Abstract
During mammalian vascular development, endothelial cells form a complex array of vessels that differ markedly in structure and function, but the molecular basis for this vascular complexity is poorly understood. Recent insights into endothelial diversity have come from the identification of molecular markers expressed on distinct endothelial cell populations. One such marker, the PAL-E antibody, has been used for almost 20 years to distinguish blood and lymphatic vessels, but the identity of the protein recognized by PAL-E has been unknown. In the present study we have used protein purification and tandem mass spectrometry analysis of tryptic peptides to identify the PAL-E antigen as a secreted form of vimentin. Vimentin has been well characterized as an intracellular intermediate filament protein expressed broadly in mesenchymal cells. In contrast, PAL-E-reactive vimentin is secreted extracellularly, its synthesis is restricted to a distinct population of blood endothelial cells and activated macrophages, and PAL-E-reactive vimentin is found in circulating human blood. PAL-E-reactive vimentin does not arise from an endothelial cell-specific mRNA transcript but is the product of cell-specific posttranslational modification. The PAL-E antibody therefore defines secretion of vimentin as a molecular distinction among endothelial cells and exposes a novel, extracellular role for vimentin in the blood vasculature.
The mammalian vascular system is highly diverse and composed of vessels with functions ranging from the transport of blood in tight, nonleaky vessels to the transport of lymph in open, highly permeable vessels (2). Although it has been recognized for centuries that structurally distinct vessels perform these varied functions, all vessels are lined by a single cell type, the endothelium. It is now believed that the endothelium lining distinct vessel types is functionally heterogeneous, but the molecular basis of endothelial heterogeneity remains largely unknown (11). A major step in understanding endothelial cell heterogeneity has been the recent identification of molecular markers unique to distinct endothelial cell types, such as those lining blood and lymphatic vessels. These markers have provided the tools required to identify and isolate distinct endothelial cell types and have recently provided insights into the function and development of blood and lymphatic endothelial populations (17). Further identification of the genes and proteins expressed exclusively in blood or lymphatic endothelial cells is therefore a critical step in understanding how these two major mammalian vascular systems develop and operate.
One of the first molecular markers found to distinguish blood and lymphatic endothelial cells was the antigen recognized by the monoclonal antibody PAL-E. Identified almost 20 years ago, the PAL-E antibody was generated by the injection of human melanoma lymph node metastases into mice (25). PAL-E antibody recognizes a protein expressed exclusively by the endothelial cells that line blood capillaries and small veins, with the notable exception of those in the brain (12, 23, 25). Tumor blood vessels and the high endothelial venules in lymph nodes are particularly PAL-E reactive (13, 25). In contrast, PAL-E is entirely nonreactive with lymphatic capillary endothelial cells and with the arterial endothelium (25). Since its identification, PAL-E has been used extensively to determine if small vessels in the skin and elsewhere are of blood or lymphatic origin (26).
Despite the extensive use of PAL-E as a means of establishing microvascular blood endothelial cell identity, the protein recognized by PAL-E antibody has been unknown. Immunofluorescence studies of PAL-E+ endothelial cells have demonstrated staining along the cell membrane (25). Endothelial surface staining with PAL-E antibody has also been observed by using flow cytometry on live cells (1), and high-resolution studies of PAL-E binding to endothelium in tissue sections performed using electron microscopy have revealed a polarized staining pattern along the luminal endothelial surface (18, 25). This work has suggested that PAL-E might bind an unidentified cell membrane protein whose expression is restricted to a subset of blood endothelial cells in vivo.
In the present study we have used biochemical purification and mass-spectrometry analysis of tryptic peptides to identify the antigen recognized by PAL-E. Surprisingly, these studies identify the PAL-E antigen as vimentin, a protein previously characterized primarily as a component of intracellular intermediate filaments expressed in all mesenchymal cells. Purified PAL-E antigen is recognized by the antivimentin monoclonal antibody V9, but, consistent with in vivo staining, immunoblot analysis reveals expression of PAL-E-reactive vimentin in endothelial cells but not in HEK-293 cells that express V9-reactive vimentin. Comparison of vimentin mRNA transcripts in the PAL-E-positive endothelial cell line HMEC-1 and the PAL-E-negative HEK-293 cell line reveals that PAL-E-reactive vimentin is not the product of an endothelium-specific vimentin transcript. Instead, PAL-E-reactive vimentin appears to arise as a result of several unanticipated posttranslational modifications that are associated with extracellular secretion of the protein.
Our studies reveal both an unusual molecular basis of endothelial heterogeneity and further evidence for a novel, extracellular role of the well-characterized protein vimentin. Although it was originally observed in activated macrophages (14), our studies suggest that vimentin is luminally secreted by blood endothelial cells throughout the body with significant accumulation in the circulating blood. The biological role played by secreted vimentin is not yet known, but the pattern of PAL-E staining in vivo suggests that this protein may mediate endothelial interactions with blood circulating cells, a function not required by lymphatic endothelium or by the PAL-E-negative endothelial cells that line arteries or form the blood-brain barrier. Future studies to address the biological function of secreted vimentin will further explain its vascular specificity and the molecular basis of the endothelial heterogeneity revealed by PAL-E antibody.
MATERIALS AND METHODS
Cells and antibodies.
The generation of the hybridoma clone expressing the PAL-E antibody has been described (25). V-9 monoclonal antibody was obtained from Oncogene Research Products. HMEC-1 cells were generously provided by F. J. Candal (Centers for Disease Control, Atlanta, Ga.). The THP-1 cell line was obtained from American Type Culture Collection. HMVEC-d dermal microvascular endothelial cells were obtained from Cambrex and maintained in microvascular endothelial cell medium 2. HMEC-1 cells were maintained in a solution containing Gibco's MCDB131 (formula no. 10372019), 10 mM l-glutamine, 10 ng of epidermal growth factor/ml, 1 μg of hydrocortisone/ml, 10% fetal bovine serum, and 1× penicillin-streptomycin.
Purification of PAL-E antigen.
Ascites fluid of PAL-E hybridoma was generated by Cocalico Biologicals, Inc. (Reamstown, Pa.). PAL-E immunoglobulin G2b (IgG2b) was purified from ascites with the Pierce (Rockford, Ill.) ImmunoPure kit and coupled to agarose beads using the Pierce AminoLink Plus immobilization kit. Fifteen milliliters of HMEC-1 cell lysate was generated from five 150-mm confluent dishes of cells as described below and spun to remove debris. Protein G-agarose beads were added and incubated with the HMEC-1 cell lysate for 3 h at 4°C to adsorb agarose-binding proteins. One hundred microliters of PAL-E-conjugated beads was then incubated with the HMEC-1 cell lysate on a rocker at 4°C overnight. The beads were washed carefully as described below, and bound protein was boiled off at 100°C for 3 min prior to gel electrophoresis.
Immunoblotting and immunoprecipitations.
HMEC-1, HMVEC, and THP-1 cell lysates were generated by washing cells three times with phosphate-buffered saline (PBS) followed by lysis with radioimmunoprecipitation assay (RIPA) buffer (1 ml of RIPA buffer/2 × 106 to 3 × 106 cells/100-mm petridish), as previously described (3). For immunoblotting experiments, cell lysates and concentrated serum-free supernatants were run in Invitrogen/Novex precast 7% gels, and immunoblotting was performed under reduced (containing 5% 2-mercaptoethanol) and nonreduced conditions. Live HMEC-1-cell immunoprecipitation was performed by first detaching HMEC-1 cells from the tissue culture plate using PBS containing 5 mM EDTA. Five microliters of ascitic fluid containing PAL-E antibody was added to a single HMEC-1 cell suspension in PBS-5 mM EDTA, incubated at 4°C for 1 h, and washed three times with PBS-5 mM EDTA to remove unbound antibody. After washing, the cells were spun down and lysed in 1 ml of RIPA buffer. The lysates were incubated with 50 μl of protein-G agarose beads (Roche) overnight at 4°C. The beads were then washed three times as outlined in the Roche protocol (http://www.roche-applied-science.com/pack-insert/1719408a.pdf), and bound proteins were boiled off at 100°C for 3 min prior to immunoblot analysis.
Identification of PAL-E antigen using tandem mass spectrometry (MS/MS) analysis.
Following protein immunoprecipitation using PAL-E-bound beads from HMEC-1 cell lysate, colloidal blue staining (Invitrogen) was performed on purified protein separated by using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under both reduced and nonreduced conditions. Under nonreduced conditions, two strong bands of 120 and 70 kDa were identified and excised from the gel, and the eluted proteins were analyzed using nano-capillary high-pressure liquid chromatography (HPLC) ion trap mass spectrometry (LC-MS/MS) and database searching by the Wistar Institute Proteomics Facility as follows. The protein band of interest was excised from the gel and destained. It was reduced by using 20 mM Tris(2-carboxyethyl)phosphine hydrochloride in 25 mM ammonium bicarbonate followed by alkylation with 40 mM iodoacetamide in 25 mM ammonium bicarbonate. After dry-down, it was rehydrated with 20 ml of 0.02-mg/ml modified trypsin (Promega) solution and incubated overnight. The next morning the supernatant was removed to a clean tube, and 20 ml of 40 mM ammonium bicarbonate was added for 30 min with shaking at 37°. The supernatants were combined, and 4 ml of undiluted acetic acid was added. A portion of the combined digest solution was subjected to LC-MS/MS using capillary HPLC with a 75-mm nanocolumn and a Thermo Electron LCQ Classic quadrapole ion trap mass spectrometer. The resulting masses and spectra were searched against the National Center for Biotechnology Information nr database, using TurboSEQUEST Browser (http://fields.scripps.edu/sequest/).
Detection of secreted PAL-E antigen.
Fresh human plasma (15 ml) was obtained from a normal human volunteer by centrifugation of heparinized blood at 3,000 × g for 15 min. Plasma was incubated with protein G agarose beads to adsorb proteins that bind nonspecifically for 3 h on a rocker at 4°C, followed by overnight incubation with PAL-E beads at 4°C. The beads were washed three times as described. The bound proteins were eluted off the beads, and immunoblot analysis was performed as described above. To detect PAL-E antigen secreted from primary endothelial cells, HMVEC (passage 6) cells were cultured in a 100-mm plate to 80% confluency and incubated with 10 ml of serum-free medium for 6 h prior to cell harvest. The HMVEC cell supernatant was collected and concentrated 10-fold by a Centricon spin column (Amicon Bioseparations). Secreted PAL-E was detected by examining 10 μl of concentrated supernatant, using PAL-E immunoblotting as described under nonreduced conditions.
PAL-E immunoelectron microscopy.
Indirect immunoelectron microscopy of human tissues was performed with PAL-E as the primary antibody and horseradish peroxidase-conjugated rabbit anti-mouse secondary antibody as previously described (7).
RESULTS
Identification of PAL-E antigen as a 120-kDa protein whose detection by PAL-E antibody is sensitive to reduced conditions.
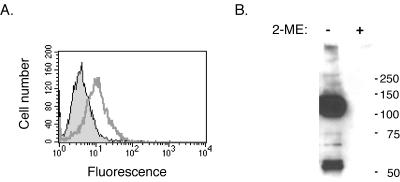
The PAL-E antibody is known to identify blood endothelial cells in frozen tissue sections but not in tissue sections following paraffin embedding, and no protein band has been identified by immunoblot analysis using reduced conditions. To determine if the PAL-E antigen is sensitive to reduced conditions, we performed immunoblot experiments using cell lysate derived from live HMEC-1 cells, microvascular endothelial cells previously shown to be PAL-E positive using flow cytometry (1). As previously reported, HMEC-1 cells exhibited surface binding of PAL-E antibody using flow cytometry (Fig. 1A), but immunoblotting under reduced conditions failed to identify an immunoreactive band (Fig. 1B, right). In contrast, PAL-E immunoblotting of HMEC-1 cell lysate performed under nonreduced conditions revealed a dominant 120-kDa band and less prominent bands between 55 and 60 kDa in size (Fig. 1B, left).
FIG. 1.
PAL-E antibody recognizes 55- and 120-kDa proteins in HMEC-1 cells under nonreduced conditions. A. Flow cytometry reveals PAL-E binding to the surface of live HMEC-1 cells. HMEC-1 cells were removed from the plate nonenzymatically, and flow cytometry was carried out with PAL-E (grey line) or isotype-matched mouse IgG (black line) as the primary antibody and fluorescein isothiocyanate-conjugated goat antimouse secondary antibody. Permeable, dead cells were excluded by using propidium iodide (data not shown). B. PAL-E recognizes 120- and 55-kDa proteins in HMEC-1 cells under nonreduced conditions. Immunoblot analysis of HMEC-1 cell lysate was performed by using the PAL-E antibody in the presence (right) or absence (left) of 2-mercaptoethanol to reduce disulfide bonds. Although no protein bands are detected under reduced conditions, two dominant bands are detected under nonreduced conditions.
Purified PAL-E-bound protein appears heterogeneous but runs as a dominant 55-kDa band under reduced conditions.
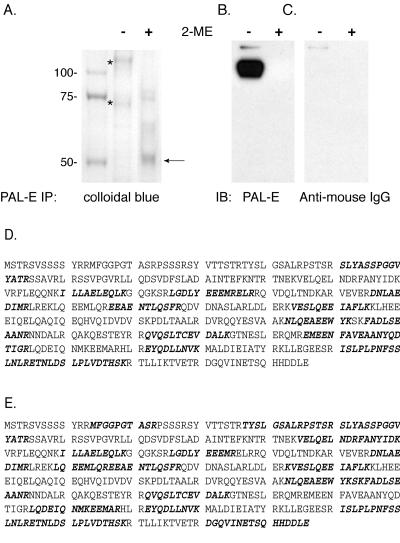
Colloidal blue staining of protein immunoprecipitated from HMEC-1 cell lysate, using PAL-E beads, revealed prominent protein bands of 120 and 70 kDa in molecular mass under nonreduced conditions (Fig. 2A). Of these bands, only the 120-kDa band was immunoreactive with PAL-E antibody in parallel immunoblots (Fig. 2B). Significantly, colloidal blue staining of PAL-E-precipitated protein under reduced conditions revealed a dominant 55-kDa band, although weaker bands of higher molecular masses were also detected (Fig. 2A). This band was not reactive with anti-mouse IgG antibody (Fig. 2C), suggesting that it represents reduced PAL-E antigen and not PAL-E antibody heavy chain released during the immunoprecipitation.
FIG. 2.
Identification of the PAL-E antigen in HMEC-1 cells as vimentin. A. Colloidal blue staining of PAL-E antigen purified from HMEC-1 cells. PAL-E bound protein was immunoprecipitated from HMEC-1 cell lysate with PAL-E-conjugated beads and analyzed by SDS-PAGE with colloidal blue staining in the presence (+) and absence (−) of the reducing agent 2-mercaptoethanol. The 120- and 70-kDa bands observed under nonreduced conditions (middle lane, indicated by asterisks) were excised for tryptic digestion and MS/MS analysis. Note the dominant 55-kDa band observed under reduced conditions (right lane, arrow). B and C. Immunoblot analysis of PAL-E immunoprecipitate using PAL-E and anti-mouse IgG antibodies. The PAL-E IP used for the colloidal blue staining shown in panel A was analyzed in a parallel immunoblot, using PAL-E+ anti-mouse IgG secondary antibody (B) or anti-mouse IgG antibody alone (C). Of the protein bands observed with colloidal blue staining in (A), only the 120-kDa band is immunoreactive with PAL-E antibody. The 55-kDa band observed under reduced conditions is not recognized by anti-mouse IgG antibody. D and E. MS/MS analysis of tryptic peptides obtained from the 120- and 70-kDa protein bands match vimentin. Tryptic peptides derived from the 120-kDa (D) and 70-kDa (E) protein bands seen in the nonreduced lane in (A) were subjected to MS/MS analysis. All high-confidence sequences matched the deduced vimentin amino acid sequence. Bold typeface indicates amino acids identified in individual peptide sequences from the two protein bands.
MS/MS analysis of tryptic peptides identifies the PAL-E antigen as vimentin.
To identify the protein(s) precipitated by the PAL-E antibody from HMEC-1 cell lysate, the 120- and 70-kDa bands observed with colloidal blue staining were isolated, and tryptic fragments were subjected to MS/MS spectra analysis and protein database searching to determine if they were known proteins. Analysis of the two bands identified 35 peptide sequences of high confidence, all of which matched the deduced vimentin amino acid sequence (Fig. 2D and E). For deduced vimentin amino acids, 33.5% and 46.4% were identified for the 120- and 70-kDa proteins, respectively, spanning virtually the entire predicted vimentin protein sequence. Significantly, all high-confidence peptides identified matched the deduced vimentin amino acid sequence, suggesting that each band was composed of only a single protein species (data not shown).
PAL-E-precipitated protein is recognized by monoclonal and polyclonal antivimentin antibodies.
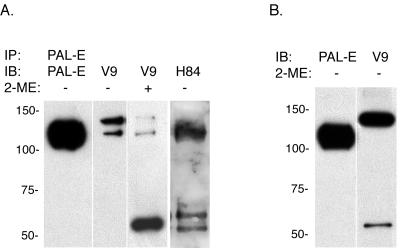
To confirm the identity of the protein detected by PAL-E antibody in HMEC-1 cells as vimentin, PAL-E-immunoprecipitated protein was immunoblotted using the monoclonal antivimentin antibody V9 and the rabbit polyclonal antivimentin antibody H84. Under nonreduced conditions these antivimentin antibodies identified 120- and 140-kDa proteins in PAL-E immunoprecipitates (Fig. 3A). Under reduced conditions, a predominant 55-kDa protein band similar to that seen in colloidal blue staining of PAL-E immunoprecipitate was detected by V9 (Fig. 3B). Interestingly, although the PAL-E antibody recognizes a 120-kDa vimentin protein under nonreduced conditions, V9 immunoblotting of PAL-E IP preferentially detected a 140-kDa band of equal strength under the same conditions. To determine if HMEC-1 cells predominantly express a 120-kDa form of vimentin or if PAL-E antibody selectively recognizes that form of the protein, we compared the detection of vimentin in HMEC-1 cell lysate under nonreduced conditions using the PAL-E and V9 monoclonal antibodies. In contrast to V9 detection of vimentin in PAL-E IP, the V9 antibody selectively recognized 140- and 55-kDa forms of vimentin in nonreduced HMEC-1 cell lysate (Fig. 3B). These results confirm that PAL-E antibody recognizes vimentin and suggest that vimentin may be found in high-molecular-mass forms to which different monoclonal antibodies bind selectively.
FIG. 3.
PAL-E-immunoprecipitated protein is recognized by the V9 antivimentin monoclonal antibody. A. Immunoblot analysis of PAL-E IP. HMEC-1 cell lysate was immunoprecipitated by using PAL-E-conjugated beads, and the precipitated proteins were analyzed using PAL-E, the V9 monoclonal antivimentin antibody, and the H84 polyclonal antivimentin antibody in the presence (+) or absence (−) of the reducing agent 2-mercaptoethanol. Note the detection of two bands of approximately 120 and 140 kDa in molecular mass by the V9 antibody. B. Immunoblot analysis of HMEC-1 cell lysate with PAL-E and antivimentin antibodies. The HMEC-1 cell lysate used for the IP in (A) was analyzed directly with PAL-E, V9, and H84 antibodies under nonreduced conditions. Note the preferential recognition of a 120-kDa protein by PAL-E and a 140-kDa protein by V9 and H84.
The vimentin recognized by PAL-E antibody does not arise from translation of an endothelial cell-specific vimentin mRNA.
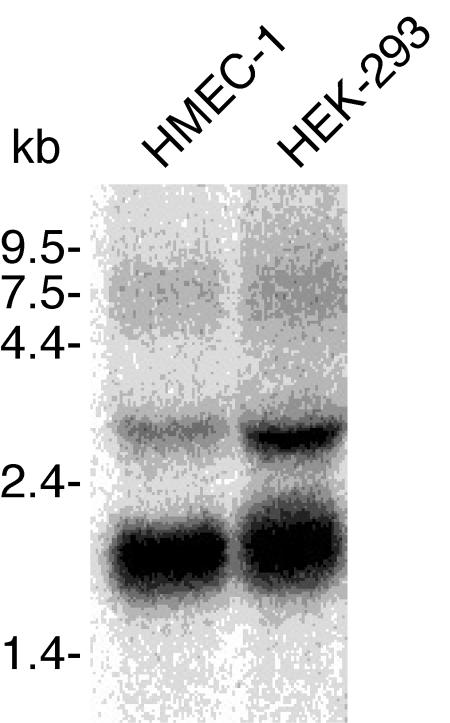
As discussed further below, there is an apparent discrepancy between the broad expression pattern of vimentin and the endothelial cell-specific staining of the PAL-E antibody. PAL-E specificity may arise due to expression of an endothelial cell-specific vimentin mRNA that encodes a unique amino acid sequence that forms the epitope recognized by PAL-E. Alternatively, PAL-E may recognize a form of vimentin generated in blood endothelial cells due to cell-specific posttranslational modification(s). To determine if PAL-E recognizes a form of vimentin encoded by an endothelial cell-specific mRNA, we compared vimentin mRNAs in PAL-E+ V9+ HMEC-1 cells with those in PAL-E− V9+ HEK-293 epithelial cells, using a probe containing sequence identified as PAL-E antigen as described above. Northern blot analysis, using a vimentin probe within the region of the protein identified by MS/MS analysis of PAL-E-purified protein, reveals identical transcripts in HMEC-1 and HEK-293 cells (Fig. 4). These results suggest that PAL-E-reactive vimentin is not the product of an endothelial cell-specific mRNA but is likely to arise as a result of posttranslational protein modification.
FIG. 4.
PAL-E-reactive vimentin is not the product of an endothelial cell-specific mRNA. Vimentin-encoding mRNAs were analyzed in PAL-E+ V9+ HMEC-1 cells and PAL-E− V9+ HEK-293 cells by Northern blotting. The probe used to detect vimentin transcripts in the two cell lines corresponded to a region encompassing amino acids 65 to 310, a region defined as contained within the PAL-E antigen by the analysis shown in Fig. 2.
PAL-E recognizes an extracellular form of vimentin distinct from that in intermediate filaments in endothelial cells.
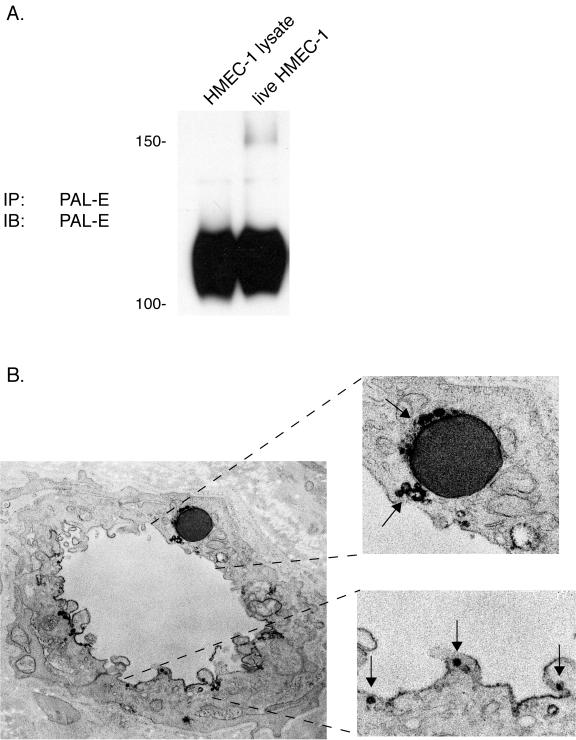
As previously reported (1) and shown in Fig. 1, PAL-E antibody binds to the extracellular surface of live HMEC-1 cells, an unexpected finding given the extensive characterization of vimentin as an intracellular intermediate filament protein. To ensure that the 120-kDa protein purified from HMEC-1 cell lysate is the same protein as that detected on the surface of live HMEC-1 cells, we performed live cell immunoprecipitation (IP) to characterize the protein recognized by PAL-E on the cells' extracellular surface. Live-cell IP using PAL-E antibody precipitated a 120-kDa protein that was indistinguishable from that identified in HMEC-1 cell lysate under nonreduced conditions (Fig. 5A), indicating that PAL-E antibody recognizes the same high-molecular-mass form of vimentin both inside and outside the cell and that PAL-E-reactive vimentin, in contrast to vimentin associated with intermediate filaments, reaches the extracellular space.
FIG. 5.
PAL-E-reactive vimentin is expressed on the luminal endothelial cell surface in association with endothelial vesicles. A. The PAL-E antigen detected on the surfaces of live HMEC-1 cells is identical to the one detected in total cell lysate. Immunoblot analysis was performed with PAL-E antibody on protein immunoprecipitated from total HMEC-1 cell lysate, using PAL-E-conjugated beads (left lane), and on protein immunoprecipitated from the surface of live HMEC-1 cells exposed to unconjugated PAL-E antibody and washed prior to lysis (“live cell” IP, right lane). Note the precipitation of identical 120-kDa proteins under both conditions. B. PAL-E-reactive vimentin is expressed in association with endothelial vesicles along the luminal cell membrane. Indirect immunoelectron microscopy was performed after binding with PAL-E antibody to a frozen human skin tissue section. Strong binding is observed around vesicles on the luminal cell membrane (lower right) and around a trapped erythrocyte (upper right) (magnification, ×76,000).
To further examine the cellular location of PAL-E-reactive vimentin, we performed immunoelectron microscopy with PAL-E antibody on human microvascular blood endothelial cells in frozen tissue sections. Extracellular PAL-E-reactive vimentin is not found in a uniform pattern along the endothelial extracellular surface but is instead expressed in a polarized fashion along the luminal cell membrane and concentrated at discrete sites in and adjacent to endothelial vesicles (Fig. 5B). PAL-E staining is also detected around a trapped erythrocyte, but PAL-E does not bind the intermediate filaments of the cell cytoskeleton (Fig. 5B). These studies suggest that PAL-E selectively recognizes an extracellular form of vimentin distinct from the vimentin in intermediate filaments.
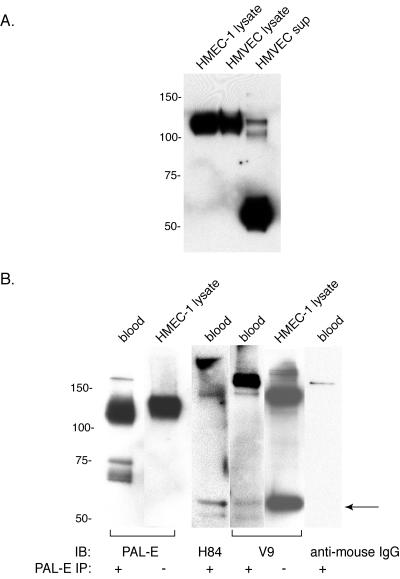
PAL-E-reactive vimentin is secreted by microvascular endothelial cells into circulating human blood.
A recent report describing the secretion of V9-reactive vimentin by activated macrophages (14) suggested that the expression of PAL-E in vesicles and on the luminal surface of blood endothelial cells might indicate luminal secretion of PAL-E-reactive vimentin. To determine if the vimentin recognized by PAL-E is secreted, HMEC-1 cells and primary microvascular endothelial cells (HMVEC) were grown in serum-free medium, and conditioned supernatant was collected, concentrated, and analyzed for the presence of PAL-E-reactive vimentin by Western blotting. Both primary HMVEC and HMEC-1 cell supernatant contained significant levels of secreted, PAL-E-reactive vimentin, although the levels were substantially higher in the primary endothelial cells (Fig. 6A). Interestingly, a large fraction of the PAL-E-reactive vimentin identified in HMVEC conditioned supernatant was detected as a 55-kDa band in contrast to the more predominant 120-kDa species isolated from the cell lysate and extracellular membrane of intact cells (Fig. 5A). Thus, the extracellular vimentin recognized by PAL-E antibody is a form of vimentin secreted by blood endothelial cells.
FIG. 6.
PAL-E-reactive vimentin is secreted by endothelial cells ex vivo and detected in human circulating blood. A. Secretion of PAL-E-reactive vimentin by primary human microvascular endothelial cells ex vivo. Primary human microvascular endothelial cells (HMVEC) and HMEC-1 cells were cultured in serum-free conditions, and immunoblot analysis was performed with PAL-E antibody under nonreduced conditions using supernatant (sup) and total cell lysate (lysate). Each lane was loaded with 10 μl of sample, corresponding to 1% of total recovered supernatant and cell lysate. Note the presence of PAL-E-reactive vimentin in HMVEC and HMEC-1 conditioned supernatant and its detection primarily as a 55-kDa protein. B. Detection of PAL-E-reactive vimentin in human blood. PAL-E antibody was used to immunoprecipitate vimentin from 15 ml of human plasma, and immunoprecipitate was analyzed with PAL-E, H84, V9, or secondary anti-mouse IgG under nonreduced conditions. Note the detection of 55- to 60-kDa vimentin species using the H84 and V9 antibodies (arrow) and the slightly smaller molecular mass of PAL-E-reactive vimentin in blood relative to that in HMEC-1 cell lysate.
The finding that PAL-E-reactive vimentin is secreted from primary HMVEC and the polarized detection of PAL-E binding on the luminal endothelial cell membrane suggested that in vivo PAL-E-reactive vimentin might be secreted into the circulating blood. Indeed, PAL-E-reactive vimentin was readily immunoprecipitated from human plasma and detected under nonreduced conditions (Fig. 6B). The molecular mass of circulating PAL-E-reactive vimentin appeared slightly smaller than that detected in HMEC-1 cell lysate and HMVEC conditioned medium (approximately 110 versus 120 kDa). Significantly, circulating vimentin immunoprecipitated by PAL-E antibody could be weakly detected by the polyclonal antivimentin antibody H84 and by the monoclonal V9 antivimentin antibody (Fig. 6B). Together with previous studies of PAL-E binding in human tissues, these results suggest that PAL-E antibody selectively identifies a form of vimentin that is luminally secreted by microvascular blood endothelial cells in vivo and that circulates in human blood. These studies also further demonstrate the marked heterogeneity of vimentin and the preferential recognition of different species by different monoclonal antivimentin antibodies.
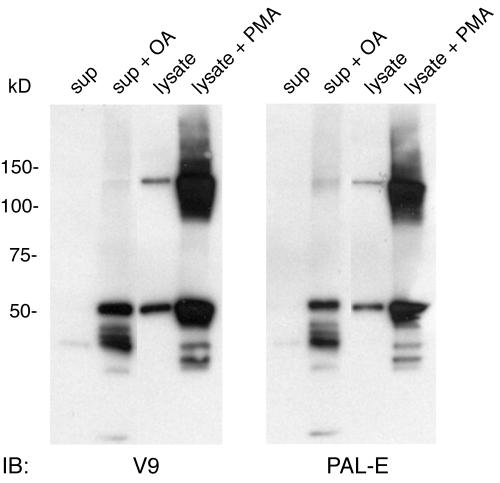
Activated macrophages secrete PAL-E-reactive vimentin.
The finding that PAL-E selectively recognizes secreted vimentin in endothelial cells and a previous report of vimentin secretion by activated macrophages suggested that those cells might also secrete PAL-E-reactive vimentin. To determine if macrophages secrete PAL-E-reactive vimentin, we performed immunoblot analysis of monocyte-derived macrophage (MDM) conditioned supernatant and cell lysate, using both the V9 and PAL-E antivimentin antibodies (14). As previously reported, MDMs cultured in 10 or 40% human serum secrete V9-reactive vimentin into the conditioned supernatant, and the level of this secretion can be dramatically increased with exposure to the protein phosphatase inhibitor okadaic acid (Fig. 7, left panel). Like endothelial cells, MDMs express two major intracellular forms of V9-reactive vimentin that appear as 140- and 55-kDa bands under nonreduced conditions, and the levels of both increase dramatically with phorbol myristate acetate (PMA) treatment (Fig. 7). The vimentin secreted by MDMs is also PAL-E reactive, and the levels of PAL-E-reactive vimentin both inside and outside the cell are regulated by modulating agents in a manner indistinguishable from that of V9-reactive vimentin (Fig. 7, right panel). As seen in primary HMVEC, the higher-molecular-mass forms of PAL-E-reactive vimentin are slightly smaller than those of V9-reactive vimentin, and the PAL-E-reactive vimentin detected in conditioned supernatant appears predominantly as a lower-molecular-mass form of the protein. These studies provide further evidence that the PAL-E antibody selectively recognizes secreted vimentin and suggest that the secreted form of vimentin detected by PAL-E antibody in endothelial cells is the same as that previously reported in activated macrophages.
FIG. 7.
The vimentin secreted by activated macrophages is PAL-E reactive. Primary human MDMs were cultured for 7 days in 10% serum, and conditioned supernatant or cell lysate was obtained as previously described (14). Total cell lysate (lysate) and concentrated conditioned supernatants (sup) were used for Western blotting with V9 and PAL-E antivimentin monoclonal antibodies under nonreduced conditions. OA indicates treatment of MDMs with okadaic acid (100 nM) for 2 h prior to incubation in serum-free medium. PMA indicates treatment with 100 nM phorbol ester for 16 h prior to cell lysis. Note that the patterns of V9-reactive and PAL-E-reactive vimentins are closely matched.
DISCUSSION
Endothelial cell heterogeneity underlies the development and function of the complex blood and lymphatic vascular networks in mammals (21). Our understanding of the molecular basis of endothelial heterogeneity has been advanced primarily through the identification of molecular markers that distinguish different endothelial cell types. One such marker, the PAL-E antigen, was discovered almost 20 years ago to be expressed by endothelial cells lining blood capillaries and venules but not by lymphatic or arterial endothelium (25). Since its discovery, PAL-E antibody has been used extensively to distinguish blood from lymphatic endothelial cells, and elevated levels of PAL-E antigen have been associated with specific vascular beds, including high endothelial venules, tumor blood vessels, and blood vessels at sites of inflammation (12, 13, 22, 23, 25). Despite its extensive characterization and association with human disease states, however, the protein recognized by the PAL-E antibody has remained unknown.
In the present study, we have identified the protein recognized by PAL-E antibody as vimentin, a surprising finding supported by several lines of evidence. PAL-E antibody primarily recognizes a 120-kDa protein on nonreduced immunoblots of HMEC-1 cell lysate, and purification of PAL-E-bound protein from the HMEC-1 endothelial cell line revealed dominant 120-kDa (immunoreactive) and 70-kDa (nonimmunoreactive) proteins. MS/MS analysis of tryptic peptides derived from these protein bands generated 35 high-confidence peptide sequences, all of which matched a single protein sequence—vimentin. When purified PAL-E bound protein was examined under reduced conditions, a dominant protein band was observed, with a molecular mass of 55 kD, the precise molecular mass of vimentin. In addition, under different conditions and in different cell types, e.g., in HMVEC conditioned supernatant and resting THP-1 cell lysate, PAL-E identified a 55-kDa protein in Western blots performed under nonreduced conditions. Finally, PAL-E-immunoprecipitated protein from HMEC-1 cells cross-reacts with the monoclonal antivimentin antibody V9 under both reduced and nonreduced conditions. Together these studies reveal that PAL-E antibody directly recognizes a form of vimentin that is highly sensitive to reduced conditions and often is associated with a high-molecular-mass form of the protein.
The identification of the protein recognized by PAL-E as vimentin is surprising because vimentin has been characterized primarily as an intracellular intermediate filament protein expressed in mesenchymal cells throughout the body (9, 10). In contrast, numerous studies of binding of PAL-E to cell lines and tissue samples have demonstrated highly specific binding to the cell surface of blood endothelium (24, 25). Thus, neither the cell-specific binding of PAL-E nor its cell surface binding matches the well-described sites of vimentin expression. A clue to the resolution of this paradox lies in the unusual surface binding pattern of PAL-E detected using immunoelectron microscopy. Rather than binding uniformly along the endothelial cell surface, PAL-E binds in a localized way to regions of the luminal cell membrane at and adjacent to sites of fusion with intracellular vesicles (Fig. 5), suggesting that PAL-E-bound protein might be associated with the cell membrane during either entrance or exit of the protein from the endothelial cell. Indeed, our studies of primary human microvascular endothelial cells and the HMEC-1 cell line reveal that endothelial cells actively secrete PAL-E-reactive vimentin into the extracellular space. Consistent with its polarized detection on the luminal side of endothelial cells in tissue sections, PAL-E-reactive vimentin is also readily detected in human circulating blood. This finding is likely to explain a recent report of vimentin associated with vitronectin and plasminogen activator inhibitor on the surface of activated platelets (20), since platelets internalize many circulating proteins, including vitronectin, that are subsequently released during platelet granule secretion after platelet activation. Thus, our findings and previous work characterizing the PAL-E antibody suggest that it selectively recognizes a secreted form of vimentin produced by blood vascular endothelial cells. The remarkable specificity of this antibody further suggests that secreted vimentin must have conformational and biochemical characteristics distinct from those of vimentin in intermediate filaments.
The existence of a secreted form of vimentin in activated macrophages has recently been reported (14), and we find that these cells also synthesize and secrete PAL-E-reactive vimentin. It therefore appears that both blood endothelial cells and macrophages are sources of this novel form of vimentin in vivo. Numerous studies have demonstrated that intracellular vimentin is phosphorylated on serine and threonine residues by a number of kinases, including protein kinase C (PKC) (27, 28). In monocyte-derived macrophages, vimentin secretion appears to be regulated by phosphorylation of the protein, and agents that increase or decrease PKC activity positively or negatively regulate vimentin secretion, respectively (14). Interestingly, in the conditioned supernatant of MDMs, vimentin detection by the secretion-specific PAL-E antibody and the broader V9 antibody are almost identical in response to PKC modulators such as PMA and okadaic acid. Thus, it seems likely that both intermediate filament vimentin and secreted vimentin are regulated by phosphorylation events. The precise role of phosphorylation in the regulation of vimentin function in vascular cells is not yet clear and must await in-depth structure-function analysis and a better understanding of the mechanism by which vimentin is secreted.
In addition to its marked sensitivity to changes in phosphorylation, our studies suggest that secreted, PAL-E-reactive vimentin differs structurally from nonsecreted, intermediate filament vimentin. The PAL-E antibody is unable to recognize reduced vimentin and frequently recognizes a form of vimentin with a molecular mass roughly double that of monomeric vimentin (120 kDa versus 50 to 60 kDa). There are several possible explanations for the unusual size and sensitivity to reduced conditions of PAL-E-reactive vimentin. First, PAL-E may recognize a complex of vimentin covalently bound to a second protein that is disrupted by reduced conditions. Such a mechanism might provide a mechanism by which the protein is secreted in the absence of a defined secretory signal. Alternatively, PAL-E may recognize an epitope present in a secreted vimentin dimer that is formed by linking the single cysteine residue of two individual vimentin molecules. Finally, endothelial cells and activated macrophages may express a unique form of vimentin that includes amino acid sequences not found in other cell types. Several lines of evidence favor PAL-E recognition of a secreted vimentin dimer that is further modified by phosphorylation. To create a high-molecular-mass covalent complex recognized by PAL-E, a second protein would have to be present at an equal molar ratio. However, high-confidence MS/MS analysis of more than 35 tryptic peptides derived from two high-molecular-mass species matched all the peptides to the known vimentin amino acid sequence. In addition, the ability of PAL-E antibody to detect a 55-kDa band is most consistent with direct recognition of the vimentin protein alone. PAL-E-reactive vimentin does not appear to result from the translation of an endothelial cell-specific vimentin mRNA, since PAL-E-expressing HMEC-1 cells and non-PAL-E-expressing HEK-293 cells exhibit identical vimentin transcripts. Finally, vimentin contains a single cysteine residue, suggesting that disulfide-linked vimentin molecules would primarily form dimers with molecular masses in the 110- to 120-kDa range, closely matching the observed molecular mass of PAL-E-reactive protein. Together these studies suggest that the high-molecular-mass protein recognized by PAL-E is a vimentin homodimer. Whether vimentin dimerization plays a role in vimentin secretion or further protein modification, such as phosphorylation, is unknown. Similarly, the mechanism by which vimentin is secreted is unclear, since the protein lacks a defined secretory signal. The amino-terminal domain of the protein has been demonstrated to mediate association with lipid membranes and may mediate its secretion through association with the endoplasmic reticulum (19), and the carboxyl terminus of the protein contains a di-acidic motif (Asp-X-Glu) like that utilized by some proteins for export from the endoplasmic reticulum to the Golgi (16), but the functional importance of these sequences for vimentin secretion remains untested.
The unique pattern of PAL-E endothelial staining in endothelial cells, the identification of circulating PAL-E-reactive vimentin, and its presence in activated macrophages are all consistent with a novel, vascular role for extracellular vimentin. Among endothelial cells in vivo, the expression of PAL-E-reactive vimentin is restricted to a subset of vessels whose endothelial cells are known to regulate passage of circulating cells from the blood into the tissues, i.e., capillaries and venules. Among normal tissue vascular beds, PAL-E-reactive vimentin expression is highest in the high endothelial venules of lymph nodes, cells specialized in the movement of circulating lymphocytes from the blood to the lymph node tissue. In contrast, PAL-E staining is absent or low in vascular beds through which circulating cells pass without restriction, such as lymphatics, and in vascular beds that do not routinely mediate passage of circulating cells, such as arteries and in the vessels that form the blood-brain barrier. While the biological function of secreted vimentin remains unknown, the pattern of expression of PAL-E-reactive vimentin suggests that the protein may play a role in mediating the movement of circulating cells across the endothelium at these sites, a process in which both activated macrophages and activated platelets participate (5, 15). In this regard it is interesting that vimentin-deficient mice do not exhibit the defects associated with the loss of other intermediate filament proteins but have impaired wound healing responses (4, 6, 8), a phenotype that has been attributed to loss of intracellular vimentin in mesenchymal cells but might also be due to impaired cellular movement across capillaries at the wound site. Our studies reveal the presence of a novel, extracellular form of vimentin in the blood vasculature and may provide new clues regarding the role played by vimentin in vivo. Further studies to determine if secreted vimentin binds a specific receptor on endothelial or circulating cells or if vimentin-deficient mice exhibit defects in the movement of circulating cells across endothelial barriers during inflammatory states will help establish the vascular role of secreted vimentin.
Acknowledgments
We acknowledge Ed Morrisey for helpful thoughts and advice throughout this project and the Proteomics Core of the Wistar Institute for analysis of purified proteins.
REFERENCES
- 1.Ades, E. W., F. J. Candal, R. A. Swerlick, V. G. George, S. Summers, D. C. Bosse, and T. J. Lawley. 1992. HMEC-1: establishment of an immortalized human microvascular endothelial cell line. J. Investig. Dermatol. 99:683-690. [DOI] [PubMed] [Google Scholar]
- 2.Carmeliet, P. 2003. Angiogenesis in health and disease. Nat. Med. 9:653-660. [DOI] [PubMed] [Google Scholar]
- 3.Chen, H., D. Locke, Y. Liu, C. Liu, and M. L. Kahn. 2002. The platelet receptor GPVI mediates both adhesion and signaling responses to collagen in a receptor density-dependent fashion. J. Biol. Chem. 277:3011-3019. [DOI] [PubMed] [Google Scholar]
- 4.Colucci-Guyon, E., M. M. Portier, I. Dunia, D. Paulin, S. Pournin, and C. Babinet. 1994. Mice lacking vimentin develop and reproduce without an obvious phenotype. Cell 79:679-694. [DOI] [PubMed] [Google Scholar]
- 5.Diacovo, T. G., K. D. Puri, R. A. Warnock, T. A. Springer, and U. H. von Andrian. 1996. Platelet-mediated lymphocyte delivery to high endothelial venules. Science 273:252-255. [DOI] [PubMed] [Google Scholar]
- 6.Eckes, B., E. Colucci-Guyon, H. Smola, S. Nodder, C. Babinet, T. Krieg, and P. Martin. 2000. Impaired wound healing in embryonic and adult mice lacking vimentin. J. Cell Sci. 113:2455-2462. [DOI] [PubMed] [Google Scholar]
- 7.Erhard, H., F. J. Rietveld, E. B. Brocker, R. M. de Waal, and D. J. Ruiter. 1996. Phenotype of normal cutaneous microvasculature. Immunoelectron microscopic observations with emphasis on the differences between blood vessels and lymphatics. J. Investig. Dermatol. 106:135-140. [DOI] [PubMed] [Google Scholar]
- 8.Evans, R. M. 1998. Vimentin: the conundrum of the intermediate filament gene family. Bioessays 20:79-86. [DOI] [PubMed] [Google Scholar]
- 9.Franke, W. W., E. Schmid, S. Winter, M. Osborn, and K. Weber. 1979. Widespread occurrence of intermediate-sized filaments of the vimentin-type in cultured cells from diverse vertebrates. Exp. Cell Res. 123:25-46. [DOI] [PubMed] [Google Scholar]
- 10.Fuchs, E., and K. Weber. 1994. Intermediate filaments: structure, dynamics, function, and disease. Annu. Rev. Biochem. 63:345-382. [DOI] [PubMed] [Google Scholar]
- 11.Garlanda, C., and E. Dejana. 1997. Heterogeneity of endothelial cells. Specific markers. Arterioscler. Thromb. Vasc. Biol. 17:1193-1202. [DOI] [PubMed] [Google Scholar]
- 12.Leenstra, S., D. Troost, P. K. Das, N. Claessen, A. E. Becker, and D. A. Bosch. 1993. Endothelial cell marker PAL-E reactivity in brain tumor, developing brain, and brain disease. Cancer 72:3061-3067. [DOI] [PubMed] [Google Scholar]
- 13.Leenstra, S., P. K. Das, D. Troost, D. A. Bosch, N. Claessen, and A. E. Becker. 1990. PAL-E, monoclonal antibody with immunoreactivity for endothelium specific to brain tumours. Lancet 335:671. [DOI] [PubMed] [Google Scholar]
- 14.Mor-Vaknin, N., A. Punturieri, K. Sitwala, and D. M. Markovitz. 2003. Vimentin is secreted by activated macrophages. Nat. Cell Biol. 5:59-63. [DOI] [PubMed] [Google Scholar]
- 15.Muller, W. A., and S. A. Weigl. 1992. Monocyte-selective transendothelial migration: dissection of the binding and transmigration phases by an in vitro assay. J. Exp. Med. 176:819-828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishimura, N., and W. E. Balch. 1997. A di-acidic signal required for selective export from the endoplasmic reticulum. Science 277:556-558. [DOI] [PubMed] [Google Scholar]
- 17.Oliver, G., and M. Detmar. 2002. The rediscovery of the lymphatic system: old and new insights into the development and biological function of the lymphatic vasculature. Genes Dev. 16:773-783. [DOI] [PubMed] [Google Scholar]
- 18.Page, C., M. Rose, M. Yacoub, and R. Pigott. 1992. Antigenic heterogeneity of vascular endothelium. Am. J. Pathol. 141:673-683. [PMC free article] [PubMed] [Google Scholar]
- 19.Perides, G., C. Harter, and P. Traub. 1987. Electrostatic and hydrophobic interactions of the intermediate filament protein vimentin and its amino terminus with lipid bilayers. J. Biol. Chem. 262:13742-13749. [PubMed] [Google Scholar]
- 20.Podor, T. J., D. Singh, P. Chindemi, D. M. Foulon, R. McKelvie, J. I. Weitz, R. Austin, G. Boudreau, and R. Davies. 2002. Vimentin exposed on activated platelets and platelet microparticles localizes vitronectin and plasminogen activator inhibitor complexes on their surface. J. Biol. Chem. 277:7529-7539. [DOI] [PubMed] [Google Scholar]
- 21.Risau, W. 1997. Mechanisms of angiogenesis. Nature 386:671-674. [DOI] [PubMed] [Google Scholar]
- 22.Ruiter, D. J., R. O. Schlingemann, J. R. Westphal, M. Denijn, F. J. Rietveld, and R. M. De Waal. 1993. Angiogenesis in wound healing and tumor metastasis. Behring Inst. Mitt 1993:258-272. [PubMed] [Google Scholar]
- 23.Ruiter, D. J., R. O. Schlingemann, F. J. Rietveld, and R. M. de Waal. 1989. Monoclonal antibody-defined human endothelial antigens as vascular markers. J. Investig. Dermatol. 93:25S-32S. [DOI] [PubMed] [Google Scholar]
- 24.Sauter, B., D. Foedinger, B. Sterniczky, K. Wolff, and K. Rappersberger. 1998. Immunoelectron microscopic characterization of human dermal lymphatic microvascular endothelial cells. Differential expression of CD31, CD34, and type IV collagen with lymphatic endothelial cells vs blood capillary endothelial cells in normal human skin, lymphangioma, and hemangioma in situ. J. Histochem. Cytochem. 46:165-176. [DOI] [PubMed] [Google Scholar]
- 25.Schlingemann, R. O., G. M. Dingjan, J. J. Emeis, J. Blok, S. O. Warnaar, and D. J. Ruiter. 1985. Monoclonal antibody PAL-E specific for endothelium. Lab. Investig. 52:71-76. [PubMed] [Google Scholar]
- 26.Sleeman, J. P., J. Krishnan, V. Kirkin, and P. Baumann. 2001. Markers for the lymphatic endothelium: in search of the holy grail? Microsc. Res. Tech. 55:61-69. [DOI] [PubMed] [Google Scholar]
- 27.Turowski, P., T. Myles, B. A. Hemmings, A. Fernandez, and N. J. Lamb. 1999. Vimentin dephosphorylation by protein phosphatase 2A is modulated by the targeting subunit B55. Mol. Biol. Cell 10:1997-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yasui, Y., H. Goto, S. Matsui, E. Manser, L. Lim, K. Nagata, and M. Inagaki. 2001. Protein kinases required for segregation of vimentin filaments in mitotic process. Oncogene 20:2868-2876. [DOI] [PubMed] [Google Scholar]