Abstract
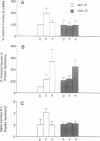
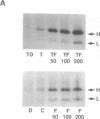
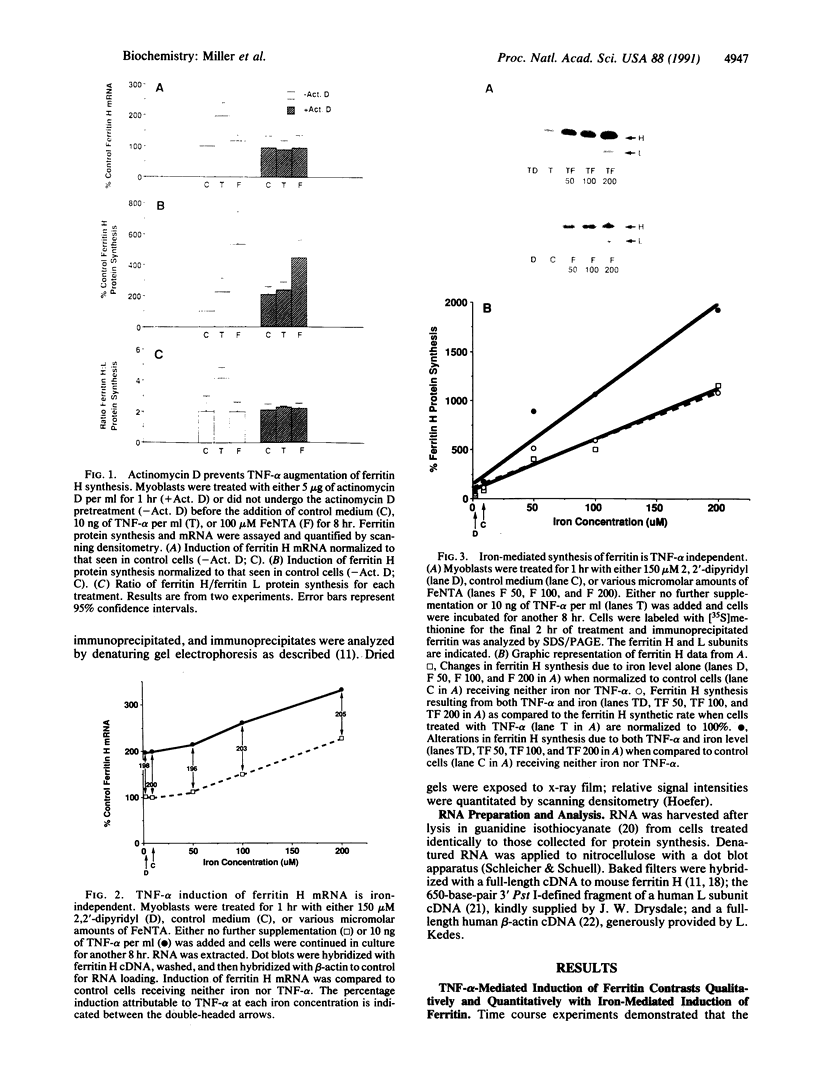
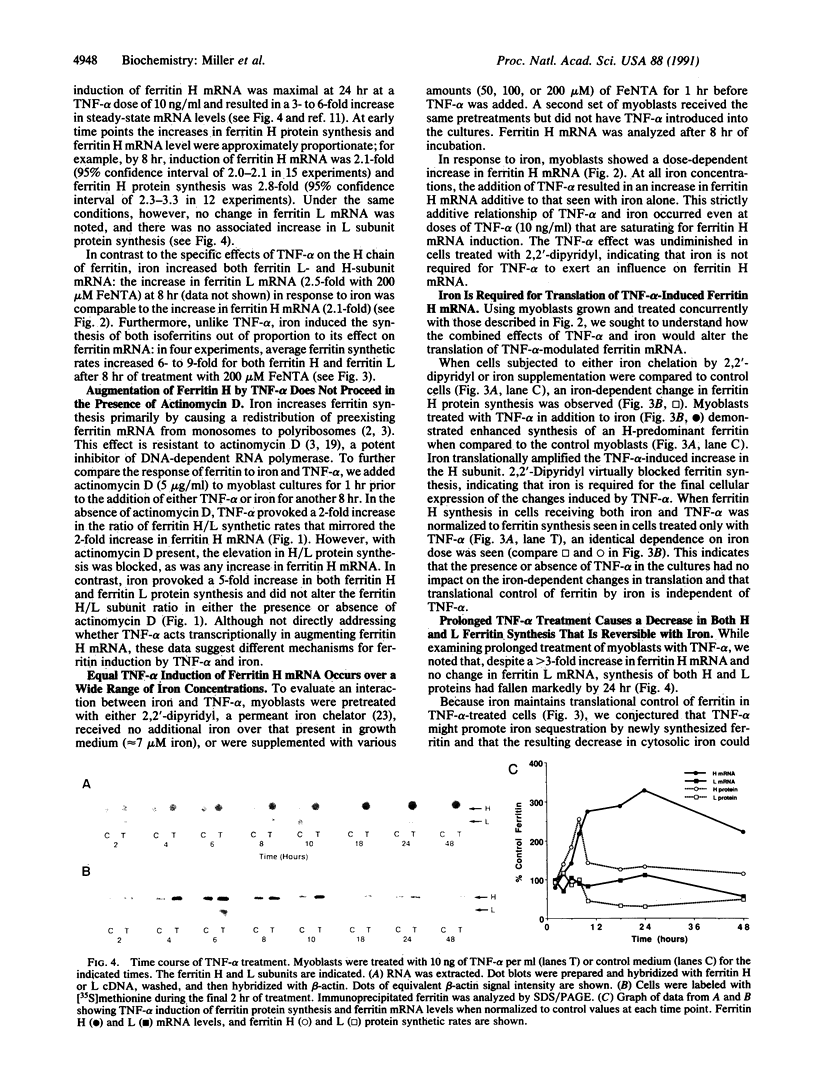
Iron increases the synthesis of the iron-storage protein, ferritin, largely by promoting translation of preexisting mRNAs for both the H and L ferritin isoforms (H, heavy, heart, acidic; L, light, liver, basic). We have recently cloned and sequenced a full-length cDNA to murine ferritin H and identified ferritin H as a gene induced by tumor necrosis factor alpha (TNF-alpha, cachectin). Using primary human myoblasts, we have now examined the relationship between TNF-alpha and iron in regulating ferritin. Four lines of evidence suggest that TNF-alpha regulates ferritin independently of iron. First, evaluation of mRNA showed that TNF-alpha increased ferritin H chain specifically, provoking no change in steady-state levels of ferritin L mRNA; iron, in contrast, increased the mRNA of both isoforms. Second, the increase in ferritin H protein synthesis observed during TNF-alpha treatment was dependent on an increase in ferritin H mRNA: actinomycin D blocked the TNF-alpha-induced changes in ferritin H but did not inhibit the translational induction of ferritin seen with iron treatment. Third, equal ferritin mRNA induction was observed in iron-loaded cells and in cells depleted of iron by a permeant chelator, 2,2'-dipyridyl. Fourth, ferritin H induction by TNF-alpha and iron was additive over the entire range of iron concentrations, even at TNF-alpha doses known to maximally stimulate ferritin H mRNA levels. Nonetheless, the role of iron in translational regulation of ferritin was retained in TNF-alpha-treated cells; effective biosynthesis of TNF-alpha-induced, H-subunit-predominant ferritin protein required iron and could be enhanced by treatment of the cells with additional iron or blocked by 2,2'-dipyridyl. Finally, we observed that the TNF-alpha-mediated increase in ferritin synthesis peaked at 8 hr and was followed by a decrease in both H and L isoferritin synthesis; the addition of iron, however, reversed the late-occurring depression in ferritin synthesis. This suggests that TNF-alpha-induced synthesis of H-rich ferritin may reduce the regulatory pool of intracellular iron, secondarily inhibiting iron-mediated translation of ferritin mRNA. We conclude that TNF-alpha acts independently of iron in its induction of ferritin H mRNA but requires the presence of iron for this effect to be fully expressed at the protein level.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aust S. D., Morehouse L. A., Thomas C. E. Role of metals in oxygen radical reactions. J Free Radic Biol Med. 1985;1(1):3–25. doi: 10.1016/0748-5514(85)90025-x. [DOI] [PubMed] [Google Scholar]
- Aziz N., Munro H. N. Both subunits of rat liver ferritin are regulated at a translational level by iron induction. Nucleic Acids Res. 1986 Jan 24;14(2):915–927. doi: 10.1093/nar/14.2.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz N., Munro H. N. Iron regulates ferritin mRNA translation through a segment of its 5' untranslated region. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8478–8482. doi: 10.1073/pnas.84.23.8478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baynes R., Bezwoda W., Bothwell T., Khan Q., Mansoor N. The non-immune inflammatory response: serial changes in plasma iron, iron-binding capacity, lactoferrin, ferritin and C-reactive protein. Scand J Clin Lab Invest. 1986 Nov;46(7):695–704. doi: 10.3109/00365518609083733. [DOI] [PubMed] [Google Scholar]
- Beutler B., Cerami A. The history, properties, and biological effects of cachectin. Biochemistry. 1988 Oct 4;27(20):7575–7582. doi: 10.1021/bi00420a001. [DOI] [PubMed] [Google Scholar]
- Birgegård G., Caro J. Increased ferritin synthesis and iron uptake in inflammatory mouse macrophages. Scand J Haematol. 1984 Jul;33(1):43–48. doi: 10.1111/j.1600-0609.1984.tb02208.x. [DOI] [PubMed] [Google Scholar]
- Bomford A., Berger M., Lis Y., Williams R. The iron content of human liver and spleen isoferritins correlates with their isoelectric point and subunit composition. Biochem Biophys Res Commun. 1978 Jul 14;83(1):334–341. doi: 10.1016/0006-291x(78)90436-9. [DOI] [PubMed] [Google Scholar]
- Boyd D., Vecoli C., Belcher D. M., Jain S. K., Drysdale J. W. Structural and functional relationships of human ferritin H and L chains deduced from cDNA clones. J Biol Chem. 1985 Sep 25;260(21):11755–11761. [PubMed] [Google Scholar]
- Cairo G., Bardella L., Schiaffonati L., Arosio P., Levi S., Bernelli-Zazzera A. Multiple mechanisms of iron-induced ferritin synthesis in HeLa cells. Biochem Biophys Res Commun. 1985 Nov 27;133(1):314–321. doi: 10.1016/0006-291x(85)91877-7. [DOI] [PubMed] [Google Scholar]
- Cazzola M., Arosio P., Bellotti V., Bergamaschi G., Dezza L., Iacobello C., Ruggeri G., Zappone E., Albertini A., Ascari E. Immunological reactivity of serum ferritin in patients with malignancy. Tumori. 1985 Dec 31;71(6):547–554. doi: 10.1177/030089168507100606. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cox F., Gestautas J., Rapoport B. Molecular cloning of cDNA corresponding to mRNA species whose steady state levels in the thyroid are enhanced by thyrotropin. Homology of one of these sequences with ferritin H. J Biol Chem. 1988 May 25;263(15):7060–7067. [PubMed] [Google Scholar]
- Dorner M. H., Silverstone A. E., de Sostoa A., Munn G., de Sousa M. Relative subunit composition of the ferritin synthesized by selected human lymphomyeloid cell populations. Exp Hematol. 1983 Oct;11(9):866–872. [PubMed] [Google Scholar]
- El-Shobaki F., Rummel W. Mucosal iron binding proteins and the inhibition of iron absorption by endotoxin. Blut. 1985 Feb;50(2):95–101. doi: 10.1007/BF00321172. [DOI] [PubMed] [Google Scholar]
- Elin R. J., Wolff S. M., Finch C. A. Effect of induced fever on serum iron and ferritin concentrations in man. Blood. 1977 Jan;49(1):147–153. [PubMed] [Google Scholar]
- Finkelstein R. A., Sciortino C. V., McIntosh M. A. Role of iron in microbe-host interactions. Rev Infect Dis. 1983 Sep-Oct;5 (Suppl 4):S759–S777. doi: 10.1093/clinids/5.supplement_4.s759. [DOI] [PubMed] [Google Scholar]
- Gunning P., Ponte P., Okayama H., Engel J., Blau H., Kedes L. Isolation and characterization of full-length cDNA clones for human alpha-, beta-, and gamma-actin mRNAs: skeletal but not cytoplasmic actins have an amino-terminal cysteine that is subsequently removed. Mol Cell Biol. 1983 May;3(5):787–795. doi: 10.1128/mcb.3.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentze M. W., Caughman S. W., Rouault T. A., Barriocanal J. G., Dancis A., Harford J. B., Klausner R. D. Identification of the iron-responsive element for the translational regulation of human ferritin mRNA. Science. 1987 Dec 11;238(4833):1570–1573. doi: 10.1126/science.3685996. [DOI] [PubMed] [Google Scholar]
- Hentze M. W., Rouault T. A., Caughman S. W., Dancis A., Harford J. B., Klausner R. D. A cis-acting element is necessary and sufficient for translational regulation of human ferritin expression in response to iron. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6730–6734. doi: 10.1073/pnas.84.19.6730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibold E. A., Munro H. N. Cytoplasmic protein binds in vitro to a highly conserved sequence in the 5' untranslated region of ferritin heavy- and light-subunit mRNAs. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2171–2175. doi: 10.1073/pnas.85.7.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi S., Luzzago A., Cesareni G., Cozzi A., Franceschinelli F., Albertini A., Arosio P. Mechanism of ferritin iron uptake: activity of the H-chain and deletion mapping of the ferro-oxidase site. A study of iron uptake and ferro-oxidase activity of human liver, recombinant H-chain ferritins, and of two H-chain deletion mutants. J Biol Chem. 1988 Dec 5;263(34):18086–18092. [PubMed] [Google Scholar]
- Mattia E., Josic D., Ashwell G., Klausner R., van Renswoude J. Regulation of intracellular iron distribution in K562 human erythroleukemia cells. J Biol Chem. 1986 Apr 5;261(10):4587–4593. [PubMed] [Google Scholar]
- Michie H. R., Spriggs D. R., Manogue K. R., Sherman M. L., Revhaug A., O'Dwyer S. T., Arthur K., Dinarello C. A., Cerami A., Wolff S. M. Tumor necrosis factor and endotoxin induce similar metabolic responses in human beings. Surgery. 1988 Aug;104(2):280–286. [PubMed] [Google Scholar]
- Miller S. C., Ito H., Blau H. M., Torti F. M. Tumor necrosis factor inhibits human myogenesis in vitro. Mol Cell Biol. 1988 Jun;8(6):2295–2301. doi: 10.1128/mcb.8.6.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Núez M. T., Gaete V., Watkins J. A., Glass J. Mobilization of iron from endocytic vesicles. The effects of acidification and reduction. J Biol Chem. 1990 Apr 25;265(12):6688–6692. [PubMed] [Google Scholar]
- Rittling S. R., Woodworth R. C. The synthesis and turnover of ferritin in rat L-6 cells. Rates and response to iron, actinomycin D, and desferrioxamine. J Biol Chem. 1984 May 10;259(9):5561–5566. [PubMed] [Google Scholar]
- Rogers J., Munro H. Translation of ferritin light and heavy subunit mRNAs is regulated by intracellular chelatable iron levels in rat hepatoma cells. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2277–2281. doi: 10.1073/pnas.84.8.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouault T. A., Hentze M. W., Caughman S. W., Harford J. B., Klausner R. D. Binding of a cytosolic protein to the iron-responsive element of human ferritin messenger RNA. Science. 1988 Sep 2;241(4870):1207–1210. doi: 10.1126/science.3413484. [DOI] [PubMed] [Google Scholar]
- Rouault T. A., Hentze M. W., Dancis A., Caughman W., Harford J. B., Klausner R. D. Influence of altered transcription on the translational control of human ferritin expression. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6335–6339. doi: 10.1073/pnas.84.18.6335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theil E. C. Ferritin: structure, gene regulation, and cellular function in animals, plants, and microorganisms. Annu Rev Biochem. 1987;56:289–315. doi: 10.1146/annurev.bi.56.070187.001445. [DOI] [PubMed] [Google Scholar]
- Torti S. V., Kwak E. L., Miller S. C., Miller L. L., Ringold G. M., Myambo K. B., Young A. P., Torti F. M. The molecular cloning and characterization of murine ferritin heavy chain, a tumor necrosis factor-inducible gene. J Biol Chem. 1988 Sep 5;263(25):12638–12644. [PubMed] [Google Scholar]
- Ulich T. R., Guo K., del Castillo J. Endotoxin-induced cytokine gene expression in vivo. I. Expression of tumor necrosis factor mRNA in visceral organs under physiologic conditions and during endotoxemia. Am J Pathol. 1989 Jan;134(1):11–14. [PMC free article] [PubMed] [Google Scholar]
- Ursini M. V., de Franciscis V. TSH regulation of ferritin H chain messenger RNA levels in the rat thyroids. Biochem Biophys Res Commun. 1988 Jan 15;150(1):287–295. doi: 10.1016/0006-291x(88)90518-9. [DOI] [PubMed] [Google Scholar]
- Wagstaff M., Worwood M., Jacobs A. Properties of human tissue isoferritins. Biochem J. 1978 Sep 1;173(3):969–977. doi: 10.1042/bj1730969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walden W. E., Daniels-McQueen S., Brown P. H., Gaffield L., Russell D. A., Bielser D., Bailey L. C., Thach R. E. Translational repression in eukaryotes: partial purification and characterization of a repressor of ferritin mRNA translation. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9503–9507. doi: 10.1073/pnas.85.24.9503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y., Miller S. C., Tsuji Y., Torti S. V., Torti F. M. Interleukin 1 induces ferritin heavy chain in human muscle cells. Biochem Biophys Res Commun. 1990 May 31;169(1):289–296. doi: 10.1016/0006-291x(90)91466-6. [DOI] [PubMed] [Google Scholar]
- White K., Munro H. N. Induction of ferritin subunit synthesis by iron is regulated at both the transcriptional and translational levels. J Biol Chem. 1988 Jun 25;263(18):8938–8942. [PubMed] [Google Scholar]