Abstract
Kinesin is a mechanochemical ATPase that induces translocation of latex beads along microtubules and microtubule gliding on a glass surface. This protein is thought to be a motor for the movement of membranous organelles in cells. Recently Hollenbeck and Swanson [Hollenbeck, P. J. & Swanson, J. A. (1990) Nature (London) 346, 864-866] showed that kinesin is involved in the positioning of tubular lysosomes in macrophages. However, the role of this protein in the movement of organelles was not yet clear. We used a polyclonal antibody against the kinesin heavy chain that inhibited kinesin-dependent microtubule gliding in vitro to study the role of kinesin in the movement of pigment granules in melanophores of the teleost black tetra (Gymnocorymbus ternetzi). Microinjection of the antibody into cultured melanophores did not produce any specific effect on the aggregation of pigment granules in melanophores, but it did result in a strong dose-dependent inhibition of the dispersion. Immunoblotting of melanophore extracts showed that the kinesin antibody reacted in these cells with a single protein component with a molecular mass of 135 kDa. Thus, kinesin is responsible for the movement of pigment granules from the center to the periphery of the melanophore.
Full text
PDF
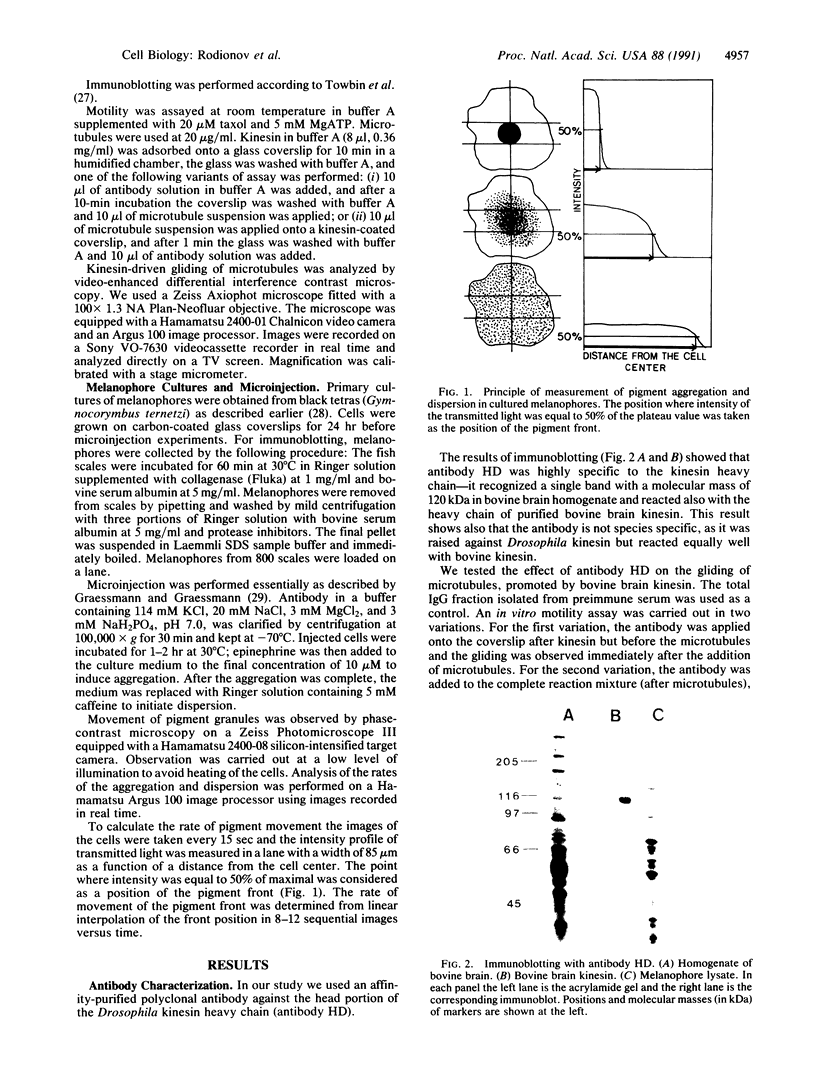
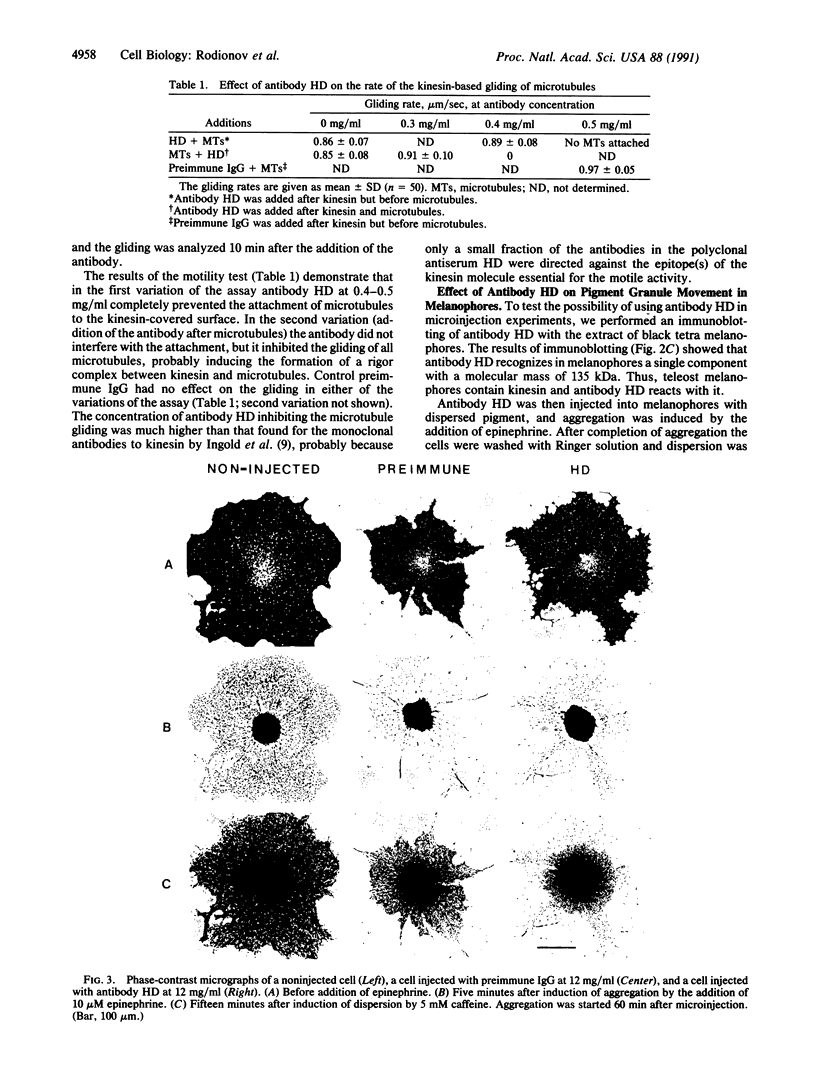


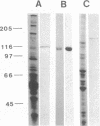
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckerle M. C., Porter K. R. Analysis of the role of microtubules and actin in erythrophore intracellular motility. J Cell Biol. 1983 Feb;96(2):354–362. doi: 10.1083/jcb.96.2.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom G. S., Wagner M. C., Pfister K. K., Brady S. T. Native structure and physical properties of bovine brain kinesin and identification of the ATP-binding subunit polypeptide. Biochemistry. 1988 May 3;27(9):3409–3416. doi: 10.1021/bi00409a043. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brady S. T., Pfister K. K., Bloom G. S. A monoclonal antibody against kinesin inhibits both anterograde and retrograde fast axonal transport in squid axoplasm. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1061–1065. doi: 10.1073/pnas.87.3.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark T. G., Rosenbaum J. L. Energy requirements for pigment aggregation in fundulus melanophores. Cell Motil. 1984;4(6):431–441. doi: 10.1002/cm.970040604. [DOI] [PubMed] [Google Scholar]
- Cohn S. A., Ingold A. L., Scholey J. M. Quantitative analysis of sea urchin egg kinesin-driven microtubule motility. J Biol Chem. 1989 Mar 15;264(8):4290–4297. [PubMed] [Google Scholar]
- Euteneuer U., McIntosh J. R. Polarity of some motility-related microtubules. Proc Natl Acad Sci U S A. 1981 Jan;78(1):372–376. doi: 10.1073/pnas.78.1.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelfand V. I. Cytoplasmic microtubular motors. Curr Opin Cell Biol. 1989 Feb;1(1):63–66. doi: 10.1016/s0955-0674(89)80038-9. [DOI] [PubMed] [Google Scholar]
- Graessmann M., Graessman A. "Early" simian-virus-40-specific RNA contains information for tumor antigen formation and chromatin replication. Proc Natl Acad Sci U S A. 1976 Feb;73(2):366–370. doi: 10.1073/pnas.73.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyoeva F. K., Leonova E. V., Rodionov V. I., Gelfand V. I. Vimentin intermediate filaments in fish melanophores. J Cell Sci. 1987 Dec;88(Pt 5):649–655. doi: 10.1242/jcs.88.5.649. [DOI] [PubMed] [Google Scholar]
- Hollenbeck P. J., Swanson J. A. Radial extension of macrophage tubular lysosomes supported by kinesin. Nature. 1990 Aug 30;346(6287):864–866. doi: 10.1038/346864a0. [DOI] [PubMed] [Google Scholar]
- Ingold A. L., Cohn S. A., Scholey J. M. Inhibition of kinesin-driven microtubule motility by monoclonal antibodies to kinesin heavy chains. J Cell Biol. 1988 Dec;107(6 Pt 2):2657–2667. doi: 10.1083/jcb.107.6.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsov S. A., Gelfand V. I. Bovine brain kinesin is a microtubule-activated ATPase. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8530–8534. doi: 10.1073/pnas.83.22.8530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsov S. A., Vaisberg E. A., Shanina N. A., Magretova N. N., Chernyak V. Y., Gelfand V. I. The quaternary structure of bovine brain kinesin. EMBO J. 1988 Feb;7(2):353–356. doi: 10.1002/j.1460-2075.1988.tb02820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lynch T. J., Taylor J. D., Tchen T. T. Regulation of pigment organelle translocation. I. Phosphorylation of the organelle-associated protein p57. J Biol Chem. 1986 Mar 25;261(9):4204–4211. [PubMed] [Google Scholar]
- McNiven M. A., Ward J. B. Calcium regulation of pigment transport in vitro. J Cell Biol. 1988 Jan;106(1):111–125. doi: 10.1083/jcb.106.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai K., Thøgersen H. C. Synthesis and sequence-specific proteolysis of hybrid proteins produced in Escherichia coli. Methods Enzymol. 1987;153:461–481. doi: 10.1016/0076-6879(87)53072-5. [DOI] [PubMed] [Google Scholar]
- Pfister K. K., Wagner M. C., Stenoien D. L., Brady S. T., Bloom G. S. Monoclonal antibodies to kinesin heavy and light chains stain vesicle-like structures, but not microtubules, in cultured cells. J Cell Biol. 1989 Apr;108(4):1453–1463. doi: 10.1083/jcb.108.4.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozdzial M. M., Haimo L. T. Bidirectional pigment granule movements of melanophores are regulated by protein phosphorylation and dephosphorylation. Cell. 1986 Dec 26;47(6):1061–1070. doi: 10.1016/0092-8674(86)90821-4. [DOI] [PubMed] [Google Scholar]
- Rozdzial M. M., Haimo L. T. Reactivated melanophore motility: differential regulation and nucleotide requirements of bidirectional pigment granule transport. J Cell Biol. 1986 Dec;103(6 Pt 2):2755–2764. doi: 10.1083/jcb.103.6.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schliwa M., Osborn M., Weber K. Microtubule system of isolated fish melanophores as revealed by immunofluorescence microscopy. J Cell Biol. 1978 Jan;76(1):229–236. doi: 10.1083/jcb.76.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroer T. A., Schnapp B. J., Reese T. S., Sheetz M. P. The role of kinesin and other soluble factors in organelle movement along microtubules. J Cell Biol. 1988 Nov;107(5):1785–1792. doi: 10.1083/jcb.107.5.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelanski M. L., Gaskin F., Cantor C. R. Microtubule assembly in the absence of added nucleotides. Proc Natl Acad Sci U S A. 1973 Mar;70(3):765–768. doi: 10.1073/pnas.70.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale R. D., Hotani H. Formation of membrane networks in vitro by kinesin-driven microtubule movement. J Cell Biol. 1988 Dec;107(6 Pt 1):2233–2241. doi: 10.1083/jcb.107.6.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale R. D. Intracellular transport using microtubule-based motors. Annu Rev Cell Biol. 1987;3:347–378. doi: 10.1146/annurev.cb.03.110187.002023. [DOI] [PubMed] [Google Scholar]
- Vale R. D., Reese T. S., Sheetz M. P. Identification of a novel force-generating protein, kinesin, involved in microtubule-based motility. Cell. 1985 Aug;42(1):39–50. doi: 10.1016/s0092-8674(85)80099-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale R. D., Schnapp B. J., Mitchison T., Steuer E., Reese T. S., Sheetz M. P. Different axoplasmic proteins generate movement in opposite directions along microtubules in vitro. Cell. 1985 Dec;43(3 Pt 2):623–632. doi: 10.1016/0092-8674(85)90234-x. [DOI] [PubMed] [Google Scholar]
- Weingarten M. D., Lockwood A. H., Hwo S. Y., Kirschner M. W. A protein factor essential for microtubule assembly. Proc Natl Acad Sci U S A. 1975 May;72(5):1858–1862. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikswo M. A., Novales R. R. Effect of colchicine on microtubules in the melanophores of Fundulus heteroclitus. J Ultrastruct Res. 1972 Nov;41(3):189–201. doi: 10.1016/s0022-5320(72)90063-9. [DOI] [PubMed] [Google Scholar]
- Yang J. T., Laymon R. A., Goldstein L. S. A three-domain structure of kinesin heavy chain revealed by DNA sequence and microtubule binding analyses. Cell. 1989 Mar 10;56(5):879–889. doi: 10.1016/0092-8674(89)90692-2. [DOI] [PubMed] [Google Scholar]
- Yang J. T., Saxton W. M., Stewart R. J., Raff E. C., Goldstein L. S. Evidence that the head of kinesin is sufficient for force generation and motility in vitro. Science. 1990 Jul 6;249(4964):42–47. doi: 10.1126/science.2142332. [DOI] [PubMed] [Google Scholar]