Abstract
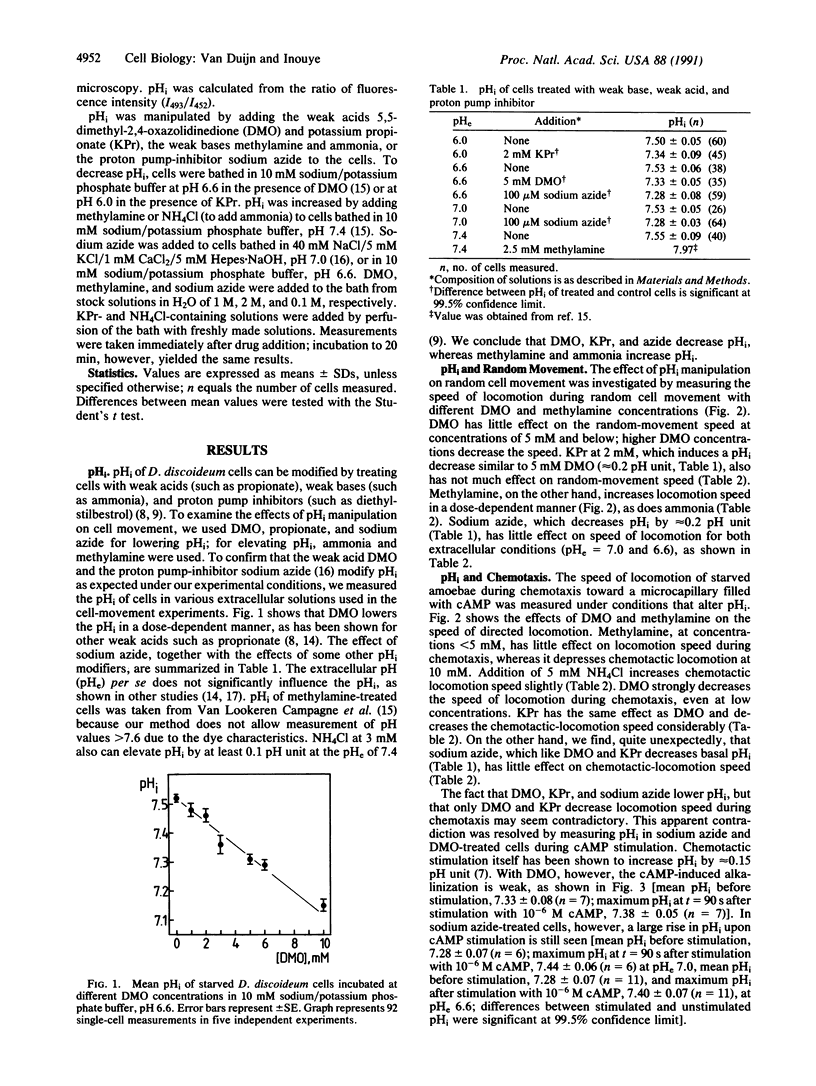
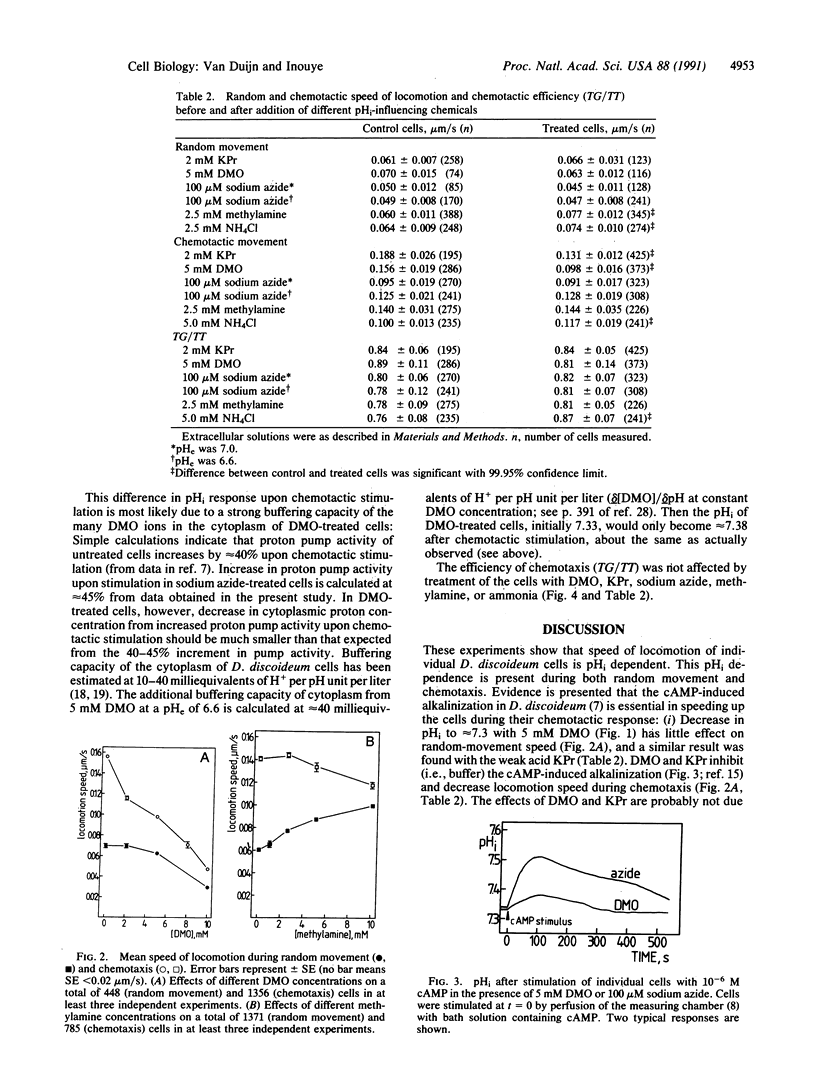
Evidence is presented that the chemoattractant-induced cytoplasmic alkalinization in the cellular slime mold Dictyostelium discoideum is essential in regulating locomotion speed during chemotaxis. Intracellular pH was manipulated with weak bases, weak acids, and proton-pump inhibition. Speed of locomotion of individual cells was measured during random and chemotactic movement. We found that (i) an increase of cytoplasmic pH increases the speed of randomly moving cells and (ii) the chemoattractant-induced rise in intracellular pH is essential for the increase in directed locomotion speed upon chemotactic stimulation. In addition, our experiments support the hypothesis that ammonia plays a key role in the thermo- and phototaxis of migrating slugs by increasing the locomotion speed of individual cells through changes in intracellular pH.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aerts R. J., De Wit R. J., Van Lookeren Campagne M. M. Cyclic AMP induces a transient alkalinization in Dictyostelium. FEBS Lett. 1987 Aug 17;220(2):366–370. doi: 10.1016/0014-5793(87)80848-7. [DOI] [PubMed] [Google Scholar]
- Aerts R. J., Durston A. J., Moolenaar W. H. Cytoplasmic pH and the regulation of the Dictyostelium cell cycle. Cell. 1985 Dec;43(3 Pt 2):653–657. doi: 10.1016/0092-8674(85)90237-5. [DOI] [PubMed] [Google Scholar]
- Bonner J. T., Chiang A., Lee J., Suthers H. B. The possible role of ammonia in phototaxis of migrating slugs of Dictyostelium discoideum. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3885–3887. doi: 10.1073/pnas.85.11.3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner J. T., Har D., Suthers H. B. Ammonia and thermotaxis: Further evidence for a central role of ammonia in the directed cell mass movements of Dictyostelium discoideum. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2733–2736. doi: 10.1073/pnas.86.8.2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M. H., Robertson A. Chemotaxis and the early stages of aggregation in cellular slime molds. J Theor Biol. 1971 Apr;31(1):119–130. doi: 10.1016/0022-5193(71)90125-1. [DOI] [PubMed] [Google Scholar]
- Condeelis J., Vahey M. A calcium- and pH-regulated protein from Dictyostelium discoideum that cross-links actin filaments. J Cell Biol. 1982 Aug;94(2):466–471. doi: 10.1083/jcb.94.2.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condeelis J., Vahey M., Carboni J. M., DeMey J., Ogihara S. Properties of the 120,000- and 95,000-dalton actin-binding proteins from Dictyostelium discoideum and their possible functions in assembling the cytoplasmic matrix. J Cell Biol. 1984 Jul;99(1 Pt 2):119s–126s. doi: 10.1083/jcb.99.1.119s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devreotes P. N., Zigmond S. H. Chemotaxis in eukaryotic cells: a focus on leukocytes and Dictyostelium. Annu Rev Cell Biol. 1988;4:649–686. doi: 10.1146/annurev.cb.04.110188.003245. [DOI] [PubMed] [Google Scholar]
- Durston A. J., Vork F. The spatial pattern of DNA synthesis in Dictyostelium discoideum slugs. Exp Cell Res. 1978 Sep;115(2):454–457. doi: 10.1016/0014-4827(78)90308-7. [DOI] [PubMed] [Google Scholar]
- Heiple J. M., Taylor D. L. Intracellular pH in single motile cells. J Cell Biol. 1980 Sep;86(3):885–890. doi: 10.1083/jcb.86.3.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ince C., van Dissel J. T., Diesselhoff M. M. A teflon culture dish for high-magnification microscopy and measurements in single cells. Pflugers Arch. 1985 Mar;403(3):240–244. doi: 10.1007/BF00583594. [DOI] [PubMed] [Google Scholar]
- Inouye K. Measurements of intracellular pH and its relevance to cell differentiation in Dictyostelium discoideum. J Cell Sci. 1985 Jun;76:235–245. doi: 10.1242/jcs.76.1.235. [DOI] [PubMed] [Google Scholar]
- Kay R. R., Gadian D. G., Williams S. R. Intracellular pH in Dictyostelium: a 31P nuclear magnetic resonance study of its regulation and possible role in controlling cell differentiation. J Cell Sci. 1986 Jul;83:165–179. doi: 10.1242/jcs.83.1.165. [DOI] [PubMed] [Google Scholar]
- Potel M. J., Mackay S. A. Preaggregative cell motion in Dictyostelium. J Cell Sci. 1979 Apr;36:281–309. doi: 10.1242/jcs.36.1.281. [DOI] [PubMed] [Google Scholar]
- Roos A., Boron W. F. Intracellular pH. Physiol Rev. 1981 Apr;61(2):296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- SAMUEL E. W. Orientation and rate of locomotion of individual amebas in the life cycle of the cellular slime mold Dictyostelium mucoroides. Dev Biol. 1961 Jun;3:317–335. doi: 10.1016/0012-1606(61)90050-1. [DOI] [PubMed] [Google Scholar]
- Scheel J., Ziegelbauer K., Kupke T., Humbel B. M., Noegel A. A., Gerisch G., Schleicher M. Hisactophilin, a histidine-rich actin-binding protein from Dictyostelium discoideum. J Biol Chem. 1989 Feb 15;264(5):2832–2839. [PubMed] [Google Scholar]
- Simchowitz L., Cragoe E. J., Jr Regulation of human neutrophil chemotaxis by intracellular pH. J Biol Chem. 1986 May 15;261(14):6492–6500. [PubMed] [Google Scholar]
- Van Duijn B., Vogelzang S. A., Ypey D. L., Van der Molen L. G., Van Haastert P. J. Normal chemotaxis in Dictyostelium discoideum cells with a depolarized plasma membrane potential. J Cell Sci. 1990 Jan;95(Pt 1):177–183. doi: 10.1242/jcs.95.1.177. [DOI] [PubMed] [Google Scholar]
- Van Lookeren Campagne M. M., Aerts R. J., Spek W., Firtel R. A., Schaap P. Cyclic-AMP-induced elevation of intracellular pH precedes, but does not mediate, the induction of prespore differentiation in Dictyostelium discoideum. Development. 1989 Feb;105(2):401–406. doi: 10.1242/dev.105.2.401. [DOI] [PubMed] [Google Scholar]
- Wang M., Roelfsema J. H., Williams J. G., Schaap P. Cytoplasmic acidification facilitates but does not mediate DIF-induced prestalk gene expression in Dictyostelium discoideum. Dev Biol. 1990 Jul;140(1):182–188. doi: 10.1016/0012-1606(90)90065-q. [DOI] [PubMed] [Google Scholar]



