Abstract
Eustrongylides spp. is considered a freshwater fish zoonotic nematode. In the present study, the prevalence of Eustrongylides spp. in six edible fish (European perch - Perca fluviatilis, goldfish - Carassius auratus, largemouth black bass - Micropterus salmoides, tench- Tinca tinca, carp - Cyprinus carpio and sand smelt - Atherina boyeri) of Trasimeno lake was surveyed. The investigations were conducted from October 2014 to September 2015 and 384 specimens per species for each season were caught in Trasimeno lake and examined for the presence of larvae in the abdominal cavity and muscle. The presence of nematodes in the abdominal cavity and musculature was revealed in three fish species. The prevalence of Eustrongylides spp. infection was 6.84, 1.89 and 0.13% in perch, largemouth black bass and sand smelt, respectively. The number of parasites per fish was only one in largemouth black bass and sand smelt and ranged from one up to three in perch. This study states that the European perch, largemouth black bass and sand smelt of Trasimeno lake are infected with zoonotic parasites; therefore, food business operators have to take appropriate measures to guarantee the health of consumers.
Key words: Eustrongylides spp, Freshwater fish, Nematode larvae, Parasitic hazards
Introduction
Changes in food habits/tastes in recent years have led to an increase in the consumption of raw fish and less cooked fish products and this new tendency has increased the risk of exposure of the consumer to parasitic hazards (Broglia and Kapel, 2011; Ljubojevic et al., 2015; Ferrantelli et al., 2014). Nematodes of the genus Eustrongylides can be the causative agent of a zoonotic disease that includes infections by nematodes having larval stages in aquatic hosts. In humans who have consumed raw or undercooked freshwater fish, five-stage Eustrongylides spp. larvae ingested along with fish can produce gastritis and intestinal perforation (Ljubojevic et al., 2015).
The life cycle of Eustrongylides spp. includes five stages from the egg to the sexually mature worm. The first larval stage develops within the egg and is shed in the faeces by the infected bird, then is ingested by aquatic oligochaetes, such as Lumbriculus variegatus, Tubifex or Limnodrilus spp., which are the first intermediate hosts. Inside the oligochaete, the parasite develops into the second and third larval stages (Bjeli - abrilo et al., 2013). The second intermediate hosts are planktivorous and benthivorous fishes in which the third-stage larvae transform and moult into the fourth larval stage and remain in the fish, most frequently in the muscles, to be ingested by wading birds such as cormorant, the definite hosts (Moravec, 1994; Spalding and Forrester, 2008; Cole, 2009). Predatory fish, such as perch, which consume infected fish, can serve as paratenic or transport hosts and are capable of infecting birds or (accidentally) humans through the consumption of raw or undercooked freshwater fish.
Eustrongylides spp. has been recognized as zoonotic parasite (Centers for Disease Control, 1982; Eberhard et al. 1989; Wittner et al., 1989; Narr et al., 1996) that may pose a public health risk to consumers. More recently, the European Commission established that food business operators must ensure that fishery products have been subjected to a visual examination with the purpose of detecting visible parasites before being placed on the market (European Commission, 2005). Recently, the first recorded presence of Eustrongylides spp. in Italy was reported in the muscle of European perch fish caught from lake Trasimeno (Dezfuli et al., 2015).
The aim of this survey was to assess the prevalence of Eustrongylides spp. in six edible freshwater fish species caught from Trasimeno lake to support the existence of emerging problems regarding the presence of Eustrongylides in these fish products, whose consumption is increasing as a result of consumer demand.
Materials and Methods
Sample collection and preparation
Fishes were collected on Trasimeno lake from monthly catches by local fishermen between October 2014 and September 2015 with no samples taken during the closed season for each species. The species considered were: perch (Perca fluviatilis, Linnaeus length 19.96± 1.37 cm), goldfish (Carassius auratus Linnaeus, average length 23.83± 1.77), largemouth black bass (Micropterus salmoides, Lacépède, average length 20.43± 1.52 cm), tench (Tinca tinca, Linnaeus, average length 38.08± 2.28 cm), carp (Cyprinus carpio, Linnaeus average length 73.8± 11.62 cm), and sand smelt (Atherina boyeri, Risso, average length 5± 0.98 cm).
A total of 1536 fish for each species considered were sampled and examined during the year (384 samples for each season). Only seven hundred sixty-eight sand smelt were sampled and examined because the catching period of this species in Trasimeno lake is limited to autumn and winter. All fish were caught using different centimetre mesh sizes of gillnets and pots (gillnets of 24, 28, 70 and 90 mm, respectively; pots of 6 mm for sand smelt) that were used in the fishing industry at Trasimeno lake. All fish samples were transported to a local approved establishment and a visual inspection was carried out as recommended by European Commission (2004 and 2005). The nematode larvae recovered from fish were prepared for scanning electron microscopy in order to characterize the parasite. To this end, the larvae were fixed for 2 h at 4°C in 2.5% glutaraldehyde in 0.1 M phosphate buffer (PB), pH 7.3. The nematode was then rinsed in PB, dehydrated through a graded ethanol series ending with absolute ethanol and critical-point dried. Finally mounted on metal stubs, coated with gold to a thickness of 15 nm and examined with a Philips XL30 scanning electron microscope (Philips, Amsterdam, The Netherlands). The determination of genus was completed by morphometric analysis according to keys provided by Moravec (1994), Anderson (2000) and Dezfuli et al. (2015).
In European perch larvae encysted in the muscles appeared as nodular structures that were removed from the fillets during the postmortem evaluation, fixed with 10% neutral buffered formalin for histological analysis. After fixation, they were dehydrated through an alcohol series ending with absolute alcohol, cleared in xylene and paraffin embedded. Five-micron-thick sections were cut from each tissue block and stained with haematoxylin and eosin (H&E), periodic acid Schiff and Masson’s trichrome.
Statistical analysis
The prevalence and 95% confidence intervals (CI) of Eustrongylides spp. were estimated in fish species by an exact method based on the binomial distribution. For the evaluation of the differences in Eustrongylides spp. prevalence among fish species and the effect of the season, chi-squared and Fisher’s exact tests were used. The results for each variable were expressed as P values and odds ratios (OR) with a CI. All statistical analyses were performed using the WINPEPI (PEPI-for Windows) freeware epidemiological software with the P value set to 0.05.
Results and Discussion
The prevalence of Eustrongylides spp. in the six fish species considered are reported in Table 1. Eustrongylides spp. were found in three species: European perch, largemouth black bass and sand smelt, with the highest prevalence (6.84%) in European perch followed by largemouth black bass (1.89%). In sand smelt, the nematode was found in only one sample of the 764 analysed (0.13% prevalence) (Figure 1). The estimated odds ratio for acquiring Eustrongylides infection in Perch was 3.81 times higher than in black bass (Table 2). The parasite prevalence did not show a seasonal pattern in perch or black bass. Seasonality might not be expected in fish caught on Trasimeno lake because Eustrongylides eggs are shed by the final hosts throughout the year and they may develop and hatch at any time. Furthermore, the nematode eggs remain viable and infective for up to two years, the larvae can survive in the intermediate host for a period of more than one-year and on arrival into the definitive host, the development is promptly completed (Bjeli - abrilo et al., 2013). The mean intensity proved to be the highest in European perch (1.3%), with the number of parasites per fish ranging from one to three. The fourth larval stage of Eustrongylides spp. recovered from European perch and largemouth black bass had an average body length of 4 cm (ranging from 30 to 50 mm) and a 0.5-1 mm maximum width.
Table 1.
Prevalence (%) of Eustrongylides spp. in six different fish species caught in Trasimeno lake.
| Common name | Scientific name | Body lenght | Prevalence (%) | CI 95% min | CI 95% max | MI |
|---|---|---|---|---|---|---|
| Perch | Perca fluviatilis | 19.90±1.37 | 6.84 | 5.57 | 8.10 | 1.30 |
| Largemouth black bass | Micropterus salmoides | 20.43±1.52 | 1.89 | 1.21 | 2.57 | 1.00 |
| Carp | Cyprinus carpio | 73.80± 11.60 | 0 | nd | nd | nd |
| Goldfish | Carassius auratus | 23.83±1.77 | 0 | nd | nd | nd |
| Tench | Tinca tinca | 38.08±2.28 | 0 | nd | nd | nd |
| Sand smelt | Atherina boyeri | 5.00±0.92 | 0.13 | 0 | 0.39 | 1.00 |
CI, confidence interval; MI, mean intensity; nd, not detected.
Figure 1.
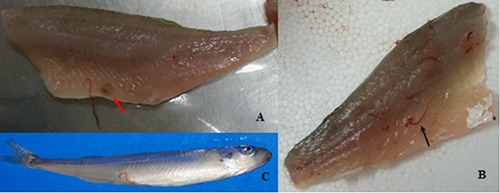
Photograph of the fish species parasitized: A) European perch fillet infected by the larvae of Eustrongylides spp. and capsula (red arrow); B) largemouth black bass fillet infected by the larvae of Eustrongylides spp. (black arrow); C) sand smelt infected by the larvae of Eustrongylides spp. showing the larva protruding from the anus.
Table 2.
Chi square test, odd ratio and odd ratio confidence intervals of perch vs largemouth black bass.
| χ2 | P | OR | CI 95% min | CI 95% max | |
|---|---|---|---|---|---|
| Perch vs larger nouth black bass | 45.07 | <0.001 | 3.81 | 2.51 | 5.79 |
OR, odd ratio; CI, confidence interval.
The cephalic extremity of the larva showed a small oral cavity surrounded by 12 spike-like cephalic papillae distributed in two concentric circles. The papillae of the inner circle were smaller, while those of the outer circle were larger and delimited by a circular grove. The posterior end of the larva was roundish and devoid of any formation. The parasite body was covered by a transversely striated cuticle. Based on their morphology, the nematodes were identified as fourth-stage larvae of Eustrongylides spp. (Figure 2).
Figure 2.
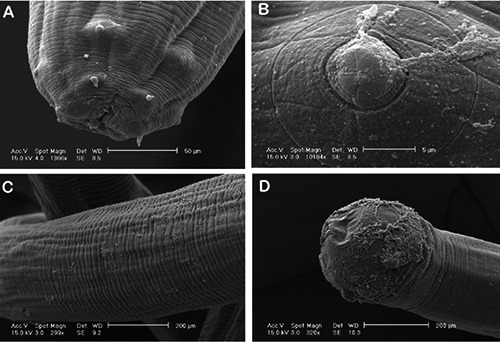
Scanning electron micrographs of Eustrongylides spp. fourth-stage larva. A) Cephalic end: oral orifice, surrounded by cephalic papillae, scale bar = 50 μm; B) cephalic papilla of external circle, high magnification, scale bar = 5 μm; C) middle part of the worm showing transverse striations of the cuticle, scale bar = 200 μm; D) rounded posterior end of the larva, scale bar = 200 μm.
The nematodes in perch were found to be encapsulated or free in the musculature and body cavity (Figures 1 and 3). The presence of capsules in perch muscle is in agreement with the observations of Dezfuli et al. (2015). Inside the nodules, a single, reddish, coiled larva was present. The histological observation revealed that the nodules were granulomas resulting from the intense host response elicited by the worm larvae. In the central part of some granulomas, the parasites often appeared structurally intact, with a well-defined cuticle. Occasionally, the larvae appeared degenerated. The capsule wall was mostly composed of fibrous connective tissue characterized by a mixed cellular infiltration mainly consisting of macrophages, lymphocytes and a low number of eosinophils. Neoformed microvessels were detected in the capsule wall. Degeneration and necrosis of adjacent striated muscle fibres were observed (Figure 3).
Figure 3.
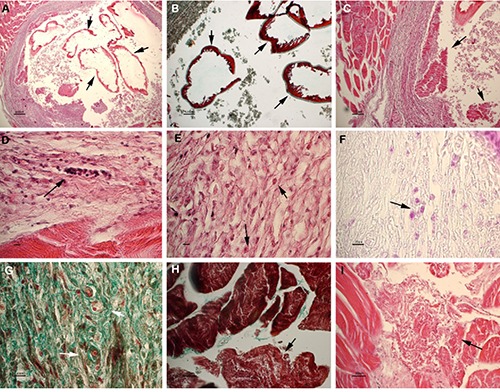
Larva encapsulated in host muscle. A-B) Transverse section of the parasite showing intact cuticular layers (arrows). A) Haematoxylin-eosin (HE), scale bar = 200 μm; B) masson trichrome stain (MT), scale bar 100= pm. C) The arrows point out degenerating and necrotic larvae comprised inside the lumen of the capsule. HE, scale bar = 100 μm. DF) Magnification of the connective capsule surrounding the larva. Arrows point out lymphocytes (D, HE, scale bar = 10 μm), eosinophils (E, HE, scale bar = 10 μm) and macrophages (F, PAS, scale bar = 20 μm) infiltrating the capsule. G) Neoformed microvessels are evident in the wall of the capsule. MT, scale bar = 20 μm. H-I) Degenerating and necrotic muscle fibers surrounding the capsule. H) MT, scale bar=30 μm. I) HE, scale bar = 30 μm.
In perch, Eustrongylides spp. probably start to migrate at the muscular level when the host is still alive. On the other hand, in largemouth black bass, the larvae were never found encapsulated but only free in the musculature. Probably in this fish species, larval migration occurs after the death of the fish (Figure 2). No larvae of Eustrongylides were found in the muscle or abdominal cavity of tench, carp and goldfish. In the sand smelt, only one subject was found to be parasitized by Eustrongylides spp. (Figure 3). The presence of Eustrongylides was also observed by Çolak (2013), who reported the presence of larvae of Eustrongylides excisus in sand smelt caught in a Iznik lake in Turkey. The author also studied the feeding habits of sand smelt and found that this species is an opportunistic carnivore that feeds on both planktonic and benthonic prey during different seasons. In particular, the study reveals a higher prevalence of Eustrongylides in winter when sand smelt feed on zoobenthos the infected oligochaete. The lack of study on the feeding habits of Trasimeno lake sand smelt make difficult to understand the sources of infection in this species. Atherina boyeri is a natural perch prey and therefore, it can be considered an important host in the developmental cycle of the nematode. It is possible that sand smelt can harbour the nematode in the intestinal wall and as they are the prey of piscivorous fish species, they can transmit the infection to predator fish, which themselves become infected by ingesting infected fishes (Moravec, 1994). Other benthos-eating species present in the lake, which are the second intermediate hosts of the nematode, can harbour the parasite and could contribute significantly to the nutrition of the predator, as reported by Bjeli - abrilo et al. (2013).
Conclusions
Eustrongylides specimens are typically very long, coiled, and red (due to the presence of haemoglobin), clearly differentiable from the tissues of the fish, even in the absence of optical instruments and the visual inspection easily allowed the removal of visible parasites. According to European Commission (European Commission, 2004, 2005) food producers should ensure that fishery products have been subjected to visual examination with the purpose of detecting visible parasites before being placed on the market. The disruption of the parasite life cycle is of the utmost importance for preventing Eustrongylides infections in freshwater fish, nonetheless this goal is difficult to achieve based on several factors: the length of time that the eggs can remain viable and intermediate hosts can remain infective; the rather rapid maturation of the parasite (once it is inside the definitive bird host); and the long time period over which intermediate and paratenic hosts can remain infected. A visual examination carried out by the food business operator followed by proper food preparation should be the best ways to avoid Eustrongylides spp. hazards to human health.
Acknowledgements
The authors would like to express their sincere gratitude to the Fisheries Consortium of the Lake Trasimeno for providing technical support.
References
- Anderson RC, 2000. Nematode parasites of vertebrates. Their development and transmission. 2nd ed. CAB International Publishing, Wallingford, UK. [Google Scholar]
- Bjeli - abrilo O, Novakov N, irkovi M, Kosti D, Popovi E, Aleksi N, Luji J, 2013. The first determination of Eustrongylides excisus Jägerskiöld, 1909: larvae (Nematoda: Dioctophymatidae) in the pike-perch Sander lucioperca in Vojvodina (Serbia). Helminthologia 50:291-4. [Google Scholar]
- Broglia A, Kapel C, 2011. Changing dietary habits in a changing world: emerging drivers for the transmission of foodborne parasitic zoonoses. Vet Parasitol 182:2-13. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control, 1982. Intestinal perforation caused by larval Eustrongylides – Maryland. MMWR Morb Mortal Wkly Rep 31:383-4. [PubMed] [Google Scholar]
- Çolak SÖ, 2013. The helminth community of the sand smelt (Atherina boyeri Risso, 1810) from Lake Iznik, Turkey. J Helminthol 87:129-34. [DOI] [PubMed] [Google Scholar]
- Cole RA, 2009. Eustrongylidosis. Available from: www.nwhc.usgs.gov/publications/field_manual/chapter_29.pdf [Google Scholar]
- Dezfuli BS, Manera M, Lorenzoni M, Pironi F, Shinn AP, Giari L, 2015. Histopathology and the inflammatory response of European perch, Perca fluviatilis muscle infected with Eustrongylides spp. (nematode). Parasite Vector 8:227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhard ML, Hurwitz H, Sun AM, Coletta D, 1989. Intestinal perforation caused by larval Eustrongylides (Nematoda: Dioctophymatoidae) in New Jersey. Am J Trop Med Hyg 40:648-50. [DOI] [PubMed] [Google Scholar]
- European Commission, 2004. Regulation of the European Parliament and of the Council of 29 April 2004 laying down specific hygiene rules for Laying down specific hygiene rules for the hygiene of foodstuffs, 853/2004/CE. In: Official Journal, L139/55, 30/4/2004. [Google Scholar]
- European Commission, 2005. Commission Regulation of 5 December 2005 N°2074/2005 laying down implementing measures for certain products under Regulation (EC) No 853/2004 of the European Parliament and of the Council and for the organization of official controls under Regulation (EC) No 854/2004 of the European Parliament and of the Council and Regulation (EC) No 882/2004 of the European Parliament and of the Council, derogating from Regulation (EC) No 852/2004 of the European Parliament and of the Council and amending Regulations (EC) No 853/2004 and (EC) No 854/2004 (EC 853/2004 rev). In: Official Journal, L 238, 22.12.2005. [Google Scholar]
- Ferrantelli V, Cicero A, Costa A, Alongi A, Palumbo P, Graci S, Giangrosso G, 2014. Anisakidae in fishing products sold in Sicily. Ital J Food Safety 3:47-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljubojevic D, Novakov N, Djordjevic V, Radosavljevic V, Pelic M, Cirkovic M, 2015. Potential parasitic hazards for humans in fish meat. Procedia Food Sci 5:172-5. [Google Scholar]
- Moravec F, 1994. Parasitic nematodes of freshwater fishes of Europe. Kluwer Academic Publishers, Berlin, Germany. [Google Scholar]
- Narr LL, O’Donnell JG, Libster B, Alessi P, Abraham D, 1996. Eustrongylidiasis – a parasitic infection acquired by eating live minnows. J Am Ost Assoc 96:400-2. [PubMed] [Google Scholar]
- Spalding MD, Forrester GJ, 2008. Eustrongylidosis. Parasitic diseases of wild birds. Wiley Blackwell Publishing, Ames, IA, USA. [Google Scholar]
- Wittner M, Turner JW, Jacquette G, Ash LR, Salgo MP, Tanowitz HB, 1989. Eustrongylidiasis – a parasitic infection acquired by eating sushi. New Engl J Med 320:1124-6. [DOI] [PubMed] [Google Scholar]


