Abstract
Within the extended receptor cycle of peroxisomal matrix import, the function of the import receptor Pex5p comprises cargo recognition and transport. While the C-terminal half (Pex5p-C) is responsible for PTS1 binding, the contribution of the N-terminal half of Pex5p (Pex5p-N) to the receptor cycle has been less clear. Here we demonstrate, using different techniques, that in Saccharomyces cerevisiae Pex5p-N alone facilitates the import of the major matrix protein Fox1p. This finding suggests that Pex5p-N is sufficient for receptor docking and cargo transport into peroxisomes. Moreover, we found that Pex5p-N can be functionally replaced by Pex18p, one of two auxiliary proteins of the PTS2 import pathway. A chimeric protein consisting of Pex18p (without its Pex7p binding site) fused to Pex5p-C is able to partially restore PTS1 protein import in a PEX5 deletion strain. On the basis of these results, we propose that the auxiliary proteins of the PTS2 import pathway fulfill roles similar to those of the N-terminal half of Pex5p in the PTS1 import pathway.
The transport of proteins into and across a membrane is an essential event in the biogenesis of any organelle. The cellular mechanisms of protein import, however, differ from organelle to organelle, and in addition, various import pathways exist for the same compartment (1, 59). Proteins which are destined for the lumen of peroxisomes are imported posttranslationally in a folded and even oligomeric state (for recent reviews, see references 20, 41, 63, and 71). The targeting of peroxisomal matrix proteins is mediated by two soluble cycling receptors, Pex5p and Pex7p, that deliver their cargo proteins to the peroxisomal matrix and then return to the cytosol to allow for another round of transport (extended receptor cycle) (13, 17, 42). With respect to the targeting signal used, at least three different import pathways for peroxisomal matrix proteins exist.
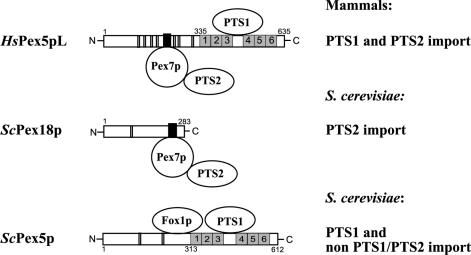
The majority of matrix proteins possess the peroxisomal targeting signal PTS1, which consists of the tripeptide S-K-L (or a conserved variant thereof) at the extreme carboxy termini of these proteins (31, 47). Proteins carrying this signal are bound in the cytosol by the PTS1 receptor Pex5p (14, 16, 28, 37, 40, 44, 72, 73, 78, 79). The PTS1 specifically interacts with the six tetratricopeptide repeat (TPR) motifs in the C-terminal half of Pex5p (29) (Fig. 1). The function of the N-terminal half of Pex5p, which is not as highly conserved as the TPR domain, is poorly understood.
FIG. 1.
Schematic presentation of components required for the cytosolic steps of protein import into peroxisomes. HsPex5pL, the long isoform of human Pex5p; ScPex18p, one of two auxiliary proteins of the PTS2 import pathway of S. cerevisiae; and ScPex5p from S. cerevisiae are shown schematically. Numbers refer to the amino- and carboxy-terminal amino acids. The PTS1 binding regions of HsPex5pL and ScPex5p, starting at amino acids 335 and 313, respectively, both contain six TPR domains (grey boxes). The conserved Pex7p binding boxes of HsPex5pL and ScPex18p are represented by black boxes. Double vertical lines indicate the positions of WXXXF pentapeptide motifs. The ScPex5p binding region for Fox1p, which does not contain either a PTS1 or a PTS2 sequence, is also indicated. For further details, see the introduction.
A minor class of peroxisomal proteins contains the PTS2 signal, which consists of the sequence (R,K)-(L,V,I)-X5-(H,Q)-(L,A,F) near the N terminus (12, 65). This signal is recognized and bound by the soluble receptor Pex7p (8, 9, 22, 53, 81), which in fungi delivers PTS2 proteins to the peroxisome in conjunction with accessory proteins. These are Pex18p and Pex21p in Saccharomyces cerevisiae (52) and Pex20p in the yeast Yarrowia lipolytica (68) and the filamentous fungus Neurospora crassa (61). Partial import defects, found in PEX5 or PEX7 null mutants, demonstrate that in yeasts the two import pathways are independent (50) (Fig. 1). The situation is different in higher eukaryotes, in which no auxiliary proteins have been identified and deletion of the PEX5 gene blocks both the PTS1 and PTS2 pathways (8, 43). The long isoform of the mammalian Pex5p shares a conserved sequence region, referred to as the Pex7p binding box, with the nonorthologous proteins Pex18p, Pex20p, and Pex21p (18, 21) (Fig. 1). However, the function of the auxiliary proteins of the PTS2 import pathway is still elusive.
Besides these two pathways for PTS1 and PTS2 proteins, at least one other import route exists for peroxisomal matrix proteins which do not contain recognizable PTS1 or PTS2 signals. For some of these proteins, it has been reported that they form complexes with PTS-containing proteins and therefore enter the peroxisomes by a “piggyback” mechanism (45, 80). However, other non-PTS1/PTS2 proteins are able to interact directly with Pex5p in a non-PTS1-dependent fashion. This was shown for Fox1p and a version of S. cerevisiae Cat2p with PTS1 deleted (38). These proteins bind to a region of Pex5p which is outside the TPR domain responsible for PTS1 recognition (Fig. 1). The corresponding targeting signal (PTS3), however, has not yet been identified.
The cytoplasmic receptor-cargo complexes dock at the peroxisomal membrane, most likely via protein-protein interactions with the integral membrane proteins Pex14p and/or Pex13p (for reviews, see references 20, 41, and 63). In several cases it could be demonstrated that conserved linear sequence motifs, the WXXXF motifs, are ligands for one or both membrane proteins (5, 49, 56). One or more copies of this motif exist within the sequences of Pex5p and auxiliary proteins of the PTS2 import pathway (21) (Fig. 1). Events following docking, e.g., the translocation of cargo proteins across the membrane and recycling of receptors, are poorly understood.
The extended shuttle model suggests that the receptor-cargo complex enters (at least partly) the lumen of the peroxisome before it dissociates and the receptor returns to the cytosol for another import cycle (13). Despite all efforts, a pore which could facilitate the transport of oligomeric protein complexes has not yet been identified. On the other hand, a number of proteins shown to be essential for matrix protein import (peroxins) are associated with the PTS receptors at the peroxisomal membrane. Direct in vitro interactions between receptors and membrane-associated peroxins were demonstrated by various techniques for Pex5p/Pex14p (28, 49, 58, 70), Pex5p/Pex13p (5, 19, 23, 24, 30, 70), Pex7p/Pex14p (7, 48, 60, 64), Pex7p/Pex13p (64), and Pex5p/Pex8p (75). Pex14p, Pex17p, and Pex13p are constituents of the docking core complex, whereas Pex10p, Pex12p, and Pex2p are referred to as the ring finger core complex (2, 32). It has been demonstrated that the association of these two subcomplexes requires an intraperoxisomal peroxin, Pex8p (2). The whole Pex5p-associated multiprotein complex is referred to as the importomer (2).
In this paper we show that the N-terminal half of Pex5p alone acts as a fully functional receptor for Fox1p. We conclude that two basic functions of protein import into peroxisomes, namely, cargo recognition and receptor transport, can be assigned to distinct regions of the PTS1 receptor Pex5p. Furthermore, using a chimeric protein consisting of Pex18p (without its Pex7p binding site) and the C-terminal half of Pex5p, we could demonstrate that Pex18p can functionally replace the N-terminal half of Pex5p in transporting PTS1 proteins into peroxisomes.
MATERIALS AND METHODS
Strains, media, and growth conditions.
All bacterial manipulations were carried out with Escherichia coli strain DH5α. The yeast strains used in this study are listed in Table 1. Strains which express proteins fused to gamma enhanced green fluorescent protein (GFP) or tobacco etch virus protease cleavage site-protein A (TEV-ProtA) were produced by transforming haploid yeast cells with the PCR products obtained by the strategy of Knop et al. (39). The sequences of the oligonucleotides used to amplify the integration cassettes are presented in Table 2. To fuse the coding region of gamma enhanced GFP in frame to genomic FOX1, the oligonucleotides KU1169 and KU1170 were used. To create strains expressing Pex5p-ProtA (synonymous with Pex5p-TEV-ProtA) and Pex5p-N-ProtA [synonymous with Pex5(1-313)p-TEV-ProtA] by genomic integration, PCR products were obtained with the primer pairs KU896-KU897 and KU896-KU1263, respectively, and transformed into wild-type and pex5Δ cells as described by Schiestl and Gietz (57). Transformants were selected for kanamycin resistance as a marker, and proper integration was confirmed by PCR and immunodetection of the respective fusion protein.
TABLE 1.
S. cerevisiae strains used
| Strain | Descriptiona | Reference |
|---|---|---|
| UTL-7A (wild type) | MATα ura3-52 trp1 leu2-3/112 | 26 |
| UTL-Pex5p-ProtA | UTL-7A PEX5-TEV-protein A-kanMX6-Tpex5 | This study |
| UTL-FOX1-GFP | UTL-7A FOX1-kanMX4-Tfox1 | This study |
| pex5Δ | MATα ura3-52 trp1 leu2-3/112 pex5::LEU2 | This study |
| pex5Δ-Pex5p-N-TEV-ProtA | PEX5(1-313)-TEV-protein A-kanMX6-Tpex5 | This study |
| pex5Δ-FOX1-GFP | pex5::LEU2 FOX1-γEGFP-kanMX4-Tfox1 | This study |
| pex5Δ-FOX1-GFP | pex5::LEU2 FOX1-γEGFP-kanMX4-Tfox1 | This study |
| pex5Δ pex7Δ | pex5::loxP/pex7::LEU2 MATα ura3-52 trp1 leu2-3/112 | This study |
| pex5Δ pex14Δ | pex5::LEU2 pex14::loxP MATα ura3-52 trp1 leu2-3/112 | This study |
| pex5Δ pex14Δ-FOX1-GFP | pex5::LEU2 rpex14::loxP FOX1-γEGFP-kanMX4-Tfox1 | This study |
| pex5Δ pex12Δ | pex5::LEU2 pex12::loxP MATα ura3-52 trp1 leu2-3/112 | This study |
| pex5Δ pex12Δ-FOX1-GFP | pex5::LEU2 pex12::loxP FOX1-γEGFP-kanMX4-Tfox1 | This study |
| pex5Δ pex8Δ | pex5::LEU2 pex8::loxP MATα ura3-52 trp1 leu2-3/112 | This study |
| pex5Δ pex8Δ-FOX1-GFP | pex5::LEU2 pex8::loxP FOX1-γEGFP-kanMX4-Tfox1 | This study |
Additional proteins expressed from plasmids are indicated by parentheses.
TABLE 2.
Oligonucleotides used
| Designation | Sequence (5′ to 3′) |
|---|---|
| KU873 | TGACCTCGAGTTGTCGTTGGTAATTCTCTCG |
| KU874 | ACTGCCGTCGACGTGTTATGATATATTGTTTATTGAT |
| KU875 | GTCAGTGTCGACCATGGACGTAGGAAGTTGCTCA |
| KU878 | CGGCAGATCTTCAAGGATTATTAAAATATTCGTTGTTAG |
| KU896 | GAAGCCAGGCATGGACCTGAAAAGATTTAAAGGAGAATTTTCGTTTCGTACGCTGCAGGTCGAC |
| KU897 | GAATTTGGGCAGTGATGCGAGAACATAAAATTGCGGAGAACCATAATCGATGAATTCGAGCTCG |
| KU1169 | TGGTGCAGCTAATGCGGAAATTTTATCGAAAATAAACAAGCGTACGCTGCAGGTCGAC |
| KU1170 | CGCAAAACAGAGGGTTCGAAGGAAAACAGGAAACCTCTACATCGATGAATTCGAGCTCG |
| KU1263 | GTATGCTTTTCAATCTAACAACGAATATTTTAATAATCCTCGTACGCTGCAGGTCGAC |
| KU1346 | AAAGATGTAAAAGAACCTAATGCTTATAAAATTGGCTG |
| KU1347 | AGCATTAGGAGCAATTCTGTCTTCAACATC |
| KU1382 | CTAGGTCGACAATGAATAGTAACCGATGCCAAACGAATGAGGT |
| KU1383 | GCAGAGATCTTCAAAACGAAAATTCTCCTTTAAATCTTTTCAGGTC |
| KU1405 | GCAGAGATCTTCTCAAAAACGAAAATTCTCCTTTAAATC |
| KU1411 | CTAGGTCGACAATGCCTAATGCTTATAAAATTGGCTG |
Complete and minimal media used for yeast culturing have been described previously (26). YNO medium contained 0.1% (wt/vol) oleic acid, 0.05% (vol/vol) Tween 40, 0.1% (wt/vol) yeast extract, and 0.67% (wt/vol) yeast nitrogen base with amino acids (pH 6.0). To induce pex mutants by oleate, either cells grown with 0.3% glucose were shifted to an oleate medium or mutant cells were grown in YNO medium supplemented with 0.1% glucose. Oleic acid plates were prepared as described previously and contain 0.1% (wt/vol) oleic acid, 0.5% (wt/vol) Tween 40, 0.1% (wt/vol) yeast extract, 0.67% (wt/vol) yeast nitrogen base, amino acids (pH 6.0), and 1% (wt/vol) agarose. Manipulation of yeast cells was performed according to standard methods (55).
Plasmids, oligonucleotides, and cloning procedures.
The N-terminal half of Pex5p (Pex5p-N) comprising amino acids 1 to 313, the C-terminal part of Pex5p consisting of amino acids 313 to 612 (Pex5p-C), and the Pex18/5p-C hybrid protein containing Pex18p(1-225) in fusion with Pex5p-C were expressed in yeast cells from low-copy-number vectors under control of the PEX5 promoter. The corresponding coding DNA regions were created by PCR with genomic DNA of the wild-type strain as a template. The oligonucleotides used in this study are listed in Table 2 and were obtained from Eurogentec (Seraing, Belgium).
The PEX5 N fragment was amplified by using the primers KU875 and KU878. The SalI/BglII PEX5(1-939) fragment was first cloned into SalI/BglII-restricted pPC86 (11), followed by digestion with SalI and BamHI, resulting in a fragment containing PEX5(1-939) and the ADC1 termination region (TADC1). The PEX5 promoter sequence region (−233 bp upstream of the PEX5 open reading frame) was amplified with the primer pair KU873-KU874. The XhoI/SalI promoter fragment and the SalI/BamHI PEX5(1-939)-TADC1 fragment were ligated into XhoI/BamHI-digested pRS416 (Stratagene, La Jolla, Calif.) in which the internal SalI recognition site was removed by SalI/XhoI restriction and religation, resulting in pWK-PEX5(1-313).
PEX5-C was amplified by using the primer pair KU1411-KU1405. The SalI/BglII-digested amplification product was cloned into SalI/BglII-digested pWK-PEX5(1-313), resulting in pDK-PEX5(313-612).
To express the Pex18/5p-C-hybrid, the coding sequences of Pex18p(1-225) and Pex5p(313-612) were amplified by using the primer pairs KU1382-KU1347 and KU1346-KU1383, respectively, followed by overlap extension PCR (34) with primer pair KU1382-KU1383. The 1,593-bp amplification product was cloned after SalI/BglII restriction into SalI/BglII-digested pWK-PEX5(1-313), resulting in pDK-Pex18/5p-C.
The yeast expression plasmid pGFP-SKL codes for a modified version of the GFP of Aequoria victorea (S65T) terminating in the PTS1, SKL. The coding sequence was isolated together with the MET25 promoter region and the cyc termination region as a SacI/KpnI fragment from plasmid pRS6MPTS1GFP, which was kindly provided by W. Girzalsky (Bochum, Germany), and cloned into pRS415 (Stratagene), resulting in pGFP-SKL.
Antibodies.
Western blots were incubated with polyclonal rabbit antibodies raised against glutathione S-transferase-GFP, catalase A, 3-ketoacyl-coenzyme A (CoA) thiolase, Fox1p, Pex3p, Pex5p, Pex10p, Pex12p, Pex13p, Pex14p, Pex17p, and fructose-1,6-bisphosphatase (all raised in our laboratory) and aconitase (a kind gift of R. Lill, University of Marburg, Marburg, Germany). Horseradish peroxidase-coupled anti-rabbit immunoglobulin G (IgG) in combination with the ECL system (Amersham Biosciences, Freiburg, Germany) was used to detect immunoreactive complexes. For immunofluorescence analysis, additional monoclonal antibodies against all fluorescent proteins (AFP, 1:250; Q-Biogene, Heidelberg, Germany), Cy3-conjugated goat anti-rabbit IgG (Alexa-Fluor 568, 1:500; Molecular Probes, Leiden, Netherlands), and fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG (Alexa-Fluor 488, 1:500) were used.
Immunopurification of membrane-associated protein complexes.
The isolations of membrane-associated Pex5p-ProtA and Pex5p-N-ProtA with IgG-coupled Sepharose were performed as described by Agne et al. (2). In addition, a washing step with the total 100,000 × g sedimented cell membranes (4°C, 1 h, Hitachi RP45AT rotor) was performed in 60 ml of buffer containing 20 mM HEPES, 100 mM potassium acetate, 5 mM magnesium acetate (pH 7.5), and protease inhibitors (1 mM phenylmethylsulfonyl fluoride, 1 mM benzamidine, 8 μM antipain, 5 mM NaF, 10 μM chymostatin), followed by another centrifugation at 100,000 × g. Two hundred milligrams of the membrane sediment was solubilized with 1% (wt/vol) digitonin (Calbiochem) and subjected to affinity chromatography. For elution of Pex5p-ProtA and Pex5p-N-ProtA containing protein complexes bound to IgG-coupled Sepharose, the resin was incubated with 50 μl of sodium dodecyl sulfate sample buffer and analyzed by immunoblotting.
Cell fractionation.
Spheroplasting of yeast cells and homogenization were performed as described by Erdmann et al. (26). A volume of 5 to 7 ml corresponding to 7 mg of total proteins of postnuclear supernatants were loaded on preformed 15.5 to 36% (wt/vol) Optiprep (Iodixanol) gradients containing 18% (wt/vol) sucrose, 5 mM MES (morpholineethanesulfonic acid) (pH 6.0), 1 mM EDTA, 1 mM KCl, and 0.1% ethanol underlaid with 1 ml of 60% (wt/vol) Optiprep solution (Nycomed, Oslo, Norway). Gradients (about 30 ml) were centrifuged for 90 min at 48,000 × g at 4°C in an SV-288 rotor (Sorvall RC5B; DuPont, Bad Nauheim, Germany) and subsequently fractionated in 26 fractions of 1.2 ml each. One half of each fraction was applied for enzyme and refractive index measurements followed by conversion to the respective density. The other half was used for Western blot analysis as described by Erdmann et al. (26). The enzyme activities of ketoacyl-CoA thiolase (EC 2.3.1.16), catalase (EC 1.11.1.6), and cytochrome c oxidase (EC 1.9.3.1) were measured according to published procedures (6, 46, 69).
Electron microscopy.
For immunoelectron microscopy, whole cells were fixed and prepared as described by Waterham et al. (76). Immunolabeling was performed on ultrathin sections of Unicryl-embedded cells, using specific polyclonal rabbit antibodies against 3-ketoacyl-CoA thiolase and glutathione S-transferase-GFP and gold-conjugated goat anti-rabbit antibodies.
Fluorescence microscopy.
For fluorescence microscopy, yeast cells were transformed with Pex5p-N-, Pex18/5p-C-, and GFP-SKL-coding plasmids. The analyses were performed according to the method of Westermann and Neupert (77), or the cells were prepared as described by Erdmann and Kunau (25) for double labeling.
The direct fluorescence of GFP was recorded at room temperature in distilled water with a Axiophot microscope (Zeiss, Oberkochen, Germany) and a 100×, 1.4 NA oil immersion objective. Both fluorescence and optical photographs were taken by using the connected hardware in combination with the Spot RT software version 3.1 (Diagnostics Instruments). For double labeling, spheroplasts were bound on polylysine-covered glass slides and labeled with polyclonal antibodies against 3-ketoacyl-CoA thiolase (25) and monoclonal antibodies against all fluorescent proteins (AFP). As second antibodies, Cy3-conjugated goat anti-rabbit IgG (Alexa-Fluor 568) and FITC-conjugated goat anti-mouse IgG (Alexa-Fluor 488) (Molecular Probes) were applied. The cells were covered with mounting solution containing n-propylgallate (35). For confocal imaging and merging, an LSM 510 system attached to an Axiovert 100 microscope (Zeiss) was employed. Adjustments of contrast and brightness were carried out with Adobe Photoshop software version 5.0, and characteristic cells were cut out and copied to Macromedia Freehand software version 8.0 or 10.0.
RESULTS
The N-terminal half of Pex5p associates with the docking complex at the peroxisomal membrane.
In accordance with the extended shuttle hypothesis (13, 17, 42), a significant fraction of the predominantly cytosolic PTS1 receptor Pex5p of S. cerevisiae is associated with peroxisomal membranes. In order to investigate which part of Pex5p is responsible for the binding at the peroxisomal membrane, we studied a C-terminally truncated version, Pex5p-N, comprising amino acid residues 1 to 313 and therefore lacking all six TPR motifs, which have been demonstrated to be necessary and sufficient for the recognition of PTS1 cargo proteins. Pex5p-N was expressed in oleic acid-grown pex5Δ cells of S. cerevisiae. Separation of whole-cell lysate by centrifugation at 25,000 × g for 20 min into an organellar fraction and a supernatant revealed that approximately 50% of Pex5p-N associated with the pellet (data not shown). This was a surprising result, because in density gradients much less Pex5p-N comigrates with mature peroxisomes (see Fig. 4). This seems to suggest that at least part of the Pex5p-N detected in fractions of lower density sediments at 25,000 × g.
FIG. 4.
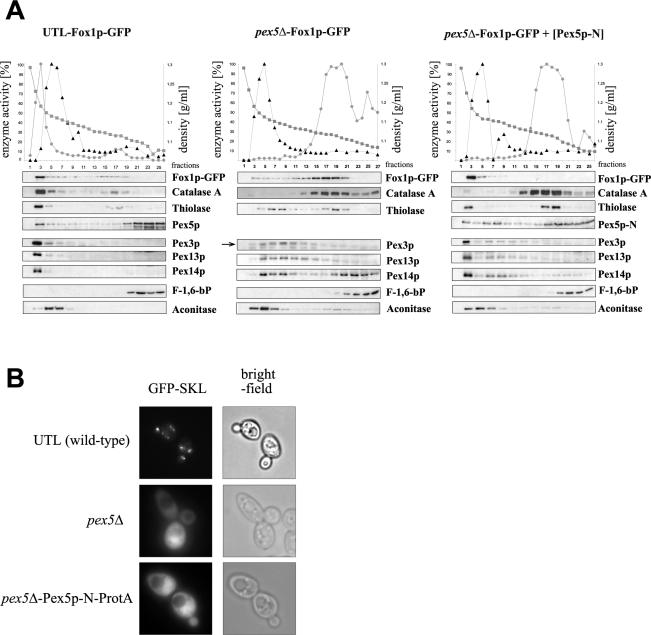
Fox1p-associated peroxisomes of Pex5p-N-expressing cells do not contain typical PTS1 marker proteins. (A) Fox1p colocalizes with catalase-free peroxisomes in PEX5 deletion cells expressing the N-terminal half of Pex5p. For subcellular fractionation of oleic acid-grown cells (14 h), postnuclear supernatants of the UTL-Fox1p-GFP, pex5Δ-Fox1p-GFP, and pex5Δ-Fox1p-GFP strains transformed with Pex5p-N expression plasmid were separated on 15.5 to 36% (wt/vol) Optiprep gradients with 18% (wt/vol) sucrose in gradient buffer. Enzyme activities in the graphs are given in percentages corresponding to the fraction with highest activity for the peroxisomal matrix enzyme catalase (circles) and the mitochondrial marker cytochrome c oxidase (triangles). The density (squares) of each fraction was calculated after refractive index measurement. Equal quantities of each gradient fraction were separated by gel electrophoresis and analyzed by immunoblotting with antibodies raised against Fox1p; the PTS1 protein catalase A; the PTS2 protein thiolase; Pex5p; peroxisomal membrane proteins Pex3p, Pex13p, and Pex14p; cytosolic fructose-1,6-bisphosphatase (F-1,6-bP); and mitochondrial aconitase. (B) The PTS1 marker protein GFP-SKL is localized to the cytosol in cells expressing Pex5p-N instead of full-length Pex5p. UTL (wild-type), pex5Δ, and pex5Δ-Pex5p-N-ProtA cells were grown for 14 h in oleic acid medium. The intracellular distribution of GFP-SKL was visualized by direct fluorescence microscopy (left panels). Bright-field microscopy demonstrated structural integrity of the cells (right panels).
To investigate which of the membrane-bound peroxins are involved in the association of Pex5p-N with the peroxisomal membrane, multiprotein complexes from cells expressing either Pex5p-N or Pex5p tagged with protein A were isolated from total cellular membranes. The strains expressing protein A fusion proteins instead of wild-type Pex5p were generated by genomic integration into the PEX5 locus (see Materials and Methods). The strain expressing full-length Pex5p-ProtA (UTL-Pex5p-ProtA) grew equally well as the wild type on oleic acid as the sole carbon source, indicating that the tag does not affect the functionality of the PTS1 receptor (data not shown). Protein complexes containing protein A-tagged Pex5p forms were isolated from digitonin-solubilized membranes by means of affinity chromatography with IgG-Sepharose, using the protein A-tagged Pex5 proteins as baits. In line with results of previous experiments in which Pex2p, Pex8p, or Pex14p was used as bait (2), both wild-type Pex5p and Pex5p-N pulled out proteins of both the docking core complex and the ring finger core complex (Fig. 2). However, in the case of Pex5p-N, the amount of the ring finger peroxins was drastically reduced compared with full-length Pex5p. In contrast, the association with the docking core complex seems not to be affected by truncation of the C-terminal half of Pex5p, as indicated by equal amounts of Pex13p, Pex14p, and Pex17p in both eluates (Fig. 2).
FIG. 2.

Pex5p-N-ProtA assembles with several membrane-associated peroxins. S. cerevisiae wild-type cells (UTL) and mutant cell lines in which the genomic copy of the PEX5 open reading frame was replaced by a DNA regions coding for Pex5p-ProtA (UTL-Pex5p-ProtA) or Pex5p-N-ProtA (pex5Δ-Pex5p-N-ProtA) fusion proteins were grown for 14 h on oleate medium. Membrane protein complexes were solubilized with 1% (wt/vol) digitonin from total cellular membranes. Equal amounts of these protein mixtures containing either protein A-tagged Pex5 proteins (UTL-Pex5p-ProtA and pex5Δ-Pex5p-N-ProtA) or nontagged Pex5p (UTL, wild type) as a control for unspecific binding were subjected to affinity chromatography with IgG-coupled Sepharose. Bound proteins were eluted with sodium dodecyl sulfate-polyacrylamide gel electrophoresis sample buffer and subjected to immunoblot analyses with antisera directed against Pex5p; suggested docking complex constituents Pex13p, Pex14p, and Pex17p; and ring finger peroxins Pex12p, Pex10p, and Pex3p as a nonconstituent of this complex.
Taken together, these findings indicate that the N-terminal half of Pex5p has the ability to dock efficiently to the peroxisomal membrane. Moreover, the binding occurs with key components of the importomer.
The N-terminal half of Pex5p functions as a peroxisome import receptor of Fox1p.
In order to investigate whether Pex5p-N not only associates with the importomer of the peroxisomal membrane but also facilitates cargo transport into the organelle, we studied the import of acyl-CoA oxidase (Fox1p) into peroxisomes. Previous studies demonstrated that the import of this peroxisomal matrix protein without a detectable PTS1 or PTS2 is strictly dependent on Pex5p, but in contrast to the PTS1 proteins, Fox1p binds to the N-terminal half of Pex5p outside the TPR region (38).
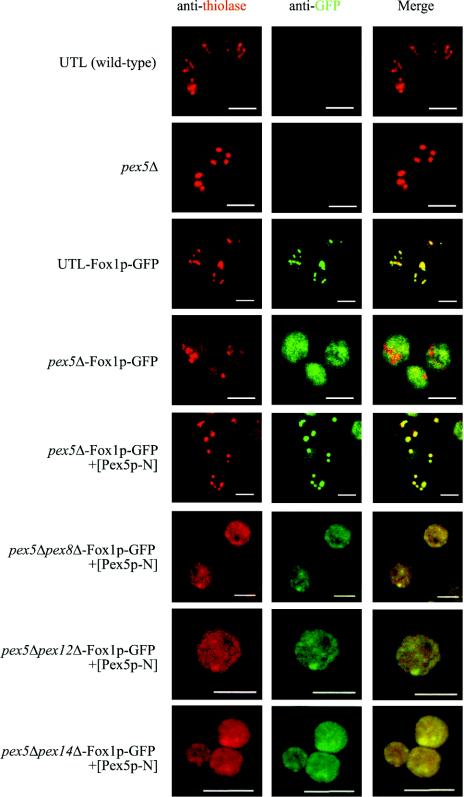
For localization studies, we generated wild-type and pex5Δ strains expressing Fox1p fused C terminally to GFP under the control of its own promoter. The wild-type strain exhibited normal growth on oleic acid medium, demonstrating that the functionality of Fox1p is not affected by the fusion (data not shown). The intracellular distribution of Fox1p-GFP was analyzed by immunofluorescence and subcellular fractionation by using density gradient centrifugation. Whereas in wild-type cells Fox1p-GFP is targeted to peroxisomes, in pex5Δ cells Fox1p-GFP is mislocalized to the cytosol. The Fox1p import defect of pex5Δ cells is clearly shown by a diffuse cytosolic staining in immunofluorescence analysis (Fig. 3), as well as by the fact that most of the Fox1p, like catalase, no longer comigrated with peroxisomal membrane proteins and thiolase during density gradient centrifugation but could be detected in fractions of lower density (Fig. 4A). Additional expression of Pex5p-N in the pex5 deletion strain restored the peroxisome targeting of Fox1p-GFP, as indicated by a punctate pattern and colocalization of Fox1p-GFP with thiolase (Fig. 3) and Pex14p (data not shown) in fluorescence studies. Pex5p-N was also expressed together with Fox1p-GFP in double-deletion strains. Immunofluorescence studies revealed a diffuse cytosolic staining for Fox1p-GFP in pex5Δ pex8Δ, pex5Δ pex12Δ, and pex5Δ pex14Δ strains (Fig. 3). This suggests that the peroxins Pex8p, Pex12p, and Pex14p are significantly involved in Pex5p-N-mediated Fox1p import. In particular, the requirement of Pex12p seems to be relevant here, because the amounts of Pex5p-N-associated ring finger peroxins at the peroxisomal membrane are drastically reduced compared with those in the full-length Pex5p membrane complex (Fig. 2).
FIG. 3.
The N-terminal half of Pex5p without the TPR region enables Fox1p targeting to peroxisomes. The localization of Fox1p-GFP, expressed from a genomic context, in cells of UTL (wild type), the pex5Δ strain, and pex5Δ pex14Δ, pex5Δ pex8Δ, and pex5Δ pex12Δ double-deletion strains, with or without Pex5p-N expression plasmid, was examined by indirect immunofluorescence with a confocal laser scanning microscope. Peroxisomes were labeled with rabbit antithiolase antibodies and Cy3-conjugated secondary antibody (left panels). Fox1p-GFP was marked with monoclonal mouse anti-GFP antibodies and FITC-conjugated secondary antibody (middle panels). Colocalization of Fox1p-GFP with thiolase is indicated by the yellow color in the right panels, obtained by overlaying the images of the left and middle panels. Bars, 5 μm.
Subcellular fractionation studies further supported the colocalization of Fox1p with various peroxisomal membrane proteins and the PTS2 protein thiolase in pex5Δ cells expressing Pex5p-N. Fox1p-GFP migrated in Optiprep-sucrose gradients to a density similar to that for mature peroxisomes, whereas the PTS1 protein catalase A remained at the top of the gradient (Fig. 4A). Consistent with the absence of the C-terminal half of Pex5p, there is no indication of a discrete localization of the PTS1 protein GFP-SKL in Pex5p-N-ProtA-expressing cells (Fig. 4B).
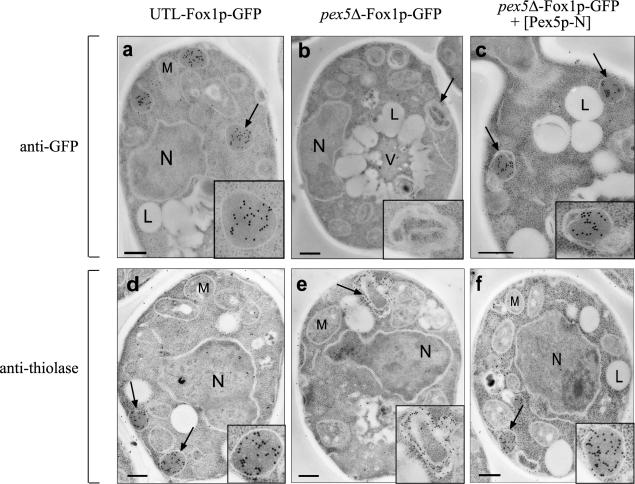
To distinguish whether Fox1-GFP is accumulated at the outer surface of the membrane or actually imported into peroxisomes, we applied immunoelectron microscopy. Oleate-grown pex5Δ mutant cells of S. cerevisiae with and without the expression of Fox1p-GFP are characterized morphologically by the absence of normal peroxisomes. Instead, these cells contain several multilamellar vesicles or membrane stacks which can be labeled by antithiolase antibodies (Fig. 5) (74) and various antibodies directed against peroxisomal membrane proteins (33). When pex5Δ-FOX1-GFP cells were transformed with the Pex5p-N-expressing plasmid, we observed a profound morphological change. All cells contain vesicles with an electron-dense matrix resembling wild-type peroxisomes in number and size (Fig. 5). Immunogold labeling with anti-GFP antibodies revealed that Fox1p, like thiolase, is localized to the matrix of these peroxisomes. These findings show that the N-terminal half of Pex5p enables not only the association with the peroxisomal membrane but also the translocation of Fox1p-GFP across it, which in turn restores normal peroxisome morphology.
FIG. 5.
The morphology of peroxisomes without PTS1 proteins is similar to that of wild-type peroxisomes. Morphological and immunocytochemical analyses were carried out with the UTL-Fox1p-GFP (a and d) and pex5Δ-Fox1p-GFP (b and e) strains and with the pex5Δ-Fox1p-GFP strain expressing Pex5p-N (c and f). All cells were grown for 12 h on oleic acid medium and fixed in glutaraldehyde. For immunogold labeling, antibodies raised against GFP (a to c) or thiolase (d to f) and gold-conjugated goat anti-rabbit secondary antibodies were used. The insets show magnifications of representative peroxisomal structures. L, lipid drops; M, mitochondria; N, nucleus. Bars, 1 μm.
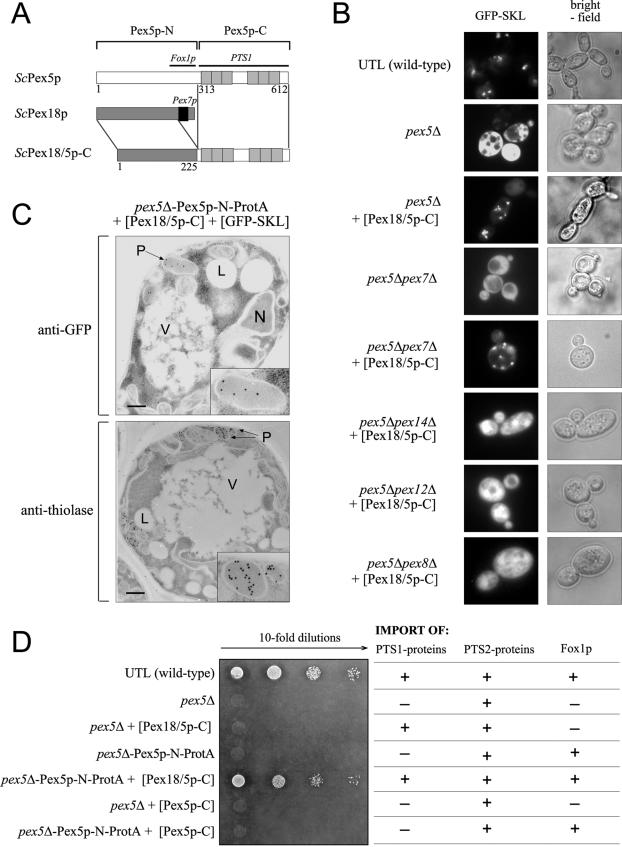
A chimeric protein consisting of Pex18(1-225)p and the C-terminal half of Pex5p can mediate the import of PTS1 proteins into peroxisomes.
Next we asked whether the modular character of the PTS1 receptor also applies to components of the PTS2 import pathway. In S. cerevisiae this import route requires, in addition to Pex7p (the PTS2 receptor), the partially redundant auxiliary peroxins Pex18p and Pex21p. Similar auxiliary proteins are Pex20p in Y. lipolytica and N. crassa and the long isoform of Pex5p in higher eukaryotes (mammals and plants). Previous studies have suggested that these nonorthologous proteins fulfill a common, although still unknown, function (48, 51, 61, 64, 68). Our results presented above led us to the question whether the auxiliary peroxins of lower eukaryotes carry out the same function in the PTS2 pathway that the N-terminal half of Pex5p fulfils in the PTS1 pathway. In order to test this hypothesis, we replaced the C-terminal Pex7p binding site of Pex18p with the C-terminal half of Pex5p, which contains the PTS1 cargo binding site (Fig. 6A). This hybrid protein, designated Pex18/5p-C, was coexpressed in a PEX5 deletion strain together with the PTS1 reporter protein GFP-SKL, and the intracellular localization of GFP-SKL was analyzed by fluorescence microscopy (Fig. 6B). The punctate pattern obtained suggested that despite the lack of the N-terminal half of Pex5p, GFP-SKL is targeted to peroxisomes. To exclude the possibility that the observed punctate pattern is caused by aggregation of GFP-SKL in the presence of Pex18/5p-C, we analyzed the localization of GFP-SKL in three additional double mutants which are known not to form import-competent peroxisomes, the pex5Δ pex8Δ, pex5Δ pex12Δ, and pex5Δ pex14Δ mutants. In each case, we observed no indication of a punctate pattern but always a diffuse staining of the cytosol (Fig. 6B). A punctate pattern was also obtained when the same experiment was carried out with a double mutant in which both receptors, Pex5p and Pex7p, were deleted. These results clearly demonstrate that the targeting of GFP-SKL to peroxisomes facilitated by the chimeric protein Pex18/5p-C does not require Pex7p.
FIG.6.
The chimeric protein Pex18/5p-C can mediate the import of PTS1 proteins into peroxisomes. (A) Construction of the chimeric protein Pex18/5p-C was carried out by replacing the Pex7p binding box of Pex18p (black box) with the C-terminal half of Pex5p containing the PTS1 binding TPR motifs (grey). (B) The intracellular localization of the plasmid-encoded PTS1 protein GFP-SKL in a pex5Δ strain is affected by expression of a Pex18/5p-C chimeric protein. Wild-type UTL-7A, pex5Δ, and pex5Δ pex7Δ cells, as well as pex5Δ, pex5Δ pex7Δ, pex5Δ pex14Δ, pex5Δ pex12Δ, and pex5Δ pex8Δ cells expressing Pex18/5p-C, were grown for 14 h in oleic acid medium and analyzed by fluorescence microscopy. Bright-field microscopy demonstrated structural integrity of the cells. (C) Morphological and immunocytochemical analysis of pex5Δ-Pex5p-N-ProtA cells expressing both the chimeric protein Pex18/5p-C and GFP-SKL. All cells were grown for 12 h in oleic acid medium and fixed in glutaraldehyde. For immunogold labeling, antibodies raised against GFP or 3-ketoacyl-CoA thiolase and gold-conjugated goat anti-rabbit antibodies were used. The insets show magnifications of representative peroxisomal structures. L, lipid drops; M, mitochondria; N, nucleus. Bars, 1 μm. (D) Expression of the chimeric protein Pex18/5p-C rescues the PTS1 import defect of cells expressing Pex5p-N instead of full-length Pex5p. As the beta-oxidation system in S. cerevisiae comprises members of all three classes of peroxisomal matrix proteins (PTS1, PTS2, and non-PTS1/2 proteins), growth on oleate is an assay for the functionality of all three import routes (e.g., growth of wild-type cells demonstrates that all import pathways are functional, as illustrated in the table). For this assay cells were grown in medium containing 0.3% glucose for 16 h. Equal numbers of cells were diluted in distilled water, and aliquots were applied as a series of 10-fold dilutions on an oleic acid plate, whereas the spots on the left correspond to 2 × 104 cells. The growth plate was subsequently incubated at 30°C for 5 days. The partial import defects of cells expressing only the chimeric Pex18/5p-C (pex5Δ cells transformed with Pex18/5p-C expression plasmid) or Pex5p-N (pex5Δ-Pex5p-N-ProtA) can be rescued when both proteins are expressed together (pex5Δ-Pex5p-N-ProtA strain transformed with Pex18/5p-C expression plasmid). The C-terminal half of Pex5p alone (Pex5p-C), not fused to Pex18p, is not able to mediate import of PTS1 proteins. This is demonstrated by the inability of the pex5Δ-Pex5p-N-ProtA strain, transformed with Pex5p-C expression plasmid, to grow on oleate.
We extended our analysis of Pex18/5p-C function by exploring whether endogenous PTS1 proteins have actually been imported into peroxisomes. To this end, we tested whether the hybrid protein is able to rescue the PTS1 import defect of the PEX5 deletion mutant by using a growth test in which oleic acid is the sole carbon and energy source. To degrade oleic acid, all enzymes of the beta-oxidation pathway must be localized to the peroxisome. These enzymes include PTS1-containing proteins such as Eci1p (enoyl-CoA isomerase) and Fox2p (multifunctional β-oxidation enzyme), the PTS2-targeted protein thiolase, and the non-PTS1/PTS2 protein Fox1p (acyl-CoA oxidase) (36). Not surprisingly, the expression of the chimeric Pex18/5p-C alone in pex5Δ cells cannot rescue the oleate nonutilizer phenotype of the pex5 mutant (Fig. 6D). As the import of the non-PTS1 matrix protein Fox1p requires its binding to the N-terminal half of Pex5p (see above), we repeated the experiments with transformants which expressed both the hybrid protein Pex18/5p-C and Pex5p-N and found that these two proteins restored the ability to grow on oleic acid as the sole carbon source (Fig. 6D). Although growth was reduced compared to that of wild-type cells, the result clearly demonstrated that the chimeric protein Pex18/5p-C facilitates the import of PTS1 proteins into peroxisomes. This conclusion was further supported by immunoelectron microscopy, showing that in cells expressing both the hybrid protein and Pex5p-N, GFP-SKL was found predominantly in peroxisomes (Fig. 6C). In contrast, a strain which expressed Pex5p-N together with Pex5p-C did not grow. These data indicate that it is indeed the Pex18p portion of the chimera that imparts the transport of cargo and not the Pex5p portion.
DISCUSSION
According to the extended receptor cycle, peroxisomal import receptors have to fulfill two distinct functions, cargo recognition and transport. It has been demonstrated that the PTS-specific recognition of cargo proteins is mediated by the C-terminal half of Pex5p (10, 29, 66, 67) and full-length Pex7p (22, 53). In this study, we explored the role of the N-terminal halves of the PTS1 receptor Pex5p and of Pex18p, one of the binding partners of the PTS2 receptor Pex7p. We present evidence that in S. cerevisiae, the N-terminal half of Pex5p is sufficient to import Fox1p into peroxisomes. Moreover, Pex18p when fused to the C-terminal half of Pex5p can substitute for the N-terminal half of Pex5p in transporting PTS1 proteins from the cytosol to the peroxisomal matrix. These findings strongly suggest that both the N-terminal half of Pex5p and Pex18p are sufficient for receptor docking and cargo transport into peroxisomes.
Evidence that the N-terminal half of Pex5p is sufficient to transport a matrix protein across the membrane.
The extended receptor cycle requires that the receptor binds and enters the peroxisomal membrane, facilitates the cargo release, and exits the peroxisome (13). To analyze the role of the first half of Pex5p (Pex5p-N) in peroxisomal protein import, we took advantage of the recent observation that the import of Fox1p, a protein that lacks PTS1, is nevertheless strictly dependent on its binding to the N-terminal half of Pex5p (38). Our demonstration that Pex5p-N is sufficient to fulfill the role of a Fox1p import receptor in vivo is based on multiple experimental approaches that include isolation of Pex5p-N-containing complexes from the peroxisomal membrane, cell fractionation studies, and morphological analyses (immunofluorescence and immunoelectron microscopy). The density gradient experiments and the immunofluorescence pattern obtained demonstrated the efficient targeting of Fox1p to peroxisomes. Immunelectron micrographs clearly showed that in pex5Δ cells, Fox1p-GFP was indeed translocated in a Pex5p-N-dependent manner into the peroxisomal matrix. These data demonstrate that the N-terminal half of Pex5p is sufficient to transport a matrix protein into the peroxisome. According to the model of the extended receptor cycle, this would suggest that this part of the PTS1 receptor performs the membrane-bound steps of this cycle. However, this remains to be experimentally shown. Another possibility is that the import of Fox1p, as a non-PTS1, non-PTS2 protein, follows a hitherto-unknown translocation route. However, this latter seems extremely unlikely, as Pex5p-N was found at the peroxisomal membrane associated with the same set of peroxins as wild-type Pex5p. This notion is further supported by the findings that deletions of the PEX8, PEX12, or PEX14 gene, all of which are essential for the import of PTS1 and PTS2 proteins (3, 4, 54), also prevent the Pex5p-N-mediated import of Fox1p. However, when we isolated the membrane-bound pool of Pex5p or Pex5p-N under native conditions, significantly reduced amounts of the ring finger peroxins Pex10p and Pex12p were associated with Pex5p-N. Thus, the C-terminal half of Pex5p together with PTS1 cargo proteins seems to influence the dynamic assembly of the importomer from its subcomplexes (2). Interestingly, in higher eukaryotes a direct interaction between the TPR region of Pex5p and the ring finger peroxin Pex12p has recently been demonstrated (27). With regard to import of Fox1p, it appears that this interaction between the TPR region of Pex5p and the ring finger core complex, although not yet demonstrated in yeast, is dispensable.
In summary, our results indicate that the basic functions of PTS receptors, cargo recognition and receptor transport, can be assigned to distinct halves of the molecule, demonstrating a modular organization of yeast Pex5p.
Pex18p can replace the transport domain of Pex5p in PTS1 protein import.
Previous studies revealed functional and structural similarities between the mammalian PTS1 receptor Pex5p and the auxiliary yeast proteins of the PTS2 pathways, Pex18p and Pex21p. For example, all three proteins possess common structural motifs, such as a stretch of conserved amino acids representing a Pex7p binding box and WXXXF sequence patterns (18, 21). In the light of the observation that Pex5p-N is sufficient for cargo transport (see above), it was tempting to test whether the same is true for the auxiliary proteins, especially as no distinct function could as yet be assigned to these proteins. Three lines of evidence indicate that this is indeed the case. The chimeric protein of Pex18p without its Pex7p binding box and Pex5p-C, comprising the C-terminal half of Pex5p, led in pex5Δ cells to (i) a punctate immunofluorescence pattern of GFP-SKL, (ii) peroxisomal import of GFP-SKL as shown by immunoelectron microscopy, and (iii) growth on oleic acid when expressed together with Pex5p-N. Pex5p-N is required for the ability to import Fox1p (acyl-CoA oxidase), which is an essential enzyme of fatty acid utilization (15). Although the import mediated by Pex18/5p-C is not as efficient as that with wild-type Pex5p, these findings nevertheless clearly demonstrate that the chimeric protein in principle transports PTS1 proteins into the peroxisome. This conclusion suggests that not only wild-type Pex18p but most likely also the other auxiliary proteins, which have been shown to be functionally redundant (21, 52, 61), are sufficient for receptor docking and cargo import into peroxisomes.
Consistent with this suggestion are reports about interactions between auxiliary proteins of the PTS2 pathway and membrane-bound peroxins. While for Pex18p itself no such interactions have yet been reported, its functional counterparts ScPex21p and YlPex20p have been shown to interact with Pex13p (21) and Pex8p (62). Both peroxins are also known binding partners of Pex5p (24, 54).
In summary, it has not yet been unequivocally demonstrated that the PTS1 import facilitated by the chimeric Pex18/5p-C involves interactions with binding partners at and in the peroxisomal membrane that are identical to those for Pex5p-mediated protein import. However, this is suggested by data obtained with double mutants demonstrating the requirement of Pex8p, Pex12p, and Pex14p for the PTS1 import mediated by the chimeric Pex18/5p-C (Fig. 6A).
Assuming that the demonstrated function of Pex18p is identical to its natural role during import of PTS2 proteins, it is tempting to conclude that the actual functional receptor in the PTS2 pathway is a hetero-oligomer of at least one of the auxiliary proteins and Pex7p. In this receptor complex, Pex7p is required for the cargo recognition and the auxiliary proteins are required for the transport of PTS2 proteins. The known interactions of Pex7p with Pex13p and Pex14p (64) seem to argue against such a strict modular organization of the PTS2 receptor complex. However, the prevailing interpretation of these Pex7p in vitro interactions as docking steps in vivo still needs experimental substantiation.
The results presented here have evolutionary implications. They provide a rationale for the previously intriguing observations that homologues of the fungal auxiliary proteins of the PTS2 import pathway have not been found in higher eukaryotes. Instead, the longer of two splice isoforms of the PTS1 receptor, Pex5pL, is able to interact directly with the PTS2 receptor via a conserved Pex7p binding site which is absent in Pex5p from lower eukaryotes (21). The acquisition of this additional property during the course of evolution enables Pex5pL to act as a point of convergence for both the PTS1 and the PTS2 import pathway prior to steps involving the peroxisomal membrane.
Acknowledgments
We thank Uschi Dorpmund, Uta Ricken, and Klaas Sjollema for technical assistance and Wolfgang Girzalsky for kindly providing a GFP-SKL-coding plasmid. We are especially thankful to Ralf Erdmann and Will Stanley for critically reading the manuscript.
This work was supported by Deutsche Forschungsgemeinschaft grants SFB394, DFGSchl 584/1-1, and DFGSchl 584/1-2.
REFERENCES
- 1.Agarraberes, F. A., and J. F. Dice. 2001. Protein translocation across membranes. Biochim. Biophys. Acta 1513:1-24. [DOI] [PubMed] [Google Scholar]
- 2.Agne, B., N. M. Meindl, K. Niederhoff, H. Einwachter, P. Rehling, A. Sickmann, H. E. Meyer, W. Girzalsky, and W. H. Kunau. 2003. Pex8p: an intraperoxisomal organizer of the peroxisomal import machinery. Mol. Cell 11:635-646. [DOI] [PubMed] [Google Scholar]
- 3.Albertini, M., W. Girzalsky, M. Veenhuis, and W.-H. Kunau. 2001. Pex12p of Saccharomyces cerevisiae is a component of a multi-protein complex essential for peroxisomal matrix protein import. Eur. J. Cell Biol. 80:257-270. [DOI] [PubMed] [Google Scholar]
- 4.Albertini, M., P. Rehling, R. Erdmann, W. Girzalsky, J. A. K. W. Kiel, M. Veenhuis, and W.-H. Kunau. 1997. Pex14p, a peroxisomal membrane protein binding both receptors of the two PTS-dependent import pathways. Cell 89:83-92. [DOI] [PubMed] [Google Scholar]
- 5.Barnett, P., G. Bottger, A. T. J. Klein, H. F. Tabak, and B. Distel. 2000. The peroxisomal membrane protein Pex13p shows a novel mode of SH3 interaction. EMBO J. 19:6382-6391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baudhuin, P. 1974. Isolation of rat liver peroxisomes. Methods Enzymol. 31:356-368. [DOI] [PubMed] [Google Scholar]
- 7.Bottger, G., P. Barnett, A. T. Klein, A. Kragt, H. F. Tabak, and B. Distel. 2000. Saccharomyces cerevisiae PTS1 receptor Pex5p interacts with the SH3 domain of the peroxisomal membrane protein Pex13p in an unconventional, non-PXXP-related manner. Mol. Biol. Cell 11:3963-3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braverman, N., G. Dodt, S. J. Gould, and D. Valle. 1998. An isoform of Pex5p, the human PTS1 receptor, is required for the import of PTS2 proteins into peroxisomes. Hum. Mol. Genet. 7:1195-1205. [DOI] [PubMed] [Google Scholar]
- 9.Braverman, N., G. Steel, C. Obie, A. Moser, H. Moser, S. J. Gould, and D. Valle. 1997. Human PEX7 encodes the peroxisomal PTS2 receptor and is responsible for rhizomelic chondrodysplasia punctata. Nat. Genet. 15:369-376. [DOI] [PubMed] [Google Scholar]
- 10.Brocard, C., F. Kragler, M. M. Simon, T. Schuster, and A. Hartig. 1994. The tetratricopeptide repeat-domain of the Pas10 protein of Saccharomyces cerevisiae is essential for binding the peroxisomal targeting signal SKL. Biochem. Biophys. Res. Commun. 204:1016-1022. [DOI] [PubMed] [Google Scholar]
- 11.Chevray, P. M., and D. Nathans. 1992. Protein interaction cloning in yeast: identification of mammalian proteins that react with the leucine zipper of Jun. Proc. Natl. Acad. Sci. USA 89:5789-5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chudzik, D. M., P. A. Michels, S. de Walque, and W. G. Hol. 2000. Structures of type 2 peroxisomal targeting signals in two trypanosomatid aldolases. J. Mol. Biol. 300:697-707. [DOI] [PubMed] [Google Scholar]
- 13.Dammai, V., and S. Subramani. 2001. The human peroxisomal targeting signal receptor, Pex5p, is translocated into the peroxisomal matrix and recycled to the cytosol. Cell 105:187-196. [DOI] [PubMed] [Google Scholar]
- 14.de Walque, S., J. A. Kiel, M. Veenhuis, F. R. Opperdoes, and P. A. Michels. 1999. Cloning and analysis of the PTS-1 receptor in Trypanosoma brucei. Mol. Biochem. Parasitol. 104:106-119. [DOI] [PubMed] [Google Scholar]
- 15.Dmochowska, A., D. Dignard, R. Maleszka, and D. Y. Thomas. 1990. Structure and transcriptional control of the Saccharomyces cerevisiae POX1 gene encoding acyl-coenzyme A oxidase. Gene 88:247-252. [DOI] [PubMed] [Google Scholar]
- 16.Dodt, G., N. Braverman, C. Wong, A. Moser, H. W. Moser, P. Watkins, D. Valle, and S. J. Gould. 1995. Mutations in the PTS1 receptor gene, PXR1, define complementation group 2 of the peroxisome biogenesis disorders. Nat. Genet. 9:115-125. [DOI] [PubMed] [Google Scholar]
- 17.Dodt, G., and S. J. Gould. 1996. Multiple PEX genes are required for proper subcellular distribution and stability of Pex5p, the PTS1 receptor: evidence that PTS1 protein import is mediated by a cycling receptor. J. Cell Biol. 135:1763-1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dodt, G., D. Warren, E. Becker, P. Rehling, and S. J. Gould. 2001. Domain mapping of human PEX5 reveals functional and structural similarities to Saccharomyces cerevisiae Pex18p and Pex21p. J. Biol. Chem. 276:41769-41781. [DOI] [PubMed] [Google Scholar]
- 19.Douangamath, A., F. V. Filipp, A. T. Klein, P. Barnett, P. Zou, T. Voorn-Brouwer, M. C. Vega, O. M. Mayans, M. Sattler, B. Distel, and M. Wilmanns. 2002. Topography for independent binding of alpha-helical and PPII-helical ligands to a peroxisomal SH3 domain. Mol. Cell 10:1007-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eckert, J. H., and R. Erdmann. 2003. Peroxisome biogenesis. Rev. Physiol. Biochem. Pharmacol. 147:75-121. [DOI] [PubMed] [Google Scholar]
- 21.Einwächter, H., S. Sowinski, W. H. Kunau, and W. Schliebs. 2001. Yarrowia lipolytica Pex20p, Saccharomyces cerevisiae Pex18p/Pex21p and mammalian Pex5pL fulfil a common function in the early steps of the peroxisomal PTS2 import pathway. EMBO Rep. 2:1035-1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elgersma, Y., M. Elgersma-Hooisma, T. Wenzel, J. M. McCaffery, M. G. Farquhar, and S. Subramani. 1998. A mobile PTS2 receptor for peroxisomal protein import in Pichia pastoris. J. Cell Biol. 140:807-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elgersma, Y., L. Kwast, A. Klein, T. Voorn-Brouwer, M. van den Berg, B. Metzig, T. America, H. F. Tabak, and B. Distel. 1996. The SH3 domain of the Saccharomyces cerevisiae peroxisomal membrane protein Pex13p functions as a docking site for Pex5p, a mobile receptor for the import of PTS1 containing proteins. J. Cell Biol. 135:97-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Erdmann, R., and G. Blobel. 1996. Identification of Pex13p, a peroxisomal membrane receptor for the PTS1 recognition factor. J. Cell Biol. 135:111-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Erdmann, R., and W.-H. Kunau. 1994. Purification and immunolocalization of the peroxisomal 3-oxoacyl-CoA thiolase from Saccharomyces cerevisiae. Yeast 10:1173-1182. [DOI] [PubMed] [Google Scholar]
- 26.Erdmann, R., M. Veenhuis, D. Mertens, and W.-H. Kunau. 1989. Isolation of peroxisome-deficient mutants of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 86:2432-2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fransen, M., C. Brees, K. Ghys, L. Amery, G. P. Mannaerts, D. Ladant, and P. P. Van Veldhoven. 2002. Analysis of mammalian peroxin interactions using a non-transcription-based bacterial two-hybrid assay. Mol. Cell. Proteomics 1:243-252. [DOI] [PubMed] [Google Scholar]
- 28.Fransen, M., S. R. Terlecky, and S. Subramani. 1998. Identification of a human PTS1 receptor docking protein directly required for peroxisomal protein import. Proc. Natl. Acad. Sci. USA 95:8087-8092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gatto, G. J., B. V. Geisbrecht, S. J. Gould, and J. M. Berg. 2000. Peroxisomal targeting signal-1 recognition by the TPR domains of human PEX5. Nat. Struct. Biol. 7:1091-1095. [DOI] [PubMed] [Google Scholar]
- 30.Gould, S. J., J. E. Kalish, J. C. Morrell, J. Bjorkman, A. J. Urquhart, and D. I. Crane. 1996. Pex13p is an SH3 protein of the peroxisome membrane and a docking factor for the predominantly cytoplasmic PTS1 receptor. J. Cell Biol. 135:85-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gould, S. J., G. A. Keller, N. Hosken, J. Wilkinson, and S. Subramani. 1989. A conserved tripeptide sorts proteins to peroxisomes. J. Cell Biol. 108:1657-1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hazra, P. P., I. Suriapranata, W. B. Snyder, and S. Subramani. 2002. Peroxisome remnants in pex3delta cells and the requirement of Pex3p for interactions between the peroxisomal docking and translocation subcomplexes. Traffic 3:560-574. [DOI] [PubMed] [Google Scholar]
- 33.Hettema, E. H., W. Girzalsky, M. van Den Berg, R. Erdmann, and B. Distel. 2000. Saccharomyces cerevisiae Pex3p and Pex19p are required for proper localization and stability of peroxisomal membrane proteins. EMBO J. 19:223-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Higuchi, R., B. Krummel, and R. K. Saiki. 1988. A general method of in vitro preparation and specific mutagenesis of DNA fragments: study of protein and DNA interactions. Nucleic Acids Res. 16:7351-7367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hille, A., J. Klumperman, H. J. Geuze, C. Peters, F. M. Brodsky, and K. von Figura. 1992. Lysosomal acid phosphatase is internalized via clathrin-coated pits. Eur. J. Cell Biol. 59:106-115. [PubMed] [Google Scholar]
- 36.Hiltunen, J. K., A. M. Mursula, H. Rottensteiner, R. K. Wierenga, A. J. Kastaniotis, and A. Gurvitz. 2003. The biochemistry of peroxisomal beta-oxidation in the yeast Saccharomyces cerevisiae. FEMS Microbiol Rev. 27:35-64. [DOI] [PubMed] [Google Scholar]
- 37.Jardim, A., W. Liu, E. Zheleznova, and B. Ullman. 2000. Peroxisomal targeting signal-1 receptor protein PEX5 from Leishmania donovani. Molecular, biochemical, and immunocytochemical characterization. J. Biol. Chem. 275:13637-13644. [DOI] [PubMed] [Google Scholar]
- 38.Klein, A. T., M. van Den Berg, G. Bottger, H. F. Tabak, and B. Distel. 2002. Saccharomyces cerevisiae acyl-CoA oxidase follows a novel, non-PTS1, import pathway into peroxisomes that is dependent on Pex5p. J. Biol. Chem. 277:25011-25019. [DOI] [PubMed] [Google Scholar]
- 39.Knop, M., K. Siegers, G. Pereira, W. Zachariae, B. Winsor, K. Nasmyth, and E. Schiebel. 1999. Epitope tagging of yeast genes using a PCR-based strategy: more tags and improved practical routines. Yeast 15:963-972. [DOI] [PubMed] [Google Scholar]
- 40.Kragler, F., G. Lametschwandtner, J. Christmann, A. Hartig, and J. J. Harada. 1998. Identification and analysis of the plant peroxisomal targeting signal 1 receptor NtPEX5. Proc. Natl. Acad. Sci. USA 95:13336-13341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lazarow, P. B. 2003. Peroxisome biogenesis: advances and conundrums. Curr. Opin. Cell Biol. 15:489-497. [DOI] [PubMed] [Google Scholar]
- 42.Marzioch, M., R. Erdmann, M. Veenhuis, and W.-H. Kunau. 1994. PAS7 encodes a novel yeast member of the WD-40 protein family essential for import of 3-oxoacyl-CoA thiolase, a PTS2-containing protein, into peroxisomes. EMBO J. 13:4908-4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matsumura, T., H. Otera, and Y. Fujiki. 2000. Disruption of the interaction of the longer isoform of Pex5p, Pex5pL, with Pex7p abolishes peroxisome targeting signal type 2 protein import in mammals. Study with a novel Pex5-impaired Chinese hamster ovary cell mutant. J. Biol. Chem. 275:21715-21721. [DOI] [PubMed] [Google Scholar]
- 44.McCollum, D., E. Monosov, and S. Subramani. 1993. The pas8 mutant of Pichia pastoris exhibits the peroxisomal protein import deficiencies of Zellweger syndrome cells—the Pas8 protein binds to the COOH-terminal tripeptide peroxisomal targeting signal, and is a member of the TPR protein family. J. Cell Biol. 121:761-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McNew, J. A., and J. M. Goodman. 1994. An oligomeric protein is imported into peroxisomes in vivo. J. Cell Biol. 127:1245-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Middleton, B. 1975. 3-Ketoacyl-CoA thiolases of mammalian tissues. Methods Enzymol. 35:128-136. [DOI] [PubMed] [Google Scholar]
- 47.Neuberger, G., S. Maurer-Stroh, B. Eisenhaber, A. Hartig, and F. Eisenhaber. 2003. Motif refinement of the peroxisomal targeting signal 1 and evaluation of taxon-specific differences. J. Mol. Biol. 328:567-579. [DOI] [PubMed] [Google Scholar]
- 48.Otera, H., T. Harano, M. Honsho, K. Ghaedi, S. Mukai, A. Tanaka, A. Kawai, N. Shimizu, and Y. Fujiki. 2000. The mammalian peroxin Pex5pL, the longer isoform of the mobile peroxisome targeting signal (PTS) type 1 transporter, translocates the Pex7p-PTS2 protein complex into peroxisomes via its initial docking site, Pex14p. J. Biol. Chem. 275:21703-21714. [DOI] [PubMed] [Google Scholar]
- 49.Otera, H., K. Setoguchi, M. Hamasaki, T. Kumashiro, N. Shimizu, and Y. Fujiki. 2002. Peroxisomal targeting signal receptor Pex5p interacts with cargoes and import machinery components in a spatiotemporally differentiated manner: conserved Pex5p WXXXF/Y motifs are critical for matrix protein import. Mol. Cell. Biol. 22:1639-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Purdue, P. E., and P. B. Lazarow. 1994. Peroxisome biogenesis: multiple pathways of protein import. J. Biol. Chem. 269:30065-30068. [PubMed] [Google Scholar]
- 51.Purdue, P. E., and P. B. Lazarow. 2001. Pex18p is constitutively degraded during peroxisome biogenesis. J. Biol. Chem. 276:47684-47689. [DOI] [PubMed] [Google Scholar]
- 52.Purdue, P. E., X. Yang, and P. B. Lazarow. 1998. Pex18p and Pex21p, a novel pair of related peroxins essential for peroxisomal targeting by the PTS2 pathway. J. Cell Biol. 143:1859-1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rehling, P., M. Marzioch, F. Niesen, E. Wittke, M. Veenhuis, and W.-H. Kunau. 1996. The import receptor for the peroxisomal targeting signal 2 (PTS2) in Saccharomyces cerevisiae is encoded by the PAS7 gene. EMBO J. 15:2901-2913. [PMC free article] [PubMed] [Google Scholar]
- 54.Rehling, P., A. Skaletz-Rorowski, W. Girzalsky, T. Voorn-Brouwer, M. M. Franse, B. Distel, M. Veenhuis, W. H. Kunau, and R. Erdmann. 2000. Pex8p, an intraperoxisomal peroxin of Saccharomyces cerevisiae required for protein transport into peroxisomes binds the PTS1 receptor Pex5p. J. Biol. Chem. 275:3593-3602. [DOI] [PubMed] [Google Scholar]
- 55.Rose, M. D., F. Winston, and P. Hieter. 1990. Laboratory course manual for methods in yeast genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 56.Saidowsky, J., G. Dodt, K. Kirchberg, A. Wegner, W. Nastainczyk, W. H. Kunau, and W. Schliebs. 2001. The di-aromatic pentapeptide repeats of the human peroxisome import receptor PEX5 are separate high affinity binding sites for the peroxisomal membrane protein PEX14. J. Biol. Chem. 276:34524-34529. [DOI] [PubMed] [Google Scholar]
- 57.Schiestl, R. H., and R. D. Gietz. 1989. High efficiency transformation of intact yeast cells using single stranded nucleic acids as a carrier. Curr. Genet. 16:339-346. [DOI] [PubMed] [Google Scholar]
- 58.Schliebs, W., J. Saidowsky, B. Agianian, G. Dodt, F. W. Herberg, and W. H. Kunau. 1999. Recombinant human peroxisomal targeting signal receptor PEX5. Structural basis for interaction of pex5 with pex14. J. Biol. Chem. 274:5666-5673. [DOI] [PubMed] [Google Scholar]
- 59.Schnell, D. J., and D. N. Hebert. 2003. Protein translocons: multifunctional mediators of protein translocation across membranes. Cell 112:491-505. [DOI] [PubMed] [Google Scholar]
- 60.Shimizu, N., R. Itoh, Y. Hirono, H. Otera, K. Ghaedi, K. Tateishi, S. Tamura, K. Okumoto, T. Harano, S. Mukai, and Y. Fujiki. 1999. The peroxin Pex14p. cDNA cloning by functional complementation on a Chinese hamster ovary cell mutant, characterization, and functional analysis. J. Biol. Chem. 274:12593-12604. [DOI] [PubMed] [Google Scholar]
- 61.Sichting, M., A. Schell-Steven, H. Prokisch, R. Erdmann, and H. Rottensteiner. 2003. Pex7p and Pex20p of Neurospora crassa function together in PTS2-dependent protein import into peroxisomes. Mol. Biol. Cell 14:810-821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smith, J. J., and R. A. Rachubinski. 2001. A role for the peroxin Pex8p in Pex20p-dependent thiolase import into peroxisomes of the yeast Yarrowia lipolytica. J. Cell Biol. 276:1618-1625. [DOI] [PubMed] [Google Scholar]
- 63.Sparkes, I. A., and A. Baker. 2002. Peroxisome biogenesis and protein import in plants, animals and yeasts: enigma and variations? Mol. Membr. Biol. 19:171-185. [DOI] [PubMed] [Google Scholar]
- 64.Stein, K., A. Schell-Steven, R. Erdmann, and H. Rottensteiner. 2002. Interactions of Pex7p and Pex18p/Pex21p with the peroxisomal docking machinery: implications for the first steps in PTS2 protein import. Mol. Cell. Biol. 22:6056-6069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Swinkels, B. W., S. J. Gould, A. G. Bodnar, R. A. Rachubinski, and S. Subramani. 1991. A novel, cleavable peroxisomal targeting signal at the amino-terminus of the rat 3-ketoacyl-CoA thiolase. EMBO J. 10:3255-3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Szilard, R. K., and R. A. Rachubinski. 2000. Tetratricopeptide repeat domain of Yarrowia lipolytica Pex5p is essential for recognition of the type 1 peroxisomal targeting signal but does not confer full biological activity on Pex5p. Biochem. J. 346:177-184. [PMC free article] [PubMed] [Google Scholar]
- 67.Terlecky, S. R., W. M. Nuttley, D. McCollum, E. Sock, and S. Subramani. 1995. The Pichia pastoris peroxisomal protein PAS8p is the receptor for the C-terminal tripeptide peroxisomal targeting signal. EMBO J. 14:3627-3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Titorenko, V. I., J. J. Smith, R. K. Szilard, and R. A. Rachubinski. 1998. Pex20p of the yeast Yarrowia lipolytica is required for the oligomerization of thiolase in the cytosol and for its targeting to the peroxisome. J. Cell Biol. 142:403-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tolbert, N. E. 1974. Isolation of subcellular organelles of metabolism on isopycnic sucrose gradients. Methods Enzymol. 31:734-746. [DOI] [PubMed] [Google Scholar]
- 70.Urquhart, A. J., D. Kennedy, S. J. Gould, and D. I. Crane. 2000. Interaction of Pex5p, the type 1 peroxisome targeting signal receptor, with the peroxisomal membrane proteins Pex14p and Pex13p. J. Biol. Chem. 275:4127-4136. [DOI] [PubMed] [Google Scholar]
- 71.van der Klei, I., and M. Veenhuis. 2002. Peroxisomes: flexible and dynamic organelles. Curr. Opin. Cell Biol. 14:500-505. [DOI] [PubMed] [Google Scholar]
- 72.van der Klei, I. J., R. E. Hilbrands, G. J. Swaving, H. R. Waterham, E. G. Vrieling, V. I. Titorenko, J. M. Cregg, W. Harder, and M. Veenhuis. 1995. The Hansenula polymorpha PER3 gene is essential for the import of PTS1 proteins into the peroxisomal matrix. J. Biol. Chem. 270:17229-17236. [DOI] [PubMed] [Google Scholar]
- 73.Van der Leij, I., M. M. Franse, Y. Elgersma, B. Distel, and H. F. Tabak. 1993. PAS10 is a tetratricopeptide-repeat protein that is essential for the import of most matrix proteins into peroxisomes of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 90:11782-11786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.van der Leij, I., M. van den Berg, R. Boot, M. M. Franse, B. Distel, and H. F. Tabak. 1992. Isolation of peroxisome assembly mutants from Saccharomyces cerevisiae with different morphologies using a novel positive selection procedure. J. Cell Biol. 119:153-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang, D., N. V. Visser, M. Veenhuis, and I. J. van der Klei. 2003. Physical interactions of the peroxisomal targeting signal 1 receptor Pex5p, studied by fluorescence correlation spectroscopy. J. Biol. Chem. 278:43340-43345. [DOI] [PubMed] [Google Scholar]
- 76.Waterham, H. R., V. I. Titorenko, P. Haima, J. M. Cregg, W. Harder, and M. Veenhuis. 1994. The Hansenula polymorpha PER1 gene is essential for peroxisome biogenesis and encodes a peroxisomal matrix protein with both carboxy- and amino-terminal targeting signals. J. Cell Biol. 127:737-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Westermann, B., and W. Neupert. 2000. Mitochondria-targeted green fluorescent proteins: convenient tools for the study of organelle biogenesis in Saccharomyces cerevisiae. Yeast 16:1421-1427. [DOI] [PubMed] [Google Scholar]
- 78.Wiemer, E. A. C., W. M. Nuttley, B. L. Bertolaet, Li, X., U. Francke, M. J. Wheelock, J. Anné, K. R., and S. Subramani. 1995. Human peroxisomal targeting signal-1 receptor restores peroxisomal protein import in cells from patients with fatal peroxisomal disorders. J. Cell Biol. 130:51-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wimmer, C., M. Schmid, M. Veenhuis, and C. Gietl. 1998. The plant PTS1 receptor: similarities and differences to its human and yeast counterparts. Plant J. 16:453-464. [DOI] [PubMed] [Google Scholar]
- 80.Yang, X., P. E. Purdue, and P. B. Lazarow. 2001. Eci1p uses a PTS1 to enter peroxisomes: either its own or that of a partner, Dci1p. Eur. J. Cell Biol. 80:126-138. [DOI] [PubMed] [Google Scholar]
- 81.Zhang, J. W., and P. B. Lazarow. 1996. PEB1 (PAS7) is an intraperoxisomal receptor for the NH2-terminal, type 2, peroxisomal targeting sequence of thiolase: Peb1p itself is targeted to peroxisomes by an NH2-terminal peptide. J. Cell Biol. 132:325-334. [DOI] [PMC free article] [PubMed] [Google Scholar]