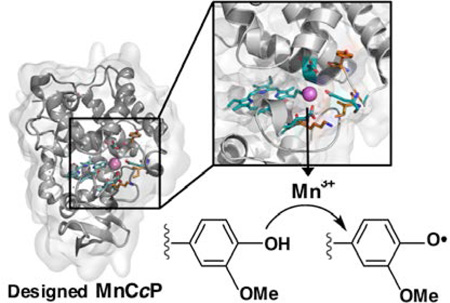
Abstract
Non-covalent second shell interactions are important in controlling metal-binding affinity and activity in metalloenzymes, but fine-tuning these interactions in designed metalloenzymes has not been fully explored. As a result, most designed metalloenzymes have low metal-binding affinity and activity. Here we identified three mutations in the second coordination shell of an engineered Mn(II)-binding site in cytochrome c peroxidase (called MnCcP.1, containing Glu45, Glu37, and Glu181 ligands) that mimics the native manganese peroxidase (MnP) and explored their effects on both Mn(II)-binding affinity and MnP activity. First, removing a hydrogen bond to Glu45 through Tyr36Phe mutation, enhanced Mn(II)-binding affinity, as evidenced by a decrease of KM of Mn(II) oxidation 2.8 fold. Second, introducing a salt bridge through Lys179Arg improved Glu35 and Glu181 coordination of Mn(II), decreasing KM 2.6 fold. Third, eliminating a steric clash that prevented Glu37 from orienting towards Mn(II) resulted in an 8.6 fold increase in kcat/KM, arising primarily from a 3.6 fold decrease in KM, with a KM value comparable to that of the native enzyme (0.28 mM vs. 0.2 mM for Pleurotus eryngii MnP PS3). We further demonstrated that while the effects of Tyr36Phe and Lys179Arg mutations are additive, other combinations of mutations were antagonistic. Finally, we showed that these MnCcP variants are functional models of MnP that mimic its activity both in Mn(II) oxidation and degradation of a phenolic lignin model compound and kraft lignin. Our results offer molecular insight into the role of non-covalent interactions around metal binding sites for improving metal binding and overall activity; such insight can be applied to rationally enhance these properties in other metalloenzymes and their models.
Keywords: Lignin degradation, protein engineering, protein design, secondary coordination, hydrogen bond
Graphical Abstract
Introduction
Proteins have evolved to catalyze numerous reactions with high efficiency and specificity. Among them, metalloproteins are of particular interest as they are responsible for catalyzing some of the most difficult reactions on earth, such as water oxidation,1 nitrogen fixation2 and lignin degradation.3 Therefore, there has been long-standing interest in elucidating the structural features responsible for strong metal binding and efficient catalysis.4–15 Toward this goal, many biochemical and biophysical studies have identified conserved amino acid residues in metalloenzyme active sites. An effective way to verify the importance of these residues is to introduce them to a designed protein to determine whether they confer new activity.4–15 Despite progress made in this field, most designed metalloproteins display much lower binding affinity for metal ions and lower activity than native enzymes.16 One contributing factor is a lack of appreciation for the role of non-covalent secondary coordination shell interactions around the metal binding site in determining the metal-binding affinity and functionality, as most studies so far have focused mainly on reproducing the primary coordination sphere of native metalloproteins. Recently, we and others have explored the roles of electrostatics, hydrogen bonding, and hydrophobic interactions around the primary coordination sphere in fine-tuning the reduction potentials of metalloproteins.17–23 While reduction potentials are an important parameter in metalloprotein function, metal-binding affinity and enzymatic activity are just as— if not more—important; however, few studies have focused on exploring the roles of such secondary shell interactions in tuning these aspects.
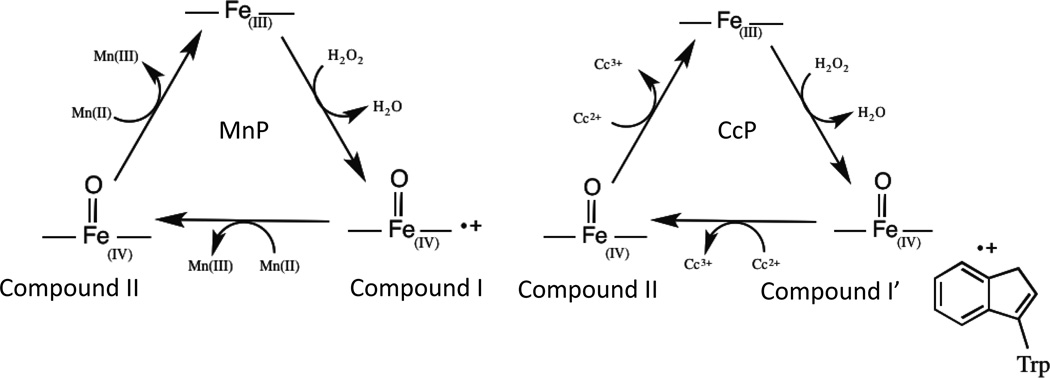
Manganese peroxidase (MnP, EC 1.11.1.13)24, 25 is a heme peroxidase that also binds Mn(II) in the active site. MnP plays a key role in degradation of lignin, the second most abundant biopolymer on earth and an attractive potential source of energy and many commodity chemicals.26 MnP oxidizes lignin indirectly via oxidation of Mn(II) to Mn(III) and secretion of Mn(III) to the extracellular environment.27–29 Secretion of Mn(III) is highly advantageous because it eliminates complicated heterogeneous catalytic kinetics and broadens the substrate scope of organisms possessing MnP. Similar to many other peroxidases, the reaction cycle of MnP begins with the oxidation of a heme cofactor by hydrogen peroxide (H2O2) (Scheme 1). It then returns to the resting state by two consecutive, one-electron oxidation steps, converting two equivalents of Mn(II) to Mn(III) during the process. The resulting Mn(III) exits the active site whereupon it is chelated by organic acids such as oxalate or lactate secreted by the organism. Mn(III) is a potent oxidizing agent that can activate lignin to initiate its degradation, mainly by generating radicals and oxidizing phenolic substrates.27–30
Scheme 1.
Reaction cycles of MnP and CcP.
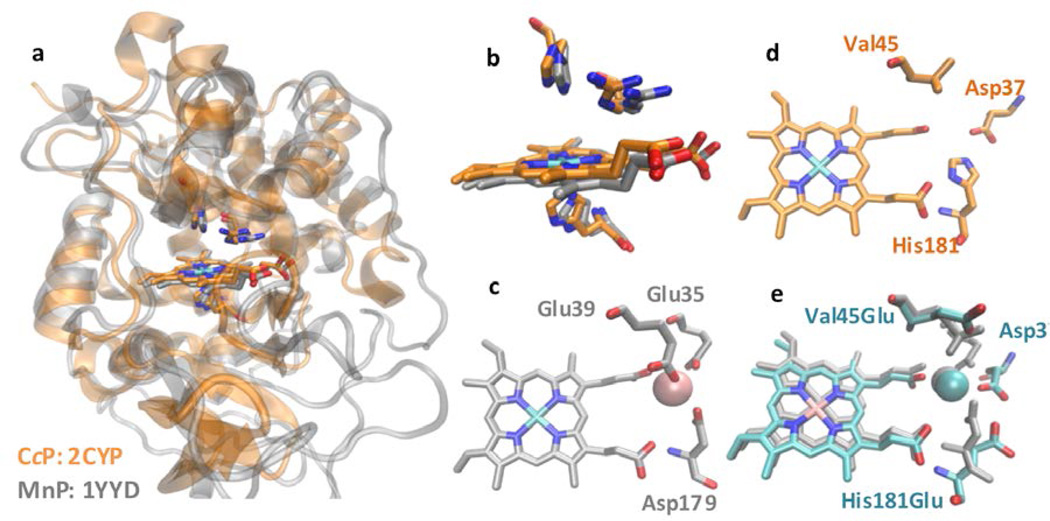
Despite the demonstrated activity of MnP for lignin degradation, its industrial-scale application has been limited due to difficulties in obtaining sufficient quantities of functional protein and in genetic manipulation of the organisms that produce it.28, 31–33 There have been several reports on efficient conversion of lignin to fuel precursors using biosynthetic models or organic catalysts with considerable success.34–40 However, most of these studies require extreme conditions such as high temperature and pressure.40, 41 In a complementary approach, we and other groups have used easy-to-prepare proteins such as cytochrome c peroxidase (CcP)24, 25 or DypB42 as a scaffold to mimic MnP. CcP is a heme peroxidase found in baker’s yeast that shares significant structural similarity with MnP, both in terms of the overall fold (Figure 1a) and the active site (Figure 1b), with the major difference being lack of a Mn(II)-binding site.43–45 The catalytic cycle of CcP is also similar to that of MnP, diverging mainly in the type of substrate oxidized (Scheme 1). In contrast to MnP, CcP can be overexpressed in large quantities in E. coli in its functional form.24, 46
Figure 1.
Structural comparison of MnP, CcP, and MnCcP.1. (a,b) Overlay of MnP (gray, PDB ID: 1YYD) and CcP (orange, PDB ID: 2CYP) using Stamp structural alignment shows high structural similarities (a), especially in the heme-binding site (b). (c,d) Comparison of Mn(II)-binding site in MnP (c) and corresponding site in MnCcP.1 (d). (e) An overlay of Mn(II) CcP.1 (cyan, PDB ID: 5D6M) and Mn(II)P (gray, PDB ID: 1YYD).
Previously, we have reported the successful construction of a Mn(II)-binding site in CcP by structural comparison of the Mn(II)-binding site in MnP (Figure 1c) with the corresponding region of CcP (Figure 1d) and making Gly41Glu, Val45Glu, and His181Asp mutations (called MnCcP).24 The protein was then further optimized through replacing Asp181 by Glu, reverting Glu41 back to Gly, and mutating Asp37 to Glu (called MnCcP.1).47 These designs allowed converting WTCcP that does not bind Mn(II) and that has no MnP activity into MnCcPs that are capable of binding Mn(II) and performing new MnP activity (Figure 1). In the process, we have identified factors contributing to MnP activity such as the nature of the reaction intermediates (e.g., Compound I’ that contains a tryptophan radical in CcP vs. Compound I that contains a porphyrin π cation radical in MnP) and the roles of redox active amino acids (e.g., Trp51 in CcP), which can lead to a more stable Compound I’ that has a slower rate of decay to Compound II and a less reactive Compound II. These factors directly influence the ability of CcP to oxidize organic substrates.48, 49 Making mutations of Trp and Tyr residues in CcP into corresponding non-redox active amino acids (e.g., Phe) has resulted in a MnCcP with higher MnP activity.48, 49
Although these MnCcP model proteins were shown to bind Mn(II) and oxidize it to Mn(III), their catalytic efficiency (0.6 mM−1s−1 for the most active variant) was still far lower than the native MnP (390–4340 mM−1s−1) (Table S1 contains some relevant kinetic parameters for MnPs and their mimics). To improve the catalytic efficiency even further, rather than focusing mainly on improving kcat as in previous studies,48, 49 we hypothesize that higher KM of the variants is an important factor contributing to low activity; hence, in this study we focus on improving KM of the reaction. We report herein a crystal structure of Mn(II)-bound MnCcP.1 (Figure 1). Based on structural comparisons between MnCcP.1 and MnP, we have identified key non-covalent interactions in the secondary coordination shell of Mn(II) for tuning Mn(II) binding and MnP activity, specifically mutations of Tyr36, Ile40, and Lys179 that allowed removal of hydrogen bonding (H-bonding) interactions, adding a salt bridge, and removing steric clashes and increasing flexibility, respectively. These mutations resulted in a protein with a ∼9-fold increase of catalytic efficiency compared to our initial design and a KM comparable to that of native MnPs. Finally, we show that MnCcP mutants studied here display degradation activity towards phenolic lignin model compounds and alkali-treated lignin.
EXPERIMENTAL PROCEDURE
Materials and reagents
All the materials and reagents used were purchased from either Sigma or Fisher chemicals unless stated otherwise.
Mutant protein generation, purification, and verification
The mutants were generated using PfuUltra II fusion DNA polymerase enzyme (Agilent) for site directed mutagenesis. PCR was performed on a plasmid containing MnCcP.147 and the following DNA sequences as forward primers and their complementary sequences as reverse primers (synthesized by IDT, Inc):
Tyr36Phe: 5’- GGG AAG ATG ACG AAT TTG ACA ACT ATA TAG GC
Ile40Gly: 5’-CGA ATA TGA CAA CTA TGG CGG CTA TGG GCC C
Tyr36Phe/Ile40Gly: 5’- GGG AAG ATG ACG AAT TTG ACA ACT ATG GCG GCT ATG GGC CC
Tyr36Phe2: 5’- GGG AAG ATG ACG AAT TTG AAA ACT ATA TAG GC
Lys179Arg(His181Glu): 5’- CGC TCT GGG CAG AAC CGA GCT CAA G −3’
The template plasmid containing the original MnCcP.1 gene was removed by treating the PCR solution with DpnI restriction enzyme (NEB), followed by transformation of the remaining plasmid containing the mutant into Turbo competent cells (NEB) using a protocol prescribed by the company. The plasmids were then purified using QIAprep Spin Miniprep Kit, and the mutations were confirmed by DNA sequencing (ACGT-Inc.).
Purified plasmids containing the mutant sequences were then transformed into BL21-DE3* competent E. coli cells (Invitrogen). Protein purification was performed based on a previously developed protocol in our lab.24, 47 Briefly, the cells were grown in a 5 mL Luria Broth media starter culture (10 g bacto tryptone (BD), 5 g yeast extract (BD), and 10 g NaCl (Fisher Chemicals) in 1 L) at 37 °C for 8 hours. Two flasks of 2 L rich media (10 g bacto tryptone, 8 g of yeast extract, 5 g NaCl) were inoculated by 1 mL of starter culture and grown for 16 hours at 37 °C. The culture was then induced by 1 mM IPTG (GoldBio) for 4 hours at 37 °C. The protein was purified from cytosol by sonication using Misonix ultrasonic liquid processor. The processing time was 6 min with 6 s pulses on and 12 s pulses off and an amplitude of 70. The supernatant was then batch-bound to a DEAE column (GE Healthcare) and eluted with a gradient of KCl in a phosphate buffer. The protein was then passed down a Sephedex S-100 gel filtration column (GE healthcare) and an additional DEAE column following reconstitution with hemin. The masses of all proteins were verified by electrospray ionization mass spectroscopy (ESI-MS) with a Quattro II (Waters) ESI mass spectrometer maintained by the School of Chemical Sciences Mass Spectrometry Laboratory (Urbana, IL). Formic acid was added to ionize the protein upon injection.
Protein crystallization, data collection and structure determination
The protein samples were concentrated using a Centricon with a centrifugal filter that has 10,000 Da molecular weight cutoff (Millipore). Proteins were crystalized using a vapor diffusion hanging drop technique. A 1 µL drop of 50 mg·mL−1 protein solution with 1 mM MnSO4 was placed on siliconized glass with 1 µL of well solution. The drop was then equilibrated against 500 µL of well solution of 0.2 M sodium iodide pH 7.0 (the number 10 from PEG/ion screen HR2-126, Hampton Research) and was incubated at 4 °C. Crystals for suitable X-ray analysis began to appear after 1 week and finished growth after 2–3 weeks. The crystals were soaked for several minutes in 30% PEG 400 in the well solution before mounting. The diffraction data were collected at beam-line X-29A of Brookhaven National Laboratory (BNL). The data were phased and refined using CCP450 and Shelx97 suites, respectively. Molecular replacement was used as phasing model using WT-CcP structure as a model (PDB ID: 2CYP). Crystallographic data are presented in Table S2.
Kinetic experiments
Kinetics experiments were performed on an Agilent 8453 diode array (Agilent) instrument by monitoring the increase in absorption at 270 nm due to increased production of Mn(III)-malonate (molar extinction coefficient = 8000 M−1cm−1 51). The experiment begins by addition of 6–10 µM protein, 400 µL of different MnSO4 concentrations in overall 3000 µL of 500 mM malonate buffer, pH=4.5. Reactions started upon addition of 25 µL of 10 mM H2O2. The data were then transferred to Origin 9.1 for analysis. The values of kcat and KM were analyzed based on Michaelis-Menten curve fitting.
The extinction coefficient of the Soret band for each protein was determined using the common pyridine hemochromogen assay as described previously.52, 53 Extinction coefficients are presented in Table S3.
Gas Chromatography-Mass spectrometry (GC-MS) studies
GC-Ms studies were performed on a 6890N network GC system from Agilent Technology with a network GC system 5973 mass selective detector. Samples were extracted by ethyl acetate and dried in a sodium sulfate column prior to injection. The measurement begins at an initial temperature of 50 °C for 3 min and ramped to 300 °C at 40 °C/min, pausing at the final temperature for 2.5 min.
Molecular dynamics and simulation
Molecular dynamics (MD) simulations were performed using NAMD254 and the results were analyzed and visualized using visual molecular dynamics (VMD)55. The structures of the mutants were modeled based on the structures of MnCcP.1 and MnP (PDB IDs: 1YYD and 1MNP, respectively). PSF files were generated using the PSFGEN suite in VMD. Proteins were solvated in a water box with a side of 15 Å. TIP3 explicit water model56 was used to describe water. The simulation systems were neutralized by addition of Na+ and Cl– ions, with a final NaCl concentration of 0.1 M. Production MD simulations were performed after 1,000–2,000 steps of minimization and a 2-ns equilibration (2 fs/step) in an NPT ensemble.
HPLC analysis
Guaiacylglycerl-β-guaiacol ether, a phenolic lignin model compound dimer was synthesized and generously provided by Ms. Margaret Brown and Dr. Michele Chang in the University of California at Berkeley. The degradation reactions of the compounds were performed in 50 mM sodium acetate buffer pH=4.5 as previously described.57 The reaction mixture contained 0.2 µM protein, 1 µM (final) model compound, 1 mM MnCl2, and initiated with 1 mM H2O2. The reaction then stirred overnight at room temperature. Samples for HPLC analysis were prepared by centrifuging the reaction for 5 min at 20,850 x g in a bench top centrifuge. 10 µL of the samples were then injected into an Agilent Eclipse XDB-C18 column (4.6*150, 5u) and ran at 0.5 mL/min. The program was as follows:
A: water with 0.1% formic acid, B: Acetonitrile with 0.1% formic acid
0–2 min, 10% B; 8–11.5 min, 90% B; 12–16 min, 10% B
Reactions were also carried out in the absence of MnCl2 as a control. The sample elution was monitored at 254 nm.
The reaction with kraft lignin (Lignin, alkali from Sigma-Aldrich) was carried out in 3 mL of 500 mM malonate buffer pH=4.5, by adding 100 µL of 0.5 mM protein to the reaction in the absence or presence of 200 µL of 500 mM MnSO4. The reaction was initiated with 100 µL of 50 mM H2O2 and mixed by shaking the container gently for 84 hours at 30 °C or 24 hours at 37 °C. The samples were prepared for HPLC analysis by addition of 40 µL of 100% CCl3COOH to 400 µL of the reaction. After mixing, the samples were centrifuged for 10 min at 4,000 rpm. Samples were then loaded onto the HPLC column. The sample elution was monitored at 275 nm.
The program was as follows:
A: water with 0.1% formic acid, B: methanol with 0.1% formic acid
0–10 min, gradient of 20–30% B; 10–24 min, gradient from 30% to 50% B; 24–50 min, gradient from 50% to 80% B (flow rate: 0.5 mL/min).
RESULTS AND DISCUSSION
Crystal structure of Mn(II)-bound MnCcP.1 and its comparison to MnP
In order to investigate structural features responsible for improving Mn(II) binding and MnP activity of the designed MnCcP, we obtained the X-ray crystal structure of MnCcP.1 containing Mn(II) at the active site (see crystallographic parameters in Table S2). An overlay of the crystal structures of MnCcP.1 and MnP indicates the close similarity of both the overall structural folds (Figure 1a) and the heme-binding sites (Figure 1b), including the proximal His and the distal His and Arg residues. This structural comparison strongly suggests that MnCcP.1 offers an excellent scaffold for engineering a Mn(II)-binding site to mimic MnP function.
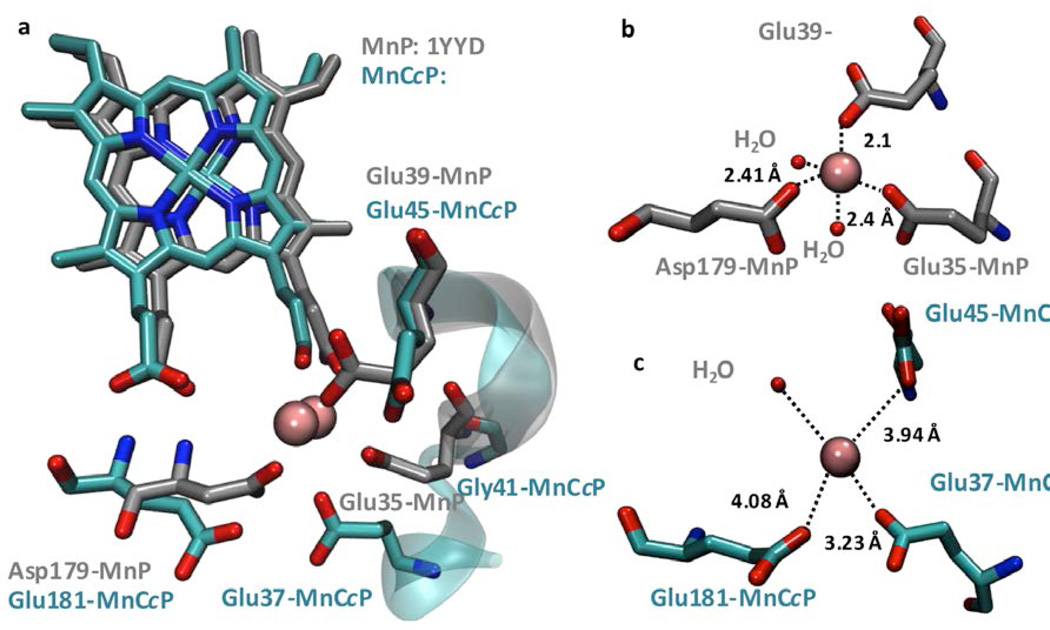
The primary Mn(II) coordination shell in MnCcP.1, consisting of the heme propionate group and three carboxylate ligands (Glu37, Glu45, and Glu181), is also similar to that in MnP (Figure 2a). However, it is interesting that despite the similar position of the ligands with respect to Mn ion, their distances vary substantially (Figure 2b,c). Similarly long distances between Mn ligands and Mn ion have also been observed in the crystal structure of another Mn-bound CcP variant25 (see figure S1). A careful inspection of the two Mn(II)-binding sites suggests several subtle differences between the carboxylate ligands to Mn(II) in the two proteins that can lead to the elongated Mn-ligand distances in MnCcP.1. The first difference that caught our attention was the positions of Glu37 in MnCcP.1 and Glu35 in MnP (Figure 2). While the relative positions of these carboxylate ligands are similar, Glu37 in MnCcP is separated from Mn(II) ion by 3.23 Å (Figure 2b) vs. only 2.40 Å in MnP (Figure 2c). Similarly, although Glu181 in MnCcP occupies the same position as Asp179 in MnP, their orientations differ, causing a significant increase in the Mn-O distance from 2.41 Å in MnP to 4.08 Å in MnCcP.1 (Figure 2). Another even more subtle difference is the position of the ligands in different structures of the same protein. A comparison of two crystal structures of Mn(II)-bound MnP (PDB ID: 1MNP58, 1YYD45) with that of Mn(II)-free MnP (PDB ID: 1YZP45) suggests that Glu39 in MnP shows the greatest variability in position and therefore in its distance to Mn(II) ion among all ligands. In contrast, the corresponding Glu45 in MnCcP.1 retained its distance in all of our crystal structures (Mn(II)-free MnCcP.1, PDB ID: 2IA847; Co(II)-MnCcP.1 PDB ID: 2ICV47; and Mn(II)-bound MnCcP.1, PDB ID: 5D6M). This consistency in distance among different structures of MnCcP.1 suggests that the Glu45 in MnCcP.1 cannot adopt the observed rotamer of Glu39 in MnP for coordinating to Mn(II). This difference between the rotamers at the same position of the two proteins resulted in a 1.84 Å difference in distance between the carboxylate ligands and Mn(II) (3.94 Å in MnCcP.1 vs. 2.10 Å in MnP, see Figure 2b,c). These observations suggest that subtle differences in the orientation of the ligand that coordinates to the metal ion and the resulting differences in geometry and metal-ligand distances may be responsible for the differences of Mn(II)-binding affinity and MnP activity between the designed MnCcP.1 and native MnP. To minimize or eliminate these differences, we sought to engineer the second-sphere interactions around these ligands in order to change the orientation of the ligands in MnCcP.1, as described below.
Figure 2.
(a) Structural comparison of the Mn(II) binding sites of MnP (gray, PDB ID: 1YYD) and MnCcP.1 (cyan, PDB ID: 5D6M). (b) Primary Mn(II) ligands in MnP. (c) Primary Mn(II) ligands in MnCcP.1. Heme is not shown in b and c. The red spheres in the figures are water molecules.
Improving Mn(II)-binding by Tyr36Phe mutation to remove a H-bond to Glu45 ligand of Mn(II)
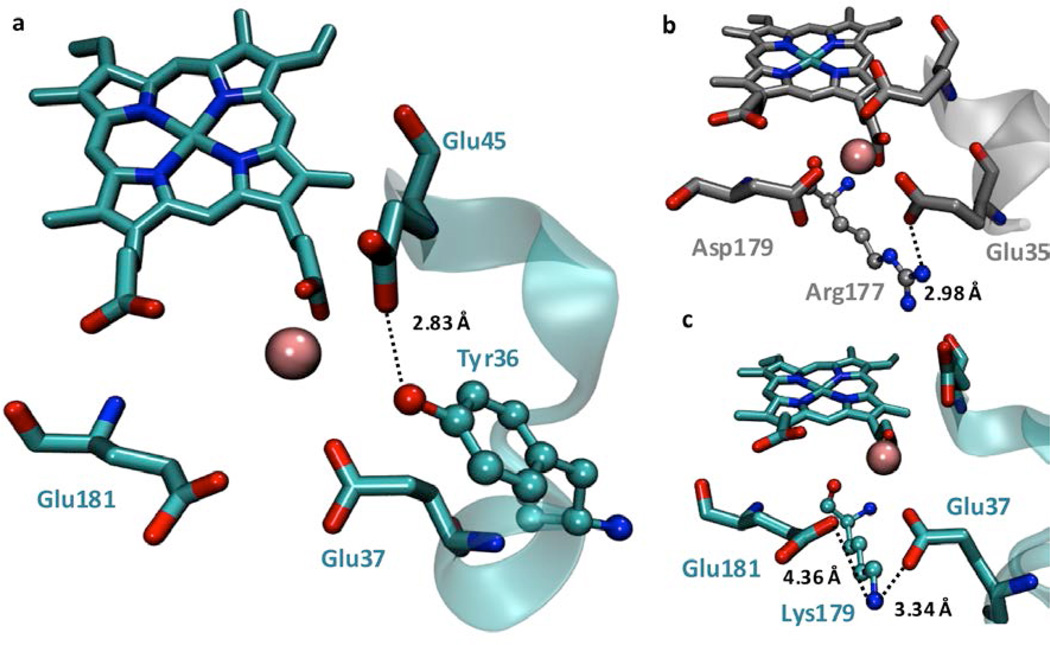
Since crystallographic analysis indicated that Glu45 in MnCcP.1 is much more rigid and more distant from Mn(II) than the corresponding Glu39 residue in MnP, we sought a way to relieve the rigidity and move Glu45 closer to Mn(II) in MnCcP.1 for stronger coordination. Structural analysis of Mn(II)-MnCcP.1 suggested that Tyr36 is within a hydrogen bonding (H-bonding) distance to the carboxylate of Glu45 in MnCcP.1 at a distance of 2.83 Å (Figure 3a) while an analogous interaction is absent at the corresponding residue Glu39 in MnP. We hypothesized that this H-bond interaction may compete with Mn(II) coordination by stabilizing the rotamer of Glu45 in MnCcP.1 that is observed in the crystal structure, resulting in low flexibility and poor orientation of the ligand and thus weakening the ability of Glu35 to coordinate Mn(II). There have been multiple reports on conserved hydrogen bonds that help position active site residues or substrates in the proper orientation to fine-tune the catalytic efficiency of enzymes or to tune reduction potentials of metal centers.20, 59, 60 Inspired by such reports, we mutated Tyr36 to Phe in MnCcP.1. We chose Phe because it differs from Tyr only by the OH group that is responsible for the H-bonding interactions, thus minimizing other structural perturbations.
Figure 3.
(a) Structure of Mn(II)-MnCcP.1 showing Tyr36 and its interaction with Glu45. (b) Salt bridge between Arg177 and Glu35 in MnP (PDB Id: 1YYD). (c) Possible salt bridge between Lys179 and Glu37/Glu181 ligands in the Mn(II)-MnCcP.1 structure (PDB ID: 5D6M).
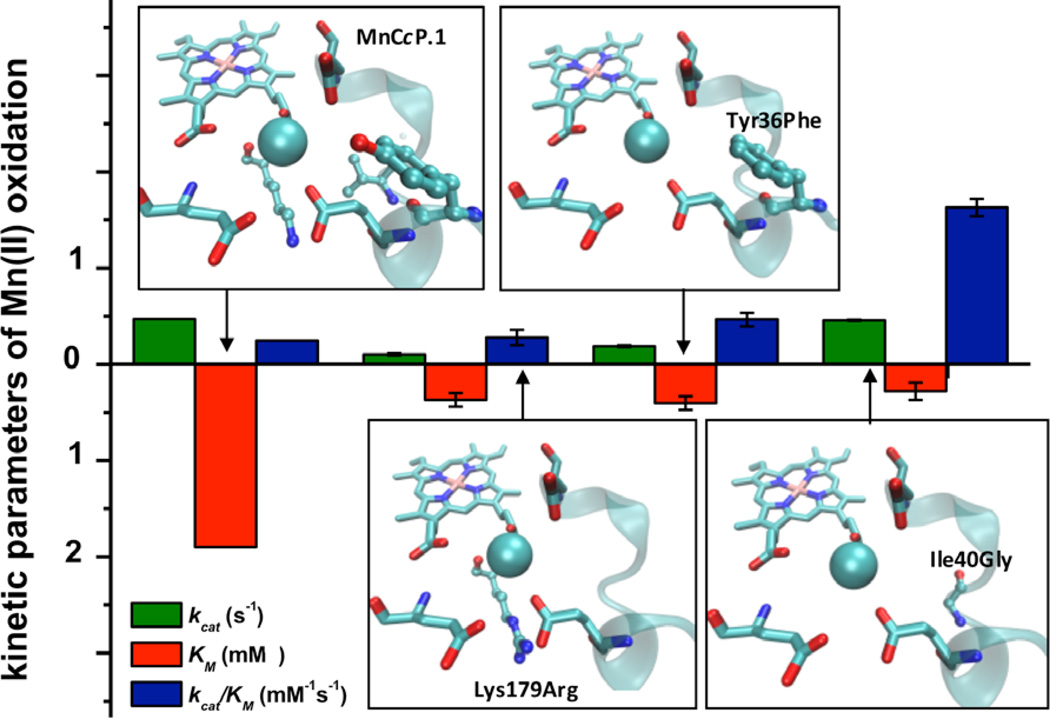
MnP activity assays showed that the Tyr36Phe mutation improved the KM by 2.8 fold (Figure 4 and Table 1), suggesting that removing the H-bonding interactions to the primary coordinating ligand, Glu45, to secondary shell residues (Tyr36) did indeed improve binding affinity to Mn(II).
Figure 4.
Different secondary shell residue positions investigated in this study and their effect on activity and metal affinity of MnCcP.1
Table 1.
Kinetic parameters of different MnP mimics. All the reactions were performed at pH 4.5.
| enzyme | KM (mM) (fold decrease)* | kcat (s−1) (fold increase) | kcat/KM(mM−1s−1) (fold increase) |
|---|---|---|---|
| MnCcP.1 | 1.02 | 0.19 | 0.19 |
| Tyr36Phe-MnCcP.1 | 0.37 (2.8) | 0.10 (0.5) | 0.28 (1.5) |
| Lys179Arg-MnCcP.1 | 0.40 (2.6) | 0.19 (1.0) | 0.47 (2.6) |
| Ile40Gly-MnCcP.1 | 0.28 (3.6) | 0.46 (2.4) | 1.63 (8.6) |
| Y36F/K179R–MnCcP.1 | 1.10 (0.9) | 0.62 (3.3) | 0.56 (2.9) |
| I40G/K179R–MnCcP.1 | 0.48 (2.1) | 0.39 (2.0) | 0.81 (4.3) |
| Y36F/I40G–MnCcP.1 | 0.28 (3.6) | 0.28 (1.5) | 1.02 (5.4) |
| Y36F/I40G/K179R–MnCcP.1 | 0.57 (1.8) | 0.46 (2.4) | 0.80 (4.2) |
The values in the parentheses are the factor of decrease or increase with respect to MnCcP.1.
Improving Mn(II)-binding by Lys179Arg mutation to introduce a salt bridge to better orient Glu37 and Glu181 ligands to Mn(II)
To investigate a way to improve Mn(II) coordination by Glu37 and Glu181 and to increase MnP activity in MnCcP.1, we performed structure-based sequence alignment of MnPs from different organisms and found that they all share a conserved Arg positioned below Glu35 in MnP (Figure 3b). We hypothesized that this Arg may play a key role in proper positioning of the Glu35 and Asp179 ligands in MnP through the formation of a salt bridge. In fact, mutagenesis studies in MnP from Phenorocheatea chrysosporium showed that replacing this Arg residue by a negatively charged residue (Glu) or a neutral residue (Gln, Asn) decreased the catalytic efficiency (kcat/KM) by four orders of magnitude.61 In MnCcP.1, Lys179 is analogous to Arg181 in MnP. Our MD simulations on Lys179Arg-MnCcP.1 showed an enhanced salt bridge interaction between this residue and Glu37 (from 3.32 to 2.72 Å) (Figure 3c). Consistent with the design, Arg179 moved further away from Glu181 in MnCcP.1 by 0.5 Å, resulting in a reorientation of Glu181 toward Mn(II) so that their distance is closer by almost 2 Å. Both Lys and Arg have positive charges, and previous studies on native MnP have shown that mutation of Arg177 to Lys effects mostly KM of the enzyme, presumably by destabilizing the optimal conformation for Mn(II) binding (Figure 4 and Table S1).62 Encouraged by the above findings, we mutated Lys179 to Arg, and we found that the resulting mutant had a 2.6 fold improvement in KM (Table 1).
Improving Mn(II)-binding and oxidation activity by Ile40Gly mutation to relieve steric clashes in the ligand-containing loop
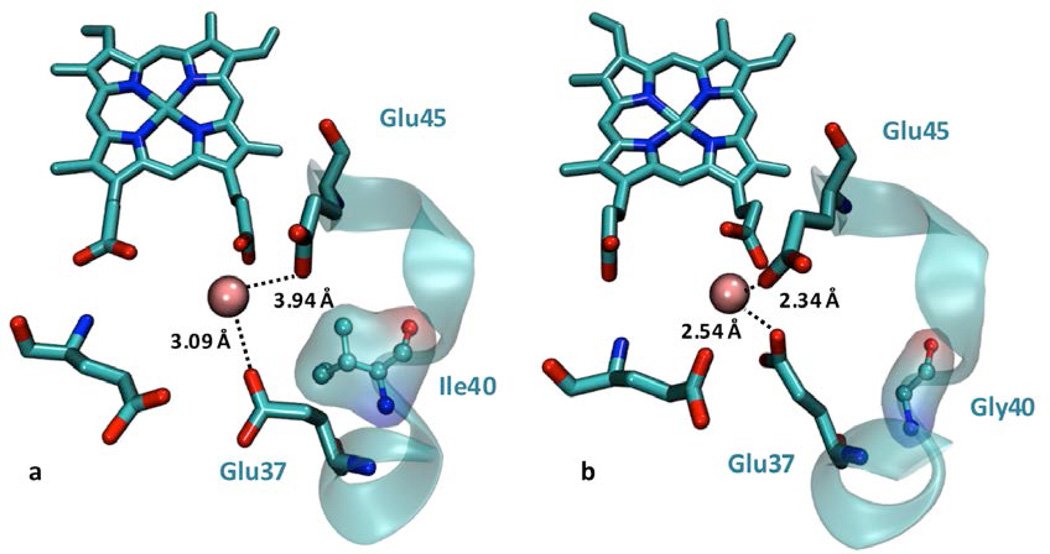
Further inspection of the overlaid structures of MnCcP.1 and MnP revealed that the loop containing Glu37 and Glu45 ligands in MnCcP.1 differs substantially from the corresponding loop containing Glu35 and Glu39 ligands in MnP, leading to different positions of these carboxylate ligands (Figure 2a). Close inspection of this loop suggested that the side chain of Ile40 in MnCcP.1 can cause a steric clash with Glu37 in MnCcP.1, preventing it from orienting towards Mn(II) (Figure 5a). In contrast, a Gly is present at the same position in many MnPs. Therefore, to relieve this steric clash, we made the Ile40Gly mutation so that the smaller size of Gly would provide the Glu37 and Glu45 ligands in the loop of MnCcP.1 the freedom to better coordinate to Mn(II) ion, decreasing KM and consequentially increasing kcat/KM. MD simulations of Ile40Gly-MnCcP.1 showed that the distances between these ligands to Mn(II) decreased from 3.23 to 2.54 Å for Glu37 and from 3.94 to 2.34 Å for Glu45 (Figure 5b). As shown in Figure 4 and Table 1, this mutation resulted in the highest catalytic efficiency among all of the mutants described in this work, arising primarily from a 3.6 fold decrease in KM. As a result of this engineering effort, the KM of Ile40Gly-MnCcP (0.28 mM) is comparable to that of the native Pleurotus eryngii MnP PS3 (0.2 mM).63 This mutant also showed a 2.4 fold increase in the catalytic activity (kcat) which, together with the decrease in KM, results in an 8.6-fold increase in the catalytic Mn(II) oxidation efficiency.
Figure 5.
Mn(II)-binding site and corresponding distances between Mn(II) and Glu37 and Glu45 in (a) Mn(II)-MnCcP.1 crystal structure (PDB ID: 5D6M) and (b) MD simulated model of Ile40Gly-MnCcP.1 variant.
Additive effect of the mutations on the activity of MnCcP.1
To investigate whether the effects of these mutations are additive, we made all possible combinations of our mutants: Tyr36Phe/Lys179Arg, Tyr36Phe/Ile40Gly, Ile40Gly/Lys179Arg, and Tyr36Phe/Ile40Gly/Lys179Arg. As shown in Table 1 and Figure 6, the MnP activity of the Tyr36Phe/Lys179Arg double mutant is equal to the combined activities of Tyr36Phe and Lys179Arg single mutant within the experimental error. This result was somewhat expected, as the Tyr36Phe and Lys179Arg mutations are involved in secondary shell interactions to different ligands (Glu45 and Glu37/Glu181, respectively).
Figure 6.
Catalytic efficiency of double and triple mutants of MnCcP.1. The observed experimental values are shown in solid red bars. On the left of each red column is shown the theoretical value if the effects of mutations were additive. The original MnCcP.1 value is shown in white.
Based on the activity results shown in Figure 4, we found Ile40Gly to be the most effective single mutation. However, when combined with either of the other single mutations (Tyr36Phe or Lys179Arg), we found that the effects of the double mutations were not additive (Figure 6), suggesting that the effect of Ile40Gly mutation on catalytic efficiency was diminished by combination with either of the other two mutations. In the case of the Ile40Gly/Lys179Arg double mutant, the decrease in the catalytic efficiency compared with Ile40Gly was mainly due to a larger KM (Table 1), suggesting that the Lys179Arg and Ile40Gly mutations work antagonistically toward enhancing Mn(II) binding. This explanation is reasonable because, while the Ile40Gly mutation increases the flexibility of the ligands in the loop to coordinate to Mn(II), the Lys179Arg mutation favors the formation of a salt bridge to the Glu37 ligand that rigidifies the loop. In contrast, while the Tyr36Phe/Ile40Gly double mutant displayed a KM similar to Ile40Gly alone, the kcat decreased by 2 fold. Since Tyr36 is positioned in the same loop as Ile40, and both mutations have the effect of increasing flexibility of the loop containing Glu37 and Glu45, it is possible that the combination of both mutations will lead to overall instability of the protein that lowers kcat.
The triple mutant Tyr36Phe/Ile40Gly/Lys179Arg displays a kcat similar to Ile40Gly alone, an effect consistent with the dominance of Ile40Gly for increasing kcat in all variants. However, KM of the triple mutant is higher than most other single and double variants except for Tyr36Phe/Lys179Arg. Such an increase in KM compared to other variants can be explained by an ultimately antagonistic effect from the combination of the three mutations even though the binding is enhanced compared to the initial MnCcP.1 variant.
Activity assays of the combined triple mutant, Tyr36Phe/Ile40Gly/Lys179Arg-MnCcP.1
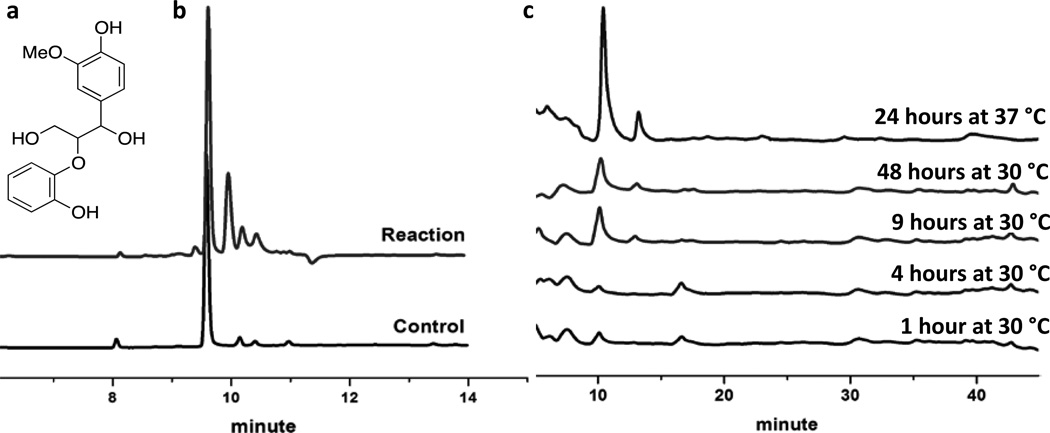
MnP exhibits its lignin depolymerization activity by oxidizing Mn(II) to Mn(III), which then diffuses out of the enzyme to degrade phenolic components of lignin, aided by organic acids secreted by the white rot fungus such as oxalate or malonate.27, 28, 64, 65 To demonstrate that our designed MnCcP.1 mutants display a similar activity toward lignin through Mn(III) released from the enzyme, we tested the activity of the triple mutant on a previously reported lignin model compound, guaiacylglycerl-β-guaiacol ether (figure 7a), and analyzed the resulting products by HPLC.57 As with other hybrid MnPs,66–69 our mutant was able to degrade guaiacylglycerl-β-guaiacol ether in the presence of Mn(II) (Figure 7b). We also assessed the activity of the triple mutant on kraft lignin with HPLC using previously reported methods.70 The protein showed degradation activity (Figure 7c). Even though the detected activity was low, these results indicate that our models are capable of lignin degradation. HPLC trace of alkali-treated lignin exposed to Tyr36Phe/Ile40Gly/Lys179Arg at different times monitored at 275 nm.
Figure 7.
(a) Phenolic lignin model compound. (b) The HPLC trace of degradation of the phenolic lignin model compound by Tyr36Phe/Ile40Gly/Lys179Arg monitored at 254 nm. (c)
CONCLUSION
By making Tyr36Phe, Lys179Arg, and Ile40Gly mutations within a region beyond the primary coordination shell of the Mn(II)-binding site in MnCcP.1, we have enhanced Mn(II)-binding affinity and MnP activity of an engineered CcP (MnCcP.1) that mimics MnP by removing a hydrogen bonding interaction, adding a salt bridge, and relieving steric clashes in the second sphere. Our endeavor has resulted in a set of variants with enhanced catalytic efficiencies over the previous design, in particular Ile40Gly-MnCcP.1 with almost 9-folds increase in the catalytic efficiency. Even though such enhancement is relatively modest in comparison with native enzymes, it is a significant step in the realm of protein design, as most designed proteins reported so far display low catalytic efficiency. Excitingly, the Ile40Gly-MnCcP.1 mutant also displays a KM value (0.28 mM) comparable to that of native Pleurotus eryngii MnP PS3 (0.2 mM),63 which shares a Mn-binding site nearly identical to other MnPs, including Phanerochaete chrysosporium MnP (see figure S2). Achieving a KM in a designed protein that is similar to native enzymes is a rare achievement and suggests that the geometry of the MnCcP Mn-binding site can be improved to better mimic that of native MnPs. More importantly, this work demonstrated that careful design of structural features in the second coordination sphere is critical to achieve the same level of KM as in the native enzymes.
We have also shown that while the combination of some mutations (e.g., Tyr36Phe and Lys179Arg) is additive, others are not. The Tyr36Phe and Lys179Arg mutations with different target residues (Glu45 and Glu37, respectively) result in additive effects while combinations of mutations that individually impact different aspects of ligand geometry at the same residues (e.g. Ile40Gly/Lys179Arg on Glu37) were found to be antagonistic. We further demonstrated that the designed CcP is a functional model of MnP that mimics its activity both in terms of Mn(II) oxidation and degradation of both a phenolic lignin model compound and alkali-treated lignin. These results indicate that the ability of primary ligands to coordinate Mn(II) is highly dependent on proper distance and orientation of the coordinating atoms, and even slight deviations from the optimal geometry can influence metal binding and activity. This study has deepened our understanding of the role of non-covalent interactions around the metal binding site and how to rationally modify them to improve metal binding and overall activity. The principles demonstrated by this study can be applied to enhance metal-binding affinity and activity of other enzymes and their biomimetic models.
Supplementary Material
Acknowledgments
The authors wish to acknowledge Prof. Michelle Chang and her student Margaret E. Brown for generously providing us with lignin model compounds. We also acknowledge Dr. Furong Sun, the director of mass-spectroscopy facility of UIUC and Dr. Zhong (Lucas) Li the director of metabolomics section at Carver Biotechnology center for their help in ESI-MS and HPLC, respectively. We thank Prof. Denmark, Department of Chemistry at UIUC, for our use of their GC-MS instrument. The authors wish to thank Dr. Igor Petrik for his help in refinement improvement.
Funding Sources
This material is based on work supported by the National Institutes of Health under award No. R01GM062211 (to Yi Lu) and P41-GM104601 (Emad Tajkhorshid and Christopher Mayne).
ABBREVIATIONS
- CcP
cytochrome c peroxidase
- MnP
manganese peroxidase
- H-bond
hydrogen bond
- H2O2
hydrogen peroxide
- MD
molecular dynamics
- VMD
Visual Molecular Dynamics
- ESI-MS
electron spray ionization mass spectroscopy
- HPLC
high performance liquid chromatography
- GC-MS
gas chromatograph-mass spectroscopy
Footnotes
ASSOCIATED CONTENT
Supporting Information
The supplemental material is available free of charge via the Internet at http://pubs.acs.org
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interests.
REFERENCES
- 1.Michel H, Behr J, Harrenga A, Kannt A. Cytochrome c oxidase: structure and spectroscopy. Annual Reviews of Biophysics. 1998;27:329–356. doi: 10.1146/annurev.biophys.27.1.329. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman BM, Lukoyanov D, Yang ZY, Dean DR, Seefeldt LC. Mechanism of nitrogen fixation by nitrogenase: the next stage. Chem. Rev. 2014;114:4041–4062. doi: 10.1021/cr400641x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dashtban M, Schraft H, Syed TA, Qin W. Fungal biodegradation and enzymatic modification of lignin. International Journal of Biochemistry and Molecular Biology. 2010;1:36. [PMC free article] [PubMed] [Google Scholar]
- 4.Reddi AR, Guzman TR, Breece RM, Tierney DL, Gibney BR. Deducing the energetic cost of protein folding in zinc finger proteins using designed metallopeptides. J. Am. Chem. Soc. 2007;129:12815–12827. doi: 10.1021/ja073902+. [DOI] [PubMed] [Google Scholar]
- 5.Reddi AR, Reedy CJ, Mui S, Gibney BR. Thermodynamic investigation into the mechanisms of proton-coupled electron transfer events in heme protein maquettes. Biochemistry. 2007;46:291–305. doi: 10.1021/bi061607g. [DOI] [PubMed] [Google Scholar]
- 6.Westerlund K, Moran SD, Privett HK, Hay S, Jarvet J, Gibney BR, Tommos C. Making a single-chain four-helix bundle for redox chemistry studies. Protein Eng. Des. Sel. 2008;21:645–652. doi: 10.1093/protein/gzn043. [DOI] [PubMed] [Google Scholar]
- 7.Pipirou Z, Guallar V, Basran J, Metcalfe CL, Murphy EJ, Bottrill AR, Mistry SC, Raven EL. Peroxide-dependent formation of a covalent link between Trp51 and the heme in cytochrome c peroxidase. Biochemistry. 2009;48:3593–3599. doi: 10.1021/bi802210g. [DOI] [PubMed] [Google Scholar]
- 8.Lin YW, Yeung N, Gao YG, Miner KD, Tian S, Robinson H, Lu Y. Roles of glutamates and metal ions in a rationally designed nitric oxide reductase based on myoglobin. Proc. Natl. Acad. Sci. U. S. A. 2010;107:8581–8586. doi: 10.1073/pnas.1000526107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson TD, Savelieff MG, Nilges MJ, Marshall NM, Lu Y. Kinetics of copper incorporation into a biosynthetic purple CuA azurin: characterization of red, blue, and a new intermediate species. J. Am. Chem. Soc. 2011;133:20778–20792. doi: 10.1021/ja205281t. [DOI] [PubMed] [Google Scholar]
- 10.Murphy EJ, Metcalfe CL, Nnamchi C, Moody PC, Raven EL. Crystal structure of guaiacol and phenol bound to a heme peroxidase. FEBS J. 2012;279:1632–1639. doi: 10.1111/j.1742-4658.2011.08425.x. [DOI] [PubMed] [Google Scholar]
- 11.Ivancich A, Donald LJ, Villanueva J, Wiseman B, Fita I, Loewen PC. Spectroscopic and kinetic investigation of the reactions of peroxyacetic acid with Burkholderia pseudomallei catalase-peroxidase, KatG. Biochemistry. 2013;52:7271–7282. doi: 10.1021/bi400963j. [DOI] [PubMed] [Google Scholar]
- 12.Casadei CM, Gumiero A, Metcalfe CL, Murphy EJ, Basran J, Concilio MG, Teixeira SC, Schrader TE, Fielding AJ, Ostermann A, Blakeley MP, Raven EL, Moody PC. Heme enzymes. Neutron cryo-crystallography captures the protonation state of ferryl heme in a peroxidase. Science. 2014;345:193–197. doi: 10.1126/science.1254398. [DOI] [PubMed] [Google Scholar]
- 13.Miner KD, Pfister TD, Hosseinzadeh P, Karaduman N, Donald LJ, Loewen PC, Lu Y, Ivancich A. Identifying the elusive sites of tyrosyl radicals in cytochrome c peroxidase: implications for oxidation of substrates bound at a site remote from the heme. Biochemistry. 2014;53:3781–3789. doi: 10.1021/bi500353p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Makhlynets OV, Raymond EA, Korendovych IV. Design of Allosterically Regulated Protein Catalysts. Biochemistry. 2015;54:1444–1456. doi: 10.1021/bi5015248. [DOI] [PubMed] [Google Scholar]
- 15.Loewen PC, Villanueva J, Switala J, Donald LJ, Ivancich A. Unprecedented access of phenolic substrates to the heme active site of a catalase: substrate binding and peroxidase-like reactivity of Bacillus pumilus catalase monitored by X-ray crystallography and EPR spectroscopy. Proteins. 2015;83:853–866. doi: 10.1002/prot.24777. [DOI] [PubMed] [Google Scholar]
- 16.Korendovych IV, DeGrado WF. Catalytic efficiency of designed catalytic proteins. Curr. Opin. Struct. Biol. 2014;27:113–121. doi: 10.1016/j.sbi.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Varadarajan R, Zewert TE, Gray HB, Boxer SG. Effects of buried ionizable amino acids on the reduction potential of recombinant myoglobin. Science. 1989;243:69–72. doi: 10.1126/science.2563171. [DOI] [PubMed] [Google Scholar]
- 18.Pascher T, Karlsson BG, Nordling M, Malmstrom BG, Vanngard T. Reduction potentials and their pH dependence in site-directed-mutant forms of azurin from Pseudomonas aeruginosa. Eur J Biochem. 1993;212:289–296. doi: 10.1111/j.1432-1033.1993.tb17661.x. [DOI] [PubMed] [Google Scholar]
- 19.Miller AF. Redox tuning over almost 1 V in a structurally conserved active site: lessons from Fe-containing superoxide dismutase. Acc Chem Res. 2008;41:501–510. doi: 10.1021/ar700237u. [DOI] [PubMed] [Google Scholar]
- 20.Marshall NM, Garner DK, Wilson TD, Gao Y-G, Robinson H, Nilges MJ, Lu Y. Rationally tuning the reduction potential of a single cupredoxin beyond the natural range. Nature. 2009;462:113–116. doi: 10.1038/nature08551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zuris JA, Halim DA, Conlan AR, Abresch EC, Nechushtai R, Paddock ML, Jennings PA. Engineering the redox potential over a wide range within a new Class of FeS proteins. J. Am. Chem. Soc. 2010;132:13120–13122. doi: 10.1021/ja103920k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lancaster KM. Encyclopedia of Inorganic and Bioinorganic Chemistry. John Wiley & Sons, Ltd; 2011. Copper Protein Variants: “Type Zero” Sites. [Google Scholar]
- 23.Hosseinzadeh P, Lu Y. Design and fine-tuning redox potentials of metalloproteins involved in electron transfer in bioenergetics. Biochimica et Biophysica Acta (BBA) - Bioenergetics. doi: 10.1016/j.bbabio.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yeung BKS, Wang X, Sigman JA, Petillo PA, Lu Y. Construction and characterization of a manganese-binding site in cytochrome c peroxidase: towards a novel manganese peroxidase. Chemistry & biology. 1997;4:215–221. doi: 10.1016/s1074-5521(97)90291-x. [DOI] [PubMed] [Google Scholar]
- 25.Wilcox SK, Putnam CD, Sastry M, Blankenship J, Chazin WJ, McRee DE, Goodin DB. Rational Design of a Functional Metalloenzyme: Introduction of a Site for Manganese Binding and Oxidation into a Heme Peroxidase. Biochemistry. 1998;37:16853–16862. doi: 10.1021/bi9815039. [DOI] [PubMed] [Google Scholar]
- 26.Boerjan W, Ralph J, Baucher M. Lignin biosynthesis. Annu. Rev. Plant Biol. 2003;54:519–546. doi: 10.1146/annurev.arplant.54.031902.134938. [DOI] [PubMed] [Google Scholar]
- 27.Wariishi H, Valli K, Gold MH. Manganese(II) oxidation by manganese peroxidase from the basidiomycete Phanerochaete chrysosporium. Kinetic mechanism and role of chelators. J. Biol. Chem. 1992;267:23688–23695. [PubMed] [Google Scholar]
- 28.Hofrichter M. Review: lignin conversion by manganese peroxidase (MnP) Enzyme Microb. Technol. 2002;30:454–466. [Google Scholar]
- 29.Annele H, Taina L, Martin H, Pekka M. Applications of Enzymes to Lignocellulosics. American Chemical Society; 2003. Manganese Peroxidase and Its Role in the Degradation of Wood Lignin; pp. 230–243. [Google Scholar]
- 30.Kishi K, Wariishi H, Marquez L, Dunford HB, Gold MH. Mechanism of Manganese Peroxidase Compound II Reduction. Effect of Organic Acid Chelators and pH. Biochemistry. 1994;33:8694–8701. doi: 10.1021/bi00195a010. [DOI] [PubMed] [Google Scholar]
- 31.Whitwam RE, Gazarian IG, Tien M. Expression of Fungal Mn Peroxidase in E. Coli and Refolding to Yield Active Enzyme. Biochem. Biophys. Res. Commun. 1995;216:1013–1017. doi: 10.1006/bbrc.1995.2721. [DOI] [PubMed] [Google Scholar]
- 32.Gu L, Lajoie C, Kelly C. Expression of a Phanerochaete chrysosporium Manganese Peroxidase Gene in the Yeast Pichia pastoris. Biotechnol. Progr. 2003;19:1403–1409. doi: 10.1021/bp025781h. [DOI] [PubMed] [Google Scholar]
- 33.Jiang F, Kongsaeree P, Schilke K, Lajoie C, Kelly C. Effects of pH and temperature on recombinant manganese peroxidase production and stability. Appl. Biochem. Biotechnol. 2008;146:15–27. doi: 10.1007/s12010-007-8039-5. [DOI] [PubMed] [Google Scholar]
- 34.Hurff SJ, Klein MT. Reaction pathway analysis of thermal and catalytic lignin fragmentation by use of model compounds. Ind. Eng. Chem. Fundam. 1983;22:426–430. [Google Scholar]
- 35.Futong C, David D. Plant Cell Wall Polymers. American Chemical Society; 1989. Biomimetic Studies in Lignin Degradation; pp. 519–528. [Google Scholar]
- 36.Alonso DM, Bond JQ, Dumesic JA. Catalytic conversion of biomass to biofuels. Green Chem. 2010;12:1493–1513. [Google Scholar]
- 37.Sergeev AG, Hartwig JF. Selective, Nickel-Catalyzed Hydrogenolysis of Aryl Ethers. Science. 2011;332:439–443. doi: 10.1126/science.1200437. [DOI] [PubMed] [Google Scholar]
- 38.Parsell TH, Owen BC, Klein I, Jarrell TM, Marcum CL, Haupert LJ, Amundson LM, Kenttamaa HI, Ribeiro F, Miller JT, Abu-Omar M. Cleavage and hydrodeoxygenation (HDO) of C-O bonds relevant to lignin conversion using Pd/Zn synergistic catalysis. Chem. Sci. 2013;4:806–813. [Google Scholar]
- 39.Strassberger Z, Alberts AH, Louwerse MJ, Tanase S, Rothenberg G. Catalytic cleavage of lignin small beta]-O-4 link mimics using copper on alumina and magnesia-alumina. Green Chem. 2013;15:768–774. [Google Scholar]
- 40.Li C, Zhao X, Wang A, Huber GW, Zhang T. Catalytic Transformation of Lignin for the Production of Chemicals and Fuels. Chem. Rev. 2015;115:11559–11624. doi: 10.1021/acs.chemrev.5b00155. [DOI] [PubMed] [Google Scholar]
- 41.Yan N, Zhao C, Dyson PJ, Wang C, Liu LT, Kou Y. Selective degradation of wood lignin over noble-metal catalysts in a two-step process. ChemSusChem. 2008;1:626–629. doi: 10.1002/cssc.200800080. [DOI] [PubMed] [Google Scholar]
- 42.Singh R, Grigg JC, Qin W, Kadla JF, Murphy MEP, Eltis LD. Improved Manganese-Oxidizing Activity of DypB, a Peroxidase from a Lignolytic Bacterium. ACS Chem. Biol. 2013 doi: 10.1021/cb300608x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Finzel BC, Poulos TL, Kraut J. Crystal structure of yeast cytochrome c peroxidase refined at 1.7-A resolution. J. Biol. Chem. 1984;259:13027–13036. [PubMed] [Google Scholar]
- 44.Sundaramoorthy M, Kishi K, Gold MH, Poulos TL. The crystal structure of manganese peroxidase from Phanerochaete chrysosporium at 2.06-A resolution. J. Biol. Chem. 1994;269:32759–32767. [PubMed] [Google Scholar]
- 45.Sundaramoorthy M, Youngs HL, Gold MH, Poulos TL. High-Resolution Crystal Structure of Manganese Peroxidase:Substrate and Inhibitor Complexes. Biochemistry. 2005;44:6463–6470. doi: 10.1021/bi047318e. [DOI] [PubMed] [Google Scholar]
- 46.Volkov AN, Wohlkonig A, Soror SH, van Nuland NA. Expression, purification, characterization, and solution nuclear magnetic resonance study of highly deuterated yeast cytochrome c peroxidase with enhanced solubility. Biochemistry. 2013;52:2165–2175. doi: 10.1021/bi400220w. [DOI] [PubMed] [Google Scholar]
- 47.Pfister T, Mirarefi A, Gengenbach A, Zhao X, Danstrom C, Conatser N, Gao Y-G, Robinson H, Zukoski C, Wang A, Lu Y. Kinetic and crystallographic studies of a redesigned manganese-binding site in cytochrome c peroxidase. J. Biol. Inorg. Chem. 2007;12:126–137. doi: 10.1007/s00775-006-0171-0. [DOI] [PubMed] [Google Scholar]
- 48.Gengenbach A, Syn S, Wang X, Lu Y. Redesign of Cytochrome c Peroxidase into a Manganese Peroxidase: Role of Tryptophans in Peroxidase Activity. Biochemistry. 1999;38:11425–11432. doi: 10.1021/bi990666+. [DOI] [PubMed] [Google Scholar]
- 49.Pfister TD, Gengenbach AJ, Syn S, Lu Y. The Role of Redox-Active Amino Acids on Compound I Stability, Substrate Oxidation, and Protein Cross-Linking in Yeast Cytochrome c Peroxidase. Biochemistry. 2001;40:14942–14951. doi: 10.1021/bi011400h. [DOI] [PubMed] [Google Scholar]
- 50.Winn MD, Ballard CC, Cowtan KD, Dodson EJ, Emsley P, Evans PR, Keegan RM, Krissinel EB, Leslie AGW, McCoy A, McNicholas SJ, Murshudov GN, Pannu NS, Potterton EA, Powell HR, Read RJ, Vagin A, Wilson KS. Overview of the CCP4 suite and current developments. Acta. Cryst. 2011;D67:235–242. doi: 10.1107/S0907444910045749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roy BP, Dumonceaux T, Koukoulas AA, Archibald FS. Purification and Characterization of Cellobiose Dehydrogenases from the White Rot Fungus Trametes versicolor. Appl Environ Microbiol. 1996;62:4417–4427. doi: 10.1128/aem.62.12.4417-4427.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.de Duve C. Spectrophotometric method for the simultaneous determination of myoglobin and hemoglobin in extracts of human muscle. Acta Chem. Scand. 1948;2:264–289. doi: 10.3891/acta.chem.scand.02-0264. [DOI] [PubMed] [Google Scholar]
- 53.Morrison M, Horie S. Determination of heme a concentration in cytochrome preparations by the hemochromogen method. Anal. Biochem. 1965;12:77–82. doi: 10.1016/0003-2697(65)90144-2. [DOI] [PubMed] [Google Scholar]
- 54.Phillips JC, Braun R, Wang W, Gumbart J, Tajkhorshid E, Villa E, Chipot C, Skeel RD, Kale L, Schulten K. Scalable molecular dynamics with NAMD. J. Comput. Chem. 2005;26:1781–1802. doi: 10.1002/jcc.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Humphrey W, Dalke A, Schulten K. VMD: visual molecular dynamics. J. Mol. Graph. 1996;14:33–38. 27–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- 56.Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW, Klein ML. Comparison of Simple Potential Functions for Simulating Liquid Water. J. Chem. Phys. 1983;79:926. [Google Scholar]
- 57.Brown ME, Barros T, Chang MC. Identification and characterization of a multifunctional dye peroxidase from a lignin-reactive bacterium. ACS Chem. Biol. 2012;7:2074–2081. doi: 10.1021/cb300383y. [DOI] [PubMed] [Google Scholar]
- 58.Sundaramoorthy M, Kishi K, Gold MH, Poulos TL. Preliminary Crystallographic Analysis of Manganese Peroxidase from Phanerochaete chrysosporium. J. Mol. Biol. 1994;238:845–848. doi: 10.1006/jmbi.1994.1338. [DOI] [PubMed] [Google Scholar]
- 59.Ju KS, Parales RE. Control of substrate specificity by active-site residues in nitrobenzene dioxygenase. Appl. Environ. Microbiol. 2006;72:1817–1824. doi: 10.1128/AEM.72.3.1817-1824.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fukumoto M, Kudou D, Murano S, Shiba T, Sato D, Tamura T, Harada S, Inagaki K. The role of amino acid residues in the active site of L-methionine gamma-lyase from Pseudomonas putida. Biosci. Biotechnol. Biochem. 2012;76:1275–1284. doi: 10.1271/bbb.110906. [DOI] [PubMed] [Google Scholar]
- 61.Gelpke MDS, Youngs HL, Gold MH. Role of arginine 177 in the MnII binding site of manganese peroxidase. Eur. J. Biochem. 2000;267:7038–7045. doi: 10.1046/j.1432-1327.2000.01798.x. [DOI] [PubMed] [Google Scholar]
- 62.Sollewijn Gelpke MD, Moenne-Loccoz P, Gold MH. Arginine 177 is involved in Mn(II) binding by manganese peroxidase. Biochemistry. 1999;38:11482–11489. doi: 10.1021/bi990943c. [DOI] [PubMed] [Google Scholar]
- 63.Sarkar S, Martinez AT, Martinez MJ. Biochemical and molecular characterization of a manganese peroxidase isoenzyme from Pleurotus ostreatus. Biochim.Biophys.Acta. 1997;1339:23–30. doi: 10.1016/s0167-4838(96)00201-4. [DOI] [PubMed] [Google Scholar]
- 64.Kuan IC, Tien M. Stimulation of Mn peroxidase activity: a possible role for oxalate in lignin biodegradation. Proc. Natl. Acad. Sci. 1993;90:1242–1246. doi: 10.1073/pnas.90.4.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Onnerud H, Zhang L, Gellerstedt Gr, Henriksson G. Polymerization of Monolignols by Redox Shuttle-Mediated Enzymatic Oxidation: A New Model in Lignin Biosynthesis I. Plant Cell. 2002;14:1953–1962. doi: 10.1105/tpc.001487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hofrichter M, Scheibner K, Schneegass I, Fritsche W. Enzymatic combustion of aromatic and aliphatic compounds by manganese peroxidase from Nematoloma frowardii . Appl. Environ. Microbiol. 1998;64:399–404. doi: 10.1128/aem.64.2.399-404.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moreira MT, Sierra-Alvarez R, Lema JM, Feijoo G, Field JA. Oxidation of lignin in eucalyptus kraft pulp by manganese peroxidase from Bjerkandera sp. strain BOS55. Bioresour. Technol. 2001;78:71–79. doi: 10.1016/s0960-8524(00)00161-9. [DOI] [PubMed] [Google Scholar]
- 68.Cullen D. The mycota : a comprehensive treatise on fungi as experimental systems for basic and applied research. Berlin; London: Springer, Agricultural applications; 2002. Molecular genetics of lignin-degrading fungi and their applications in organopollutant degradation; p. 71. [Google Scholar]
- 69.Mendonça Maciel M, Castro e Silva A, Camarão Telles Ribeiro H. Industrial and biotechnological applications of ligninolytic enzymes of the basidiomycota: A review. Electron. J. Biotechno. 2010:13. [Google Scholar]
- 70.Bugg TDH, Ahmad M, Hardiman EM, Rahmanpour R. Pathways for degradation of lignin in bacteria and fungi. Nat. Prod. Rep. 2011;28:1883–1896. doi: 10.1039/c1np00042j. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.