Haemost. 1986;56:18–22. [PubMed] [Google Scholar]
- 18.Huang ZF, Higuchi D, Lasky N, Broze GJ., Jr Tissue factor pathway inhibitor gene disruption produces intrauterine lethality in mice. Blood. 1997;90:944–951. [PubMed] [Google Scholar]
- 19.Healy AM, Rayburn HB, Rosenberg RD, Weiler H. Absence of the blood-clotting regulator thrombomodulin causes embryonic lethality in mice before development of a functional cardiovascular system. Proc Natl Acad Sci USA. 1995;92:850–854. doi: 10.1073/pnas.92.3.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu JK, Scheffield WP, Blajchman MA. Molecular cloning and cell-free expression of mouse antithrombin III. Thromb Haemost. 1992;68:291–296. [PubMed] [Google Scholar]
- 21.Tsuzuki S, et al. Molecular cloning, genomic organization, promoter activity, and tissue-specific expression of the mouse ryudocan gene. J Biochem. 1997;122:17–24. doi: 10.1093/oxfordjournals.jbchem.a021724. [DOI] [PubMed] [Google Scholar]
- 22.Igakura T, et al. A null mutation in basigin, an immunoglobulin superfamily member, indicates its important roles in peri-implantation development and spermatogenesis. Dev Biol. 1998;194:152–165. doi: 10.1006/dbio.1997.8819. [DOI] [PubMed] [Google Scholar]
- 23.Terauchi Y, et al. Increased insulin sensitivity and hypoglycaemia in mice lacking the p85 alpha subunit of phosphoinositide 3-kinase. Nat Genet. 1999;21:230–235. doi: 10.1038/6023. [DOI] [PubMed] [Google Scholar]
- 24.Liu MA. Overview of DNA vaccines. Ann NY Acad Sci. 1995;772:15–20. doi: 10.1111/j.1749-6632.1995.tb44727.x. [DOI] [PubMed] [Google Scholar]
- 25.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. - [DOI] [PubMed] [Google Scholar]
- 26.Yamamoto K, Loskutoff DJ. Fibrin deposition in tissues from endotoxin-treated mice correlates with decreases in the expression of urokinase-type but not tissue-type plasminogen activator. J Clin Invest. 1996;97:2440–2451. doi: 10.1172/JCI118691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosenberg RD, Aird WC. Vascular-bed-specific hemostasis and hypercoagulable states. N Engl J Med. 1999;340:1555–1564. doi: 10.1056/NEJM199905203402007. [DOI] [PubMed] [Google Scholar]
- 28.Jalbert LR, et al. Inactivation of the gene for anticoagulant protein C causes lethal perinatal consumptive coagulopathy in mice. J Clin Invest. 1998;102:1481–1488. doi: 10.1172/JCI3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maruyama I, Bell CE, Majerus PW. Thrombomodulin is found on endothelium of arteries, veins, capillaries, and lymphatics, and on syncytiotrophoblast of human placenta. J Cell Biol. 1985;101:363–371. doi: 10.1083/jcb.101.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamanobe F, Mochida S, Ohno A, Ishikawa K, Fujiwara K. Recombinant human tissue factor pathway inhibitor as a possible anticoagulant targeting hepatic sinusoidal walls. Thromb Res. 1997;85:493–501. doi: 10.1016/s0049-3848(97)00038-8. [DOI] [PubMed] [Google Scholar]
- 31.Suh TT, et al. Resolution of spontaneous bleeding events but failure of pregnancy in fibrinogen-deficient mice. Genes Dev. 1995;9:2020–2033. doi: 10.1101/gad.9.16.2020. [DOI] [PubMed] [Google Scholar]
- 32.McColl M, et al. Low thrombosis rate seen in blood donors and their relatives with inherited deficiencies of antithrombin and protein C: correlation with type of defect, family history, and absence of the factor V Leiden mutation. Blood Coagul Fibrinolysis. 1996;7:689–694. doi: 10.1097/00001721-199610000-00005. [DOI] [PubMed] [Google Scholar]
- 33.Tait RC, et al. Prevalence of antithrombin deficiency in the healthy population. Br J Haematol. 1994;87:106–112. doi: 10.1111/j.1365-2141.1994.tb04878.x. [DOI] [PubMed] [Google Scholar]