Abstract
Mouse embryo fibroblasts deficient for the c-Jun proto-oncogene (c-Jun−/− MEF) undergo p53-dependent premature senescence in conventional culture. This phenotype becomes evident only after several cell divisions, suggesting that senescence may result from exposure to unknown environmental factors. Here, we show that c-Jun−/− MEF can proliferate successfully in low oxygen (3% O2), indicating that premature senescence under conventional culture conditions is a consequence of hyperoxic stress. c-Jun−/− MEF exhibit higher basal levels of DNA damage compared to normal fibroblasts in high but not low oxygen, implying that senescence results from chronic accumulation of spontaneous DNA damage. This accumulation may be attributable, at least in part, to inefficient repair, since DNA damage induced by γ ionizing radiation and H2O2 persists for longer in c-Jun−/− MEF than in wild-type MEF. Unexpectedly, p53 expression, phosphorylation, and transcriptional activity are largely unaffected by oxygen exposure, indicating that the accumulation of spontaneous DNA damage does not result in chronic activation of p53 as judged by conventional criteria. Finally, we find that c-Jun associates with nuclear foci containing γH2AX and ATM following irradiation, suggesting a potential role for c-Jun in DNA repair processes per se.
c-Jun was originally identified as the normal cellular counterpart of the viral Jun (v-Jun) oncoprotein (23). It is the prototypical member of the Jun family of proteins that form stable homo- or heterodimeric complexes with Fos family members which bind to AP-1 DNA recognition elements. Studies with mouse knockout models have demonstrated that c-Jun is required for cell proliferation. c-Jun-deficient mouse embryo fibroblast cultures (c-Jun−/− MEF) show marked proliferation defects and undergo premature senescence in vitro (19, 31, 37). Genetic evidence demonstrates that these growth defects are dependent on p53, since simultaneous deletion of p53 rescues the growth defect of c-Jun−/− MEF in vitro (31). These studies imply that the c-Jun and p53 pathways are functionally linked, although the molecular nature of the cross talk between them is incompletely understood. Two alternative mechanisms have been proposed; in the first c-Jun negatively regulates p53 expression (31) or function (32), while in the second c-Jun is required to sustain expression of cyclin D1 (37). These models are mechanistically quite distinct, since they invoke either an intrinsic defect in mitogenic signaling processes or inappropriate activation of a negative cell cycle regulator which is normally induced only under conditions of damage or stress as the proximal cause of premature senescence, respectively.
The senescence phenotype of c-Jun−/− MEF becomes apparent after only a short time in culture (19, 31, 37), suggesting that this growth arrest may be due to environmental stress. The major environmental stress encountered by cells in culture is exposure to hyperoxia. Under normal physiological conditions in the embryo, cells in vivo are exposed to a maximum of 5% oxygen, in contrast to the 21% oxygen that cells experience when placed in conventional atmospheric culture. Further evidence that in vitro culture generates genotoxic stress is the finding that mouse fibroblasts isolated from day 14 gestation embryos express p53, whereas cells in the corresponding day 14 embryos do not show any detectable p53 expression (15).
These observations led to the hypothesis that hyperoxia might limit life span in mouse cells as a consequence of DNA damage accumulation. Recently, this concept has been substantiated in a report demonstrating that hyperoxia and DNA damage induced by oxidative stress are major determinants of proliferative capacity in mouse cells (26). It seems likely that significant background levels of hyperoxia-induced DNA damage are continually offset by active repair processes during atmospheric culture, since MEF that lack DNA damage sensors, such as ATM, sustain high levels of chromosomal breakage (33). Furthermore, MEF deficient for a number genes involved in signaling and/or repairing DNA damage also display premature senescence in vitro, indicating that the effects of culture hypoxia can be exacerbated by defects in DNA repair processes. Examples include cells deficient for ATM, Brca1, Hus1, Ku80, and DNA ligase IV (7, 10, 24, 36, 39).
Given the similarities between the senescence phenotype of c-Jun−/− MEF and MEF with defects in either DNA damage recognition or repair, we hypothesized that irreversible growth arrest in cells lacking c-Jun might be attributable to more rapid accumulation of DNA damage under hyperoxic culture conditions. Here, we show that c-Jun-deficient MEF can proliferate successfully under low oxygen conditions and that the growth arrest observed in conventional culture is associated with spontaneous DNA damage accumulation. We also show that DNA damage induced by ionizing radiation (IR) or hydrogen peroxide (H2O2) persists for longer than normal in cells lacking c-Jun, suggestive of diminished DNA repair capacity. Finally, we found that c-Jun itself can associate with sites of DNA damage, as judged by colocalization with nuclear foci containing γH2AX and ATM.
MATERIALS AND METHODS
MEF isolation and culture.
MEF were isolated from day 10.5 gestation embryos, washed in phosphate-buffered saline, and disaggregated with an 18-gauge needle. Following three washes in Dulbecco's modified Eagle medium (DMEM), the suspension was plated in a 60-mm dish in DMEM containing 15% fetal calf serum. MEF were isolated at 37°C and 5% CO2 in either 3 or 21% oxygen. After 24 h cells were trypsinized and replated to enrich for fibroblasts in DMEM containing 10% fetal calf serum. Until the genotypes were confirmed, each embryo was cultured separately. After 2 days, cells with an identical genotype were pooled and designated passage 1 MEF. For long-term growth experiments cells were passaged every 3 days and mean population doublings (MPD) were calculated by counting cell numbers. A total of 105 cells (or all available if less than 105 in the case of senescent cultures) were reseeded at each subculture. Established cell lines were generated by serial passage in either 3 or 21% O2, using a 3T3-like protocol.
Retroviral infection.
pBabe and pBabe c-Jun vectors were used to produce retrovirus in the Phoenix/Eco packaging cell line. pBabe c-Jun S63/73A and pBabe D284-286 were kind gifts of R. Wisdom, and pBabe Tam67-GFP was a kind gift of R. Hennigan; they have been described elsewhere (18, 37). Proliferating passage 1 MEF were incubated in viral supernatant in the presence of 5 μg of Polybrene/ml overnight to permit infection followed by selection in medium containing hygromycin (50 μg/ml).
Western blotting.
Whole-cell extracts were prepared from cultures as described previously (22). Extracts were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and analyzed by Western blotting as described previously (6). The antisera used for Western blotting were as follows: anti-p53 (Santa Cruz pAB240), anti-Ser15 phosphorylated p53 (Cell Signaling #9284), anti-p21 (Santa Cruz C-19), anti-c-Jun (Becton Dickinson 610327), anti-Erk (Sigma M5670), anti-cyclin D1 (Santa Cruz sc-200044), anti-cyclin D2 (Neomarkers Ab-3), anti-cyclin D3 (Santa Cruz sc-6283), anti-cyclin E (Santa Cruz sc-481), anti-cyclin A (Santa Cruz sc-596), and anti-Cdk2 (Santa Cruz sc-163).
Reporter assays.
The pGL3-Mdm2 or pGL3-p21 firefly luciferase reporter construct was transfected into MEF in combination with a renilla luciferase construct (pRL-SV40) (Promega). Cells were transfected overnight with 5 μg of DNA and harvested 48 h later. Cells were lysed, and firefly and renilla luciferase activities were measured with a luminometer. Firefly luciferase activities were normalized to both renilla activity (transfection efficiency) and protein concentration.
Flow cytometry.
For DNA content analysis, trypsinized cultures were fixed in 70% ethanol prior to staining with propidium iodide and analysis using a Becton-Dickinson FACScan flow cytometer.
Comet assays.
A Trevigen comet assay kit was used to perform both neutral and alkali denaturing comet assays following the manufacturer's instructions. Where cells were cultured under low oxygen conditions, buffers and cells were flushed with nitrogen until cells were immobilized in agarose and lysed to prevent oxidative damage during manipulation following transfer from low oxygen. Samples were stained with SYBR green prior to analysis by fluorescence microscopy. One hundred cells were scored per sample.
Immunocytochemistry.
Cells were cultured on glass coverslips at 3% O2 for 24 h prior to fixation in 3.7% formaldehyde as described previously (2). The antibodies used were as follows: anti-c-Jun (Becton Dickinson 610327), anti-H2AX pSer 139 (Upstate #07-164), and anti-ATM (Santa Cruz H248). All primary and secondary antibodies were used at a 1/100 dilution.
RESULTS
Premature senescence of c-Jun−/− MEF is due to hyperoxic stress.
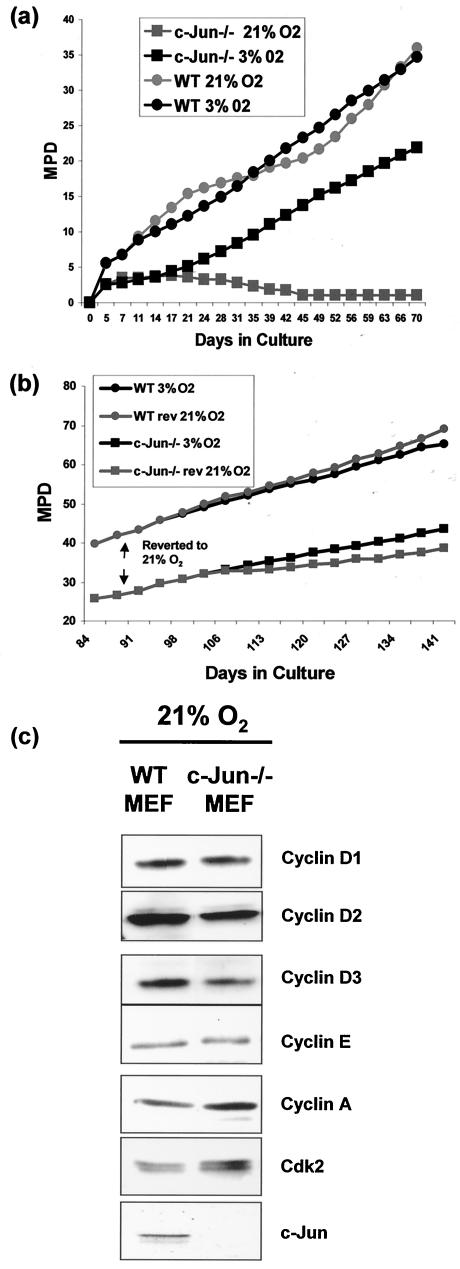
To test the hypothesis that exposure to hyperoxia might be a factor in the premature senescence of c-Jun−/− MEF, we prepared cells from c-Jun−/− and wild-type (WT) embryos, divided each culture into two, and cultured one half under normal (21% O2) conditions and the second under low oxygen (3% O2) conditions. We observed that culture in high or low oxygen had little effect on the long-term proliferative capacity of the WT MEF (Fig. 1a, ) although cells cultured in 21% O2 exhibited characteristic biphasic growth kinetics (Fig. 1a) which we believe represents crisis followed by the emergence of an immortal clone(s) (26). As described previously (19, 31, 37), c-Jun−/− MEF proliferation was negligible in 21% O2, with little or no increase in cell number after approximately 2 weeks (Fig. 1a). Strikingly, however, sister cultures maintained in 3% O2 multiplied vigorously (Fig. 1a), clearly demonstrating that c-Jun−/− MEF are capable of proliferation under appropriate conditions. To confirm that the effects of oxygen tension were due solely to the absence of c-Jun, we restored c-Jun expression in c-Jun−/− MEF by retroviral transduction (see Fig. 4 for details) and found that the proliferative capacity of the reconstituted cells under hyperoxic conditions was similar to that of WT MEF (data not shown).
FIG. 1.
Premature senescence associated with c-Jun deficiency can be rescued by culture at low oxygen. (a) WT (filled circles) and c-Jun−/− (filled squares) MEF were cultured at either 21% oxygen (grey symbols) or 3% oxygen (black symbols) continuously for 70 days (24 passages). Cells were split every 3 days, and the total numbers ofcells were counted and mean population doublings (MPD) were determined. Following isolation at 3% oxygen, cells were grown until 2 × 105 cells of each genotype were available (approximately 3 to 4 days), and then 1 × 105 cells of each genotype were plated at either high or low oxygen and assigned an MPD of 0. (b) WT (filled circles) and c-Jun−/− (filled squares) MEF cultured at low oxygen were split into two aliquots after 90 days of culture; one flask was subsequently passaged at 21% oxygen (grey symbols), and the other was passaged at 3% oxygen (black symbols). Cells were split every 3 days, and the total numbers of cells were counted and MPD were determined. (c) Western blotting analysis of cyclin D1, D2, D3, E, A, Cdk2, and c-Jun expression in cell extracts prepared from early-passage WT and c-Jun−/− MEF cultured in 21% O2.
FIG. 4.
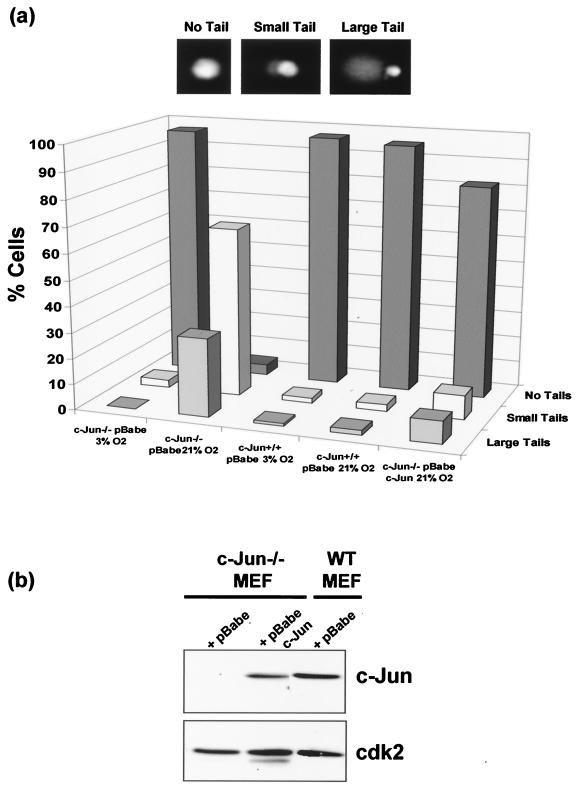
c-Jun deficiency results in increased levels of spontaneous DNA damage. (a) c-Jun−/− MEF, c-Jun−/− MEF reconstituted with ectopic c-Jun, and WT MEF were cultured at either 3 or 21% oxygen for 4 days prior to analysis. For control purposes the WT and c-Jun−/− MEF were infected with the same empty retroviral vector (pBabe) used to express ectopic c-Jun in the reconstituted cells. A denaturing comet assay was performed, and DNA was stained and examined by fluorescence microscopy. (b) Western blotting analysis of c-Jun expression levels in the cell cultures used for panel a.
To determine if c-Jun−/− MEF cultured at 3% O2 remained susceptible to growth inhibition by culture at 21% O2, we transferred cells cultured at 3% O2 for 90 days to 21% O2 and monitored the growth of the “reverted” cells. WT MEF were not greatly affected by switching from 3 to 21% O2; if anything, they grew marginally more rapidly at 21% O2 (Fig. 1b). In contrast, c-Jun−/− MEF cultured at 3% O2 slowed in growth after transfer to 21% O2 (Fig. 1b), although this effect was not so acute as that observed for MEF exposed to 21% O2 immediately after explantation (Fig. 1a). The reason for this is not known; however, these results are consistent with those of Parrinello et al. (26), who also observed relative resistance to hyperoxia-induced growth arrest after extensive passage of WT MEF under low oxygen conditions.
Although low-oxygen culture overcame the acute growth arrest normally observed with hyperoxia, c-Jun−/− MEF still displayed a mild proliferation defect. After 90 days in culture, WT MEF had undergone approximately 15 additional cumulative population doublings compared to c-Jun−/− MEF (Fig. 1a and b). Despite this, these results demonstrate that c-Jun−/− MEF are not inherently incapable of proliferation and that the acute growth arrest which normally occurs under conventional culture conditions (19, 31, 37) is a specific consequence of hyperoxic stress.
Premature senescence of c-Jun−/− MEF is not associated with increased p53 expression or function.
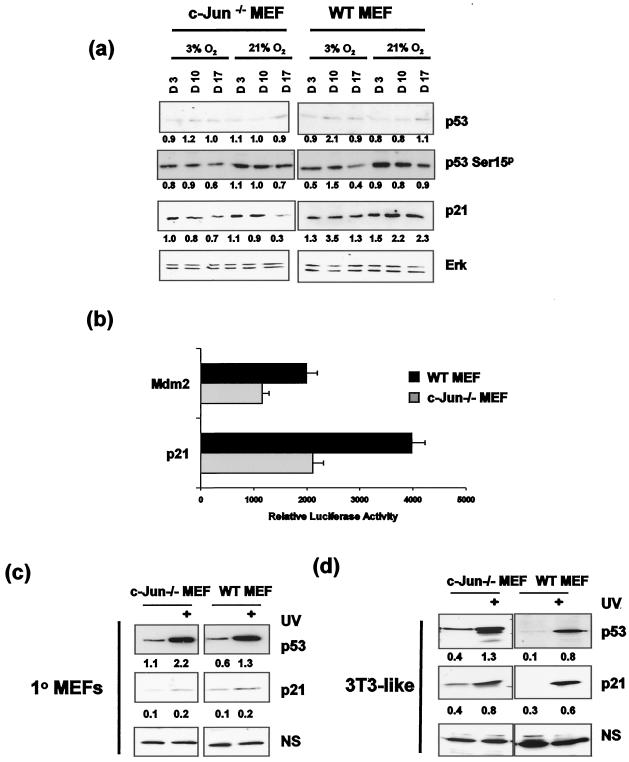
Growth arrest under hyperoxic conditions might be explained by a defect in the expression of one or more rate-limiting cell cycle components required for G1/S progression; however, by Western blotting we observed that the levels of cyclins D1, D2, D3, E, A, and Cdk2 were similar in early-passage WT and Jun−/− MEF cultured at 21% O2 (Fig. 1c). Previous studies, however, have established that premature senescence of c-Jun−/− MEF can be overcome when p53 is deleted, implying a functional link between Jun and p53 (31). To determine whether the growth arrest observed at 21% O2 was associated with increased p53 expression or function, WT and c-Jun−/− MEF were initially isolated in 3% O2, cultured at either 3 or 21% O2 for up to 3 weeks, and analyzed for p53 expression. This revealed that culture at 21% O2 did not result in a significant increase in p53 expression or in the product of a p53 target gene, p21/CIP1, in c-Jun−/− MEF compared to WT MEF under conditions where growth arrest was evident (Fig. 2a). To address the possibility that p53 might nevertheless be activated via posttranslational modification, we examined the levels of serine 15 phosphorylation of p53 (S15p), a modification induced in response to certain forms of DNA damage (34). We did not observe any large or consistent increase in the level of p53 S15p in either c-Jun−/− or WT MEF cultured at 21% O2, indicating that changes in p53 expression or phosphorylation are unlikely to be solely responsible for the premature growth arrest observed in primary c-Jun−/− MEF.
FIG. 2.
c-Jun deficiency does not alter p53 modification or function. (a) Early-passage c-Jun−/− and WT MEF were cultured at either 3 or 21% oxygen, and samples were harvested for protein analysis at days 3, 10, and 17 (i.e., passage 2 [P2], P4, and P6). Whole-cell extracts were prepared and analyzed by SDS-PAGE and Western blotting for p53, Ser15p p53, p21, and Erk expression. Band intensities were quantified by laser densitometry, and relative expression levels were calculated in arbitrary units (shown below the corresponding bands), using ERK values to correct for variations in protein loading. (b) Mdm2 and p21 luciferase constructs were transfected into early-passage WT and c-Jun−/− MEF cultured at 21% O2, and luciferase activity was measured 48 h later. Results represent the means of three independent transfections ± standard errors of the means. (c) Early-passage 1° MEF (c) and late passage 3T3-like cell lines generated from these cultures (d) were cultured in 21% O2 and either not treated or treated with 80 J of UV/m2. Cells were harvested 8 h following UV treatment, and whole-cell extracts were prepared. Following SDS-PAGE analysis, membranes were probed for either p53 or p21 expression. Relative expression levels were quantified and calculated in arbitrary units (shown below the corresponding bands) as for panel a, using a nonspecific band (NS) to correct for variations in protein loading.
To determine whether p53 function was altered in c-Jun−/− MEF, we analyzed the transcriptional activity of two gene promoters whose activity is dependent on p53, Mdm2 and p21/CIP1. Since the Mdm2 gene promoter is also known to be regulated by AP-1 (29), a version of the promoter containing a mutated AP-1 binding site (21) was used to eliminate any potential complication arising from the c-jun genotype. As shown in Fig. 2b, the Mdm2 and p21 promoters were less active in c-Jun−/− MEF than in WT MEF, demonstrating that p53 transcriptional activity was, if anything, diminished rather than elevated in the absence of c-Jun.
To investigate whether p53 protein could be induced in response to damage and signal to its downstream targets, we treated early-passage WT and c-Jun−/− primary (1°) MEF with UV irradiation. This revealed that p53 levels were induced approximately twofold in both cell types following UV irradiation while p21 expression, although only very weakly induced, was again similar in both cell backgrounds (Fig. 2c). Taken together, these results demonstrate that neither p53 expression nor function is significantly elevated in primary c-Jun−/− MEF, implying that if c-Jun loss affects p53 function then it does so in a manner which cannot be detected as fluctuations in expression, phosphorylation, or transcriptional activity.
The results described here are at variance with previous reports which concluded that either p53 expression (31) or function (32) was elevated in the absence of c-Jun. The major difference between these studies and those reported here is that the previous studies predominantly examined established cell lines. To determine whether this could account for the differences, we established 3T3-like cell lines from our primary cultures and analyzed p53 and p21 levels. As shown in Fig. 2d, c-Jun-deficient 3T3-like cell lines display modestly elevated basal levels of p53 and its downstream target p21/CIP1 compared to their WT counterparts. Similar results were obtained with three independently derived cell lines. These results imply that modulation of p53 expression as a result of c-Jun loss is observed only after cells have acquired indefinite proliferative capacity.
Growth arrest in c-Jun−/− MEF is associated with accumulation of cells in G2/M.
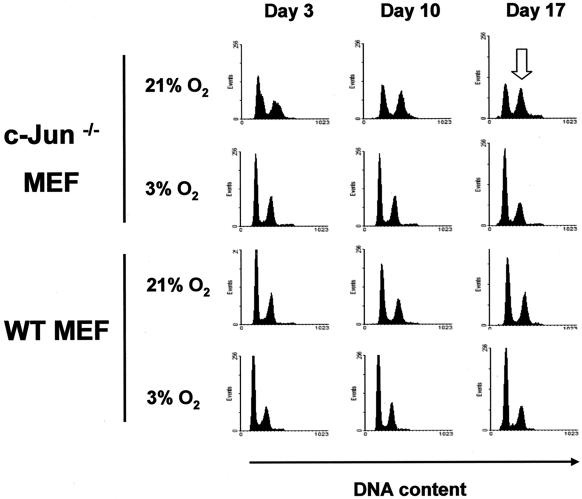
To determine whether changes in cell cycle control accompany the decline in growth of c-Jun−/− MEF, we compared the cell cycle profiles of early-passage MEF cultured for up to 3 weeks at either 21 or 3% O2. The cell cycle distributions of WT MEF cultured at 21 and 3% O2 were extremely similar and did not change significantly over time (Fig. 3, lower panels). In contrast, c-Jun−/− MEF cultured at 21% O2 displayed a gradual increase in the proportion of G2/M cells with time compared to cells maintained at 3% O2 (Fig. 3). Cells with defects in mitogenic signaling processes are generally considered to accumulate in the G1 phase of the cell cycle, whereas arrest in the G2/M phase of the cell cycle is frequently indicative of a DNA damage response (1). These results demonstrate that premature senescence induced by hyperoxic stress in c-Jun−/− MEF was associated with accumulation in the G2/M phase of the cell cycle, suggesting that arrest might be associated with higher levels of endogenous DNA damage.
FIG. 3.
Lack of c-Jun results in an accumulation in G2/M. Early-passage c-Jun−/− and WT MEF were cultured at either 3 or 21% oxygen over a 17-day period as described in the legend to Fig. 2a. Subconfluent cultures of cells were harvested at days 3, 10, and 17 (i.e., P2, P4, and P6) and fixed prior to being stained with propidium iodide. DNA content was analyzed by flow cytometry. The open arrow indicates enrichment of G2/M cells in c-Jun−/− cultures after 17 days of culture.
Lack of c-Jun results in accumulation of spontaneous DNA damage.
Hyperoxic culture conditions can result in oxidative damage to DNA (14). To determine whether the growth arrest of c-Jun−/− MEF was associated with accumulation of oxidative DNA damage, we analyzed the levels of endogenous DNA damage on a single-cell basis using a denaturing comet assay (16). As shown in Fig. 4a, cells were scored as containing either no detectable DNA damage (no tail), low levels of DNA damage (small tails), or high levels of DNA damage (large tails).
WT MEF show very little endogenous DNA damage regardless of oxygen exposure during culture (Fig. 4a). This was also true for c-Jun−/− MEF cultured at 3% O2; however, c-Jun-deficient cells cultured at 21% O2 for 4 days contained a high proportion of cells with damaged DNA (Fig. 4a). This was specifically attributable to the absence of c-Jun, since ectopic expression of c-Jun at close to physiological levels in deficient cells by retroviral infection (Fig. 4b) restored the low basal level of DNA damage seen in WT MEF during hyperoxic culture (Fig. 4a, far right columns). These results demonstrate that under culture conditions where cell growth was blocked, c-Jun deficiency is associated with the accumulation of DNA damage.
To determine what type of damage we were detecting, a comet assay was performed under neutral conditions to detect double-strand breaks. We observed no similar excess of spontaneous endogenous double-strand breaks in c-Jun−/− MEF at 21% O2 under these conditions (data not shown), suggesting that the damage observed under denaturing conditions was most likely due to single-strand breaks generated by alkali-labile abasic sites or incomplete excision repair.
Prolonged persistence of induced DNA damage in the absence of c-Jun.
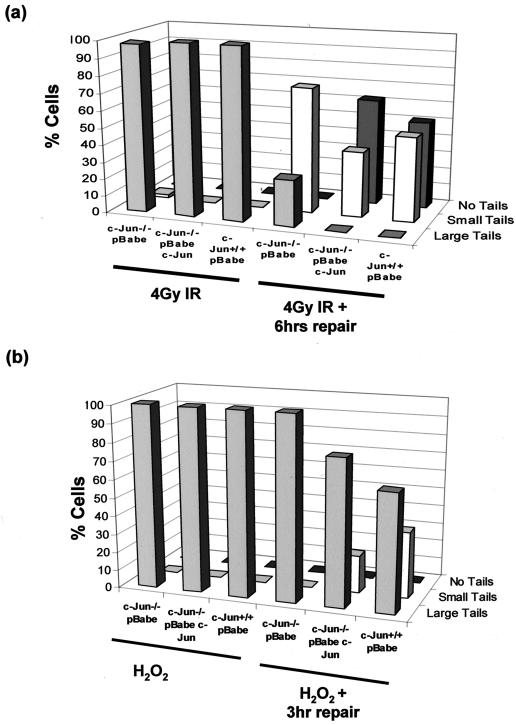
To investigate whether the increased levels of spontaneous DNA damage in c-Jun−/− MEF was a consequence of more rapid DNA damage accumulation or a delay or defect in the rate of DNA repair, we used a neutral comet assay to detect the generation and repair of induced DNA damage in cells cultured at 3% O2 following exposure to IR. Shortly after exposure to 4 Gy of IR, essentially all cells contained high levels of damage, signified by large comet tails, regardless of genotype (Fig. 5a). Six hours after irradiation, however, DNA damage had been substantially repaired in both WT and c-Jun-reconstituted cultures as judged by the reappearance of a large proportion of cells lacking tails (Fig. 5a). Cultures of c-Jun−/− MEF, by contrast, did not contain any cells without tails at 6 h, although some repair had evidently occurred, as judged by a reduction in the proportion of large tails and an increase in the proportion of small tails (Fig. 5a). The persistence of irradiation-induced damage in c-Jun−/− MEF was also associated with a prolonged G2/M arrest, as documented by flow cytometry and more robust activation of the DNA damage-activated Chk1 protein kinase (data not shown). Oxidative damage induced by H2O2 treatment was also found to persist for longer in c-Jun−/− MEF compared to WT and reconstituted MEF (Fig. 5b).
FIG. 5.
Prolonged persistence of DNA damage induced by IR and H2O2 in c-Jun-deficient MEF. (a) A neutral comet assay was performed on c-Jun−/− MEF, c-Jun−/− cells reconstituted with ectopic c-Jun, and WT MEF cultured at 3% O2. Cells were treated with 4Gy of IR to induce double-strand breaks and harvested immediately or left for 6 h to repair damage prior to analysis. Cells were scored as having no tail, small tails, or large tails, and results were plotted as the percentage of cells in each group. The results are representative of three independent experiments. (b) Denaturing comet analysis of c-Jun−/− MEF, c-Jun−/− MEF reconstituted with ectopic c-Jun, and WT MEF treated with 100 μM H2O2 for 15 min. Cells were either harvested immediately or washed and incubated in fresh medium for 3 h to allow repair prior to analysis.
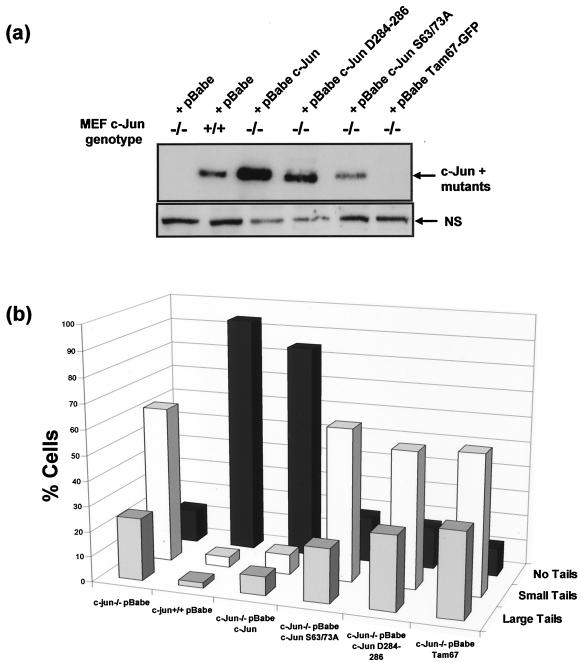
We also investigated whether the DNA binding and/or transcriptional activation capacity of c-Jun affected the level of DNA damage by reconstituting c-Jun−/− cells with mutant forms of c-Jun deficient for one or both functions. To this end, we used retroviral vectors to transduce c-Jun−/− MEF with c-Jun mutants lacking either a functional DNA binding domain (c-Jun D284-286) or transactivation domain (Tam67-GFP [green fluorescent protein]) or in which the regulatory Jun N-terminal kinase (JNK) phosphorylation sites within the transactivation domain had been replaced with nonphosphorylatable alanine residues (c-Jun S63/73A). Expression of the mutant c-Jun D284-286 and c-Jun S63/73A proteins was confirmed by Western blotting (Fig. 6a). Tam67-GFP was not reactive with the anti-c-Jun antiserum used; however, its presence in the transduced cells was confirmed by fluorescence microscopy (data not shown).
FIG. 6.
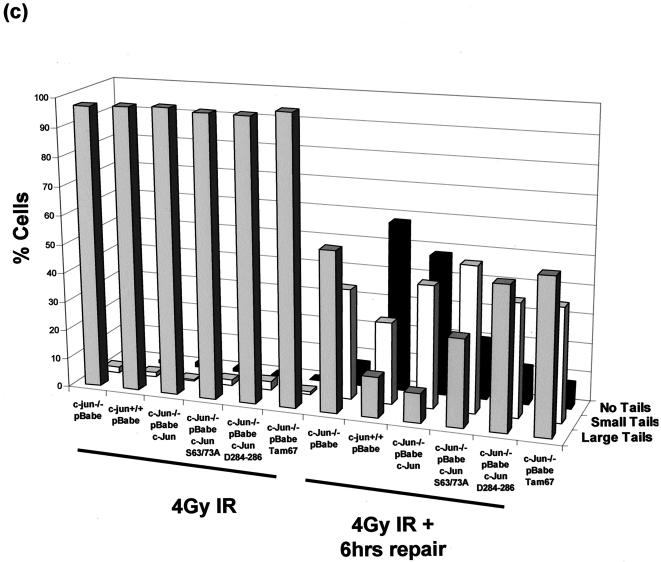
Restoration of normal DNA damage levels requires functional c-Jun DNA binding and transactivation domains. (a) WT and c-Jun−/− MEF were transduced with an empty retroviral vector (pBabe) or a vector encoding either c-Jun, c-Jun mutant D284-286 or S63/73A, or Tam67-GFP. Protein extracts were prepared from selected cultures and analyzed by Western blotting for expression of endogenous and ectopic c-Jun proteins. (b) WT or c-Jun−/− MEF reconstituted with c-Jun or the indicated mutants were cultured in 21% O2 for 7 days, after which the cells were harvested and analyzed for basal levels of DNA damage using a denaturing comet assay and scored by fluorescence microscopy. (c) WT or c-Jun−/− MEF reconstituted with c-Jun or the indicated mutants cultured in 3% O2 were irradiated with 4 Gy of IR, after which the cells were either harvested immediately or left for 6 h to allow repair prior to analysis. Cells were analyzed for DNA damage using a neutral comet assay and scored by fluorescence microscopy.
As shown in Fig. 6b, comet analysis revealed that c-Jun−/− MEF reconstituted with all three mutants exhibited higher basal levels of DNA damage when cultured in 21% O2 than cells expressing ectopic wild-type c-Jun. Furthermore, irradiation-induced damage persisted in cells expressing c-Jun D284-286 and Tam67-GFP in 3% O2 as long as in cells completely deficient for c-Jun, although c-Jun S63/73A appeared to be intermediate between WT and c-Jun−/− MEF (Fig. 6c).
c-Jun co-localizes with γH2AX and ATM at sites of DNA damage.
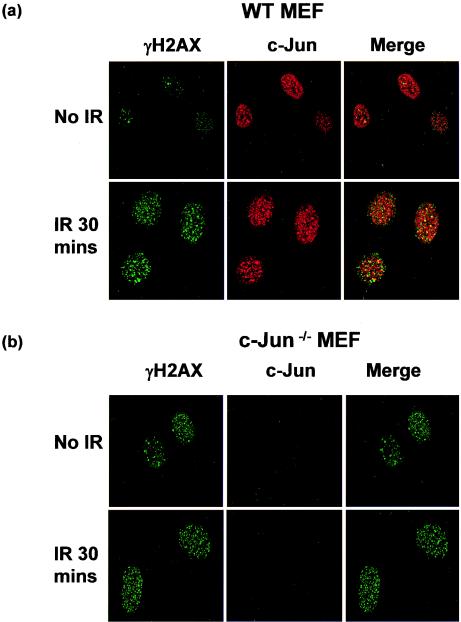
The Brca1 (8) and c-Myc (5) transcription factors have been shown to interact directly with components of the DNA repair machinery. To determine if this might also be true for c-Jun, we irradiated cells and examined whether c-Jun localized to sites of induced DNA damage. Irradiation rapidly induces phosphorylation of H2AX on serine 139 to generate γH2AX, which subsequently accumulates in discrete foci at sites of DNA damage (3). We therefore examined the formation of γH2AX foci in WT and c-Jun−/− MEF following IR treatment and compared the distribution of these structures with the distribution of c-Jun by immunocytochemistry.
As shown in Fig. 7a, irradiation induced a marked increase in γH2AX staining in WT MEF cultured in 21% O2, and strikingly, c-Jun was found to colocalize with a significant proportion of the γH2AX foci shortly after DNA damage (Fig. 7a). Limited colocalization was also observed between c-Jun and the smaller number of γH2AX foci present in unirradiated cells (Fig. 7a). γH2AX staining also increased in c-Jun−/− MEF cultured in 21% O2 after irradiation, although the basal level appeared to be somewhat higher, as would be expected in cells bearing higher levels of spontaneous DNA damage (Fig. 7b). c-Jun−/− MEF, however, were completely devoid of c-Jun staining, thereby validating the specificity of the anti-c-Jun antibody (Fig. 7b).
FIG. 7.
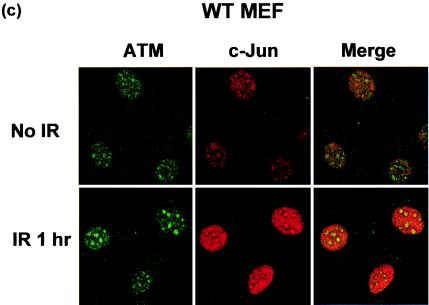
c-Jun colocalizes to sites of DNA damage. WT and c-Jun−/− MEF cultured in 21% O2 for 4 days were treated with 4 Gy of IR and left for 30 min. Cells were then fixed and stained with anti-phosphorylated H2AX (γH2AX; green) and anti-c-Jun (red) antibodies (a and b) or WT MEF were stained with anti-ATM (green) or anti-Jun (red) antibodies (c). Images were analyzed and merged by confocal microscopy and are representative of the majority of cells in the cultures.
H2AX is known to be phosphorylated by the ATM protein kinase following DNA damage (3); therefore, we also compared the subcellular localization of c-Jun and ATM. ATM was also observed in discrete foci following irradiation in both WT and c-Jun−/− MEF (data not shown), and again c-Jun was observed to colocalize with ATM in a portion of these structures in WT cells following damage (Fig. 7c). The close association of c-Jun with structures containing components of DNA damage recognition or repair pathways raises the possibility that c- Jun could affect such processes directly.
DISCUSSION
Previous studies have shown that c-Jun−/− MEF undergo premature senescence in culture which is dependent on p53 (31, 32), although the underlying mechanism remained unresolved. Similarities between the senescence phenotype of c-Jun−/− MEF and MEF deficient for genes involved in DNA damage pathways suggested that growth arrest in c-Jun−/− MEF might be attributable to DNA damage accumulation. To test this, we cultured c-Jun−/− MEF under conditions which limit oxidative DNA damage, the major environmental insult cells experience during conventional culture (15). As we have shown, protecting cells from hyperoxia alleviated premature senescence, thereby demonstrating that c-Jun−/− MEF are capable of proliferation under specific conditions. When we examined the phenotype of senescent c-Jun−/− MEF in detail we found that growth arrest was associated with, and we believe attributable to, elevated levels of spontaneous DNA damage.
Our results are consistent with a recent report which demonstrates that normal mouse cell senescence occurs as a consequence of the accumulation of DNA damage resulting from hyperoxic culture conditions (26). This study also established that premature senescence resulting from deletion of the DNA repair protein DNA-PKcs can be overcome by culture in 3% O2, indicating that diminished repair can exacerbate oxygen-dependent premature senescence, presumably by accelerating the rate at which oxidative damage accumulates during hyperoxic culture. Since DNA damage leads to multiple checkpoint-mediated cell cycle arrests (25), one might expect senescent MEF to arrest at both the G1/S and G2/M transitions of the cell cycle. Consistent with this prediction, we observed accumulation of G2/M-arrested cells in our senescent cultures of c-Jun−/− MEF, and very recently Wada et al. have also reported G2/M arrest in senescent cultures of MEF deficient for either c-Jun or the upstream JNK pathway regulator MKK7 (35). These findings are unexpected, since G2/M arrest was not detected in earlier studies of c-Jun-deficient cells, which instead documented arrest primarily in the G1/S phase of the cell cycle (31, 37). Although the reasons for this apparent discrepancy are not completely clear, G2/M-arrested cells constitute only a subpopulation of the senescent cultures (Fig. 3) (35), which may have been obscured as a result of variations in cell culture conditions and/or other experimental methodologies in these earlier studies.
The absence of any obvious relationship between DNA damage accumulation and activation of p53 was also unexpected. Previous reports have demonstrated that growth arrest of c-Jun−/− MEF was dependent on p53, and since c-Jun−/− MEF accumulate DNA damage it might be anticipated that this would activate p53. However, we analyzed p53 expression, phosphorylation, and transcriptional activity and observed no evidence of activation in early-passage 1° c-Jun−/− MEF, suggesting that if c-Jun deficiency modifies p53 function it does so in a manner that cannot be detected by conventional criteria. Historically, p53 function has generally been studied following acute insults which result in high levels of DNA damage. We, in contrast, have examined spontaneously occurring DNA damage resulting from chronic exposure to hyperoxia. c-Jun−/− MEF presumably undergo senescence after a certain threshold of spontaneous oxidative damage has accumulated; however, this is evidently not sufficient to trigger a conventional p53 response. Our observations, however, do not exclude the possibility that low-level chronic oxidative damage modulates p53 activity in a manner distinct from acute DNA damage. In fact, this seems likely and furthermore can provide an alternative explanation for the previously documented genetic interaction between c-Jun and p53 in senescence (31).
One potential mechanism that could account for the accumulation of DNA damage in c-Jun−/− MEF would be if c-Jun was required for the expression of genes that protect cells from DNA damage or are required for efficient DNA repair. We considered the possibility that c-Jun might regulate genes involved in redox homeostasis, since imbalances in reactive oxygen species production and removal could lead to elevated levels of DNA damage. However, using a redox-sensitive indicator dye (CM-H2DCF-DA), we found no significant difference in intracellular redox potential between WT and c-Jun−/− MEF, suggesting that excess reactive oxygen species production is unlikely to be a major cause of DNA damage accumulation (data not shown).
The prolonged persistence of DNA damage induced by IR or H2O2 in c-Jun−/− MEF is however strongly suggestive of a defect in DNA repair. Recently, the c-Myc proto-oncogene has been shown to play a role in regulating the expression of two genes involved in DNA repair: the human Werner syndrome protein gene (WRN gene) (13) and, more recently, the Nbs1 gene (5). Furthermore, a number of studies have implicated the JNK signaling pathway acting via c-Jun or the related transcription factor ATF2 in modulation of DNA repair and/or cell survival in response to various forms of genotoxic damage (17, 27, 28). Consistent with these observations, we found that mutant forms of c-Jun which are deficient for DNA binding or transactivation or which were nonresponsive to JNK regulation were unable to restore normal regulation of basal or induced DNA damage in c-Jun−/− MEF. c-Jun is involved in regulating the activity of many individual gene promoters through AP-1 response elements, and although there is limited evidence that some of these might influence DNA repair (12, 28, 30), a direct link between specific c-Jun target genes and repair processes remains to be established.
Alternatively, it is also conceivable that c-Jun might have a direct role in DNA repair processes per se. The finding that c-Jun, like ATM and γH2AX, localizes to sites of DNA damage is broadly consistent with this idea. Furthermore c-Jun is known to interact physically or functionally with two proteins involved in DNA repair: redox factor 1 (Ref-1; also known as APEX), an endonuclease specific for abasic sites (38), and thymine DNA glycosylase (4), both of which are involved in base excision repair (BER). BER is responsible for the repair of modified lesions on DNA, including those produced by oxidative damage (14). We investigated whether c-Jun−/− MEF displayed deficiencies in BER by using in vitro assays; however, extracts from c-Jun−/− MEF were as active as those from WT MEF in the recognition and excision of oxidatively modified bases from DNA (data not shown).
Precedents exist for interactions between other transcription factors and components of the repair machinery, particularly in the context of transcription-coupled repair (20). Transcriptional activators may stimulate DNA repair at sites of active transcription either by facilitating access through chromatin remodeling or by directly recruiting repair proteins to actively transcribed genes (11). c-Jun binds to a DNA recognition sequence common to many gene promoters and theoretically could promote transcription-coupled repair by either of these mechanisms. Such a relatively subtle role could explain why c-Jun−/− MEF are capable of a low level of repair and why the repair defect can be tolerated under permissive conditions. In low oxygen, where damage is presumably minimal, c-Jun−/− MEF may be able to repair at a rate which is compatible with proliferation, but when switched to a more damaging hyperoxic environment, the cells may be unable to prevent the accumulation of unrepaired lesions in essential genes.
Each of these scenarios could be reconciled with the current data; however, further studies including direct measurement of repair capacity per se will be required to determine conclusively whether c-Jun regulates DNA repair, and if so, to understand the molecular mechanism. The present study nevertheless demonstrates a novel and previously unrecognized role for c-Jun in sustaining cell proliferation by limiting the genotoxic consequences of hyperoxic culture.
Mutations which impair the recognition or repair of DNA damage frequently predispose to cancer, and therefore loss, as opposed to gain, of c-Jun function might conceivably promote neoplasia through accumulation of genetic damage. Although we are not aware of any specific evidence to support this scenario, it is intriguing that certain other AP-1 family proteins, such as JunB and c-Fos, have been implicated in tumor suppression (9). Further work will be required to establish if these other AP-1 proteins share c-Jun's capacity to protect cells from genotoxic stress, and if so, to determine whether this function can influence carcinogenesis.
Acknowledgments
We thank I. Morgan and K. Parkinson for comments on the manuscript and R. Wisdom and R. Hennigan for generous provision of reagents.
This work was supported by Cancer Research UK.
REFERENCES
- 1.Baus, F., V. Gire, D. Fisher, J. Piette, and V. Dulic. 2003. Permanent cell cycle exit in G2 phase after DNA damage in normal human fibroblasts. EMBO J. 22:3992-4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Black, E. J., W. Clark, and D. A. Gillespie. 2000. Transient deactivation of ERK signalling is sufficient for stable entry into G0 in primary avian fibroblasts. Curr. Biol. 10:1119-1122. [DOI] [PubMed] [Google Scholar]
- 3.Burma, S., B. P. Chen, M. Murphy, A. Kurimasa, and D. J. Chen. 2001. ATM phosphorylates histone H2AX in response to DNA double-strand breaks. J. Biol. Chem. 276:42462-42467. [DOI] [PubMed] [Google Scholar]
- 4.Chevray, P. M., and D. Nathans. 1992. Protein interaction cloning in yeast: identification of mammalian proteins that react with the leucine zipper of Jun. Proc. Natl. Acad. Sci. USA 89:5789-5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiang, Y. C., S. C. Teng, Y. N. Su, F. J. Hsieh, and K. J. Wu. 2003. c-Myc directly regulates the transcription of the NBS1 gene involved in DNA double-strand break repair. J. Biol. Chem. 278:19286-19291. [DOI] [PubMed] [Google Scholar]
- 6.Clark, W., and D. A. Gillespie. 1997. Transformation by v-Jun prevents cell cycle exit and promotes apoptosis in the absence of serum growth factors. Cell Growth Differ. 8:371-380. [PubMed] [Google Scholar]
- 7.Cressman, V. L., D. C. Backlund, A. V. Avrutskaya, S. A. Leadon, V. Godfrey, and B. H. Koller. 1999. Growth retardation, DNA repair defects, and lack of spermatogenesis in BRCA1-deficient mice. Mol. Cell. Biol. 19:7061-7075. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 8.Deng, C. X., and R. H. Wang. 2003. Roles of BRCA1 in DNA damage repair: a link between development and cancer. Hum. Mol. Genet. 12:R113-R123. [DOI] [PubMed] [Google Scholar]
- 9.Eferl, R., and E. F. Wagner. 2003. AP-1: a double-edged sword in tumorigenesis. Nat. Rev. Cancer 3:859-868. [DOI] [PubMed] [Google Scholar]
- 10.Frank, K. M., N. E. Sharpless, Y. Gao, J. M. Sekiguchi, D. O. Ferguson, C. Zhu, J. P. Manis, J. Horner, R. A. DePinho, and F. W. Alt. 2000. DNA ligase IV deficiency in mice leads to defective neurogenesis and embryonic lethality via the p53 pathway. Mol. Cell 5:993-1002. [DOI] [PubMed] [Google Scholar]
- 11.Frit, P., K. Kwon, F. Coin, J. Auriol, S. Dubaele, B. Salles, and J. M. Egly. 2002. Transcriptional activators stimulate DNA repair. Mol. Cell 10:1391-1401. [DOI] [PubMed] [Google Scholar]
- 12.Gjerset, R. A., S. Lebedeva, A. Haghighi, S. T. Turla, and D. Mercola. 1999. Inhibition of the Jun kinase pathway blocks DNA repair, enhances p53-mediated apoptosis and promotes gene amplification. Cell Growth Differ. 10:545-554. [PubMed] [Google Scholar]
- 13.Grandori, C., K. J. Wu, P. Fernandez, C. Ngouenet, J. Grim, B. E. Clurman, M. J. Moser, J. Oshima, D. W. Russell, K. Swisshelm, S. Frank, B. Amati, R. Dalla-Favera, and R. J. Monnat, Jr. 2003. Werner syndrome protein limits MYC-induced cellular senescence. Genes Dev. 17:1569-1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grollman, A. P., and M. Moriya. 1993. Mutagenesis by 8-oxoguanine: an enemy within. Trends Genet. 9:246-249. [DOI] [PubMed] [Google Scholar]
- 15.Halliwell, B. 2003. Oxidative stress in cell culture: an under-appreciated problem? FEBS Lett. 540:3-6. [DOI] [PubMed] [Google Scholar]
- 16.Hartmann, A., E. Agurell, C. Beevers, S. Brendler-Schwaab, B. Burlinson, P. Clay, A. Collins, A. Smith, G. Speit, V. Thybaud, and R. R. Tice. 2003. Recommendations for conducting the in vivo alkaline Comet assay. 4th International Comet Assay Workshop. Mutagenesis 18:45-51. [DOI] [PubMed] [Google Scholar]
- 17.Hayakawa, J., C. Depatie, M. Ohmichi, and D. Mercola. 2003. The activation of c-Jun NH2-terminal kinase (JNK) by DNA-damaging agents serves to promote drug resistance via activating transcription factor 2 (ATF2)-dependent enhanced DNA repair. J. Biol. Chem. 278:20582-20592. [DOI] [PubMed] [Google Scholar]
- 18.Hennigan, R. F., and P. J. Stambrook. 2001. Dominant negative c-jun inhibits activation of the cyclin D1 and cyclin E kinase complexes. Mol. Biol. Cell 12:2352-2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson, R. S., B. van Lingen, V. E. Papaioannou, and B. M. Spiegelman. 1993. A null mutation at the c-jun locus causes embryonic lethality and retarded cell growth in culture. Genes Dev. 7:1309-1317. [DOI] [PubMed] [Google Scholar]
- 20.Leadon, S. A. 1999. Transcription-coupled repair of DNA damage: unanticipated players, unexpected complexities. Am. J. Hum. Genet. 64:1259-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maclaren, A., W. Clark, E. J. Black, D. Gregory, H. Fujii, and D. A. Gillespie. 2003. v-Jun stimulates both cdk2 kinase activity and G1/S progression via transcriptional repression of p21 CIP1. Oncogene 22:2383-2395. [DOI] [PubMed] [Google Scholar]
- 22.MacLaren, A., W. Clark, and D. A. Gillespie. 2000. v-Jun sensitizes cells to apoptosis by a mechanism involving mitochondrial cytochrome C release. Oncogene 19:5906-5918. [DOI] [PubMed] [Google Scholar]
- 23.Maki, Y., T. J. Bos, C. Davis, M. Starbuck, and P. K. Vogt. 1987. Avian sarcoma virus 17 carries the jun oncogene. Proc. Natl. Acad. Sci. USA 84:2848-2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nussenzweig, A., C. Chen, V. da Costa Soares, M. Sanchez, K. Sokol, M. C. Nussenzweig, and G. C. Li. 1996. Requirement for Ku80 in growth and immunoglobulin V(D)J recombination. Nature 382:551-555. [DOI] [PubMed] [Google Scholar]
- 25.Nyberg, K. A., R. J. Michelson, C. W. Putnam, and T. A. Weinert. 2002. Toward maintaining the genome: DNA damage and replication checkpoints. Annu. Rev. Genet. 36:617-656. [DOI] [PubMed] [Google Scholar]
- 26.Parrinello, S., E. Samper, A. Krtolica, J. Goldstein, S. Melov, and J. Campisi. 2003. Oxygen sensitivity severely limits the replicative lifespan of murine fibroblasts. Nat. Cell Biol. 5:741-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Potapova, O., S. Basu, D. Mercola, and N. J. Holbrook. 2001. Protective role for c-Jun in the cellular response to DNA damage. J. Biol. Chem. 276:28546-28553. [DOI] [PubMed] [Google Scholar]
- 28.Potapova, O., A. Haghighi, F. Bost, C. Liu, M. J. Birrer, R. Gjerset, and D. Mercola. 1997. The Jun kinase/stress-activated protein kinase pathway functions to regulate DNA repair and inhibition of the pathway sensitizes tumor cells to cisplatin. J. Biol. Chem. 272:14041-14044. [DOI] [PubMed] [Google Scholar]
- 29.Ries, S., C. Biederer, D. Woods, O. Shifman, S. Shirasawa, T. Sasazuki, M. McMahon, M. Oren, and F. McCormick. 2000. Opposing effects of Ras on p53: transcriptional activation of mdm2 and induction of p19ARF. Cell 103:321-330. [DOI] [PubMed] [Google Scholar]
- 30.Scherer, S. J., S. M. Maier, M. Seifert, R. G. Hanselmann, K. D. Zang, H. K. Muller-Hermelink, P. Angel, C. Welter, and M. Schartl. 2000. p53 and c-Jun functionally synergize in the regulation of the DNA repair gene hMSH2 in response to UV. J. Biol. Chem. 275:37469-37473. [DOI] [PubMed] [Google Scholar]
- 31.Schreiber, M., A. Kolbus, F. Piu, A. Szabowski, U. Mohle-Steinlein, J. Tian, M. Karin, P. Angel, and E. F. Wagner. 1999. Control of cell cycle progression by c-Jun is p53 dependent. Genes Dev. 13:607-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shaulian, E., M. Schreiber, F. Piu, M. Beeche, E. F. Wagner, and M. Karin. 2000. The mammalian UV response: c-Jun induction is required for exit from p53-imposed growth arrest. Cell 103:897-907. [DOI] [PubMed] [Google Scholar]
- 33.Sherr, C. J., and R. A. DePinho. 2000. Cellular senescence: mitotic clock or culture shock? Cell 102:407-410. [DOI] [PubMed] [Google Scholar]
- 34.Siliciano, J. D., C. E. Canman, Y. Taya, K. Sakaguchi, E. Appella, and M. B. Kastan. 1997. DNA damage induces phosphorylation of the amino terminus of p53. Genes Dev. 11:3471-3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wada, T., N. Joza, H. Y. Cheng, T. Sasaki, I. Kozieradzki, K. Bachmaier, T. Katada, M. Schreiber, E. F. Wagner, H. Nishina, and J. M. Penninger. 2004. MKK7 couples stress signalling to G2/M cell-cycle progression and cellular senescence. Nat. Cell Biol. 6:215-226. [DOI] [PubMed] [Google Scholar]
- 36.Weiss, R. S., T. Enoch, and P. Leder. 2000. Inactivation of mouse Hus1 results in genomic instability and impaired responses to genotoxic stress. Genes Dev. 14:1886-1898. [PMC free article] [PubMed] [Google Scholar]
- 37.Wisdom, R., R. S. Johnson, and C. Moore. 1999. c-Jun regulates cell cycle progression and apoptosis by distinct mechanisms. EMBO J. 18:188-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xanthoudakis, S., and T. Curran. 1992. Identification and characterization of Ref-1, a nuclear protein that facilitates AP-1 DNA-binding activity. EMBO J. 11:653-665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu, Y., E. M. Yang, J. Brugarolas, T. Jacks, and D. Baltimore. 1998. Involvement of p53 and p21 in cellular defects and tumorigenesis in Atm−/− mice. Mol. Cell. Biol. 18:4385-4390. [DOI] [PMC free article] [PubMed] [Google Scholar]