Abstract
Background
Targeted therapies (TTs) have revolutionized metastatic renal cell carcinoma (mRCC) treatment in the past decade, largely replacing immunotherapy including high-dose interleukin-2 (HD IL-2) therapy. We evaluated trends in HD IL-2 use for mRCC in the TT era.
Methods
Our cohort comprised a weighted estimate of all patients undergoing HD IL-2 treatment for mRCC from 2004 to 2012 using the Premier Hospital Database. We assessed temporal trends in HD IL-2 use including patient, disease, and hospital characteristics stratified by era (pre-TT uptake: 2004–2006, uptake: 2007–2009, and post-TT uptake: 2010–2012) and fitted multivariable regression models to identify predictors of treatment toxicity and tolerability.
Results
An estimated 2,351 patients received HD IL-2 therapy for mRCC in the United States from 2004 to 2012. The use decreased from 2004 to 2008. HD IL-2 therapy became increasingly centralized in teaching hospitals (24% of treatments in 2004 and 89.5% in 2012). Most patients who received HD IL-2 therapy were men, white, younger than 60 years, had lung metastases, and were otherwise healthy. Vasopressors, intensive care unit admission, and hemodialysis were necessary in 53.4%, 33.0%, and 7.1%, respectively. Factors associated with toxicities in multivariable analyses included being unmarried, male sex, and multiple metastatic sites. African Americans and patients with single-site metastases were less likely to receive multiple treatment cycles.
Conclusions
HD IL-2 therapy is used infrequently for mRCC in the United States, and its application has diminished with the uptake of TT. Patients are being increasingly treated in teaching hospitals, suggesting a centralization of care and possible barriers to access. A recent slight increase in HD IL-2 therapy use likely reflects recognition of the inability of TT to effect a complete response.
Keywords: High-dose interleukin-2, Immunotherapy, Renal cell carcinoma, Kidney cancer, Therapy trends, Toxicity
1. Introduction
Renal cell carcinoma affects approximately 65,000 individuals annually in the United States, of whom 20% have synchronous metastases [1]. High-dose interleukin-2 (HD IL-2) was approved for the management of metastatic renal cell carcinoma (mRCC) by the Food and Drug Administration in 1992. It can elicit complete responses in 5% to 10% of patients [2–5]. However, its use is limited by the high rate of severe acute toxicities and the need for experienced staff to administer it [5–8].
Along with interferon alpha (IFN-α), HD IL-2 was the previous standard treatment for mRCC. However, novel targeted therapies (TTs) including vascular endothelial growth factor antagonists and mammalian target of rapa-mycin inhibitors have demonstrated superior response rates and are better tolerated among a broader population of patients [9–12]. Consequently, the past decade has seen a paradigm shift in the management of mRCC, as TTs have become the de facto standard of care.
The introduction of TT for mRCC has certainly affected contemporary use of HD IL-2. In this study, we assessed the treatment patterns of HD IL-2 use since the introduction of the TT. We used a population-based cohort, focusing on overall incidence of HD IL-2 use as well as temporal trends as a function of patient, tumor, and hospital characteristics.
2. Methods
2.1. Data source
Data were extracted from the Premier Hospital Database (Premier Inc., Charlotte, NC), an all-payer hospital database developed for quality and use benchmarking in the United States. The database includes more than 600 hospitals, translating to approximately 20% of annual discharges in the United States. The Premier database contains validated sampling weights, allowing estimation of nationally representative incidence estimates [13]. Each patient has a unique identifier, permitting longitudinal analyses. The study received institutional review board exemption given the deidentified nature of the data.
2.2. Study cohort
We used the International Classification of Disease Ninth Revision codes to capture admissions of patients with renal cell carcinoma (189.0), limiting inclusion to those patients receiving HD IL-2 therapy and excluding patients with melanoma (172.x), for which HD IL-2 therapy may also be used. We selected the study period of 2004 to 2012 to encompass the years immediately before and after the introduction of TT. We excluded patients who had previously received any HD IL-2 treatments before 2004 and captured all subsequent cycles for patients through 2013.
2.3. Outcomes
We described baseline characteristics of patients who underwent HD IL-2 treatments for mRCC in the United States during the study period as well as characteristics of treating hospitals. Baseline patient characteristics included age, sex, race, marital status, Charlson comorbidity index (CCI), insurance status (Medicare, Medicaid, private, other, or none), number of metastatic sites, and location(s) of metastases. Hospital characteristics included teaching status (teaching or nonteaching), number of beds, and geographic region (northeast, midwest, west, or south). Temporal trends in these characteristics were assessed, stratifying by periods defined relative to the approval and widespread uptake of TT in the United States: the preuptake (2004–2006), uptake (2007–2009), and postuptake eras (2010–2012).
We used failure to receive >1 cycle of HD IL-2 treatment as a surrogate for significant treatment toxicity or disease progression during treatment. One treatment cycle was defined as 2 hospital admissions for HD IL-2 within a 30-day period. The dosing schedule for HD IL-2 therapy was defined as intravenous infusion every 8 hours over 15 minutes on days 1 through 5 and days 15 through 19, with a maximum of 12 to 15 doses per admission and 2 admissions constituting a single treatment cycle [5,8]. Up to 3 treatment cycles, each separated by 6 to 8 weeks, are usually recommended for patients who demonstrate a response and tolerate treatment [14]. Although definitions of treatment cycle may vary across institutions, failure to receive more than 1 cycle as defined earlier suggests lack of response, progression, or toxicity. Additional measures of toxicity or tolerability occurring during admission for HD IL-2 treatment included intensive care unit (ICU) admission, hypotension requiring vasopressors, and initiation of hemo-dialysis. We explored associations between patient and treatment characteristics with toxicities/tolerability measures.
2.4. Statistical analyses
Descriptive statistics were performed for baseline patient and hospital characteristics. Categorical variables and continuous variables were compared using the chi-square and the Wilcoxon rank sum tests, respectively. Temporal trends in HD IL-2 treatments were explored by trend analysis. Logistic regression models explored associations between baseline patient and hospital characteristics and treatment toxicity or tolerability. Because of potentially similar practice patterns and outcomes within hospitals, all analyses used multilevel models accounted for hospital clustering [15]. We used 10-fold cross-validation to avoid overfitting. Statistical analyses were performed using Stata 13 (StataCorp LP, College Station, TX). All tests were 2 sided with P < 0.05 defining statistical significance.
3. Results
3.1. Study cohort
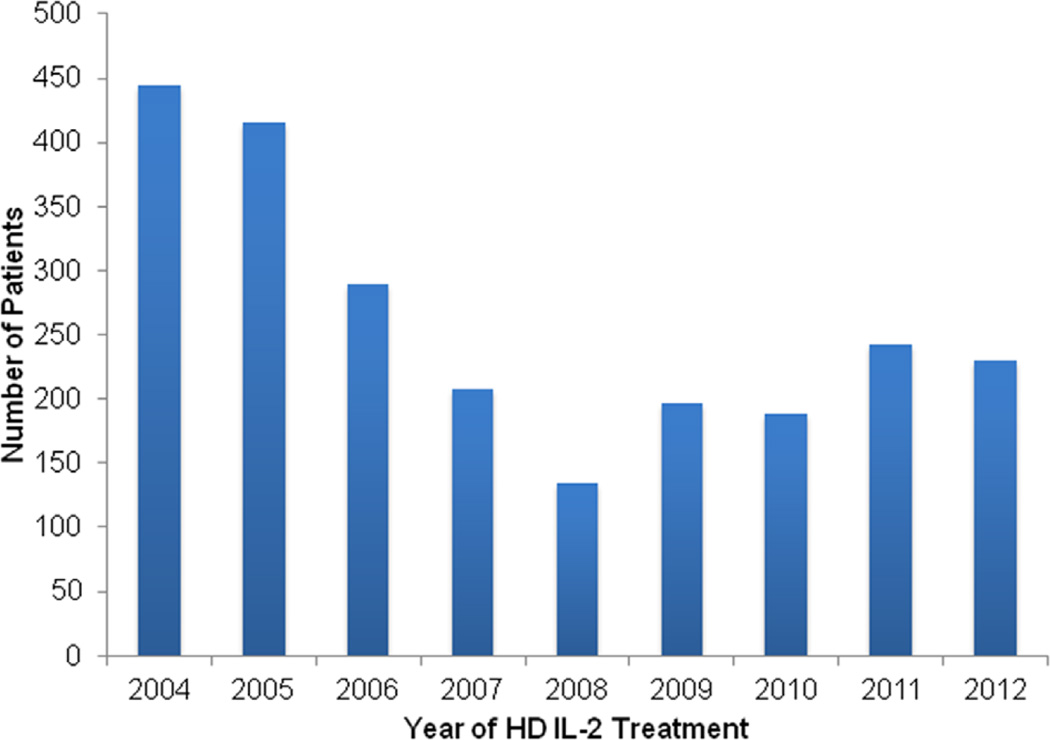
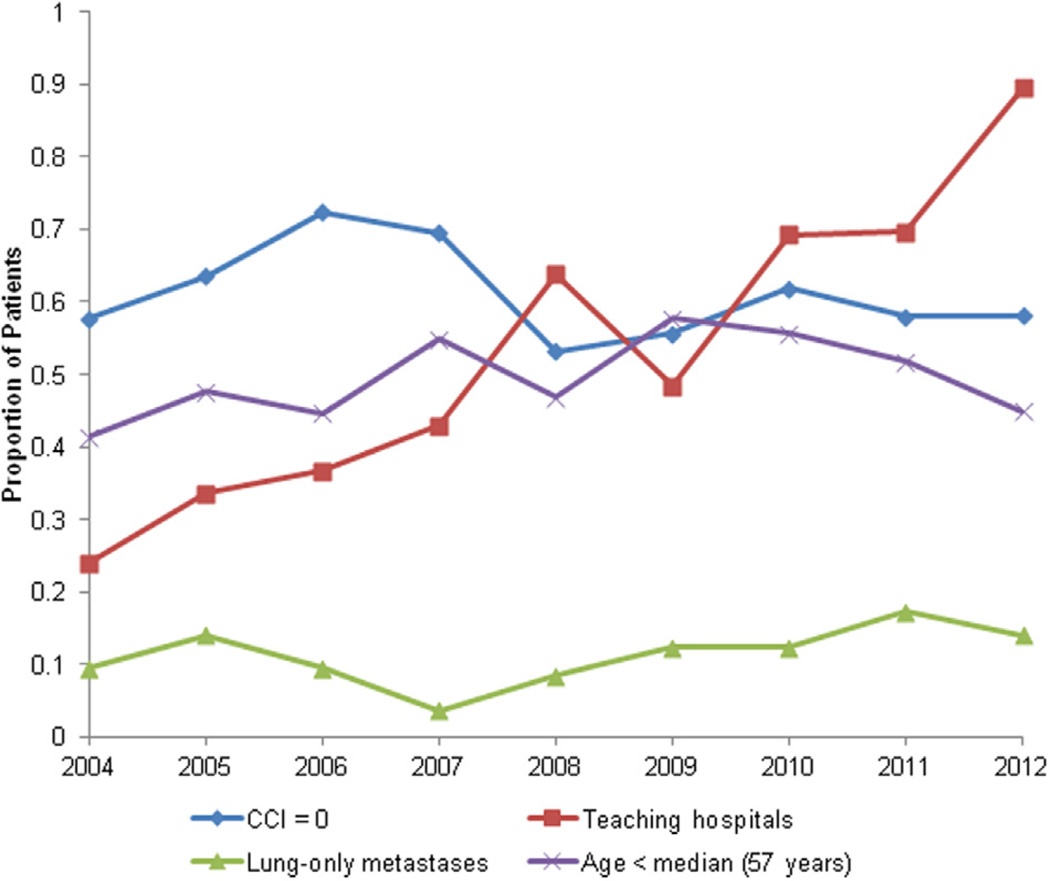
Table describes baseline patient and hospital characteristics stratified by era of TT adoption. A total weighted cohort of 2,351 patients with mRCC received HD IL-2 therapy in the United States from 2004 through 2012. The estimated number of patients treated annually with HD IL-2 in the United States is shown in Fig. 1. Use of HD IL-2 was highest in 2004 (n = 444) and lowest in 2008 (n = 135), with a subsequent increase in use from 2009 onwards (n = 230 in 2012). Most of the patients (75%) were men and the median age was 57 years. Most patients were white (70.7%) and had minimal comorbidities (64.72%, CCI = 0). Most of the patients (60.9%) had lung metastases, whereas a small proportion (11.7%) had lung-only metastases. We observed no significant temporal trends in patient characteristics over the study period (Fig. 2).
Table.
Characteristics of patients receiving and hospitals distributing high-dose interleukin-2 therapy for metastatic renal cell carcinoma in the United States
| Years of initial HD IL-2 treatment | P value | |||
|---|---|---|---|---|
| 2004–2006 | 2007–2009 | 2010–2012 | ||
| Patient characteristic no. (%) | ||||
| Total | 1,025 | 597 | 730 | |
| Age | ||||
| < 50 | 245 (24.0) | 151 (25.4) | 158 (21.6) | 0.99 |
| 50–59 | 341 (33.2) | 225 (37.8) | 280 (38.3) | |
| 60–69 | 359 (35.0) | 189 (31.6) | 247 (33.9) | |
| ≥ 70 | 80 (7.8) | 32 (5.3) | 45 (6.1) | |
| Sex | ||||
| Male | 736 (71.9) | 511 (85.6) | 524 (71.7) | 0.049 |
| Female | 288 (28.1) | 86 (14.3) | 206 (28.2) | |
| Race | ||||
| White | 797 (77.8) | 369 (61.8) | 496 (68.0) | 0.20 |
| Black | 27 (2.6) | 0 (0) | 19 (2.6) | |
| Hispanic | 79 (7.7) | 37 (6.1) | 0 (0) | |
| Other | 122 (11.9) | 191 (32.0) | 214 (29.3) | |
| Marital status | ||||
| Married | 746 (72.8) | 432 (72.4) | 418 (57.3) | 0.083 |
| Unmarried | 265 (25.8) | 124 (20.8) | 193 (26.5) | |
| Unknown | 14 (1.4) | 41 (6.8) | 118 (16.2) | |
| Health care payer | ||||
| Medicare | 185 (18.0) | 104 (17.4) | 149 (20.4) | 0.51 |
| Medicaid | 38 (3.7) | 26 (4.3) | 15 (2.0) | |
| Managed Care | 762 (74.4) | 413 (69.2) | 537 (73.6) | |
| Commercial | 40 (3.9) | 54 (9.1) | 29 (4.0) | |
| Charlson comorbidity index | ||||
| 0 | 696 (67.9) | 367 (61.5) | 458 (62.8) | 0.67 |
| 1 | 167 (16.3) | 77 (13.0) | 137 (18.8) | |
| 2 | 102 (9.9) | 96 (16.1) | 82 (11.2) | |
| ≥ 3 | 60 (5.9) | 56 (9.4) | 51 (7.1) | |
| Number of metastatic sites | ||||
| 1 | 566 (55.2) | 223 (37.4) | 345 (47.3) | 0.45 |
| 2 | 229 (22.4) | 155 (26.0) | 162 (22.3) | |
| 3 | 207 (20.2) | 97 (16.2) | 67 (9.2) | |
| ≥ 4 | 59 (5.7) | 18 (3.0) | 4 (0.6) | |
| Unknown | 90 (8.7) | 47 (7.9) | 73 (10.0) | |
| Location of metastases | ||||
| Lymph nodes | 238 (23.3) | 140 (23.5) | 129 (17.7) | 0.74 |
| Lungs | 647 (63.2) | 372 (62.4) | 412 (56.4) | 0.22 |
| Bone | 319 (31.1) | 141 (23.6) | 136 (18.7) | 0.40 |
| Liver | 225 (22.0) | 49 (8.2) | 67 (9.1) | 0.034 |
| Brain | 85 (8.3) | 44 (7.3) | 28 (3.9) | 0.50 |
| Treatment characteristic no. (%) | ||||
| Number of HD IL-2 cycles | ||||
| 1 | 522 (51.0) | 262 (43.9) | 342 (46.9) | 0.11 |
| 2 | 314 (30.6) | 174 (29.1) | 289 (40.0) | |
| 3 | 109 (10.6) | 157 (27.0) | 98 (13.4) | |
| ≥ 4 | 80 (7.8) | 4 (0.7) | 0 (0) | |
| Hospital type | ||||
| Teaching | 313 (30.6) | 295 (49.4) | 558 (76.4) | 0.014 |
| Community | 711 (69.4) | 302 (50.6) | 172 (23.5) | |
| Number of hospital beds | ||||
| < 400 | 222 (21.7) | 62 (10.4) | 30 (4.1) | 0.11 |
| 400–600 | 534 (52.1) | 374 (62.7) | 580 (79.5) | |
| > 600 | 268 (26.2) | 161 (26.9) | 119 (16.3) | |
| Hospital region | ||||
| Northeast | 143 (14.0) | 70 (11.7) | 133 (18.2) | 0.97 |
| Midwest | 140 (13.7) | 87 (14.7) | 108 (14.8) | |
| South | 406 (39.6) | 216 (36.2) | 232 (31.8) | |
| West | 335 (32.7) | 224 (37.5) | 257 (35.2) | |
Fig. 1.
Estimated annual number of patients treated with high-dose interleukin-2 for mRCC in the United States from 2004 to 2012 from the Premier hospital database.
Fig. 2.
Estimated annual baseline patient and hospital characteristics of high-dose interleukin-2 treatments for mRCC in the United States from 2004 to 2012 from the Premier hospital database.
HD IL-2 was administered primarily in hospitals of intermediate size (63.3%, with 400–600 beds). The median length of hospital stay per HD IL-2 course was 5 days. Approximately half (47.9%) of the patients underwent initiation of only 1 HD IL-2 treatment cycle, whereas 34.1%, 17.4%, and 0.6% had initiation of 2, 3, and 4 cycles, respectively. Overall, half (50.4%) of the patients received HD IL-2 at community hospitals; however, during the study period, HD IL-2 treatments became increasingly concentrated in teaching hospitals, from 24.0% in 2004 to 89.5% in 2012 (P = 0.017 for trend) (Fig. 2). No other significant trends in hospital characteristics were observed.
3.2. Toxicity and tolerability
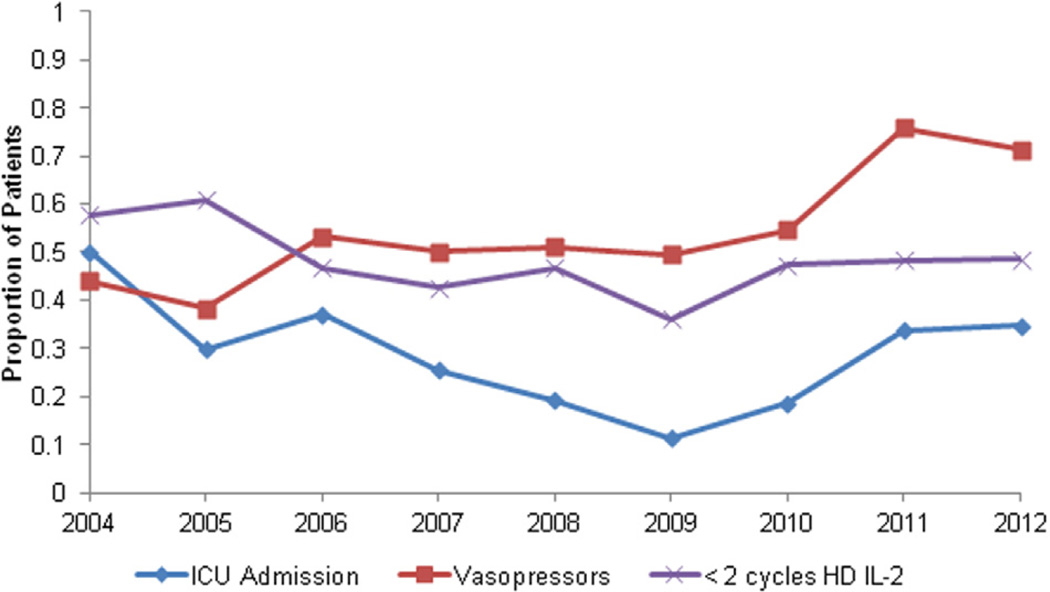
Annual rates for surrogates of toxicity and tolerability outcomes are shown in Fig. 3. Surrogates included vasopressor use, ICU admission, and hemodialysis. Our review suggests toxicities among a substantial portion of the cohort and that the incidence did not change considerably over time.
Fig. 3.
Estimated annual toxicity and tolerability measures among patients treated with HD IL-2 for mRCC in the United States from 2004 to 2012 from the Premier hospital database.
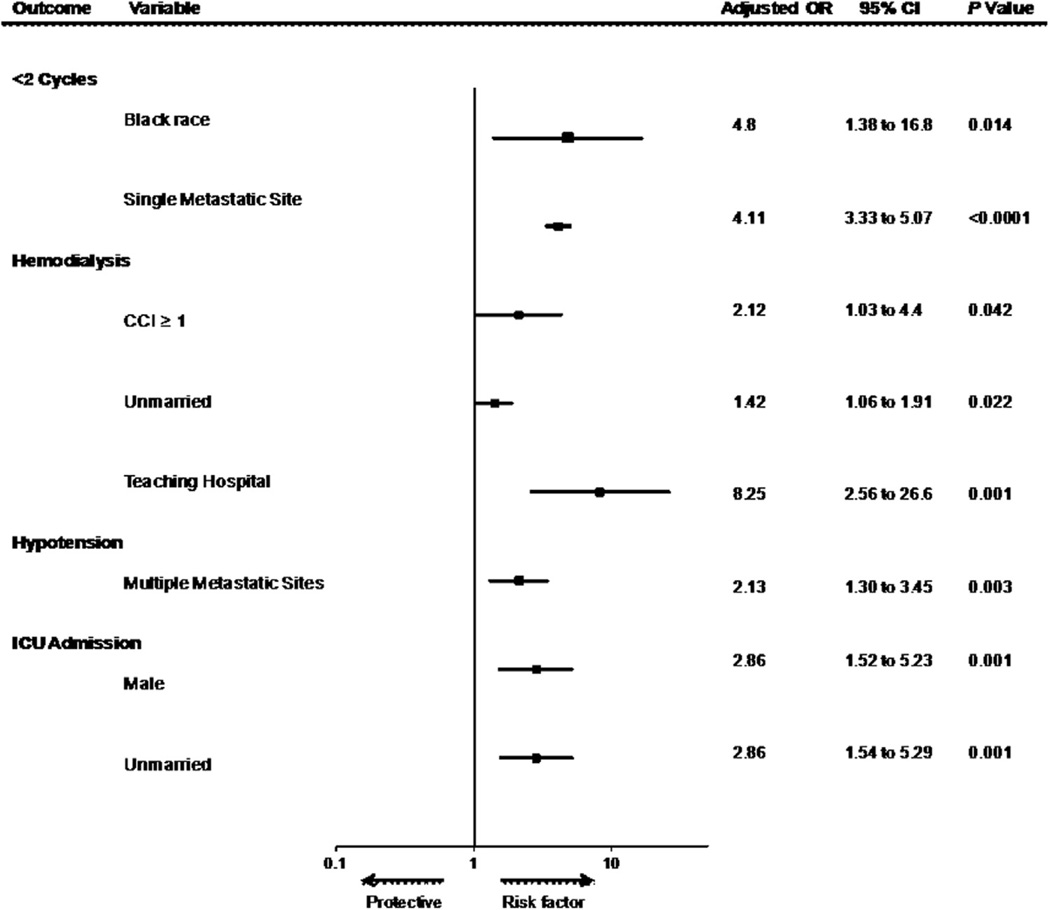
Characteristics associated with toxicity and tolerability outcomes in multivariable regression models are shown in Fig. 4. During admission for HD IL-2 therapy, 53.4% of patients received vasopressors. Vasopressor use was independently associated with the presence of multiple meta-static sites (odds ratio [OR] = 2.13, 95% CI: 1.30–3.45; P = 0.003). The rate of ICU admission was 33.0% and was associated with male sex (OR = 2.86, 95% CI: 1.52–5.23; P = 0.001) and unmarried status (OR = 2.86, 95% CI: 1.54–5.29; P = 0.001). Hemodialysis was performed in 7.1% and was associated with unmarried status (OR = 1.42, 95% CI: 1.06–1.91; P = 0.022), comorbidities (CCI > 0, OR = 2.12, 95% CI: 1.03–4.4; P = 0.042), and teaching hospital as site of treatment (OR = 8.25, 95% CI: 2.56–25.60; P = 0.001). Failure to receive >1 treatment cycle was associated with black race and the presence of a single metastatic site.
Fig. 4.
Hospital and patient characteristics associated with high-dose interleukin-2 toxicities and tolerability in multivariable analyses. Multivariable regression analyses were performed for the outcome measures listed in the left column. Covariates were patient age, sex, race (white [reference], black, Hispanic, and other), marital status, Charlson comorbidity index (CCI) (0 or ≥1), number of metastatic sites, hospital type (teaching vs. nonteaching), hospital size (<400 beds [reference], 400–600 beds, and >600 beds), geographic region, and total number of HD IL-2 doses.
4. Discussion
This study represents the first population-based assessment of contemporary HD IL-2 use for the management of mRCC in the United States. Our findings show that, in the modern TT era, HD IL-2 is used very infrequently in patients with mRCC. Given the toxicities and intensity of administration, it is not surprising that patients receiving HD IL-2 are disproportionately young and healthy. High rates of vasopressor use and ICU admission suggest HD IL-2 toxicity rates may remain high even in this highly selected cohort. Despite an increase in the national incidence of mRCC [16], during the study period, we observed an initial decrease in HD IL-2 use followed by a subsequent gradual increase beginning in 2009.
Enthusiasm for the TT likely accounts for the observed initial decrease in HD IL-2 use, and the nadir in use in 2008 is temporally associated with broad national experience with TT. The first targeted agent approved for use in mRCC gained Food and Drug Administration approval in December 2005 [9]. Multiple phase 3 trials have subsequently demonstrated effectiveness of additional single-agent TT [10,12,17–19]. These agents are associated with improved response rates, are significantly easier to administer, and are generally well tolerated when compared with HD IL-2. However, complete responses are rarely seen with TT.
We found that approximately half of the patients received only 1 HD IL-2 treatment cycle, possibly owing to the high toxicity rates observed, as 53.4% of patients required vasopressors, 33.0% were admitted to the ICU, and 7.1% received hemodialysis. Previous studies demonstrated that HD IL-2 toxicities are common and may affect almost every organ system, often causing treatment delay or discontinuation. However, with appropriate patient and center selection and aggressive management, toxicities are typically manageable and reversible [7,20]. Prior studies on the efficacy of HD IL-2 limited enrollment to patients with good performance status (Eastern Cooperative Oncology Group 0 or 1) [3,4,6]. We did not observe significant temporal trends in the baseline characteristics of patients selected for HD IL-2 therapy. Rather, patients were relatively young and healthy throughout the study period, suggesting that strict patient selection was routine even before the introduction of the targeted agents.
We observed a rapid and significant centralization of HD IL-2 therapy to teaching hospitals, which accounted for 24% of HD IL-2 treatments in 2004 and 89.5% in 2012. In the context of the high toxicity of HD IL-2, as well as the increased availability of TT, HD IL-2 therapy has likely been increasingly limited to experienced centers. The possible consequences of this shift on patient outcomes and equitable access to treatment warrant evaluation.
In addition to the appropriate selection of healthier patients, our analysis suggests possible patient selection biases. In this cohort, 75% of patients were men when compared with approximately 60% of patients with mRCC in the United States [21]. African Americans were less likely than white individuals to receive more than one treatment cycle despite lack of any observed association in this analysis between race and other surrogates of treatment toxicity. It is not possible to determine whether this observation results from selection biases or differences in patient preferences or disease progression. However, earlier findings by Saigal et al. [22], who used data from the Surveillance, Epidemiology, and End Results (SEER) Program from years 1992 to 2002, found that women and African Americans with mRCC were less likely to receive HD IL-2 therapy, suggesting possible selection biases. Finally, patients with a single metastatic site were less likely to receive multiple HD IL-2 cycles than patients with multiple metastatic sites did. A possible explanation is a tendency toward more aggressive HD IL-2 treatment of patients with a greater burden of disease, although this remains speculative.
The increasing use of HD IL-2 therapy since 2009 (Fig. 1) is possibly because of the recognition that TT, unlike HD IL-2 [2–5], are not curative and because of the resurgence of interest in novel cancer immunotherapies. After initial excitement and the approval of 7 different TT agents, enthusiasm may have waned, as there has been a plateau in their efficacy, which was generally limited to 9- to 12-month median progression-free survival rates and median overall survival of approximately 2 years when used in sequence [23,24]. Early results with the programmed death 1 inhibitors such as nivolumab in mRCC have reignited interest in cancer immunotherapy [25–27]. These agents reactivate CD8+ effector T cells to target the tumor and have shown preliminary efficacy in the form of response rates (22%–30%) and overall survival (~2 y) in treatment-refractory patients [25,28]. Complete responses with these agents are rare but durable. With all immunotherapies, the potential for the development of memory response against the tumor is notable and is generally unattainable with the TT.
4.1. Limitations
The Premier database was selected, as it provides claims-based data, including specific dosing information, for all inpatient costs including HD IL-2 treatments. However, as it does not capture patients with mRCC who received other treatments, we could not assess factors predictive of HD IL-2 use. We lacked histologic data and could therefore not evaluate HD IL-2 use by mRCC subtype. Oncologic outcomes related to HD IL-2 use could not be explored owing to the inherent nature of this claims database analysis. Trends in the use of additional systemic or surgical therapies before or after HD IL-2 therapy could not be evaluated. Although we observed no temporal trends in patient age or CCI, we could not evaluate other measures of comorbidity such as Karnofsky performance status. Furthermore, surrogate outcomes associated with HD IL-2 toxicity and tolerability were explored, but it is not clear to what extent these measures reflect true toxicities vs. prophylactic measures, and neither a comprehensive inventory of toxicities nor an assessment of toxicity severity was possible using this data set. Although we speculate on reasons for low HD IL-2 use in recent years, we did not explore other potential factors such as treatment costs.
5. Conclusions
HD IL-2 treatment has diminished in the contemporary era, reflecting an increasing reliance on TT in mRCC. Currently, it is administered in a small minority of patients with mRCC and generally excellent performance status and is associated with severe acute toxicities that may limit its use and necessitates careful patient selection. However, as the patient-level characteristics over the study period have not significantly changed, the use of IL-2 is increasing in teaching hospitals, reflecting a centralization of care. A slight increase in the receipt of HD IL-2 therapy from its nadir in 2008 likely reflects a renewed enthusiasm for immune checkpoint blockers in the context of a rapidly evolving treatment landscape.
Acknowledgments
Funding: This work was supported by a Grant from the National Cancer Institute, USA (P30CA072720) and Kidney SPORE (5P50CA101942-09).
Footnotes
Disclosures: Dr. Choueiri serves on advertising boards for Pfizer, GlaxoSmithKline, Novartis, Merck, and Bristol-Myers Squibb and has research affiliations with Pfizer, GlaxoSmithKline, Novartis, Merck, Bristol-Myers Squibb, Exelixis, and TRACON. All other coauthors report no disclosures.
References
- 1.Desantis C, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64:252–271. doi: 10.3322/caac.21235. http://dx.doi.org/10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 2.Fisher RI, Rosenberg SA, Fyfe G. Long-term survival update for high-dose recombinant interleukin-2 in patients with renal cell carcinoma. Cancer J Sci Am. 2000;6(Suppl. 1):S55–S57. [PubMed] [Google Scholar]
- 3.McDermott DF, Regan MM, Clark JI, et al. Randomized phase III trial of high-dose interleukin-2 versus subcutaneous interleukin-2 and interferon in patients with metastatic renal cell carcinoma. J Clin Oncol. 2005;23:133–141. doi: 10.1200/JCO.2005.03.206. http://dx.doi.org/10.1200/JCO.2005.03.206. [DOI] [PubMed] [Google Scholar]
- 4.Yang JC, Sherry RM, Steinberg SM, et al. Randomized study of high-dose and low-dose interleukin-2 in patients with metastatic renal cancer. J Clin Oncol. 2003;21:3127–3132. doi: 10.1200/JCO.2003.02.122. http://dx.doi.org/10.1200/JCO.2003.02.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klapper JA, Downey SG, Smith FO, et al. High-dose interleukin-2 for the treatment of metastatic renal cell carcinoma: a retrospective analysis of response and survival in patients treated in the surgery branch at the National Cancer Institute between 1986 and 2006. Cancer. 2008;113:293–301. doi: 10.1002/cncr.23552. http://dx.doi.org/10.1002/cncr.23552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fyfe BG, Fisher RI, Rosenberg SA, Sznol M, Parkinson DR, Louie AC. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose. J Clin Oncol. 1995;13:688–696. doi: 10.1200/JCO.1995.13.3.688. [DOI] [PubMed] [Google Scholar]
- 7.Poust JC, Woolery JE, Green MR. Management of toxicities associated with high-dose interleukin-2 and biochemotherapy. Anticancer Drugs. 2013;24:1–13. doi: 10.1097/CAD.0b013e32835a5ca3. http://dx.doi.org/10.1097/CAD.0b013e32835a5ca3. [DOI] [PubMed] [Google Scholar]
- 8.Hanzly M, Aboumohamed A, Yarlagadda N, et al. High-dose interleukin-2 therapy for metastatic renal cell carcinoma: a contemporary experience. Urology. 2014;83:1129–1134. doi: 10.1016/j.urology.2014.02.005. http://dx.doi.org/10.1016/j.urology.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 9.Szczylik C, Oudard S, Siebels M, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 10.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 11.Escudier B, Bellmunt J, Négrier S, et al. Phase III trial of bevacizumab plus interferon alfa-2a in patients with metastatic renal cell carcinoma (AVOREN): final analysis of overall survival. J Clin Oncol. 2010;28:2144–2150. doi: 10.1200/JCO.2009.26.7849. http://dx.doi.org/10.1200/JCO.2009.26.7849. [DOI] [PubMed] [Google Scholar]
- 12.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 13.Watanabe KK. Final Report. Charlotte, NC: Premier Inc.; 2001. Projection Methodology: National Projections from Premier Perspective's Hospital Inpatient Data (1999) [Google Scholar]
- 14.George S, Pili R, Carducci MA, Kim JJ. Role of immunotherapy for renal cell cancer in 2011. J Natl Compr Canc Netw. 2011;9:1011–1018. doi: 10.6004/jnccn.2011.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Panageas KS, Schrag D, Riedel E, Bach PB, Begg CB. The effect of clustering of outcomes on the association of procedure volume and surgical outcomes. Ann Intern Med. 2003;139:658–665. doi: 10.7326/0003-4819-139-8-200310210-00009. [DOI] [PubMed] [Google Scholar]
- 16.Sun M, Thuret R, Abdollah F, et al. Age-adjusted incidence, mortality, and survival rates of stage-specific renal cell carcinoma in North America: a trend analysis. Eur Urol. 2011;59:135–141. doi: 10.1016/j.eururo.2010.10.029. http://dx.doi.org/10.1016/j.eururo.2010.10.029. [DOI] [PubMed] [Google Scholar]
- 17.Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28:1061–1068. doi: 10.1200/JCO.2009.23.9764. http://dx.doi.org/10.1200/JCO.2009.23.9764. [DOI] [PubMed] [Google Scholar]
- 18.Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449–456. doi: 10.1016/S0140-6736(08)61039-9. http://dx.doi.org/10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 19.Escudier B, Pluzanska A, Koralewski P, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma:a randomised, double-blind phase III trial. Lancet. 2007;370:2103–2111. doi: 10.1016/S0140-6736(07)61904-7. [DOI] [PubMed] [Google Scholar]
- 20.Rosenberg SA, Yang JC, Topalian SL, et al. Treatment of 283 consecutive patients with metastatic melanoma or renal cell cancer using high-dose bolus interleukin 2. J Am Med Assoc. 1994;271:907–913. [PubMed] [Google Scholar]
- 21.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. http://dx.doi.org/10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 22.Saigal CS, Deibert CM, Lai J, Schonlau M. Disparities in the treatment of patients with IL-2 for metastatic renal cell carcinoma. Urol Oncol. 2010;28:308–313. doi: 10.1016/j.urolonc.2008.09.022. http://dx.doi.org/10.1016/j.urolonc.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Albiges L, Choueiri T, Escudier B, et al. A systematic review of sequencing and combinations of systemic therapy in metastatic renal cancer. Eur Urol. 2014:1–11. doi: 10.1016/j.eururo.2014.04.006. http://dx.doi.org/10.1016/j.eururo.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 24.Singer EA, Gupta GN, Marchalik D, Srinivasan R. Evolving therapeutic targets in renal cell carcinoma. Curr Opin Oncol. 2013;25:273–280. doi: 10.1097/CCO.0b013e32835fc857. [DOI] [PubMed] [Google Scholar]
- 25.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brahmer JR, Tykodi SS, Chow LQM, et al. Safety and activity of anti–PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. http://dx.doi.org/10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harshman LC, Choueiri TK, Drake C, Hodi S. Subverting the B7-H1/PD-1 pathway in advanced melanoma and kidney cancer. CancerJ. 2014;20:272–280. doi: 10.1097/PPO.0000000000000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harshman LC, Drake CG, Choueiri TK. PD-1 blockade in renal cell carcinoma: to equilibrium and beyond. Cancer Immunol Res. 2014;2:1132–1141. doi: 10.1158/2326-6066.CIR-14-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]