Abstract
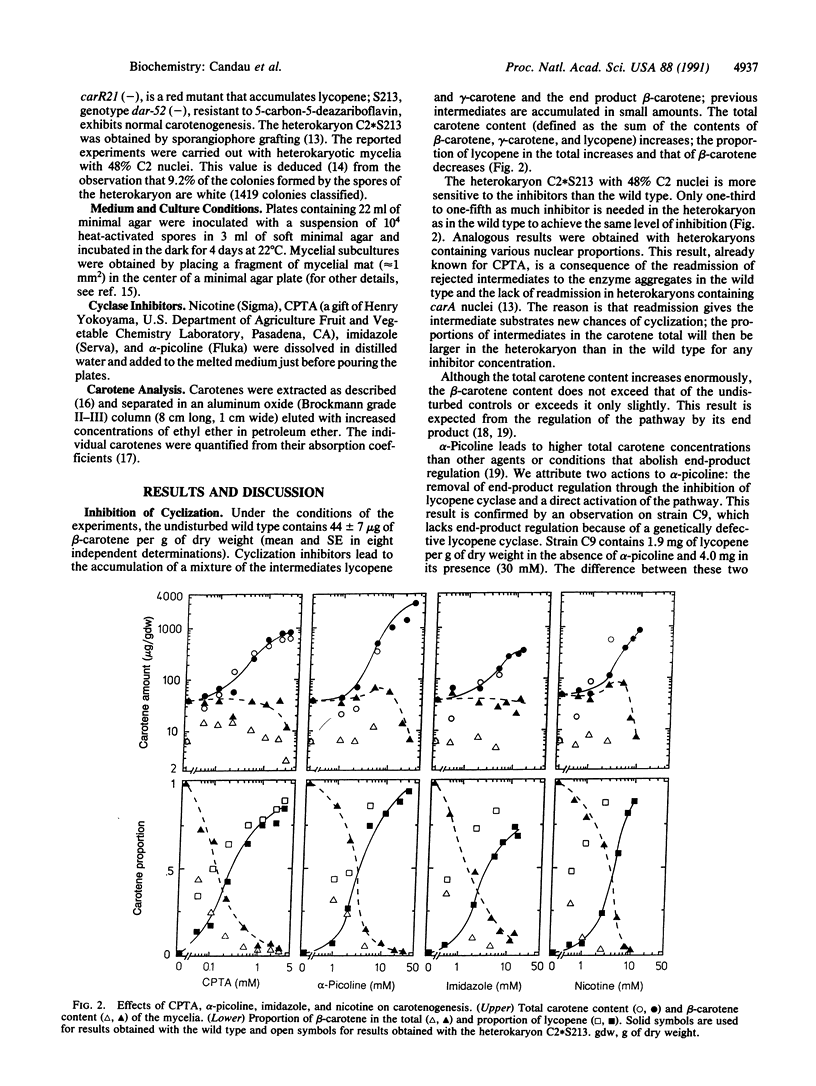
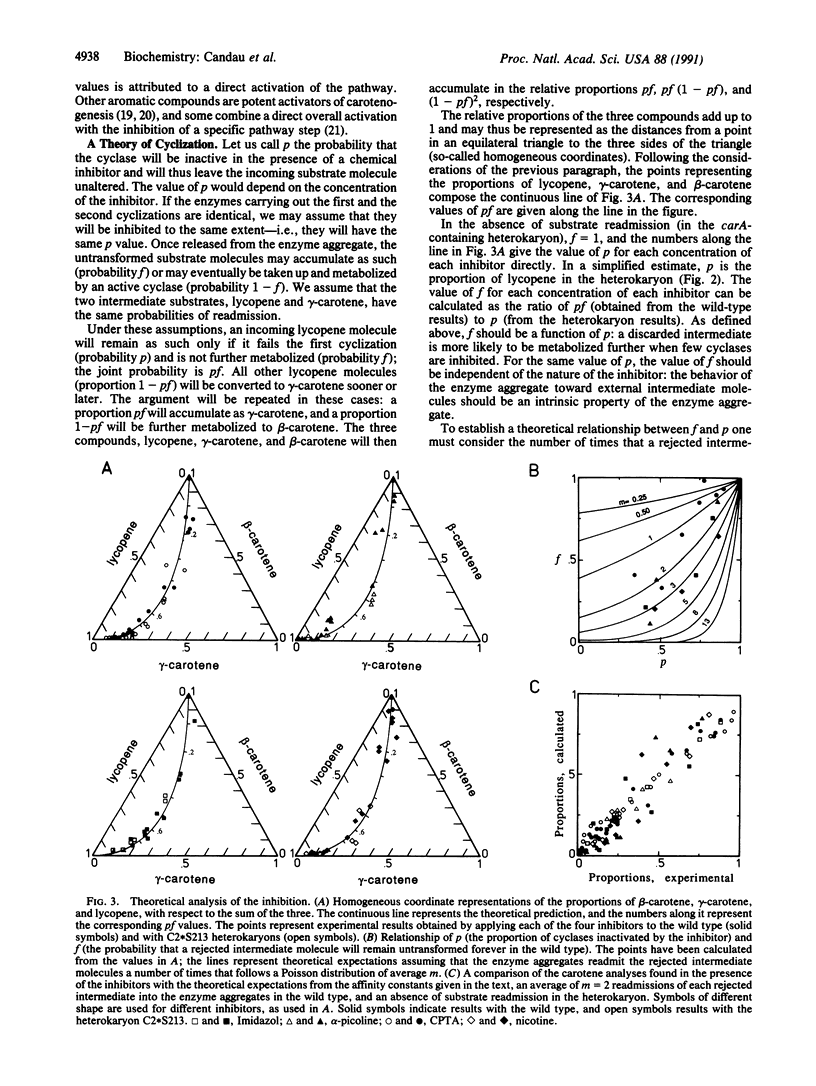
The existence and the mode of operation of certain enzyme aggregates may be established from the concentrations of intermediates measured in the presence of specific inhibitors. beta-Carotene, the most abundant carotenoid pigment in the fungus Phycomyces blakesleeanus, arises from ring formation at both ends of lycopene. The inhibitors nicotine, imidazole, alpha-picoline, and 2-(4-chlorophenylthio)triethylamine lead to the simultaneous accumulation of lycopene, beta-carotene, and the one-ring intermediate gamma-carotene. The quantitative analytical values obey precise mathematical relationships: those expected from the operation of an enzyme aggregate with two cyclases equally sensitive to the inhibitors. The intermediates lycopene and gamma-carotene rejected by chemically inhibited enzymes may be readmitted to other cyclases in the wild type but not in heterokaryons containing a carA mutation. We have calculated the fraction of inhibited cyclase under each condition, the affinity constant of each inhibitor for the cyclase, and the probability that a rejected intermediate molecule will be readmitted and further metabolized. The probabilities for lycopene and gamma-carotene are identical and independent of the inhibitor responsible for the rejection. Our calculations suggest that each rejected intermediate molecule is readmitted to the enzyme aggregates two or three times on the average.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aragon C. M., Murillo F. J., de la Guardia M. D., Cerda-Olmedo E. An enzyme complex for the dehydrogenation of phytoene in Phycomyces. Eur J Biochem. 1976 Mar 16;63(1):71–75. doi: 10.1111/j.1432-1033.1976.tb10208.x. [DOI] [PubMed] [Google Scholar]
- Coggins C. W., Jr, Henning G. L., Yokoyama H. Lycopene accumulation induced by 2-(4-chlorophenylthio)-triethylamine hydrochloride. Science. 1970 Jun 26;168(3939):1589–1590. doi: 10.1126/science.168.3939.1589. [DOI] [PubMed] [Google Scholar]
- Davies B. H. Carotene biosynthesis in fungi. Pure Appl Chem. 1973;35(1):1–28. doi: 10.1351/pac197335010001. [DOI] [PubMed] [Google Scholar]
- De la Guardia M. D., Aragón C. M., Murillo F. J., Cerdá-Olmedo E. A carotenogenic enzyme aggregate in Phycomyces: evidence from quantitive complementation. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2012–2015. doi: 10.1073/pnas.68.9.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisenberg M., Cerdá-Olmedo E. Segregation of heterokaryons in the asexual cycle of Phycomyces. Mol Gen Genet. 1968;102(3):187–195. doi: 10.1007/BF00385973. [DOI] [PubMed] [Google Scholar]
- Howes C. D., Batra P. P. Mechanism of photoinduced carotenoid synthesis. Further studies on the action spectrum and other aspects of carotenogenesis. Arch Biochem Biophys. 1970 Mar;137(1):175–180. doi: 10.1016/0003-9861(70)90424-8. [DOI] [PubMed] [Google Scholar]
- Kacser H., Sauro H. M., Acerenza L. Enzyme-enzyme interactions and control analysis. 1. The case of non-additivity: monomer-oligomer associations. Eur J Biochem. 1990 Feb 14;187(3):481–491. doi: 10.1111/j.1432-1033.1990.tb15329.x. [DOI] [PubMed] [Google Scholar]
- Murillo F. J., Cerdá-Olmedo E. Regulation of carotene synthesis in Phycomyces. Mol Gen Genet. 1976 Oct 18;148(1):19–24. doi: 10.1007/BF00268541. [DOI] [PubMed] [Google Scholar]
- Sauro H. M., Kacser H. Enzyme-enzyme interactions and control analysis. 2. The case of non-independence: heterologous associations. Eur J Biochem. 1990 Feb 14;187(3):493–500. doi: 10.1111/j.1432-1033.1990.tb15330.x. [DOI] [PubMed] [Google Scholar]
- Simpson C. N., Maskell J. P., Williams J. D. The effect of clavulanic acid on the susceptibility of Bacteroides fragilis to three acyl-ureidopenicillins, ampicillin and carbenicillin. J Antimicrob Chemother. 1984 Aug;14(2):133–138. doi: 10.1093/jac/14.2.133. [DOI] [PubMed] [Google Scholar]
- Srere P. A. Complexes of sequential metabolic enzymes. Annu Rev Biochem. 1987;56:89–124. doi: 10.1146/annurev.bi.56.070187.000513. [DOI] [PubMed] [Google Scholar]
- Welch G. R., Keleti T., Vértessy B. The control of cell metabolism for homogeneous vs. heterogeneous enzyme systems. J Theor Biol. 1988 Feb 21;130(4):407–422. doi: 10.1016/s0022-5193(88)80206-6. [DOI] [PubMed] [Google Scholar]



