Abstract
Brd4 is a mammalian protein that contains a double bromodomain. It binds to chromatin and regulates cell cycle progression at multiple stages. By immunopurification and mass spectrometry, we identified a Rap GTPase-activating protein (GAP), signal-induced proliferation-associated protein 1 (SPA-1), as a factor that interacts with Brd4. SPA-1 localizes to the cytoplasm and to a lesser degree in the nucleus, while Brd4 resides in the nucleus. Bifluorescence complementation revealed that Brd4 and SPA-1 interact with each other in the nucleus of living cells. Supporting the functional importance of the interaction, Brd4 enhanced Rap GAP activity of SPA-1. Furthermore ectopic expression of SPA-1 and Brd4 redirected subcellular localization of the partner and disrupted normal cell cycle progression. These effects were, however, reversed by coexpression of the two proteins, indicating that a proper balance between Brd4 and SPA-1 in G2 is required for cell division. This work reveals a novel link between Brd4 and a GTPase-dependent mitogenic signaling pathway.
The BET family is a distinct group of bromodomain proteins that carry two tandem bromodomains and the ET domain (10, 20, 29) (see Fig. 3). The bromodomain is a conserved motif that is present in many nuclear regulatory factors and is composed of four α helices. Structural studies of the bromodomains in PCAF and TAF250 indicate that they have a binding affinity for acetylated lysines and acetylated histone H4 (11, 19), leading to the proposal that the domain acts as a chromatin-targeting module (42). It was recently shown that mammalian BET proteins also bind to acetylated histones in living cells (9, 22). Drosophila melanogaster Fsh is the oldest known member of the BET family (12, 15). In Saccharomyces cerevisiae, there are two BET family proteins, Bdf1 and Bdf2, that are similar in structure (7). While each gene alone is not essential, disruption of both bdf1 and bdf2 is lethal (30). Bdf1 is part of a general transcription factor complex, playing a role in transcription (27). In addition, genetic analysis indicates that it plays a role in cell growth regulation (7) and chromatin remodeling (24). In mammals there are five genes that encode members of the BET family, designated brd1 through brd5. Brd2, previously known as Ring 3/frgs1, is a component of the transcription mediator (21) and may have a kinase activity (8).
FIG. 3.
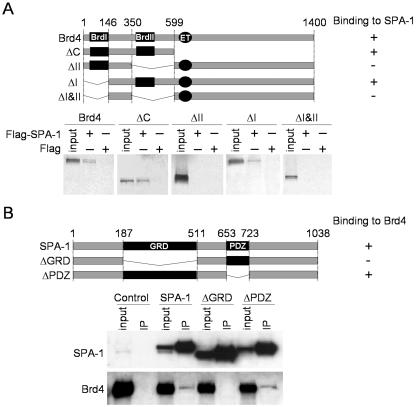
Domain analysis. (A) Diagram of Brd4 deletions and in vitro Brd4-SPA-1 pull-down assay. Two bromodomains are marked as BrdI and BrdII. The ET domain is also shown. Baculovirus recombinant Flag-SPA-1 was bound to M2-Flag agarose (Sigma). Ten microliters of SPA-1 bound to M2-Flag agarose was incubated with 10 μl of 35S-labeled Brd4 or Brd4 mutants. Bound material was separated on SDS-4 to 20% polyacrylamide gels, and bands were visualized by autoradiography. Inputs represent 50% of total Brd4. (B) Diagram of SPA-1 deletions and in vivo Brd4-SPA-1 interaction domain analysis. The Rap GAP-related domain (GRD) and postsynaptic density protein PSD-95 domain (PDZ) are indicated. Extracts from NIH 3T3 cells transfected with indicated GFP-Flag-SPA-1 constructs were immunoprecipitated with anti-Flag antibody and tested by immunoblotting. Inputs represent 7% of extracts.
Brd4 encodes a 200-kDa nuclear phosphoprotein (see Fig. 3A for structure) which is expressed broadly in many tissues and is induced in response to growth stimuli. Brd4 regulates cell growth by multiple mechanisms acting at different stages of the cell cycle. Both overexpression and underexpression of Brd4 are detrimental to cell growth (10, 16, 29). Brd4 interacts with acetylated chromatin with a rapid “on and off” mode of binding in vivo (9). While transiently binding to chromatin, Brd4 is likely to interact with other proteins to exert multiple functions. For example, it was found to interact with a component of DNA replication machinery, RFC, thereby contributing to the regulation of G1/S transition (29).
Rap1 and Rap2 are small GTPases of the Ras superfamily that are structurally very similar to Ras (4, 39). GTPases function as biochemical transducers that regulate growth, differentiation, survival, and adhesion throughout eukaryotic cells; they switch between an inactive GDP-bound and an active GTP-bound conformation. This cycling is mediated by guanine nucleotide exchange factor, the activator, and GTPase-activating proteins (GAPs), the inactivators (5). Rap GTPases are shown to antagonize with the activity of Ras to provide antimitogenic and antitransforming signaling (23). However, Rap can positively contribute to mitogenic signaling (1, 41) and exert a positive and negative effect on cell growth as well depending on the cell type (38). As with other small GTPases, Rap activities are controlled in a reciprocal manner by GTP exchange factors (GEFs) and GAPs. These factors convert Rap proteins either to the active, GTP-bound form or to the inactive, GDP-bound form. The Rap1 protein may have different cellular functions depending on the isoform and its subcellular localization, as well as the proliferative capacity and differentiation state of the particular cell type. For example Rap1 proteins have been localized in secretory granules of rat salivary gland (13), neutrophils (28), and platelets (26); in the Golgi complex of rat fibroblasts (2); in the endocytic compartment of fibroblasts and skeletal muscle cells (35); and in the perinuclear region of COS-1 cells (32). More recently Rap1 has been found to be highly expressed in the nucleus of squamous cell carcinomas, making it the second (after Ran) of over 100 small GTP binding proteins to be identified in the nucleus. In these cells the active GTP-bound Rap translocates to the nucleus whereas inactive Rap-GDP is retained in the cytoplasm, much of which is in a perinuclear distribution. The striking prominence of Rap1 expression in the nucleus of SCC cells suggests that activated Rap1 plays a role in the malignancy process (31).
The signal-induced proliferation-associated protein 1 (SPA-1) gene encodes a protein bearing a domain highly homologous to the catalytic region of human Rap1 GAP, a GAP-related domain (GRD) at the N terminus, and PEST sequences followed by a leucine zipper motif at the C terminus (25) (see Fig. 3B for structure). SPA-1 is one of several recently identified GAPs that are specific for Rap1 and Rap2. It is highly expressed in lymphoid cells and is shown to play a role in cell growth and adhesion. Little SPA-1 is expressed in quiescent lymphocytes, but it is induced following various types of mitogenic stimulation (14, 40). SPA-1-deficient mice develop myeloproliferative stem cell disorders with abnormal Rap GTP accumulation (18).
In this paper we show that Brd4 interacts with SPA-1 in vivo and in vitro and increases Rap GAP activity of SPA-1. Providing the functional significance of the interaction, we show that Brd4 and SPA-1 regulate each other's subcellular localization and affect cell cycle progression from G2 through M.
MATERIALS AND METHODS
Immunopurification of Brd4 complex.
HeLa cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum. Nuclear extracts were prepared as follows. Pelleted cells from 10 liters of culture were placed in 5 volumes of ice-cold hypotonic buffer (10 mM HEPES [pH 7.9], 10 mM KCl, 1.5 mM MgCl2, 1 mM dithiothreitol) containing proteinase inhibitor cocktail (Boehringer); phenylmethylsulfonyl fluoride (PMSF; 1 mM); and phosphatase inhibitors Na3VO4 (1 mM), Na2MoO4 (100 μM), and NaF (10 mM) and then lysed with a Dounce homogenizer. Nuclei were separated by centrifugation at 3,900 rpm (Sorval RC5C), and nuclear proteins were extracted with 20 mM HEPES (pH 7.9)-0.42 M NaCl-1.5 mM MgCl2-0.2 mM EDTA (pH 8)-25% glycerol-1 mM dithiothreitol-PMSF (1 mM)-proteinase and phosphatase inhibitors as described above. Nuclear extracts were dialyzed in buffer containing 20 mM HEPES (pH 7.9), 0.1 M KCl, 0.2 mM EDTA (pH 8), and 20% glycerol. One hundred milligrams of HeLa nuclear extracts was fractionated on a Q-Sepharose column containing ∼2.5 ml of resin equilibrated with 0.1 M KCl in buffer B (20 mM Tris-HCl [pH 8.0], 5 mM MgCl2, 10% glycerol, 1 mM PMSF, 0.1% Tween 20, 10 mM β-mercaptoethanol) and successively step eluted with buffer B containing 0.1 M KCl and 0.3 M KCl. Fractions collected from the 0.3 M KCl elution were dialyzed against buffer B containing 0.15 M KCl for 3 h. Two milligrams of proteins was incubated with 200 μl of anti-Brd4 or preimmune immunoglobulin G (IgG)-conjugated protein A-Trisacryl (Pierce) (2 mg of serum per ml of resin) for 4 h at 4°C with rotation. After washing, the bound proteins were eluted with 20 mM Tris-HCl buffer (pH 8) containing 2% sodium dodecyl sulfate (SDS) and resolved on an SDS-10% polyacrylamide gel. The 130-kDa band was excised from a Coomassie blue-stained gel and was analyzed by mass spectrometry with liquid chromatography-tandem mass spectrometry spectra as previously described (33). To assess the molecular masses of the Brd4 and SPA-1 complexes, 500 μg of the 0.3 M KCl-Q-Sepharose fraction was subjected to gel filtration analysis on a Superdex 200 column with use of the SMART system (Pharmacia Biotech, Uppsala, Sweden). Fractions were resolved on an SDS-10% polyacrylamide gel and analyzed by immunoblotting.
Coimmunoprecipitation assays.
For coimmunoprecipitation experiments, 3 × 106 HeLa cells were transfected with 6 μg of the plasmids named pCMV2-Flag-Brd4 (29) and pEGFP-SPA-1 [constructed by ligation of a BamHI-XhoI fragment of pBluescript KS (+) SPA-1 (25) into the BglII-SalI site of pEGFP-C1] or empty vector for pCMV2-Flag (Kodak) and pEGFP-C1 (Clontech) for 24 h with use of Polyfect (Qiagen). Three hundred micrograms of nuclear extracts was incubated with 20 μl of anti-Flag M2 affinity gel (Sigma) and anti-green fluorescent protein (anti-GFP) affinity gel (Santa Cruz) at 4°C for 4 h, and precipitated materials were analyzed by immunoblotting as described previously (29). Rabbit antibodies to Brd4 and SPA-1 were described previously (10, 25). Antibodies to GFP were obtained from Santa Cruz Biotechnology.
For domain analysis 3 × 106 NIH 3T3 cells were transfected with 4 μg of pEGFP-Flag-SPA-1, pEGFP-Flag-ΔGRD, pEGFP-Flag-ΔPDZ, or pEGFP-C1-Flag, constructed by ligation of a BamHI-XhoI fragment of pBluescript KS (+) SPA-1 (25) or SPA-1 deletion mutants, into the BglII-SalI site of pEGFP-C1, for 24 h with use of Polyfect. Four hundred micrograms of nuclear extracts was incubated with 20 μl of anti-Flag M2 affinity gel (Sigma), and precipitates were analyzed by immunoblotting as described above.
Expression and purification of proteins.
Rap1A, Rap2A, and glutathione S-transferase (GST)-RalGDS-Rap1b-binding domain (RBD) were expressed as GST fusion proteins and purified from Escherichia coli by single-step purification with glutathione-Sepharose 4B (Amersham Pharmacia Biotech) as described previously (25). The vectors containing 6×His-Brd4-Flag, 6×His-ΔC-Flag, and 6×His-ΔII-Flag deletion mutants were produced by adding the Flag tag to the end of the Brd4 gene, which was inserted into pAcHLT-c baculovirus vector at the EcoRI/XmaI site. The 6×His-Flag-tagged SPA-1 as well as 6×His-Brd4-Flag, 6×His-ΔC-Flag, and 6×His-ΔII-Flag deletion mutants was expressed by an Sf9-baculovirus expression system. To generate recombinant virus, 0.5 μg of Baculogold virus DNA was mixed with 2 μg of the respective pAcHLT-c baculovirus vector and cotransfected into Sf9 cells. Supernatant was collected, and recombinant virus was amplified. Sf9 cells (3 × 108) were infected with the recombinant virus at 10 PFU/cell and incubated at 27°C. After 72 h the cells were harvested, washed with phosphate-buffered saline (PBS), and lysed with lysing buffer (20 mM Tris-HCl [pH 8.0], 0.1% Tween, 0.5 M KCl, 0.2 mM EDTA [pH 8.0], 10% glycerol). The recombinant proteins were purified by double-step purification with Ni-nitrilotriacetic acid agarose (Qiagen) and Flag-M2 agarose (Sigma).
Brd4-SPA-1 interaction in vitro.
A baculovirus vector, pBacSpa1, tagged with six histidines and Flag at the C terminus, was constructed as described in reference 25 and was expressed in Sf9 cells. Lysates from baculovirus-infected Sf9 cells were purified on Ni-nitrilotriacetic acid agarose (Qiagen) (3), and recombinant Flag-SPA-1 was bound to M2-Flag agarose (Sigma). Ten microliters of SPA-1 bound to M2-Flag agarose was incubated with 10 μl of 35S-labeled Brd4 or Brd4 deletions (29) produced by the in vitro transcription-translation reaction (TNT Quick Coupled Transcription/Translation System; Promega) for 3 h at 4°C in PBS supplemented with 0.1% Triton, 0.1% NP-40, and 10% glycerol. After being washed four times, bound material was separated on an SDS-4 to 20% polyacrylamide gel, and bands were visualized by autoradiography.
Rap GTPase and Rap activation assays.
The GTPase activity was measured as described previously (25) Briefly, Rap proteins (3 μg) were added to 4.4 nM [γ-32P]GTP (MP Biomedicals) and incubated at 30°C for 30 min. Different amounts of recombinant SPA-1 (0.1 to 10 μg) were added, and samples were incubated at 30°C for 20 min. In some experiments recombinant Brd4 or Brd4 deletion mutants were incubated at the same time as was SPA-1 in different molar amounts in relation to SPA-1, which was kept constant (0.5 μg), and the samples were incubated at 30°C for 20 min. The percentage of hydrolyzed GTP was measured.
To study the in vivo effect of Brd4 on Rap GAP activity, the RBD of RalGDS fused to GST was used to isolate active Rap1 and Rap2 (37). NIH 3T3 cells (1.6 × 106) were transfected with 2 μg of pEGFP-SPA-1 alone or together with 2 μg of pEGFP-Brd4 or pEGFP-Brd4 deletion mutants, with the total amount of plasmids adjusted to 4 μg with control plasmid pEGFP-C1 (Clontech), for 24 h with use of Polyfect. Cells were lysed in 50 mM Tris-HCl (pH 7.6)-0.5% Triton X-100-150 mM NaCl, and each lysate was incubated for 1 h at 4°C with 20 μl of glutathione Sepharose 4B (Amersham Pharmacia Biotech) bound to GST-RalGDS-RBD or control GST fusion proteins. Bound materials were analyzed by immunoblotting. Rap1 and Rap2 antibodies were obtained from Transduction Laboratories and Santa Cruz, respectively.
Immunofluorescence staining.
For SPA-1 and Rap2 immunostaining, 105 NIH 3T3 cells were seeded on a coverslip and incubated in Dulbecco's modified Eagle's medium supplemented with 10% bovine calf serum. Cells were fixed in 4% paraformaldehyde for 10 min at room temperature and permeabilized with 0.5% Triton X-100 in PBS for 10 min. After incubation in blocking buffer (1% bovine serum albumin in PBS) for 10 min, cells were incubated with mouse antibody to SPA-1 (1:100 in blocking buffer) or to Rap2 (Transduction Laboratories; 1:100 in blocking buffer) for 60 min at room temperature. Cells were incubated with biotinylated anti-mouse IgG (1:400) for 60 min in blocking buffer and with Cy3-conjugated streptavidin (1:400) (both from Amersham) for 30 min in blocking buffer. Staining of Brd4 was described previously (10). In experiments designed to visualize the change of localization of SPA-1 and Brd4, 2 × 105 NIH 3T3 cells were transfected with 1.5 μg of pEGFP-Brd4, pEGFP-ΔC, pEGFP-ΔI&II, pEGFP-SPA-1, or control vector pEGFP-C1 for 24 h with use of Polyfect. Subsequently cells were stained with SPA-1 or Brd4 antibody as described above. Stained cells were viewed with a Leica TCS SP2 confocal microscope.
BiFC.
To perform bimolecular fluorescence complementation (BiFC) (17), we first constructed expression vectors encoding SPA-1 and Brd4 fused to N- and C-terminal fragments of pEYFP [pY(N)-SPA-1, pY(C)-Brd4]. To prepare a Y(N) vector, the enhanced yellow fluorescent protein (EYFP) sequence in pEYFP-C1 (Clontech) was replaced by the N-terminal half of EYFP obtained by digestion with NheI and BglII. Fragments of SPA-1 and SPA-1 deletion mutants, obtained by digestion with BamHI and XhoI, were inserted into the BglII/SalI site of the Y(N) multicloning site, downstream from the N-terminal portion of YFP. The Y(C) plasmid was prepared by replacing the EYFP sequence in the pEYFP-N1 vector (Clontech) with the C-terminal half of EYFP obtained by BamHI and NotI digestion. Brd4 cDNAs, cut by HindIII and SacII, were inserted into Y(C) upstream of the C-terminal portion of YFP, after removal of the Brd4 stop codon. NIH 3T3 cells (105) were seeded on a coverslip and transfected the next day with 0.4 μg each of pY(N)-SPA-1 and pY(C)-Brd4 constructs for 24 h with use of Polyfect. In some experiments pEBFP vector (0.4 μg) was cotransfected to verify transfection. Cells were allowed to stand at 30°C for 1 h, and the emission of YFP fluorescence was detected on a confocal microscope with excitation at 500 nm and emission at 535 nm.
Cell cycle analysis.
Synchronized HeLa cells were transfected with SPA-1, Brd4, and Brd4 mutant expression vectors alone or in combination during G1 or G2 phase as follows. HeLa cells were synchronized by double thymidine block (10) and released. For transfection of G1 cells, released cells were incubated in complete medium for an additional 10 h, allowing them to reach mitosis. Mitotic cells were harvested by mechanical shaking, replated, and allowed to progress to G1 phase for 2 h. Cells were transfected with Polyfect with indicated Brd4 or SPA-1 vectors (3 μg) and interleukin-2 receptor α (IL-2Rα) plasmids and incubated for an additional 8 h to allow expression of exogenous proteins. Transfected cells were harvested and sorted on anti-IL-2Rα antibody magnetic beads and stained with propidium iodide, and cell cycle profiles were analyzed by flow cytometry (29). For transfection of cells in G2, cells released from the double thymidine block were incubated in complete medium for 8 h, allowing cells to reach G2 phase, and transfected with indicated plasmids (3 μg). After transfection, cells were incubated for 10 h, allowing them to proceed through M and to G1. Transfected cells were harvested, and cell cycle profiles were analyzed as described above. The purity of transfected cells after sorting with IL-2Rα was ∼75%. Immunoblotting was performed with total extracts from the harvested cells to verify ectopic Brd4, Brd4 mutant, and SPA-1 expression. As loading controls the same filters were immunoblotted with antibodies to α-tubulin (Sigma).
RESULTS
Endogenous Brd4 interacts with SPA-1 in HeLa cells.
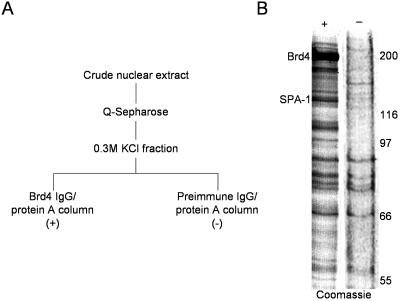
To identify proteins with which Brd4 interacts, endogenous Brd4 was immunopurified with anti-Brd4 IgG conjugated to protein A from HeLa cell nuclear extracts that had been prefractionated on Q-Sepharose. As a control, extracts were processed in the same manner with preimmune IgG (Fig. 1A). SDS-polyacrylamide gel electrophoresis analysis of proteins eluted from anti-Brd4 antibody-conjugated beads revealed several specific bands. The major band, consistently found with the Brd4 column but not with the control column, was of ∼130 kDa (Fig. 1B). A tandem mass spectrometric analysis of the band identified four peptides that precisely matched those of the human SPA-1 (data not shown).
FIG. 1.
Immunoaffinity purification of the endogenous Brd4 complex in HeLa cells. (A) Purification scheme. One hundred milligrams of HeLa nuclear extracts was fractionated on a Q-Sepharose column containing ∼2.5 ml of resin and eluted with buffer containing 0.3 M KCl. Two milligrams of eluted proteins was incubated with 200 μl of anti-Brd4 or preimmune IgG-conjugated protein A-Trisacryl. (B) Coomassie blue staining of proteins eluted from anti-Brd4 column (+) or preimmune IgG column (−) and resolved on an SDS-10% polyacrylamide gel. Brd4 and SPA-1 bands are indicated.
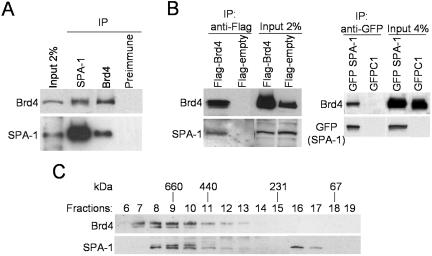
To verify that SPA-1 interacted with Brd4, the two proteins were immunoprecipitated with respective antibodies and tested for the presence of the other protein by immunoblotting (Fig. 2A). Anti-SPA-1 antibody coprecipitated endogenous Brd4 along with SPA-1. Likewise, anti-Brd4 antibody coprecipitated SPA-1 and Brd4. Preimmune sera did not precipitate either SPA-1 or Brd4. To further establish the interaction of the two proteins, coimmunoprecipitation analysis was performed with cells transfected with Flag-tagged Brd4 or GFP-tagged SPA-1 (Fig. 2B). Anti-Flag antibody precipitated endogenous SPA-1 from cells transfected with Flag-Brd4 but not with empty Flag vector. Similarly, anti-GFP antibody precipitated endogenous Brd4 along with GFP-SPA-1, from cells transfected with GFP-SPA-1, but not with empty GFP vector. These results demonstrate that the endogenous Brd4 interacts with SPA-1 and that ectopic Brd4 and SPA-1 can interact with the endogenous partner.
FIG. 2.
Brd4-SPA-1 interaction in vivo. (A) Coimmunoprecipitation of endogenous Brd4 and SPA-1. Extracts from HeLa cells were immunoprecipitated with the antibodies indicated on the top and tested for Brd4 and SPA-1 by immunoblotting. Input represents 2% of extracts. (B) Coimmunoprecipitation of ectopically expressed Brd4 or SPA-1. Extracts from HeLa cells transfected with Flag-Brd4, GFP-SPA-1, or control empty vectors were precipitated with anti-Flag or GFP antibody and blotted with the antibody indicated on the left (the band corresponding to GFP alone produced from control vector was not included in the figure). Input represents 2 or 4% of the extracts used for precipitation. (C) Molecular mass estimation of the Brd4 complex. Q-Sepharose-purified nuclear extracts (500 μg) of the 0.3 M KCl-Q-Sepharose fraction were subjected to gel filtration analysis on a Superdex 200 column by use of the SMART system (Pharmacia Biotech). Fractions were resolved on an SDS-10% polyacrylamide gel and analyzed by immunoblotting. The estimated molecular mass is shown on top.
Immunopurification assays described above suggested that Brd4 occurs as a multiprotein complex. To further substantiate the interaction and to estimate the size of the Brd4 complex, nuclear extracts were fractionated according to size by gel filtration analysis (Fig. 2C). Immunoblot assay of fractionated samples found Brd4 distributed over fractions 7 through 13, peaking at fractions 8 and 9, which corresponded to a complex with the molecular mass of ∼660 to 700 kDa. The majority of SPA-1 was found in fractions 8 to 10, cofractionating with Brd4. These results are consistent with coimmunopurification data as described above and indicate that SPA-1 is part of a large Brd4 complex in HeLa cells. It is of note that some SPA-1 was found in the fractions of smaller molecular masses, most likely representing the free, monomeric protein. In some experiments both Brd4 and SPA-1 (e.g., Fig. 2C) were seen as closely migrating doublets, which likely represent posttranslational modifications of the two molecules.
Direct interaction of Brd4 with SPA-1.
We sought to determine whether Brd4 directly binds to SPA-1 and, if it does, to which domain. For this purpose in vitro pull-down assays were performed. Recombinant Flag-tagged SPA-1 bound to anti-Flag-antibody agarose was incubated with in vitro-translated, 35S-labeled full-length Brd4 or Brd4 deletions (Fig. 3A). Flag-tagged SPA-1 pulled down full-length Brd4, as well as ΔC, lacking the C-terminal region. In addition, the first bromodomain deletion, ΔI, was pulled down with Flag-tagged SPA-1, although to a lesser degree than was full-length Brd4 or ΔC. In contrast, Flag-tagged SPA-1 did not pull down the second bromodomain deletion, ΔII, and the deletion of both bromodomains, ΔI&II. As expected, control anti-Flag antibody agarose did not pull down any of the Brd4 proteins. These data suggest that Brd4 binds to SPA-1 and that the second bromodomain is essential for interaction, although the first bromodomain appears to contribute to the binding to some degree.
To assess a domain of SPA-1 important for interacting with Brd4, Flag-tagged SPA-1 deletions (Fig. 3B) were expressed in NIH 3T3 cells and interaction was examined by coimmunoprecipitation assay. Brd4 was precipitated by full-length SPA-1 and ΔPDZ, but not by ΔGRD, which lacks the Rap GAP homology region. As expected, Brd4 was not precipitated from extracts expressing control, free GFP-Flag. These results confirm that SPA-1 binds to Brd4 and indicate that the GRD is essential for the interaction with Brd4. The same outcome was observed by an in vitro binding assay with use of Flag-tagged Brd4 and 35S-labeled SPA-1 deletions (data not shown).
Brd4 enhances Rap GAP activity of SPA-1 in vitro and in vivo.
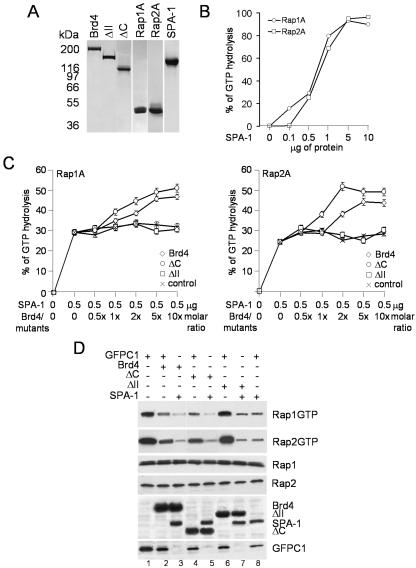
SPA-1 is an activator of small GTPases specific for Rap1 and Rap2 (25). We first measured the levels of GTP hydrolysis by SPA-1 on Rap1A and Rap2A with increasing amounts of SPA-1 (0, 0.1, 05, 1, 5, and 10 μg). Coomassie blue staining (Fig. 4A) of recombinant reagents used for these experiments shows that they are homogenous and relatively pure. In agreement with previous published data (25) SPA-1 increased hydrolysis of both Rap1A and Rap2A, in a concentration-dependent manner, leading to almost 100% hydrolysis of both Raps at SPA-1 doses greater than 5 μg (Fig. 4B). We then tested whether Brd4 regulates SPA-1 activation of Rap GTPase. We performed a GTPase activity assay using 0.5 μg of SPA-1, which gave about 30% of Rap GTP hydrolysis, at which reactions are in a linear range; these conditions allowed us to estimate any modulation in its activity due to Brd4. Recombinant Rap1A or Rap2A was incubated with SPA-1 in the presence or absence of increasing molar ratios of Brd4 or Brd4 mutants ΔC and ΔII, and percent GTP hydrolysis was measured as described above. Addition of increasing amounts of Brd4 markedly enhanced GAP activity of SPA-1 over that of control, from 29 to 46% for Rap1A and from 25 to 45% for Rap2A (Fig. 4C). Figure 4C also shows results with Brd4 deletions. ΔII, which did not interact with SPA-1 (as shown in Fig. 3), did not significantly increase Rap GAP activity over that of control. Conversely, ΔC, retaining binding activity for SPA-1, increased Rap GAP activity in a manner similar to that of full-length Brd4 (from 29 to 50% for Rap1A and from 25 to 50% for Rap2A). These results suggest that Brd4 enhances Rap GAP activity of SPA-1 by direct interaction.
FIG. 4.
Effects of Brd4 on Rap GAP activity of SPA-1 in vitro and in vivo. (A) Coomassie blue staining of recombinant proteins. Purified recombinant SPA-1, Brd4, ΔC, and ΔII, as well as Rap1A and Rap2A, were electrophoresed in a 4 to 20% gradient SDS-polyacrylamide gel and used for the in vitro assay. (B) GAP activity of SPA-1 on Rap1A and Rap2A. SPA-1 activity was measured by incubating 3 μg of Rap 1A or Rap2A with indicated amounts of SPA-1 as described under Materials and Methods. (C) SPA-1 GAP activity assayed in the presence of Brd4 or Brd4 deletion mutants. Increasing molar ratios of Brd4 or Brd4 mutants were incubated with 0.5 μg of SPA-1. Control represents SPA-1 activity on Rap1A or Rap2A in the presence of bovine serum albumin. Values represent the averages from three independent experiments. (D) Rap activation assay in NIH 3T3 cells. NIH 3T3 cells (1.6 × 106) were transfected with 2 μg of pEGFP-SPA-1 alone (lane 8) or together with 2 μg of pEGFP-Brd4 (lane 3) or pEGFP-Brd4 deletion mutants (lanes 5 and 7), with the total amount of plasmids being adjusted to 4 μg with control plasmid pEGFP-C1. Lysates were precipitated with GST-RalGDS-RBD or control GST, and bound materials were analyzed by immunoblotting with antibodies to Rap1 and Rap2. Brd4, Brd4 deletion mutants, and SPA-1 in lysates were detected by immunoblotting with anti-GFP antibody.
We sought to ascertain whether Brd4 enhances activity of SPA-1 also in vivo. To this end, NIH 3T3 cells were transfected with Brd4 and SPA-1, and GTP-bound Rap1 and Rap2 were detected by affinity binding assays with the GST-RalGDS resin, which binds only to the GTP-bound Rap (37). In Fig. 4D, transfection of SPA-1 led to a reduction in the amount of active GTP-bound Rap1 and Rap2, relative to that in the control transfection (compare lanes 8 and 1), consistent with the Rap GAP activity of SPA-1. Brd4 transfection reduced the amount of GTP-bound Rap1 and Rap2, presumably due to an interaction with the endogenous SPA-1 (lane 2). Significantly, cotransfection of Brd4 and SPA-1 led to a greater reduction in the levels of GTP-bound Rap1 and Rap2 (lane 3) compared to transfection of either protein alone. To assess the specificity of Brd4, we examined the effect of ΔII and mutant ΔC alone or in combination with SPA-1. In agreement with the in vitro data in Fig. 4C, ΔC reduced the levels of GTP-bound Rap1 and Rap2 in a manner similar to full-length Brd4 (lane 4 or lane 5 when cotransfected with SPA-1), whereas ΔII did not (lane 6). Immunoblot analysis of lysates confirmed that the total levels of Rap1 and Rap2 were not affected by transfection of Brd4, Brd4 mutants, and/or SPA-1 and that transfection of Brd4, Brd4 mutants, and SPA-1 resulted in overexpression of the respective proteins. These results indicate that Brd4 increases SPA-1's Rap GAP activity and stimulates the generation of inactive, GDP-bound Rap in vitro and in vivo.
Brd4 and SPA-1 interact in the nucleus of living cells.
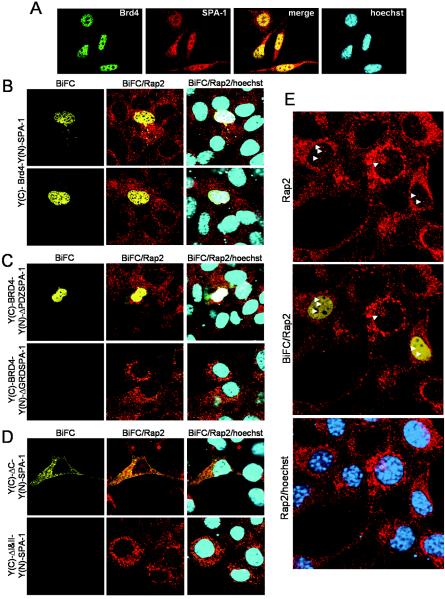
It is reported that SPA-1 largely localizes in the cytoplasm, while Brd4 localizes to the nucleus (10, 25). In agreement, immunostaining of NIH 3T3 cells revealed exclusive nuclear staining for Brd4 and cytoplasmic and nuclear staining for SPA-1 (Fig. 5A). The staining was eliminated by blocking the antibodies with an excess of antigens (recombinant proteins), verifying the specificity of antibodies (data not shown). To determine the interaction site of the two proteins in vivo, we performed BiFC experiments, which allow for visualization of two interacting molecules in living cells (17). In this method full-length YFP is split in half, each half containing the N-terminal [Y(N)] or the C-terminal [Y(C)] YFP fragments. They are fused to partner proteins and transfected into cells. Y(N) and Y(C) constructs are nonfluorescent by themselves. However, when the two partners interact, YFP fluorescence can be recaptured. We constructed Y(C)-Brd4 and Y(N)-SPA-1. When transfected in NIH 3T3 cells, neither Y(C)-Brd4 nor Y(N)-SPA-1 alone produced a YFP signal, as expected. However, coexpression of the two proteins generated a clear YFP signal in the nucleus, due to complementation of the two YFP fragments (Fig. 5B). In some experiments cells were cotransfected with pBFP to assess the efficiency of BiFC and to verify transfection of nonfluorescent Y(N) and Y(C) constructs. Transfection efficiencies were high, ranging from 30 to 45% in all the experiments. Of them, about 50% of transfected cells emitted YFP, indicating that Y(N)-SPA-1 and Y(C)-Brd4 efficiently complemented each other (data not shown). To confirm the specificity of the interaction, we tested SPA-1 and Brd4 deletion mutants. Y(N)-ΔGRDSPA-1, when coexpressed with Y(C)-Brd4, did not give a YFP signal, whereas Y(N)-ΔPDZSPA-1 complemented Y(C)-Brd4 (Fig. 5C), consistent with the SPA-1 domain assignment in Fig. 3B. In Fig. 5D, deletion Brd4 mutant Y(C)-ΔC, when coexpressed with Y(N)-SPA-1, generated a YFP signal, whereas Y(C)-ΔI&II did not complement, again consistent with Brd4 domain assignment (Fig. 3A). With Y(C)-ΔC the interaction was seen mostly in the cytoplasm, the basis of which is not clear. Both Y(C)ΔI and Y(C)ΔII did not complement Y(N)-SPA-1 (data not shown), although ΔI binds SPA-1 in vitro (Fig. 3A). These results demonstrate that the Brd4-SPA-1 interaction occurs in the nucleus and that the interaction requires intact Brd4 bromodomains and SPA-1 GRD.
FIG. 5.
Brd4 and SPA-1 interact in the nucleus. (A) Confocal images of endogenous Brd4 (green) and SPA-1 (red). NIH 3T3 cells (105) grown on a coverslip were stained with Brd4 or SPA-1 antibodies. Merged images show superimposition of green and red signals. DNA was counterstained with Hoechst stain (blue). (B, C, and D) BiFC assay. NIH 3T3 cells (105) seeded on a coverslip were transfected with the combination of plasmids indicated on the left (0.4 μg of each vector) for 24 h. The emission of YFP fluorescence was detected on a confocal microscope. (B) Yellow fluorescence is detectable in the nuclei of NIH 3T3 cells cotransfected with Y(C)-Brd4 and Y(N)-SPA-1 due to complementation of the two YFP fragments. (C) Y(N)-ΔGRDSPA-1, when coexpressed with Y(C)-Brd4, does not show a YFP signal, whereas Y(N)-ΔPDZSPA-1 complements Y(C)-Brd4 and shows a yellow signal. (D) Brd4 mutant Y(C)-ΔC, when coexpressed with Y(N)-SPA-1, generates a YFP signal, whereas Y(C)-ΔI&II does not. The same cells were fixed and stained with Rap2 antibody (red). Merged images show superimposition of yellow and red signal or yellow, red, and blue (Hoechst stain) signal. (E) Rap2 staining and merged images of BiFC-Rap2 or Rap2-Hoechst stain. Cells were transfected with Y(C)-Brd4 and Y(N)-SPA-1 and subsequently stained with Rap2 antibody, to indicate the presence of Rap2 in the nucleus (shown by arrowheads), which partially overlaps the BiFC signal.
In view of the recent report that some Rap occurs in the nucleus (31), we studied whether the Brd4-SPA-1 complex colocalizes with Rap. Cells exhibiting BiFC were stained with Rap2 antibodies. As shown by red staining in Fig. 5B to E, Rap2 predominantly localizes in the perinuclear region and the cytoplasm. In addition, Rap2 staining was found in the nucleus of most cells, albeit to a lesser extent (see arrowheads in Fig. 5E). Staining was seen as small dots scattered over some nuclear space partially overlapping with BiFC signals. These data appears to indicate that a small fraction of Rap2 that resides in the nucleus might colocalize with the Brd4-SPA-1 complex.
Ectopic expression of Brd4 or SPA-1 influences the other protein's subcellular localization.
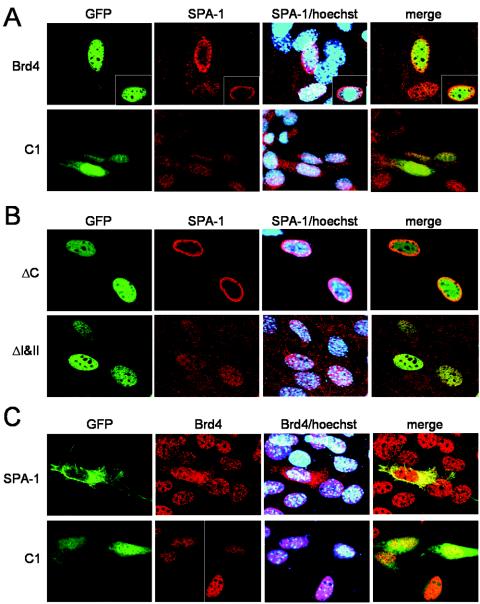
Having found that SPA-1 interacts with Brd4 in the nucleus, suggesting that the cytoplasmic SPA-1 does not take part in this interaction, we wondered whether ectopic Brd4 or SPA-1 expression had an effect on subcellular localization of the other protein. In Fig. 6A, NIH 3T3 cells were transfected with GFP-Brd4 for 24 h and localization of endogenous SPA-1 was examined by immunostaining of fixed cells with anti-SPA-1 antibody. SPA-1 localization was dramatically altered upon ectopic expression of GFP-Brd4, showing large accumulation in the perinuclear region (Fig. 6A). In some cases, a certain amount of SPA-1 was found in the nucleus near the nuclear membrane, overlapping with Brd4-GFP, the latter localizing to the nucleus (see inset). As expected, SPA-1 localization was not affected upon transfection of control GFP, which was seen over the entire cells. Transfection of unrelated proteins, such as PCAF and p300, also containing a bromodomain did not affect SPA-1 localization (data not shown). The alteration of SPA-1 localization correlated with Brd4's ability to interact with SPA-1: the Brd4 deletion (ΔI&II), which failed to bind to SPA-1, did not change SPA-1 distribution, whereas ΔC, which retained binding activity for SPA-1, caused SPA-1 to redistribute to the perinuclear region (Fig. 6B). These results indicate that Brd4 can regulate subcellular localization of SPA-1, presumably as a result of interaction. Similar redistribution of SPA-1 was observed when cells were transfected with Flag-tagged Brd4 (data not shown). In Fig. 6C, we tested the alternate possibility in which ectopic SPA-1 might change the localization of Brd4. Remarkably, upon GFP-SPA-1 expression, a large fraction of Brd4 moved to the cytoplasm. In contrast, Brd4 remained in the nucleus upon transfection of control GFP. These results indicate that Brd4 and SPA-1 can mutually regulate subcellular localization, presumably as a result of interaction.
FIG. 6.
Ectopic expression of either Brd4 or SPA-1 influences the other's localization. NIH 3T3 cells (2 × 105) were transfected with 1.5 μg of plasmid indicated on the left. (A) Cells transfected with control pEGFP-C1 or pEGFP-Brd4. (B) Cells transfected with pEGFP-ΔC and pEGFP-ΔI&II. SPA-1 (red) was visualized by immunostaining. DNA was counterstained with Hoechst stain (blue). (C) Cells transfected with pEGFP-C1 or pEGFP-SPA-1. Endogenous Brd4 (red) was visualized by immunostaining. Merged images show superimposition of red and blue or of red, blue, and green signals.
Dysregulation of Brd4 and SPA-1 expression disrupts G2/M progression.
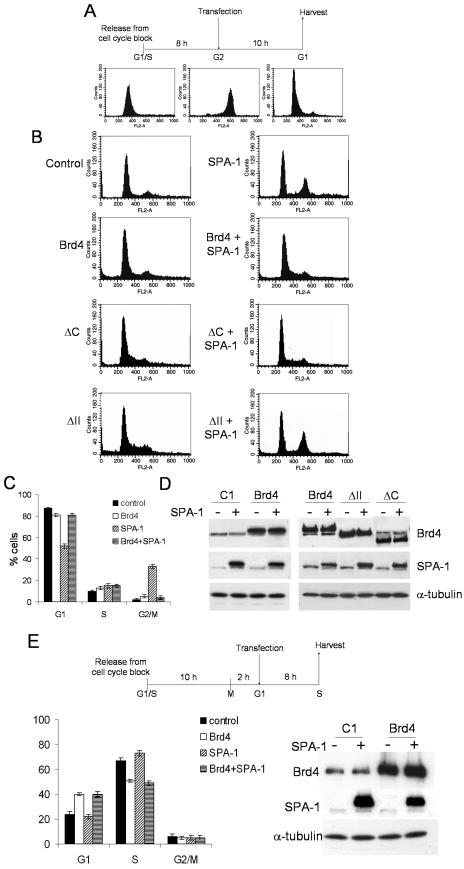
Given that both Brd4 and SPA-1 are mitogen-inducible proteins and that both Brd4 and SPA-1 have a role in cell growth, we studied the effects of ectopic expression of the two proteins on cell cycle progression. HeLa cells were synchronized by double thymidine block, released, and allowed to proceed in culture for 8 h. At the time when the majority of the cells were in G2, cells were transfected with Brd4 or SPA-1 or both vectors. Cells were incubated for an additional 10 h before harvest when control cells moved to M and G1 phase (diagram of experiments in Fig. 7A). Figure 7B shows cell cycle profiles of transfected cells analyzed by flow cytometry. Ectopic expression of SPA-1 markedly inhibited progression through mitosis, whereas that of Brd4 did not have a measurable effect (about 30% of the cells remained in G2/M upon ectopic expression of SPA-1 compared to less than 5% for control cells or after ectopic expression of Brd4; see diagram in Fig. 7C), indicating a critical role of SPA-1 in mitotic entry. Significantly, when SPA-1 and Brd4 were coexpressed in G2, the inhibitory effect of SPA-1 was markedly attenuated, apparently reestablishing progression through mitosis. In order to further validate these data, we examined cell cycle progression upon ectopic expression of Brd4 mutants, alone or in combination with SPA-1. Cell cycle profiles in Fig. 7B show that neither ΔC nor ΔII, when ectopically expressed alone, affects cell cycle progression. However, ΔC, when coexpressed with SPA-1, was able to reverse the inhibitory effect of SPA-1 in mitotic entry, behaving like wild-type Brd4, whereas ΔII did not have this ability. These results suggest that disruption of a proper balance in the expression of Brd4 and SPA-1 results in a deregulation of G2/M transition and that interaction between Brd4 and SPA-1 plays a major role in mitotic progression.
FIG.7.
Brd4 and SPA-1 regulate G2/M progression. (A) Scheme of cell synchronization and transfection. HeLa cells were transfected in G2, 8 h after release from double thymidine block. Cells were harvested 10 h after transfection, stained with propidium iodide, and analyzed by flow cytometry. (B) Cell cycle profiles of cells transfected with indicated plasmids (3 μg). (C) The percentages of cells in different phases of the cell cycle are shown for indicated transfections. Values represent the averages of three independent experiments. (D) Immunoblotting of total extracts from the harvested cells to detect ectopic Brd4, Brd4 mutants, and SPA-1 expression. As a loading control the same filters were immunoblotted with antibodies to α-tubulin. (E) HeLa cells were synchronized by double thymidine block and transfected with Brd4-expressing vectors in G1, 12 h after release from double thymidine block. Cells were harvested 8 h after transfection, stained with propidium iodide, and analyzed by flow cytometry. The percentages of G1, S, and G2/M populations are shown for indicated transfections on the left. Values represent the averages of three independent experiments. Immunoblot analyses to verify overexpression of Brd4 and SPA-1 are shown on the right.
We next studied whether SPA-1 interferes with cell cycle progression only during G2/M or during other stages. HeLa cells synchronized in G1 were transfected with SPA-1 or Brd4 or both vectors (12 h after release; Fig. 7E shows a diagram of the experiment) and allowed to proceed for an additional 8 h in culture so that a large fraction of cells moved to S phase. Cell cycle profiles of harvested cells in Fig. 7E show that SPA-1 did not have an inhibitory effect on G1/S progression, while Brd4 did, as reported elsewhere (29). Coexpression of the two proteins in G1 did not affect S-phase entry to a significant degree. These results indicate that Brd4 and SPA-1 coordinately regulate G2/M transition and that their interaction as well as the maintenance of the balance of the expression of these two proteins is important for cell cycle progression.
DISCUSSION
Interaction with SPA-1 and GTPase activity.
We show that Brd4 interacts with SPA-1 in vivo by immunoaffinity purification experiments and reciprocal coimmunoprecipitation of the endogenous or ectopically expressed proteins (Fig. 1 and 2). In vitro studies performed with recombinant Brd4 and recombinant SPA-1 showed that the interaction is direct and mediated by the second bromodomain of Brd4 and the GRD of SPA-1 (Fig. 3). Supporting a functional interaction, Brd4 increased the activity of SPA-1 in the hydrolysis of GTP bound to Rap1 or Rap2 (Fig. 4). Further, Brd4 deletions that lost the ability to interact with SPA-1 also lost the ability to modulate the GTPase-activating properties of SPA-1, providing additional credence to the interaction of the two proteins.
Subcellular distribution of Brd4 and SPA-1.
Since the interaction was detected with column-purified nuclear extracts, the major site of interaction between Brd4 and SPA-1 is most probably within the nucleus, the possibility substantiated by BiFC (Fig. 5). Nevertheless, it is of note that much of SPA-1 is present in the cytoplasm and Brd4 is present in the nucleus. This indicates that only a portion of SPA-1 interacts with Brd4 and in a transient manner. In light of Brd4's affinity for acetylated chromatin (9), the site of interaction is presumably near or at the chromatin.
It is possible that intracellular distribution of SPA-1 is tightly regulated depending, for instance, on posttranslational modifications, which are likely to be regulated by the interaction with Brd4. SPA-1, which carries a potential nuclear localization signal, once in the nucleus may be modified and exported to the cytosol. Whether Brd4 might be involved in SPA-1 posttranslational modifications is one of the objects of our ongoing studies. Given the role of both proteins in cell cycle regulation, it is also possible that the interaction is restricted to a particular stage of the cell cycle. Although not all Brd4 and SPA-1 appear to be in a stable complex at any given time, this interaction appears to have a lasting influence on SPA-1's localization, directing SPA-1 to the perinuclear region, one of the sites of Rap activity (Fig. 6). It is possible that Brd4 and SPA-1 influence each other's localization not only by direct interaction but by an indirect mechanism. Significantly, a recent report shows that Rap1 proteins are expressed in the nucleus of certain cell types (31). In view of this report, Brd4 and SPA-1 may influence the activity of Rap in the nucleus, thereby influencing Ras-dependent and -independent mitogenic signaling (1, 2). This situation is in part reminiscent of another nuclear GTPase, Ran, whose exchange factor RCC1 is bound to the chromatin. RCC1 localization creates a Ran GTP gradient that regulates growth of mitotic spindle and nucleocytoplasmic transport (6). The importance of the localization of GTPase regulators has also been shown recently for several GAPs which determine the gradient of activity of Ras family GTPases in the cell (34) and for Epac (a GEF for Rap1), whose subcellular localization is cell cycle dependent and determines correct mitosis progression (36).
Role in cell cycle regulation.
Because both Brd4 and SPA-1 are mitogen-inducible proteins and because evidence for a role in cell cycle progression has been presented for both proteins, we studied how overexpression of these proteins modifies cell cycle progression. An unmistakable inhibitory effect was seen when SPA-1 (but not Brd4) was overexpressed during G2 phase, causing strong mitotic arrest (Fig. 7). Importantly, this mitotic arrest was abolished when Brd4, or its deletion mutant ΔC, which still retains binding activity for SPA-1, was coexpressed along with SPA-1, allowing cells to progress through M to the G1 phase of the next cell cycle. These results lead to a view that the functions of the two proteins are linked and that their expression levels (and hence their activities) need to be properly balanced in order to support normal cell cycle progression, this balance being particularly critical for G2/M transition. It can be envisaged that SPA-1 exerts its negative effect on G2/M progression by interfering with the activity of Brd4. In other words, the observed inhibition of mitosis by SPA-1 may be, at least in part, attributed to the influence of SPA-1 on Brd4 localization-function. This idea is consistent with the observation in Fig. 6C where Brd4 was expelled from the nucleus upon ectopic expression of SPA-1, likely leading to the loss of Brd4 function required for G2/M progression, the idea being further supported by the restoration of M-phase progression upon Brd4 transfection.
On the other hand, as a consequence of Brd4 ectopic expression, the perinuclear localization of SPA-1 might ultimately interfere with Rap function and its mitogenic signaling, resulting in the inhibition of S-phase entry. In the past few years, significant evidence has emerged that dysregulation of Rap1 activation is responsible for the development of malignancy. Cells from SPA-1-deficient mice exhibit accumulation of Rap1-GTP and develop myeloid leukemia (18). It has also been shown (31) that growth factors stimulate nuclear translocation and activation of Rap1 in tumor cells, with Rap1 playing a role in the uncontrolled growth of human squamous cell carcinomas. In our study the increase in Brd4 and SPA-1 levels leads to an increase in Rap GDP levels, which may ultimately cause defects in cell cycle progression.
In summary, this work reveals a link between a chromatin binding protein, Brd4, and GTPase signaling mediated by SPA-1, pointing to a novel mechanism that regulates entry into mitosis.
Acknowledgments
We thank Anup Dey for helpful discussions, D. Altschuler, J. L. Bos for reagents, T. K. Kerppola for advice on BiFC, G. Humphrey for suggestions on gel filtration, and J. McCormack for technical assistance.
REFERENCES
- 1.Altschuler, D. L., and F. Ribeiro-Neto. 1998. Mitogenic and oncogenic properties of the small G protein Rap1b. Proc. Natl. Acad. Sci. USA 95:7475-7479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beranger, F., B. Goud, A. Tavitian, and J. de Gunzburg. 1991. Association of the Ras-antagonistic Rap1/Krev-1 proteins with the Golgi complex. Proc. Natl. Acad. Sci. USA 88:1606-1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blanco, J. C., S. Minucci, J. Lu, X. J. Yang, K. K. Walker, H. Chen, R. M. Evans, Y. Nakatani, and K. Ozato. 1998. The histone acetylase PCAF is a nuclear receptor coactivator. Genes Dev. 12:1638-1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bos, J. L. 1997. Ras-like GTPases. Biochim. Biophys. Acta 1333:M19-M31. [DOI] [PubMed] [Google Scholar]
- 5.Bourne, H. R., D. A. Sanders, and F. McCormick. 1990. The GTPase superfamily: a conserved switch for diverse cell functions. Nature 348:125-132. [DOI] [PubMed] [Google Scholar]
- 6.Carazo-Salas, R. E., G. Guarguaglini, O. J. Gruss, A. Segref, E. Karsenti, and I. W. Mattaj. 1999. Generation of GTP-bound Ran by RCC1 is required for chromatin-induced mitotic spindle formation. Nature 400:178-181. [DOI] [PubMed] [Google Scholar]
- 7.Chua, P., and G. S. Roeder. 1995. Bdf1, a yeast chromosomal protein required for sporulation. Mol. Cell. Biol. 15:3685-3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denis, G. V., C. Vaziri, N. Guo, and D. V. Faller. 2000. RING3 kinase transactivates promoters of cell cycle regulatory genes through E2F. Cell Growth Differ. 11:417-424. [PMC free article] [PubMed] [Google Scholar]
- 9.Dey, A., F. Chitsaz, A. Abbasi, T. Misteli, and K. Ozato. 2003. The double bromodomain protein Brd4 binds to acetylated chromatin during interphase and mitosis. Proc. Natl. Acad. Sci. USA 100:8758-8763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dey, A., J. Ellenberg, A. Farina, A. E. Coleman, T. Maruyama, S. Sciortino, J. Lippincott-Schwartz, and K. Ozato. 2000. A bromodomain protein, MCAP, associates with mitotic chromosomes and affects G2-to-M transition. Mol. Cell. Biol. 20:6537-6549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dhalluin, C., J. E. Carlson, L. Zeng, C. He, A. K. Aggarwal, and M. M. Zhou. 1999. Structure and ligand of a histone acetyltransferase bromodomain. Nature 399:491-496. [DOI] [PubMed] [Google Scholar]
- 12.Digan, M. E., S. R. Haynes, B. A. Mozer, I. B. Dawid, F. Forquignon, and M. Gans. 1986. Genetic and molecular analysis of fs(1)h, a maternal effect homeotic gene in Drosophila. Dev. Biol. 114:161-169. [DOI] [PubMed] [Google Scholar]
- 13.D'Silva, N. J., D. H. DiJulio, C. M. Belton, K. L. Jacobson, and E. L. Watson. 1997. Immunolocalization of rap1 in the rat parotid gland: detection on secretory granule membranes. J. Histochem. Cytochem. 45:965-973. [DOI] [PubMed] [Google Scholar]
- 14.Hattori, M., N. Tsukamoto, M. S. Nur-e-Kamal, B. Rubinfeld, K. Iwai, H. Kubota, H. Maruta, and N. Minato. 1995. Molecular cloning of a novel mitogen-inducible nuclear protein with a Ran GTPase-activating domain that affects cell cycle progression. Mol. Cell. Biol. 15:552-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haynes, S. R., B. A. Mozer, N. Bhatia-Dey, and I. B. Dawid. 1989. The Drosophila fsh locus, a maternal effect homeotic gene, encodes apparent membrane proteins. Dev. Biol. 134:246-257. [DOI] [PubMed] [Google Scholar]
- 16.Houzelstein, D., S. L. Bullock, D. E. Lynch, E. F. Grigorieva, V. A. Wilson, and R. S. Beddington. 2002. Growth and early postimplantation defects in mice deficient for the bromodomain-containing protein Brd4. Mol. Cell. Biol. 22:3794-3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu, C. D., Y. Chinenov, and T. K. Kerppola. 2002. Visualization of interactions among bZIP and Rel family proteins in living cells using bimolecular fluorescence complementation. Mol. Cell 9:789-798. [DOI] [PubMed] [Google Scholar]
- 18.Ishida, D., K. Kometani, H. Yang, K. Kakugawa, K. Masuda, K. Iwai, M. Suzuki, S. Itohara, T. Nakahata, H. Hiai, H. Kawamoto, M. Hattori, and N. Minato. 2003. Myeloproliferative stem cell disorders by deregulated Rap1 activation in SPA-1-deficient mice. Cancer Cell 4:55-65. [DOI] [PubMed] [Google Scholar]
- 19.Jacobson, R. H., A. G. Ladurner, D. S. King, and R. Tjian. 2000. Structure and function of a human TAFII250 double bromodomain module. Science 288:1422-1425. [DOI] [PubMed] [Google Scholar]
- 20.Jeanmougin, F., J. M. Wurtz, B. Le Douarin, P. Chambon, and R. Losson. 1997. The bromodomain revisited. Trends Biochem. Sci. 22:151-153. [DOI] [PubMed] [Google Scholar]
- 21.Jiang, Y. W., P. Veschambre, H. Erdjument-Bromage, P. Tempst, J. W. Conaway, R. C. Conaway, and R. D. Kornberg. 1998. Mammalian mediator of transcriptional regulation and its possible role as an end-point of signal transduction pathways. Proc. Natl. Acad. Sci. USA 95:8538-8543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanno, T., Y. Kanno, R. M. Siegel, M. K. Jang, M. J. Lenardo, and K. Ozato. 2004. Selective recognition of acetylated histones by bromodomain proteins visualized in living cells. Mol. Cell 13:33-43. [DOI] [PubMed] [Google Scholar]
- 23.Kitayama, H., Y. Sugimoto, T. Matsuzaki, Y. Ikawa, and M. Noda. 1989. A ras-related gene with transformation suppressor activity. Cell 56:77-84. [DOI] [PubMed] [Google Scholar]
- 24.Krogan, N. J., M. C. Keogh, N. Datta, C. Sawa, O. W. Ryan, H. Ding, R. A. Haw, J. Pootoolal, A. Tong, V. Canadien, D. P. Richards, X. Wu, A. Emili, T. R. Hughes, S. Buratowski, and J. F. Greenblatt. 2003. A Snf2 family ATPase complex required for recruitment of the histone H2A variant Htz1. Mol. Cell 12:1565-1576. [DOI] [PubMed] [Google Scholar]
- 25.Kurachi, H., Y. Wada, N. Tsukamoto, M. Maeda, H. Kubota, M. Hattori, K. Iwai, and N. Minato. 1997. Human SPA-1 gene product selectively expressed in lymphoid tissues is a specific GTPase-activating protein for Rap1 and Rap2. Segregate expression profiles from a rap1GAP gene product. J. Biol. Chem. 272:28081-28088. [DOI] [PubMed] [Google Scholar]
- 26.Lapetina, E. G., J. C. Lacal, B. R. Reep, and L. Molina y Vedia. 1989. A ras-related protein is phosphorylated and translocated by agonists that increase cAMP levels in human platelets. Proc. Natl. Acad. Sci. USA 86:3131-3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lygerou, Z., C. Conesa, P. Lesage, R. N. Swanson, A. Ruet, M. Carlson, A. Sentenac, and B. Seraphin. 1994. The yeast BDF1 gene encodes a transcription factor involved in the expression of a broad class of genes including snRNAs. Nucleic Acids Res. 22:5332-5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maridonneau-Parini, I., and J. de Gunzburg. 1992. Association of rap1 and rap2 proteins with the specific granules of human neutrophils. Translocation to the plasma membrane during cell activation. J. Biol. Chem. 267:6396-6402. [PubMed] [Google Scholar]
- 29.Maruyama, T., A. Farina, A. Dey, J. Cheong, V. P. Bermudez, T. Tamura, S. Sciortino, J. Shuman, J. Hurwitz, and K. Ozato. 2002. A mammalian bromodomain protein, Brd4, interacts with replication factor C and inhibits progression to S phase. Mol. Cell. Biol. 22:6509-6520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matangkasombut, O., R. M. Buratowski, N. W. Swilling, and S. Buratowski. 2000. Bromodomain factor 1 corresponds to a missing piece of yeast TFIID. Genes Dev. 14:951-962. [PMC free article] [PubMed] [Google Scholar]
- 31.Mitra, R. S., Z. Zhang, B. S. Henson, D. M. Kurnit, T. E. Carey, and N. J. D'Silva. 2003. Rap1A and rap1B ras-family proteins are prominently expressed in the nucleus of squamous carcinomas: nuclear translocation of GTP-bound active form. Oncogene 22:6243-6256. [DOI] [PubMed] [Google Scholar]
- 32.Mochizuki, N., S. Yamashita, K. Kurokawa, Y. Ohba, T. Nagai, A. Miyawaki, and M. Matsuda. 2001. Spatio-temporal images of growth-factor-induced activation of Ras and Rap1. Nature 411:1065-1068. [DOI] [PubMed] [Google Scholar]
- 33.Ogryzko, V. V., T. Kotani, X. Zhang, R. L. Schiltz, T. Howard, X. J. Yang, B. H. Howard, J. Qin, and Y. Nakatani. 1998. Histone-like TAFs within the PCAF histone acetylase complex. Cell 94:35-44. [DOI] [PubMed] [Google Scholar]
- 34.Ohba, Y., K. Kurokawa, and M. Matsuda. 2003. Mechanism of the spatio-temporal regulation of Ras and Rap1. EMBO J. 22:859-869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pizon, V., F. Mechali, and G. Baldacci. 1999. RAP1A GTP/GDP cycles determine the intracellular location of the late endocytic compartments and contribute to myogenic differentiation. Exp. Cell Res. 246:56-68. [DOI] [PubMed] [Google Scholar]
- 36.Qiao, J., F. C. Mei, V. L. Popov, L. A. Vergara, and X. Cheng. 2002. Cell cycle-dependent subcellular localization of exchange factor directly activated by cAMP. J. Biol. Chem. 277:26581-26586. [DOI] [PubMed] [Google Scholar]
- 37.Reedquist, K. A., and J. L. Bos. 1998. Costimulation through CD28 suppresses T cell receptor-dependent activation of the Ras-like small GTPase Rap1 in human T lymphocytes. J. Biol. Chem. 273:4944-4949. [DOI] [PubMed] [Google Scholar]
- 38.Ribeiro-Neto, F., J. Urbani, N. Lemee, L. Lou, and D. L. Altschuler. 2002. On the mitogenic properties of Rap1b: cAMP-induced G1/S entry requires activated and phosphorylated Rap1b. Proc. Natl. Acad. Sci. USA 99:5418-5423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takai, Y., T. Sasaki, and T. Matozaki. 2001. Small GTP-binding proteins. Physiol. Rev. 81:153-208. [DOI] [PubMed] [Google Scholar]
- 40.Tsukamoto, N., M. Hattori, H. Yang, J. L. Bos, and N. Minato. 1999. Rap1 GTPase-activating protein SPA-1 negatively regulates cell adhesion. J. Biol. Chem. 274:18463-18469. [DOI] [PubMed] [Google Scholar]
- 41.Vossler, M. R., H. Yao, R. D. York, M. G. Pan, C. S. Rim, and P. J. Stork. 1997. cAMP activates MAP kinase and Elk-1 through a B-Raf- and Rap1-dependent pathway. Cell 89:73-82. [DOI] [PubMed] [Google Scholar]
- 42.Winston, F., and C. D. Allis. 1999. The bromodomain: a chromatin-targeting module? Nat. Struct. Biol. 6:601-604. [DOI] [PubMed] [Google Scholar]