Abstract
In terms of functional anatomy, where does learning occur when, for a basic visual discrimination task, performance improves with practice (perceptual learning)? We report remarkable long-term learning in a simple texture discrimination task where learning is specific for retinal input. This learning is (i) local (in a retinotopic sense), (ii) orientation specific but asymmetric (it is specific for background but not for target-element orientation), and (iii) strongly monocular (there is little interocular transfer of learning). Our results suggest that learning involves experience-dependent changes at a level of the visual system where monocularity and the retinotopic organization of the visual input are still retained and where different orientations are processed separately. These results can be interpreted in terms of local plasticity induced by retinal input in early visual processing in human adults, presumably at the level of orientation-gradient sensitive cells in primary visual cortex.
Full text
PDF
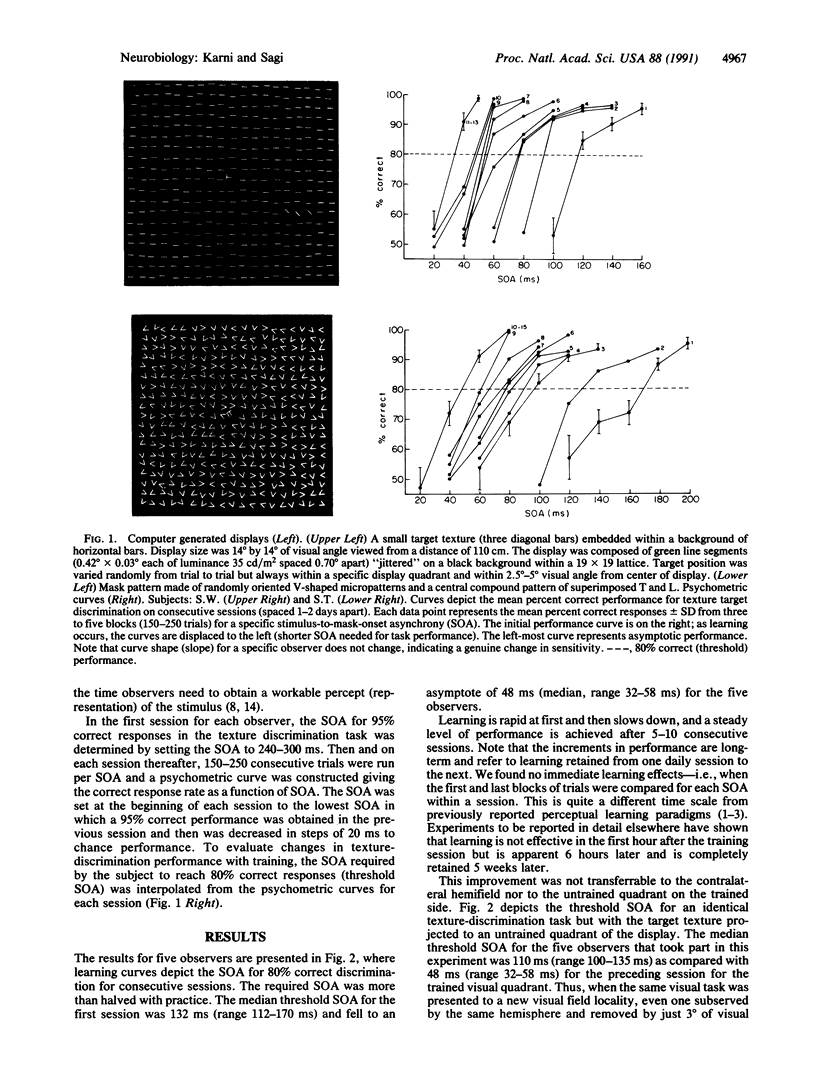
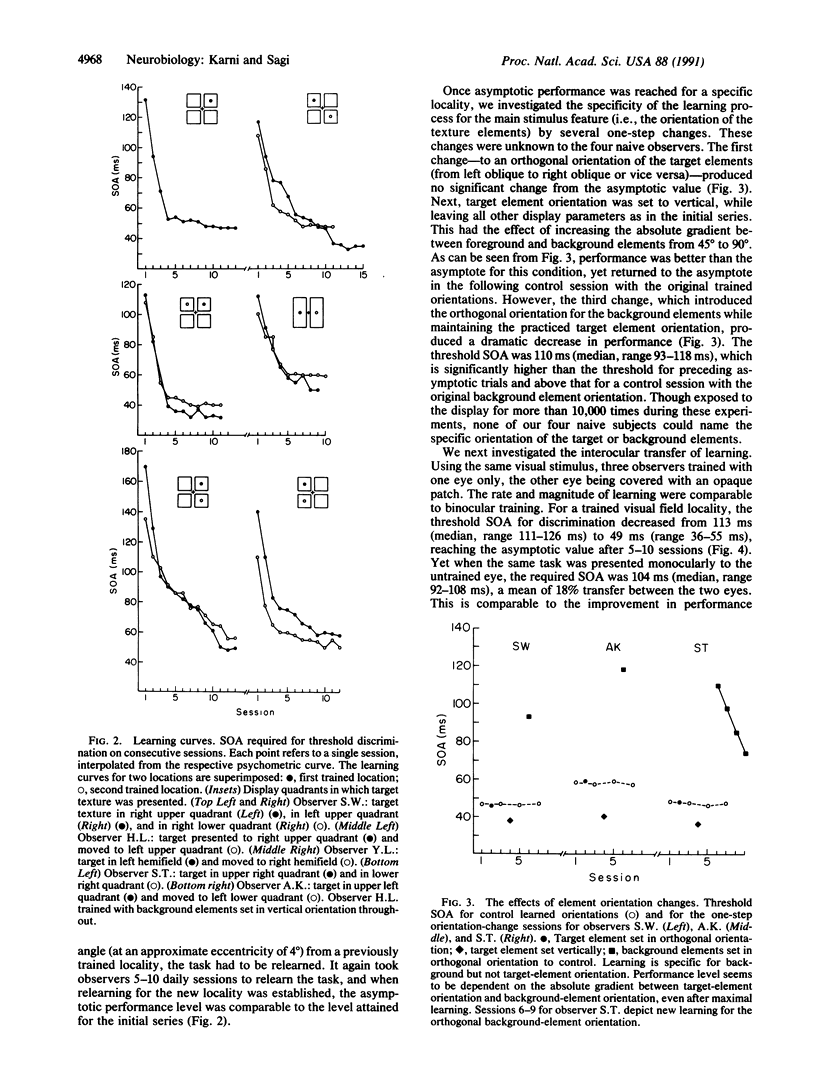
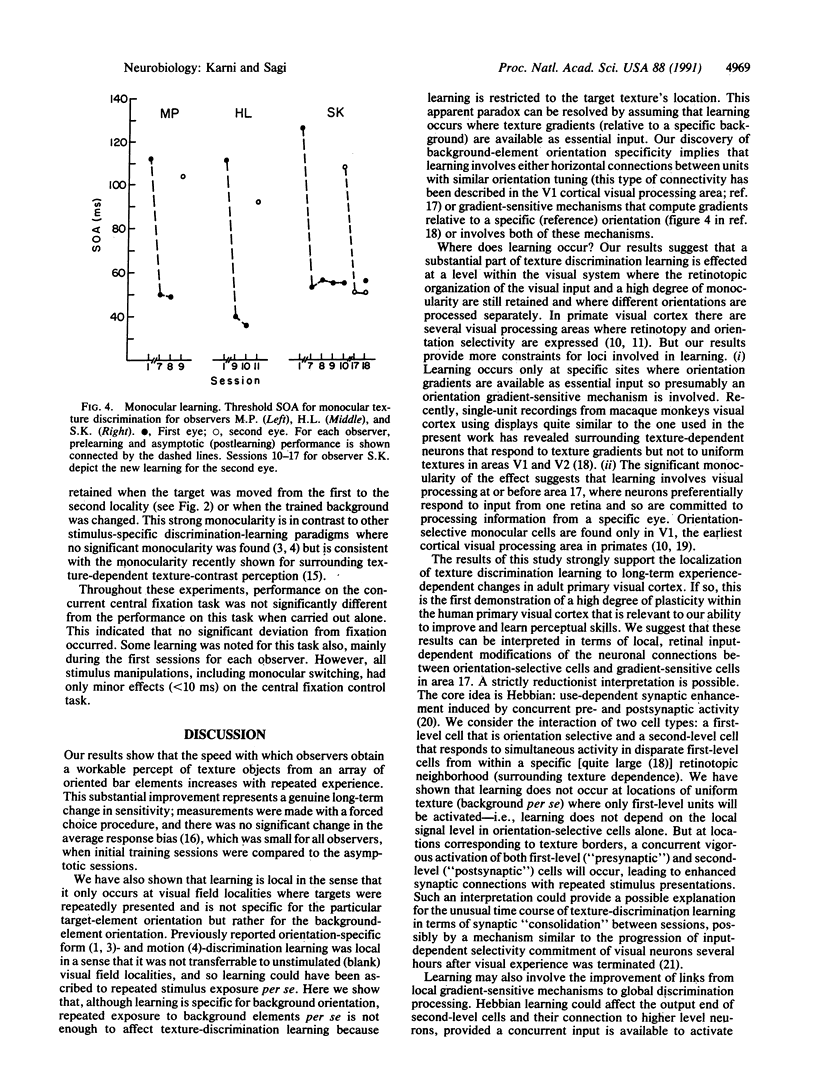

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ball K., Sekuler R. Direction-specific improvement in motion discrimination. Vision Res. 1987;27(6):953–965. doi: 10.1016/0042-6989(87)90011-3. [DOI] [PubMed] [Google Scholar]
- Bergen J. R., Julesz B. Parallel versus serial processing in rapid pattern discrimination. Nature. 1983 Jun 23;303(5919):696–698. doi: 10.1038/303696a0. [DOI] [PubMed] [Google Scholar]
- Brown T. H., Chapman P. F., Kairiss E. W., Keenan C. L. Long-term synaptic potentiation. Science. 1988 Nov 4;242(4879):724–728. doi: 10.1126/science.2903551. [DOI] [PubMed] [Google Scholar]
- Buisseret P., Gary-Bobo E., Imbert M. Ocular motility and recovery of orientational properties of visual cortical neurones in dark-reared kittens. Nature. 1978 Apr 27;272(5656):816–817. doi: 10.1038/272816a0. [DOI] [PubMed] [Google Scholar]
- Chubb C., Sperling G., Solomon J. A. Texture interactions determine perceived contrast. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9631–9635. doi: 10.1073/pnas.86.23.9631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentini A., Berardi N. Learning in grating waveform discrimination: specificity for orientation and spatial frequency. Vision Res. 1981;21(7):1149–1158. doi: 10.1016/0042-6989(81)90017-1. [DOI] [PubMed] [Google Scholar]
- Frégnac Y., Shulz D., Thorpe S., Bienenstock E. A cellular analogue of visual cortical plasticity. Nature. 1988 May 26;333(6171):367–370. doi: 10.1038/333367a0. [DOI] [PubMed] [Google Scholar]
- Gilbert C. D., Wiesel T. N. Columnar specificity of intrinsic horizontal and corticocortical connections in cat visual cortex. J Neurosci. 1989 Jul;9(7):2432–2442. doi: 10.1523/JNEUROSCI.09-07-02432.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurnsey R., Browse R. A. Micropattern properties and presentation conditions influencing visual texture discrimination. Percept Psychophys. 1987 Mar;41(3):239–252. doi: 10.3758/bf03208222. [DOI] [PubMed] [Google Scholar]
- Heggelund P., Imamura K., Kasamatsu T. Reduced binocularity in the noradrenaline-infused striate cortex of acutely anesthetized and paralyzed, otherwise normal cats. Exp Brain Res. 1987;68(3):593–605. doi: 10.1007/BF00249802. [DOI] [PubMed] [Google Scholar]
- Hubel D. H. Exploration of the primary visual cortex, 1955-78. Nature. 1982 Oct 7;299(5883):515–524. doi: 10.1038/299515a0. [DOI] [PubMed] [Google Scholar]
- Livingstone M. S., Hubel D. H. Anatomy and physiology of a color system in the primate visual cortex. J Neurosci. 1984 Jan;4(1):309–356. doi: 10.1523/JNEUROSCI.04-01-00309.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee S. P., Westheimer G. Improvement in vernier acuity with practice. Percept Psychophys. 1978 Sep;24(3):258–262. doi: 10.3758/bf03206097. [DOI] [PubMed] [Google Scholar]
- Nothdurft H. C. Orientation sensitivity and texture segmentation in patterns with different line orientation. Vision Res. 1985;25(4):551–560. doi: 10.1016/0042-6989(85)90159-2. [DOI] [PubMed] [Google Scholar]
- Ramachandran V. S., Braddick O. Orientation-specific learning in stereopsis. Perception. 1973;2(3):371–376. doi: 10.1068/p020371. [DOI] [PubMed] [Google Scholar]
- Sagi D. Detection of an orientation singularity in Gabor textures: effect of signal density and spatial-frequency. Vision Res. 1990;30(9):1377–1388. doi: 10.1016/0042-6989(90)90011-9. [DOI] [PubMed] [Google Scholar]
- Sagi D., Julesz B. "Where" and "what" in vision. Science. 1985 Jun 7;228(4704):1217–1219. doi: 10.1126/science.4001937. [DOI] [PubMed] [Google Scholar]
- Sagi D., Julesz B. Fast noninertial shifts of attention. Spat Vis. 1985;1(2):141–149. doi: 10.1163/156856885x00152. [DOI] [PubMed] [Google Scholar]
- Singer W., Tretter F., Yinon U. Evidence for long-term functional plasticity in the visual cortex of adult cats. J Physiol. 1982 Mar;324:239–248. doi: 10.1113/jphysiol.1982.sp014109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeki S. M. Functional specialisation in the visual cortex of the rhesus monkey. Nature. 1978 Aug 3;274(5670):423–428. doi: 10.1038/274423a0. [DOI] [PubMed] [Google Scholar]




