Abstract
Selective serotonin reuptake inhibitor (SSRI) exposure during pregnancy can result in symptoms of serotonin syndrome or serotonin withdrawal. In contrast to other SSRIs, reports of serotonin behavioral syndrome following in utero exposure to escitalopram and citalopram are limited. We describe a case of suspected toxicity following in utero exposure to 20 mg escitalopram throughout pregnancy. The infant was transferred to our neonatal intensive unit at 9 hours of life for further evaluation of lethargy, weak cry, bradycardia, and non-reactive pupils. Hypoxic ischemic encephalopathy was suspected upon presentation, despite APGAR scores of 8 and 9. Upon admission, symptoms progressed to signs of hypertonia, irritability, high-pitched cry, and posturing. The patient was loaded with phenobarbital for empiric management of suspected seizures versus drug withdrawal. Both electroencephalogram and computed tomography scan results were normal; however, an electrocardiogram revealed a prolonged QTc interval of 531 milliseconds. Signs of irritability and QTc prolongation continued through day of life (DOL) 5. The infant was discharged on DOL 10 with no further symptoms. We hypothesize that this represented a case of serotonin toxicity due to in utero exposure to escitalopram and recommend close monitoring for neonatal behavioral syndrome symptoms and QTc prolongation in infants exposed to escitalopram during pregnancy.
INDEX TERMS: adverse drug effect, behavioral syndrome, citalopram, escitalopram, in utero, neonatal behavioral syndrome, pregnancy, selective serotonin reuptake inhibitor
INTRODUCTION
Depression during pregnancy is common, resulting in use of antidepressant medications throughout pregnancy. Despite the negative consequences with use of antidepressants, the risks of untreated depressive symptoms pose an additional challenge, resulting in approximately 13% of pregnant women taking an antidepressant throughout pregnancy in 2003 per the American College of Obstetricians and Gynecologists.1 Selective serotonin reuptake inhibitor (SSRI) exposure during pregnancy can result in symptoms of serotonin syndrome or serotonin withdrawal. The symptoms of each often overlap, making it difficult to distinguish between the two, thus these symptoms are often referred to as neonatal behavioral syndrome (NBS).2 Neonates with NBS primarily display central nervous system (CNS) (particularly irritability), motor (significant for agitation, increased/decreased tone, tremors/jitteriness/shivering, and hyperreflexia), respiratory (including increased respiratory rate and respiratory distress), and gastrointestinal symptoms (prominent for emesis, diarrhea, and poor suck).2 In contrast to other SSRIs, such as paroxetine and fluoxetine,2 reports of NBS following in utero exposure to escitalopram and citalopram are limited. Citalopram has been associated with serious CNS and cardiac toxicities with older children and adults,3,4 while escitalopram has been reported to be a relatively safer agent. We describe a case of suspected escitalopram toxicity following in utero exposure in a term infant.
CASE
A 3030-g male infant was born via spontaneous vaginal delivery at 40 weeks' gestational age to a 22-year-old gravidity 1 and parity 1 (G1P1) Caucasian mother at an outlying hospital. The delivery was complicated by nuchal cord and significant meconium-stained amniotic fluid. APGAR scores were 8 and 9 at 1 and 5 minutes, respectively. Maternal screening was negative for chlamydia, syphilis, hepatitis B antigen, gonorrhea, human immunodeficiency virus, and group B streptococcus; non-immune for rubella; and unknown for herpes simplex virus. Mother's past medical history was significant for depressive disorder and she was treated with escitalopram 20 mg throughout pregnancy. No other medications were reported during pregnancy. Substance abuse, including nicotine, alcohol, and illicit drugs, was denied by the mother. Despite initial APGAR scores, the neonate exhibited a weak cry, non-reactive pupils, bradycardia, hypertonia, lethargy, and suspected seizures shortly after birth. After performing an initial septic workup evaluation, the infant was transferred to our institution for further evaluation and management.
The infant arrived at our institution at approximately 9 hours of age with signs and symptoms of hypertonia, irritability, high-pitched crying, and posturing. Due to shallow breathing and possible meconium aspiration, a chest radiologic study was performed demonstrating streaky perihilar densities consistent with retained lung fluid. Empiric antibiotics consisting of ampicillin and gentamicin were continued for sepsis/meningitis coverage. Evaluations to rule out an ischemic injury revealed an isolated elevation in creatine kinase (CK) at 735 international units/L. Other pertinent laboratory values were evaluated and determined within normal limits, which included serum creatinine 0.8 mg/dL, aspartate transaminase 77 units/L, alanine transaminase 20 units/L, and lactic acid 1.9 mmol/L. A head ultrasonography was performed to rule out any ischemia brain injury or brain edema. No evidence of germinal matrix bleed, hydrocephalus, or edema was observed.
At 20 hours of age, the infant developed a shrill, high-pitched cry with severe hypertonicity and was loaded with phenobarbital (PB) 20 mg/kg for suspected seizures. A head computed tomography performed at roughly 22 hours of life revealed a small extra-axial area of hyper-density in the left posterior fossa adjacent to the skull, probably representing a small subdural hematoma lying adjacent to the left transverse venous sinus. Electroencephalogram tracings were normal with no interictal abnormalities observed. On day of life (DOL) 3, a maintenance regimen of PB was initiated at 4 mg/kg/day. Later that evening, the patient exhibited recurrent seizures and the infant was loaded with levetiracetam (10 mg/kg) and a maintenance regimen (10 mg/kg/day) was started (Figure 1). Blood and cerebrospinal fluid cultures remained negative on DOL 3 and antibiotics were discontinued. The last day of clinical seizure activity was on DOL 4. At the time that escitalopram toxicity was suspected, approximately DOL 5, a blood sample was sent out to an external laboratory for parent drug citalopram and desmethylcitalopram testing.
Figure 1.
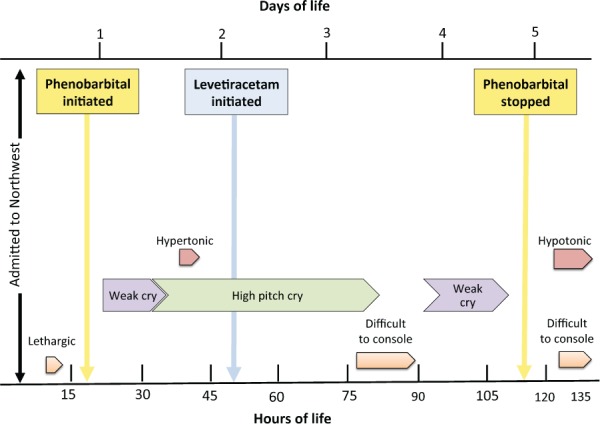
Neonatal behavioral syndrome symptoms: frequency of symptoms and drug administrations in relation to hours and days of life.
As reported from the outlying institution, the infant exhibited intermittent periods of bradycardia. Despite the patient's bradycardia, the episodes did not appear to correlate with the timing of suspected seizure activity. Additionally, the patient experienced perpetual tachycardia throughout the hospital stay (Figure 2). Patient was noted to have a murmur; echocardiogram revealed a normal finding with a patent foramen ovale with left to right shunt. An electrocardiogram (ECG) was performed at 40 hours of life, revealing a prolonged QTc interval of 531 milliseconds (normal range ≤ 450 milliseconds). Continuous ECG recordings exhibited a normal sinus rhythm with normal heart rate variability, with no evidence of atrioventricular block. Episodes of bradycardia continued to occur intermittently and a QTc prolongation was observed through DOL 7 (Figure 2). An ECG on DOL 11 was normal and no further episodes of bradycardia were observed.
Figure 2.
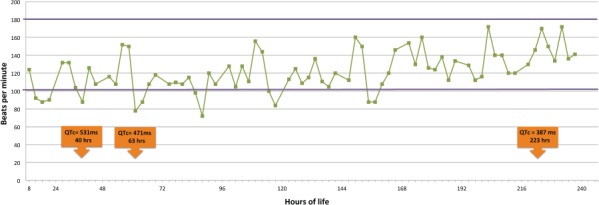
Neonatal heart rate and QTc intervals. Heart rate patterns and QTc prolongation episodes as a function of time.
 neonatal heart rate normal range
neonatal heart rate normal range
In addition to the observed intermittent seizure activity/hypertonia and bradycardia, the patient presented with lethargy and a weak cry. This progressed to a high-pitch cry and inconsolability. Signs of irritability continued through DOL 7 at which time PB was discontinued. On DOL 12, the infant was discharged home with levetiracetam (10 mg/kg/day) with referral to a pediatric neurologist. Post discharge, concentrations for citalopram and desmethylcitalopram were both less than 10 ng/mL (normal range, 30–200 ng/mL).
DISCUSSION
Although there are no prior reports of escitalopram exposure and toxicity in the literature, such toxicity has been reported with citalopram. Selective serotonin reuptake inhibitors and their metabolites cross the human placenta and can be detected in fetal serum and cord concentrations. Neonatal umbilical cord concentrations of citalopram as low as 11 ng/mL have been detected and may therefore increase the risk for serotonergic adverse effects in the neonate.5,6 Elefheriou and colleagues7 reported the first case of citalopram use during pregnancy causing neonatal toxicity, which presented with symptoms of lethargy, tachycardia, QTc prolongation, altered consciousness, hypertonia, and seizures. The infant was born at term and had been exposed to citalopram 20 mg/day from the 32th week of gestation until delivery. He was born via uneventful spontaneous vaginal delivery with APGAR score of 8, 8, and 10, at 1, 5, and 10 minutes, respectively. Similar to our patient, the infant developed severe muscle rigidity within 15 minutes of birth and jitteriness. He was also evaluated to rule out ischemia and CNS abnormalities with the only abnormality being an isolated elevation in creatinine kinase (1023 units/L). The patient required treatment with diazepam for 9 days for tremors and hypertonia and was hospitalized for 15 days for suspected citalopram toxicity followed by withdrawal. Drug concentrations for citalopram and desmethylcitalopram at 31 hours of age were 73 ng/mL and 26 ng/mL, respectively (normal range, 30–200 ng/mL). Levels dropped below therapeutic range but were detectable through DOL 9.
Adult overdoses of citalopram have been characterized as a triad of clinical symptoms: change in mental status, autonomic stimulation, and neuromuscular abnormalities.3,4 Manifestations typically occur within hours, with most reported cases in those patients using multiple serotonergic drugs. In pediatric overdoses, citalopram's CNS (causing single and multiple discrete seizures) and cardiac toxicity (resulting in conduction disturbances, dysrhythmia, and ECG changes) appear to be higher than that observed for all other SSRIs.3 Hayes and colleagues4 compared toxicity with citalopram and escitalopram in 795 acute overdoses reported to 6 poison control centers. Clinical effects frequently reported with both citalopram and escitalopram ingestions include tachycardia, drowsiness, hypertension, and vomiting. More serious toxicities including seizures, tremors, QTc prolongation, hyperthermia, hypotension, hyperreflexia, and myoclonus were more common with citalopram overdoses. Seizures and tremors were more prevalent with citalopram overdoses, while QTc prolongation occurred with both citalopram and escitalopram, although both occurred in a small number of patients.4 This report included 57 children younger than 6 years; however, no difference in toxicity rates between citalopram and escitalopram were detected. Laboratory drug concentrations are not routinely drawn, thus, conclusions cannot be drawn regarding serum concentrations and risk of toxicity, specifically QTc prolongation.
Citalopram is a racemic mixture containing the R-enantiomer as well as the therapeutically active S-enantiomer; escitalopram on the other hand, only contains the S-enantiomer. The S-enantiomer is 150 times more potent than that of the R-enantiomer. Although there is growing evidence in adults that the racemic mixture, citalopram, containing the R-enantiomer carries a greater risk of toxicity,4 this has yet to be observed in children.3 The effect on QT interval is theorized to be the result of citalopram and/or its metabolite didesmethylcitalopram on sodium and calcium channels.8,9 Citalopram-induced seizures are thought to be the result of either a sudden increase in brain serotonin concentrations or an increase in gamma-Aminobutyric acid transmission in the hippocampus to increase seizure threshold.10,11 In adults, QT prolongation has been observed at therapeutic doses, while CNS toxicity appears to be dose related.4 No identifiable dose threshold for serious toxicity has been observed in children.3 Serotonin syndrome symptoms or toxicity in adults does not appear to correlate with levels of SSRI serum concentrations, and no specific laboratory findings have been identified for diagnosis; however, an isolated elevation in CK has been observed.12 When obtaining serum concentrations, it is critical to acknowledge that the test interpretation requires knowledge of which enantiomer (R or S) is prescribed, as the assay does not distinguish the enantiomers.
Differentiation of serotonin withdrawal and serotonin toxicity in a newborn may be difficult to distinguish, since CNS symptoms frequently overlap. The onset of symptoms is often the best indicator of the likelihood of the cause, with toxicity presenting more abruptly in contrast to withdrawal symptoms, which have a delayed onset. The presence of QTc prolongation in our patient, in addition to the CNS symptoms, makes toxicity the more probable cause, since QTc prolongation has not been reported with withdrawal as observed in a dose-dependent response with citalopram use in adults.13 The isolated CK elevation observed is also indicative of a serotonin syndrome or toxicity. Although escitalopram is thought to be a safer option than citalopram, escitalopram use during pregnancy is not without its own concerns.
CONCLUSION
This report describes a case of a term infant exposed to 20 mg escitalopram in utero. The patient presented with suspected toxicity exhibited by QTc prolongation, lethargy, tachycardia, hypertonicity, and subsequent hypotonicity. Close observation for symptoms of NBS and QTc prolongation is recommended for approximately 5 days following in utero exposure to citalopram or escitalopram. Monitoring parameters for infants exposed to escitalopram in utero should consist of cardiac (i.e., conduction disturbances, dysrhythmia, and ECG changes), autonomic (i.e., heart rate, blood pressure, and temperature), and neuromuscular findings (i.e., muscle tone, tremors), and mental status changes. Although escitalopram is thought to be a safer alternative to citalopram, the risk for CNS and cardiac toxicity in the newborn following in utero exposure should still be suspected.
Acknowledgments
Presented at the Pediatric Pharmacy Advocacy Group Conference, Minneapolis, Minnesota, May 2015.
Abbreviations
- CK
creatine kinase
- CNS
central nervous system
- CT
computed tomography
- DOL
day of life
- ECG
electrocardiogram
- NBS
neonatal behavioral syndrome
- PB
phenobarbital
- SSRI
selective serotonin reuptake inhibitor
Footnotes
Disclosure The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria.
REFERENCES
- 1. A joint report from APA and ACOG. Depression during pregnancy: treatment recommendations. The American Congress of Obstetricians and Gynecologists Web site. Washington, DC: August 21, 2009. http://www.acog.org. Accessed May 26, 2016. [Google Scholar]
- 2. Moses-Kolko EL, Bogen D, Perel J, . et al. Neonatal signs after late in utero exposure to serotonin reuptake inhibitors: literature review and implications for clinical applications. JAMA. 2005; 293( 19): 2372– 2383. [DOI] [PubMed] [Google Scholar]
- 3. Klein W, Benson BE, Lee SC, . et al. Comparison of citalopram and other selective serotonin reuptake inhibitor ingestions in children. Clin Toxicol. 2012; 50( 5): 418– 423. [DOI] [PubMed] [Google Scholar]
- 4. Hayes B, Klein W, Clark RF, . et al. Comparison of toxicity of acute overdoses with citalopram and escitalopram. J Emerg Med. 2010; 39( 1): 44– 48. [DOI] [PubMed] [Google Scholar]
- 5. Heikkinen T, Ekblad U, Laine K.. Transplacental transfer of citalopram, fluoxetine and their primary demethylated metabolites in isolated perfused human placenta. BJOG. 2002; 109( 9): 1003– 1008. [DOI] [PubMed] [Google Scholar]
- 6. Laine K, Heikkinen T, Ekblad U, . et al. Effects of exposure to selective serotonin reuptake inhibitors during pregnancy on serotonergic symptoms in newborns and cord blood monoamine and prolactin concentrations. Arch Gen Psychiatry. 2003; 60( 7): 720– 726. [DOI] [PubMed] [Google Scholar]
- 7. Eleftheriou G, Butera R, Cottini FC, . et al. Neonatal toxicity following maternal citalopram treatment. Fetal Pediatr Pathol. 2013; 32( 5): 362– 366. [DOI] [PubMed] [Google Scholar]
- 8. Catalano G, Catalano MC, Epstein MA, . et al. QTc interval prolongation associated with citalopram overdose: a case report and literature review. Clin Neuropharmacol. 2001; 24( 3): 158– 162. [DOI] [PubMed] [Google Scholar]
- 9. Engebretsen KM, Harris CR, Wood JE.. Cardiotoxicity and late onset seizures with citalopram overdose. J Emerg Med. 2003; 25( 2): 163– 166. [DOI] [PubMed] [Google Scholar]
- 10. Waring WS, Gray JA, Graham A.. Predictive factors for generalized seizures after deliberate citalopram overdose. Br J Clin Pharmacol. 2008; 66( 6): 861– 865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Choi HC, Kim YI, Song HK, . et al. Effects of selective serotonin reuptake inhibitors on GABAergic inhibition in the hippocampus of normal and pilocarpine induced epileptic rats. Brain Res. 2010; 1357: 131– 141. [DOI] [PubMed] [Google Scholar]
- 12. Mason PJ, Morris VA, Balcezak TJ.. Serotonin syndrome: presentation of 2 cases and review of the literature. Medicine (Baltimore). 2000; 79( 4): 201– 209. [DOI] [PubMed] [Google Scholar]
- 13. McClelland J, Mathys M.. Evaluation of QTc prolongation and dosage effect with citalopram. Ment Health Clin. 2016; 6( 4): 165– 170. [DOI] [PMC free article] [PubMed] [Google Scholar]


