Abstract
An extremely rare case of non-mucinous lepidic-predominant invasive adenocarcinoma (LPA) showing extensive aerogenous spread with a pneumonic presentation is reported. A 73-year-old woman was referred to our hospital because of an infiltrative shadow on chest xray. Chest computed tomography revealed extensive ground glass opacities in the right lower lobe, which was accompanied by infiltrative shadow with a pneumonic presentation. Invasive mucinous adenocarcinoma was presumed, and a partial resection of the right lower lobe was done. Histopathological examination revealed lepidic growth-predominant invasive adenocarcinoma with Clara type tumor cells, and there were innumerable aerogenous metastases also consisting of Clara cells. Because Alcian Blue and periodic acid-Schiff staining disclosed no mucus, the tumor was diagnosed as a non-mucinous LPA. The patient showed a poor response to 5 courses of pemetrexed, and she died one year after the diagnosis due to cancer progression. Nonmucinous LPA showed a rare presentation characterized by extensive aerogenous spread followed by a poor prognosis.
Key words: Non-mucinous lepidic-predominant adenocarcinoma, Aerogenous spread, Invasive mucinous adenocarcinoma
Introduction
Invasive mucinous adenocarcinomas (IMAs) are frequently accompanied by intrapulmonary metastases via aerogenous spread, and they are known to present with multifocal and centrilobular opacities on computed tomography (CT), reflecting aerogenous spread.1-4 IMAs also present with ground glass opacities (GGOs) with ill-defined margins and infiltrative shadows within (so-called pneumonic presentation), which originate from mucin filling the alveoli.3,4
An extremely rare case of lepidic-predominant invasive adenocarcinoma (LPA) showing extensive aerogenous spread with a pneumonic presentation despite being non-mucinous is reported.
Case Report
Presentation
A 73-year-old woman, a never smoker, came to her home doctor because of cough, wheezing and bronchorrhea lasting for the last 6 months. Infiltrative shadow in the right lower lung field was shown on chest X-ray. Although she was treated as pneumonia for 2 months, her symptoms did not relieve, that she referred to our hospital for further examination. Chest CT revealed multifocal and centrilobular GGO in bilateral lung. Particularly, ill-defined GGO in the right lower lobe was extensive and accompanied by infiltrative shadow with air-bronchogram presenting socalled pneumonic presentation (Figure 1A). No significant lymph node swelling was seen. Laboratory studies disclosed following elevated values: carcinoembryonic antigen (CEA) 14.7 ng/mL, lactate dehydrogenase 216 IU/L, KL-6 3990U/mL, SP-D 135ng/mL. Bronchoscopic findings demonstrated marked respiratory secretion in bilateral bronchus, but no visible lesions were detected. Considering the characteristic presentations including bronchorrhea as a clinical presentation, extensive GGO on chest CT and elevated value of CEA, invasive mucinous adenocarcinoma (IMA) was strongly suspected other than interstitial pneumonitis, organized pneumonia and pulmonary proteinosis as differeintial diagnosis. Because transbronchial lung biopsy aimed for the GGO in the right lower lobe failed to confirm diagnosis, biopsy under video-assisted thoracic surgery was conducted. Intraoperative findings included rather stiffen pulmonary parenchyma but no visible nodules on visceral or parietal pleura. Partial resection of peripheral portion of the right lower lobe which corresponded to GGO area on chest CT was done. The frozen section diagnosis of the surgical specimen was proven to be adenocarcinoma that we diagnosed as surgical stage of sT3N0M1a.
Figure 1.
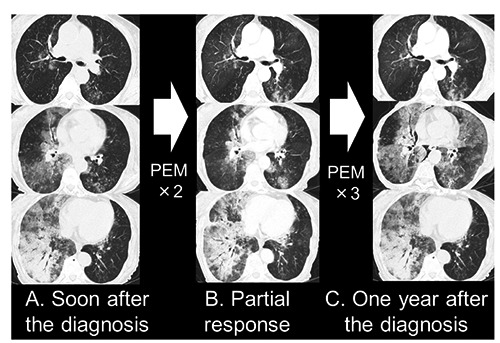
A) Chest computed tomography shows multifocal and centrilobular ground glass opacities (GGOs) in bilateral lungs. The GGO in the right lower lobe is particularly extensive and accompanied by an infiltrative shadow with an air bronchogram, the so-called pneumonic presentation. B) Two courses of pemetrexed (PEM) brought partial response temporally. C) Following three courses resulted in drastic progressive disease.
Pathological findings
The greater portion of the ill-defined tumor was occupied with a lepidic growth structure in which tumor cells grew along the surface of alveolar walls (Figure 2A). However, in contrast to adenocarcinoma in situ, an obvious invasive area, which was greater than 5 mm in maximal diameter, was observed (Figure 2B). On higher magnification, the lepidic structure showed tumor cells with hobnail projections facing the alveolar lumen; the tumor cells were considered to be Clara cell type (Figure 2C). Detached from the main tumor, there were innumerable lepidic or papillary lesions that were segregated from each other (arrows) (Figure 2D), and some of these lesions located close to peripheral bronchiole airways (arrow) (Figure 2E). That indicates detached tumor cells transferred from the main tumor via bronchiole airways and grew up at peripheral pulmonary parenchyma. These isolated lesions consisting of Clara type tumor cells, similar to those observed in the main tumor, were deemed to be aerogenous metastases (Figure 2F). Because the resected specimen included only the peripheral portion of the right lower lobe that corresponded to the GGO part on CT, histopathological evidence reflecting the infiltrative shadow in the rather deep pulmonary parenchyma observed on CT was not available. Although IMA was presumed in the preoperative differential diagnosis, the most unexpected point that needs to be emphasized is that specific stainings, including Alcian Blue (Figure 3A) and periodic acid-Schiff (PAS), disclosed no mucus either in the alveolar lumen or in the cytoplasm of the tumor cells. Based on immunohistochemistry (IHC) findings, including that the tumor cells were positive for TTF-1 (Figure 3B) and CK-7 (Figure 3C) and negative for CK-20 (Figure 3D), the tumor was diagnosed as a non-mucinous lepidic-predominant invasive adenocarcinoma.
Figure 2.

A) An ill-defined tumor [hematoxylin and eosin (H&E) staining; magnification 12 × ] is predominantly occupied with lepidic growth structure. B) In the lepidic growth structure, an obvious invasive area, which was greater than 5mm in maximal diameter, was observed (H&E staining; magnification 200 ×). C) On higher magnification, the lepidic structure shows Clara type tumor cells with hobnail projections (H&E staining; magnification 400×). D) Innumerable isolated lepidic or papillary growth lesions detached from the main tumor (arrows) (H&E staining; magnification 12 ×). E) Some of these lesions locate close to peripheral bronchiole airways (arrow) (H&E staining; magnification 12x). F) These isolated lesions consist of Clara type tumor cells, that they are confirmed to be aerogenous metastases (H&E staining; magnification 400 ×).
Figure 3.

A) Alcian Blue discloses no mucus either in the alveolar lumen or the cytoplasm of the tumor cells. The tumor cells are: B) TTF-1-positive; C) CK-7-positive; and D) CK- 20-negative.
Genetic analysis
The polymerase chain reaction-reverse sequence specific oligonucleotide (PCR-rSSO) method showed no KRAS mutation (including codons 12, 13, 59, 61, 117, 146), which supported the diagnosis of non-mucinous lepidic-predominant invasive adenocarcinoma. Other genetic analyses were also negative for epidermal growth factor receptor (EGFR) mutations on PCR testing and anaplastic lymphoma kinase fusion genes on fluorescent in situ hybridization.
Postoperative course
Considering the tumor’s genetic status and the patient’s poor performance status, singleagent pemetrexed (PEM) (500 mg/m2) was administered soon after the diagnosis. The 2 initial courses of PEM brought partial remission of the pneumonic presentation on CT (Figure 1B), but the patient showed a poor response to the following 3 courses, resulting in progressive disease (Figure 1C). Further treatment was not applied due to decreased performance status, and the patient died one year after the diagnosis because of respiratory failure attributed to cancer progression.
Discussion
This extremely rare case demonstrated that non-mucinous LPA can present with extensive aerogenous spread with a pneumonic presentation on CT that is rather commonly the representative characteristic of IMAs.
The preoperative clinical features of the present case included prominent bronchorrhea and multicentric and centrilobular GGOs with a pneumonic presentation on chest CT, very similar to the typical features of IMAs,1-4 but unexpectedly, the resected specimen showed histopathological characteristics of non-mucinous LPAs with significant aerogenous spread that was considered to be a quite unusual finding. In general, typical IMAs frequently present with aerogenous spread, recognized as multicentric and centrilobular GGOs on CT,1-4 while typical non-mucinous LPAs barely show aerogenous spread, especially to the extensive degree seen in the present case.3 In the current case, innumerable disrupted lepidic or papillary growth lesions consisting of Clara type tumor cells with identical morphology to that of the main tumor were confirmed to be aerogenous spread reflecting the multicentric GGOs on CT. The pneumonic presentation defined by ill-defined GGOs and infiltrative shadows with air bronchograms is another representative radiological characteristic of IMAs also explained by mucin production and aerogenous spread.3,4 As mentioned above, although the radiological appearance of the present case corresponded to that of IMAs, histopathological findings showed Clara type tumor cells that are specific to non-mucinous lepidic growth adenocarcinomas.2 Travis refers to immunohistochemical and genetic differences between IMAs and non-mucinous LPAs that positive rates of CK-20 and TTF-1 in IMAs are 50 and 15% respectively, whereas positive rates of these IHC markers in non-mucinous LPAs are 5 and 65% respectively. In addition to that, 75% of IMAs are KRAS-positive, while only 15% of non-mucinous LPAs are KRAS-positive.2 In the present case, the IHC pattern revealed positive staining for TTF-1 and CK-7 and negative staining for CK-20. Moreover, KRAS mutation was not detected by genetic analysis. According to Travis, these findings also suggested non-mucinous LPA. Above all, Alcian Blue and PAS stainings showed no mucinous component, which led to the definite diagnosis of non-mucinous LPA.
There are only a few known cases in which non-mucinous LPA presented with extensive aerogenous spread. Kodama reported a case of non-mucinous bronchoalveolar carcinoma (BAC) with extensive aerogenous spread showing a pneumonic presentation.5 Kodama suggested that the infiltrative shadow seen in the pneumonic presentation might originate from cuboidal or round tumor cells he discovered floating in the alveolar space.5 Colby also reported a similar case in which detached tumor cells filled the alveolar space, although they did not describe the detailed tumor cell morphology.6 In the present case, it was not possible to detect any histopathological findings reflecting infiltrative shadow in the pneumonic presentation that existed in the deep pulmonary parenchyma, because the surgical specimen was obtained from the peripheral portion of the right lower lobe corresponding to the GGO area on CT.
Although the pneumonic presentation itself is not an unusual finding in ordinary nonmucinous LPAs, as Duruisseaux reported that non-mucinous LPAs accoundted 32% of lung adenocarcinomas presenting pneumonic presentation,4 main etiology of pneumonic presentation may be different between ordinary nonmucinous LPAs and IMAs respectively. Commonly, in contrast with that the etiology of infiltrative shadows in the pneumonic presentation observed in ordinary non-mucinous LPAs are mainly explained by consolidated tumor or collapsed pulmonary parenchyma, infiltrative shadows in IMAs mainly originate from mucin production by tumor cells filling the alveoli.3,4 In the former two cases of nonmucinous LPA presented with extensive aerogeneous spread, detached tumor cells floating in the alveolar space were considered to be the origin of the infiltrative shadow in the pneumonic presentation; therefore, it is possible that non-mucinous LPAs with extensive aerogeneous spread including the present case may have an etiology of the pneumonic presentation similar to that of IMAs. To the best of our knowledge, and with the exception of the present case, the two above-mentioned cases are the only documented reports of non-mucinous LPA with extensive aerogenous spread.5,6
Several studies have addressed the mechanisms of aerogenous spread observed in lepidic growth adenocarcinomas, formerly called BACs. Kodama reported that aerogenous spread of BACs was significantly correlated with laminin-5 expression, which is responsible for construction of the basement membrane.7 While other studies found E-cadherin downregulation, type IV collagenase expression with low levels of alpha-2 integrin receptor expression may be related to the ability of the mucinous BAC tumor cells to detach from the basement membrane and spread aerogenously.8,9 There is a possibility that the present case might reflect the pathogenic factors of aerogenous spread discovered by various investigators above.
Aerogenous spread itself is defined as a poor prognostic factor, since it represents the aggressive clinical course of IMAs showing rapid and extensive progression.2,3,10 The present case showing a poor response to cytotoxic chemotherapy followed by a poor clinical outcome indicates that aerogenous spread may be a prominent factor related to a poor prognosis also in cases of non-mucinous LPAs.
Conclusions
Non-mucinous LPA can present with extensive aerogenous spread with a pneumonic presentation on CT. The present case showed a poor response to cytotoxic chemotherapy and was followed by a poor prognosis with extremely aggressive progression of aerogeneous spread.
Acknowledgements
The authors would like to express their appreciation for the technical assistance provided by Ms. Toyoko Mizuno.
References
- 1.Gaikwad A, Souza CA, Inacio JR, et al. Aerogenous metastases: a potential game changer in the diagnosis and management of primary lung adenocarcinoma. AJR Am J Roentgenol 2014;203:570-82. [DOI] [PubMed] [Google Scholar]
- 2.Travis WD, Brambilla E, Burke AP, et al. WHO classification of tumours of the lung, pleura, thymus and heart. 4th ed. Lyon, France: Cedex; 2015. pp 38-40. [DOI] [PubMed] [Google Scholar]
- 3.Truini A, Santos Pereira P, Cavazza A, et al. Classification of different patterns of pulmonary adenocarcinomas. Expert Rev Respir Med 2015;9:571-86. [DOI] [PubMed] [Google Scholar]
- 4.Duruisseaux M, Antoine M, Rabbe N, et al. The impact of intracytoplasmic mucin in lung adenocarcinoma with pneumonic radiological presentation. Lung Cancer 2014;83:334-40. [DOI] [PubMed] [Google Scholar]
- 5.Kodama T, Kameya T, Shimosato Y, et al. Cell incohesiveness and pattern of extension in a rare case of bronchioloalveolar carcinoma. Ultrastruct Pathol 1980;1:177-88. [DOI] [PubMed] [Google Scholar]
- 6.Colby TV, Koss MN, Travis WD. Bronchioloalveolar carcinoma: atlas of tumor pathology. Tumors of the lower respiratory tract. Washington DC: AFIP; 1995. pp 203-34. [Google Scholar]
- 7.Kodama K, Ishii G, Miyamoto S, et al. Laminin 5 expression protects against anoikis at aerogenous spread and lepidic growth of human lung adenocarcinoma. Int J Cancer 2005;116:876-84. [DOI] [PubMed] [Google Scholar]
- 8.Hidaka N, Nagao T, Asoh A, et al. Expression of E-cadherin, alpha-catenin, beta-catenin, and gamma-catenin in bronchioloalveolar carcinoma and conventional pulmonary adenocarcinoma: an immunohistochemical study. Mod Pathol 1998;11: 1039-45. [PubMed] [Google Scholar]
- 9.Ohori NP, Yousem SA, Griffin J, et al. Comparison of extracellular matrix antigens in subtypes of bronchioloalveolar carcinoma and conventional pulmonary adenocarcinoma. An immunohistochemical study. Am J Surg Pathol 1992;16:675-86. [DOI] [PubMed] [Google Scholar]
- 10.Geles A, Gruber-Moesenbacher U, Quehenberger F, et al. Pulmonary mucinous adenocarcinomas: architectural patterns in correlation with genetic changes, prognosis and survival. Virchows Arch 2015;467:675-686. [DOI] [PubMed] [Google Scholar]


