Abstract
The nuclear receptor transcription factor, nor-1, is expressed during mammalian development predominantly in the nervous system and is induced in a cell-specific manner in nonneuronal cells in response to a variety of extracellular stimuli. To elucidate the essential developmental functions of this transcription factor, we have analyzed the consequences of its elimination on central nervous system development in mice. Here we show that null mutant mice lacking nor-1 respond with increased limbic seizure activity to the excitotoxic glutamate receptor agonist kainic acid. We demonstrate that these abnormalities are associated with defective postnatal hippocampal development exemplified by abnormal axonal guidance of dentate gyrus granule and mossy cells, disorganization of the pyramidal cell layer, and early postnatal death of pyramidal neurons in the CA1 field of the hippocampus. Our data indicate that nor-1 plays a critical role in neuronal survival and axonal guidance in the developing murine hippocampus and that hippocampal dysgenesis in nor-1−/− mice may be an underlying cause of seizure susceptibility.
nor-1 (NR4A3) is a member of the nuclear receptor family of transcription factors, whose developmental and physiological functions are poorly understood. nor-1 (NR4A3) and two highly homologous transcription factors, nurr1 (NR4A2) and nur77 (NR4A1), constitute the NR4A subfamily of nuclear receptors (2). Unlike most nuclear receptors, the regulator function of NR4A receptors is constitutively active and does not require ligand modulation (17, 40, 43). All three proteins are products of immediate-early genes whose transcription is rapidly induced in response to a variety of extracellular stimuli, including mitogens, apoptotic signals, and neurotransmitters (reviewed in reference 21). The induced nuclear receptors undergo site-specific phosphorylation to modulate their transcriptional activity (8, 14, 27) and bind to specific cis-acting DNA elements containing the sequence motif AAAGGTCA to regulate the expression of defined sets of target genes in a stimulus- and cell-specific manner (17, 26, 43).
Previous studies have suggested that members of the NR4A subfamily of nuclear receptors, including nor-1, play key roles in mediating neuronal differentiation during central nervous system (CNS) development and in activity-dependent maintenance of neuronal plasticity in the adult CNS. For example, expression of nurr1 in the ventral midbrain is essential for embryonic specification of dopaminergic neurons (5, 33, 46). All three NR4A receptors are expressed in the limbic system, including hippocampal pyramidal neurons of the adult CNS (32, 47), suggesting a potential involvement in the regulation of long-term changes in gene expression associated with learning and memory. In addition, their expression is rapidly and transiently induced in both pyramidal neurons and dentate granule cells of the hippocampus in experimental animal models of temporal lobe epilepsy (TLE), including chemically and electrically induced limbic seizure activity (7, 28, 42, 44).
Pathological seizure activity in both human TLE and experimental rodent models of TLE is associated with region-specific cell death and lasting synaptic reorganization of hippocampal circuitry of the mature CNS (3, 4, 39). These changes require new protein synthesis and are associated with a rapid upregulation of immediate-early gene products, among which are several classes of transcription factors whose activity is thought to mediate the long-term changes in synaptic plasticity associated with pathological seizure activity (22). Consistent with this hypothesis, several members of the AP-1 class of transcription factors, including c-jun and c-fos, are induced by seizures, and inhibition of c-fos (41) or the c-jun-activating kinase jnk3 (45) results in a reduction in seizure activity and associated pathological synaptic plasticity. The rapid seizure-dependent upregulation of NR4A receptors in the hippocampus predicts that this subfamily of nuclear receptor transcription factors may also contribute to the regulation of specific gene programs whose function is to modulate activity-dependent long-term synaptic plasticity. However, elucidation of the specific roles of these receptors in hippocampal plasticity is complicated by their overlapping spatiotemporal expression and activity-dependent induction patterns in the adult hippocampus and by their ability to regulate overlapping genes through interaction with common cis-acting DNA elements. The potential for functional redundancy among NR4A nuclear receptors in mediating epileptogenesis is also underscored by the observation that the development of electrically induced seizure activity (kindling) and its associated synaptic reorganization are unaffected in mice lacking nur77 (48).
To further explore the role of NR4A nuclear receptors in the hippocampus, we have analyzed the selective contribution of nor-1 to hippocampal development and seizure-associated synaptic plasticity by examination of the consequences of its elimination in mice. Previously, our laboratory demonstrated that null mutation of the nor-1 gene in mice (nor-1−/−) did not affect postnatal viability but resulted in abnormal vestibular function due to defective outgrowth of the semicircular canals of the inner ear (30). In the present study, we demonstrate that inhibition of nor-1 expression also results in defective axonal growth and region-specific cell death in the hippocampus during the early postnatal stages of development. The developmental defects persist to adulthood and are associated with lasting changes in hippocampal excitability and increased susceptibility of nor-1−/− mice to chemically induced seizures.
MATERIALS AND METHODS
Animals.
The generation and genotyping of mice lacking a functional nor-1 gene (nor-1−/−) has been previously described (30). The mice contained the lacZ reporter gene inserted into the nor-1 locus. Mixed-strain C57BL/6/129Sv mice were used in these studies from two independent embryonic stem cell clone lines. Only heterozygous animals (interbred >8 generations) were used in breeding, and nor-1−/− offspring and their wild-type littermates were used in the experiments.
All protocols involving the use of animals were in compliance with the National Research Council's Guide for the Care and Use of Laboratory Animals (23a) and the Baylor College of Medicine Institutional Animal Care and Use Committee. The tests were performed with the experimenter being blind with respect to the genotype.
Induction of limbic seizures.
Kainic acid (KA; Sigma) was administered intraperitoneally in saline at 15, 20, or 25 mg/kg of body weight. After KA injection, seizure activity was observed for 45 min and scored and staged by a blinded observer as previously described (23) according to the following stages: stage 0, no effect; 1, motionless; 2, rigid posture with forelimb and/or tail extension; 3, myoclonic jerks of head and neck; 4, forelimb clonus and partial rearing; 5, forelimb clonus, rearing, and falling; 6, generalized tonic-clonic convulsions with loss of postural tone.
Terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) assay for apoptosis.
The brains were fixed overnight in 10% formalin, processed to paraffin, and cut to 6 μm. For staining, the sections were rehydrated and digested with proteinase K (40 ng/ml) for 7 min. After washes with phosphate-buffered saline, the sections were blocked with TdT buffer (30 mM Tris [pH 7.4], 140 mM Na-cacodylate, 1 mM CaCl2) and incubated with TdT-dUTP mix (8 μl of UTP and 6 μl of TdT [Roche] in 1,200 μl of TdT buffer) for 1 h at 37°C. The signal was detected by using Vectastain Elite and peroxidase substrate kits (Vector). Methyl green was used as a counterstain.
Immunohistochemistry.
Specimens were fixed in Bouin's fixative overnight, washed with 70% ethanol, processed for paraffin sectioning, and cut to 6 μm. Staining was done in accordance with standard avidin-biotin immunohistochemical procedures (Vector). Rabbit polyclonal immunoglobulin Gs against calretinin and calbindin (1:500 dilution) were from Calbiochem.
β-Galactosidase staining.
Brains were embedded in Tissue-Tec OCT compound and cut to 20 μm. The sections were fixed in 2% formaldehyde-0.2% glutaraldehyde for 15 min and rinsed with phosphate-buffered saline. The staining solution consisted of 1.3 mM MgCl2, 15 mM NaCl, 44 mM HEPES (pH 7.9), 3 mM potassium ferro- and ferricyanides, and 0.5 mg of 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside/ml in H2O.
Silver staining.
Sevier-Munger silver staining (Polyscientific) was performed according to the manufacturer's recommendation on Bouin's fixed 6-μm sections.
In situ hybridization.
Twenty-micrometer frozen coronal sections were cut and processed using nurr1 and nur77 probes as described elsewhere (32), with the exception of using digoxigenin-labeled probes (10 ng/slide). For signal development, a substrate solution (BCIP/NBT kit IV; Vector) was applied and incubated in a moist chamber in the dark.
Cell counts and statistical analysis.
For cell counting in the CA1 hippocampal field, ×400 images were captured from coronal brain sections (nine images from each animal) at the level of the arch of the dorsal hippocampus, and all cells in the image were counted using the Imagetools program. Clearly visible split nuclei were included in the count. TUNEL-positive cells were counted from the whole dorsal CA1 area (nine sections from each animal). Data collected from each experimental group are expressed with the standard error of mean. Student's t test was used for statistical analysis (n = 6 except for 12-month-old animals, for which n = 4).
RESULTS
nor-1−/− mice have increased susceptibility to KA-induced limbic seizures.
Observation of adult nor-1−/− mice revealed that a small percentage exhibited brief freezing spells with tonic posturing that were exacerbated with handling. To elucidate the neurological basis of this behavior, we examined the seizure susceptibility of nor-1−/− mice to a convulsant stimulus. Adult wild-type (nor-1+/+) and nor-1−/− mice were injected intraperitoneally with KA (25 mg/kg), which elicits limbic seizures by stimulating glutamate receptors and by increasing the release of excitatory amino acids (20). The development of behavioral seizures was scored as previously described (23), and the latency of progression to a stage 6 seizure was used to assess seizure susceptibility. While both nor-1+/+ and nor-1−/− mice exhibited freezing spells that progressed to head bobbing, forepaw tremor, and loss of postural control, nor-1−/− mice had a significantly shorter latency to continued convulsions than nor-1+/+ mice (Fig. 1A). In addition, they developed several maximal seizures during the experiment, while most of the nor-1+/+ mice developed only one stage 6 tonic-clonic convulsion (Fig. 1B). nor-1−/− mice also exhibited enhanced sensitivity to KA relative to nor-1+/+ mice; four out of seven nor-1−/− mice died after continuous tonic-clonic convulsions, whereas only one out of eight nor-1+/+ mice died. In addition, when injected with a lower dosage of KA (15 mg/kg), six out of eight nor-1−/− mice and only two out of nine nor-1+/+ mice developed seizures (data not shown). These results demonstrate an essential role for nor-1 in modulating the severity of convulsant stimulus-induced limbic seizure activity.
FIG. 1.
Increased susceptibility of nor-1−/− mice to KA-induced seizures. The latency to a stage 6 behavioral score was used to assess seizure susceptibility. (A and B) nor-1−/− mice showed a heightened susceptibility to KA-induced seizures by reaching stage 6 tonic-clonic convulsion faster than the nor-1+/+ mice (A) and developed several seizures during the time observed (45 min) (B). Error bars represent the means ± standard errors of the mean. *, P < 0.05 (Student's t test). (C to F) nor-1 expression in the developing hippocampus. Sagittal (C) and coronal (D to F) sections of heterozygous nor-1lacZ brains were processed for β-galactosidase staining to establish the expression pattern for nor-1 in the hippocampus. At embryonic day 14.5, nor-1 was expressed in the primitive plexiform layer of the hippocampus (Hc) and the outermost lamina in the neocortex (Cx). At P0, all the principal hippocampal cell layers had formed, and nor-1 expression was evident in the pyramidal cell layer of the subiculum (S) and CA1-CA3. In the dentate gyrus, nor-1 was expressed in the granule cell layer (Gcl) and in the hilar (H) mossy cells. nor-1 was retained in all these areas in the adult hippocampus, shown at the dorsal (E) and ventral (F) levels. Bar, 500 μm (C) and 250 μm (D to F).
To determine whether nor-1 is expressed during development of the limbic system, we examined its expression during the embryonic and postnatal stages of hippocampal development. For these analyses, we used β-galactosidase as a reporter for nor-1 promoter activity in heterozygous nor-1 mutant mice carrying the lacZ reporter gene inserted into the nor-1 locus (30). Expression of β-galactosidase at embryonic day 14.5 was observed in the primitive plexiform layer of the hippocampus, which is a continuation of the outermost lamina in the neocortex (Fig. 1C). By postnatal day 0 (P0), after the granule cell layers of the dentate gyrus begin to develop at late gestation, nor-1 expression was observed in both the pyramidal and granule cell layers (Fig. 1D). The majority of granule cells develop postnatally during the first 3 weeks of life, and nor-1 expression persisted in these cells in the adult hippocampus after their postnatal maturation (Fig. 1E). In addition, nor-1 expression was also observed in the hilar mossy cells of the dentate gyrus, which form a second major synaptic relay system within this structure (Fig. 1F).
Disorganization and postnatal neuronal cell loss in the pyramidal layer of the hippocampus in nor-1−/− mice.
To examine the structural integrity of the hippocampus, coronal sections of the hippocampus of adult nor-1−/− mice were compared with those of nor-1+/+ littermates. Analysis of these sections indicated that both the pyramidal and granule cell layers were formed in nor-1−/− mice (Fig. 2B); however, the pyramidal cell layer appeared less dense than that observed in nor-1+/+ littermates (Fig. 2A). No overt abnormalities in either organization or cell number were observed in the granule cell layer of the dentate gyrus (Fig. 2C and D). In contrast, further examination of the hippocampal subfields at higher magnification revealed that a compact pyramidal cell layer of Ammon's horn was not formed in any of the nor-1−/− mice analyzed (n = 15), as shown at the levels of CA3 (Fig. 2F) and CA1 (Fig. 2H) when compared with nor-1+/+ littermates (Fig. 2E and G). Further, pyramidal cell disorganization was accompanied by an apparent decrease in the total number of cells in the CA1 region of nor-1−/− mice (Fig. 2H) relative to that in nor-1+/+ mice (Fig. 2G), but not in the CA3 region (Fig. 2F versus E).
FIG. 2.
Abnormal pyramidal cell layer in nor-1−/− mice, as shown in H&E-stained coronal sections of nor-1+/+ hippocampus (A, C, E, and G) and nor-1−/− hippocampus (B, D, F, and H). Both the pyramidal (CA1 to CA3) and granule cell (Gcl) layers were formed in nor-1−/− mice (B). The granule cell layer of the hippocampus appeared normal in the nor-1−/− mice (D); however, the pyramidal cell layer appeared disorganized both at the level of CA3 (F) and CA1 (H). Bars, 100 μm (A and B) and 25 μm (C to H).
To confirm the apparent decrease in pyramidal cell numbers in the CA1 field and to establish the developmental cause of these defects, we quantitated the numbers of pyramidal neurons in the CA1 region in nor-1+/+ and nor1−/− mice (Fig. 3) during early postnatal development. Examination of coronal sections of newborn animals (P0) showed that the nor-1−/− CA1 pyramidal cell layer (Fig. 3E) was comparable to that in nor-1+/+ mice (Fig. 3A), with similar cell numbers observed in both genotypes (Fig. 3K). By P7, however, the disorganized phenotype of the CA1 pyramidal cell layer became evident in nor-1−/− animals (Fig. 3F). The cellular loss was maximal at the second (Fig. 3G) and third (Fig. 3H) postnatal weeks and persisted to adulthood (Fig. 3K). Interestingly, this loss of the pyramidal CA1 neurons in the nor-1−/− mice was restricted to the early postnatal stages of development and did not progress with age (Fig. 3K).
FIG. 3.
Cell loss due to increased apoptosis during development of the CA1 pyramidal cells. The CA1 pyramidal layer appeared normal in newborn nor-1−/− mice (E) compared to nor-1+/+ mice (A). However, disorganization of the cell layer that was observed at P7 (F) became more severe by P14 (G) and P21 (H). In addition, a decrease in cell number was significant at P14 and persisted in the adult nor-1−/− mice (K). *, P < 0.05 (Student's t test). TUNEL labeling analysis of nor-1+/+ and nor-1−/− CA1 regions showed a small but significant increase in the number of apoptotic cells at P0, and cell death was highly increased at P5 in the nor-1−/− mice (J and L). Inset in J is a 1,250× magnification of the TUNEL-positive cells. By P7, apoptosis in the nor-1−/− CA1 pyramidal cell layer was decreased to the level of the nor-1+/+ mice (L). Bars, 25 μm (A to H) and 50 μm (I and J).
To investigate whether the loss of CA1 pyramidal cells in the nor-1−/− mice was a result of postnatal cell death, we next performed TUNEL staining to monitor the degree of apoptosis occurring in CA1 pyramidal neurons at the early postnatal stages before the cell loss in these mice became evident. The results (Fig. 3I, J, and L) indicated a significant increase in apoptotic cells in the CA1 region of nor-1−/− mice relative to that in nor-1+/+ mice as early as P0 (Fig. 3L). The increases in apoptosis in nor-1−/− sections were maximal at P5 (Fig. 3L) and, from P7 onwards, only occasional TUNEL-positive cells were found in sections from both genotypes. Thus, the decrease in pyramidal neurons observed in the CA1 region of nor-1−/− mice appears to be the result of enhanced cell death that is restricted to the early postnatal stages of development.
Finally, in contrast to the CA1 field, similar analysis of the CA3 region of nor-1−/− mice indicated that despite a clear disorganization of pyramidal cells, no cellular loss was observed in this region (data not shown), indicating a specific role for nor-1 in the survival of CA1 pyramidal cells.
Defective axonal guidance in the dentate gyrus of nor-1−/− mice.
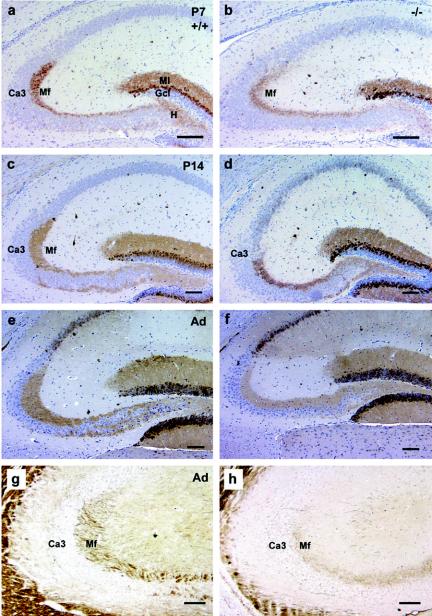
Because both mossy and granule cells of the dentate gyrus play a critical role in regulation of hippocampal circuits associated with epileptogenesis, we next examined the integrity of both populations of neurons and their axonal projections. Mossy fibers represent the axonal projections of the dentate granule cells that develop postnatally and form synapses with apical dendrites of CA3 pyramidal cells and with excitatory mossy cells in the dentate hilus (1). To examine the integrity of this system, we used calbindin immunostaining to label granule cells and their axonal and dendrite projections (36). These analyses (Fig. 4A to F) revealed normal immunostaining patterns in the granule cell somata and of their dendrites in the molecular layer in both neonatal (P7) (Fig. 4A and B) and P14 (Fig. 4C and D) and adult (Fig. 4E and F) nor-1+/+ and nor-1−/− mice. However, a significant decrease in the density of immunostaining in mossy fiber projections to the CA3 area was observed in the nor-1−/− mice at P7 and persisted through adulthood (Fig. 4E and F). This reduction in mossy fiber projections to the CA3 pyramidal neurons in the nor-1−/− mice was confirmed by neuronal process-specific Sevier-Munger silver staining, which showed a marked decrease in staining of axonal projections in nor-1−/− mice (Fig. 4H) relative to that in nor-1+/+ mice (Fig. 4G).
FIG. 4.
Reduced outgrowth of granule cell mossy fibers (arrows in E and F) into CA3 stratum lucidum. Whereas calbindin staining in the granule cell bodies (Gcl) and molecular layer (Ml) was normal, the granule cell mossy fibers (Mf) extending toward CA3 pyramidal cell layer were reduced at P7 (B), P14 (D), and in the adult (F) nor-1−/− mice. This reduction in the outgrowth of mossy fibers in the nor-1−/− mice was confirmed by Sevier-Munger silver staining (H). nor-1+/+ controls are shown in panels A, C, E, and G. Bars, 100 μm.
In order to assess the morphology of the second major excitatory pathway in the dentate gyrus, the hilar mossy cell system, we used immunostaining for calretinin, whose expression in the adult hippocampus is localized to hilar mossy cells and widely distributed subsets of GABAergic interneurons (18). Glutamatergic mossy cells in the dentate hilus give rise to the commissural-associational system that sends axonal projections to both the contralateral and ipsilateral dentate gyrus, where they form synaptic connections with both excitatory granule cells and inhibitory interneurons located in the inner molecular layer of the dentate gyrus (15). At P14, calretinin staining was observed in hilar mossy cell soma, newly generated immature granule cells located in the subgranular zone at the hilar border (18), and in mossy cell axonal projections located in the inner molecular layer (IML) of the dentate gyrus of wild-type mice (Fig. 5A). This staining pattern was similar to that in nor-1−/− mice (Fig. 5B), with the exception of the mossy cell axon belt, which showed markedly reduced staining relative to that in nor-1+/+ mice. As development proceeded to adulthood, calretinin immunostaining was extinguished in mature granule cells but was increased in the mossy cell axon belt that comprises the supra- and infrapyramidal blades of the dentate gyrus of nor-1+/+ mice (Fig. 5C and E). In contrast, calretinin staining was almost completely lost in the suprapyramidal blade (Fig. 5D and F) and was decreased in the inferior pyramidal blade (Fig. 5D) of the nor-1−/− dentate gyrus.
FIG. 5.
Reduced outgrowth of mossy cell axons. By P14, calretinin-positive mossy cell axons had reached the inner molecular layer (Iml) of the granule cell layer (Gcl) in the nor-1+/+ dentate gyrus (A), whereas only a weak calretinin staining could be seen in the nor-1−/− mice (B). An even more obvious decrease in these calretinin-positive mossy cell axons was seen in the adult nor-1−/− dentate gyrus (D). (F) Higher magnification of the suprapyramidal blade (Spb), which was almost devoid of calretinin-positive axons in the nor-1−/− dentate gyrus. No difference was found between nor-1+/+ and nor-1−/− mice in the number of calretinin-positive mossy cell bodies in the hilus of the ventral hippocampus, where the majority of these cells are located (G and H), or in the commissural fibers that cross the midline at the level of the hippocampal commissure (I and J), indicating a specific reduction in mossy cell associational fibers in the nor-1−/− mice. Bars, 100 μm (A to D and G to J) and 25 μm (E and F).
To determine whether the loss of calretinin staining in the mossy cell axons was due to reduced differentiation of mossy cells, we also examined the distribution of calretinin-positive cells in sections obtained from the ventral hippocampus, where the majority of these neurons reside. In contrast to the reduced calretinin immunoreactivity observed in the mossy cell axons innervating the IML, there was no reduction in the number of mossy cell soma in the nor-1−/− (Fig. 5H) relative to that in the nor-1+/+ (Fig. 5G) ventral hippocampus. These data indicate that in the absence of nor-1, mossy cells appear to migrate to their appropriate positions but are defective in axonal outgrowth. Finally, to determine whether the commissural axons from mossy cells were capable of crossing the midline to the contralateral hippocampus, we visualized these projections by calretinin staining at the level of the hippocampal commissure. Comparison of nor-1+/+ (Fig. 5I) and nor-1−/− (Fig. 5J) mice confirmed that these axons crossed the midline, indicating that the defect in mossy cell axon outgrowth appears to specifically disrupt mossy fiber innervation of the suprapyramidal blade of the IML of the dentate gyrus (11, 24, 25).
Since we have used mixed-background 129Sv/C57BL/6 mice in our studies, we next asked whether the major defects we observed in nor-1−/− mice relative to wild-type littermates may have been due in part to strain variations between the parental strains. In these experiments, we compared hippocampal regions relating to each of the defects in adult parental C57BL/6 and 129Sv mouse strains. No differences in the pyramidal CA1 region between strains were observed by using hematoxylin and eosin (H&E) staining (Fig. 6A and B). Secondly, immunohistochemical analysis with calretinin (Fig. 6C and D) and calbindin (Fig. 6E and F) showed no overt differences in the mossy cell axon bundle in the suprapyramidal blade of the dentate gyrus or in the suprapyramidal mossy fiber pathway to the CA3 region, respectively.
FIG. 6.
Analysis of the parental C57BL/6 (A, C, and E) and 129Sv (B, D, and F) strain hippocampus. (A and B) No overt differences were found between these two strains in the CA1 field using H&E staining. (F) In addition, immunohistochemical analysis using calretinin (C and D) and calbindin (E and F) showed no overt differences in the mossy cell axons in the suprapyramidal blade of the dentate gyrus or in the suprapyramidal mossy fiber pathway to the CA3 region, respectively.
Nonredundancy of function of NR4A receptors during hippocampal development.
To determine whether nor-1 plays a distinct functional role in hippocampal development relative to its closely related subfamily members, nurr1 and nur77, we used in situ hybridization to examine the comparative spatiotemporal expression of the two NR4A receptors in the hippocampus at P5, when the neurodevelopmental defects become apparent in nor-1−/− mice. Our results showed that while nur77 was not expressed in the hippocampus at this developmental stage (data not shown), nurr1 expression could be observed in the CA1 field of the pyramidal cell layer (Fig. 7A) of nor-1+/+ mice and was retained in this region in nor-1−/− mice (Fig. 7B). This early postnatal pattern of expression of nurr1 in CA1 pyramidal neurons was consistent with that previously reported by others (47) but was unable to compensate for the loss of nor-1 in preventing cell death of CA1 pyramidal neurons. The expression of nurr1 persisted in pyramidal neurons of the adult hippocampus (Fig. 7C), and the expression of nur77 overlapped with nurr1 in this region (Fig. 7D). In contrast, the level of both receptors in the adult dentate gyrus was extremely low to undetectable, consistent with the lack of expression of either receptor observed in this region during early postnatal development. Treatment with KA resulted in robust induction of both nurr1 (Fig. 7E) and nur77 (Fig. 7G) in the dentate gyrus of nor-1+/+ mice as has been reported previously (7, 16). Significantly, however, this induction was unaffected in nor-1−/− mice (Fig. 7F and H), indicating that nurr1 and nur77 induction in the adult hippocampus is unable to compensate for earlier developmental defects that contribute to enhanced seizure susceptibility in nor-1−/− mice.
FIG. 7.
nurr1 and nur77 expression in the hippocampus. nurr1 was expressed in the pyramidal cell layer at P5 (A), and its expression was unchanged in nor-1−/− mice (B). nurr1 (C) and nur77 (D) are expressed in adult hippocampus. Both nurr1 and nur77 were induced by KA injection (20 mg/kg) in the dentate gyrus in adult nor-1+/+ mice (E and G) and to the same extent in the nor-1−/− mice (F and H). Bars, 50 μm (A and B), 150 μm (C and D), and 75 μm (E and H).
We conclude that the specific requirement for nor-1 in early postnatal hippocampal development appears to be due in part to its selective expression relative to the additional NR4A receptors in the dentate gyrus and also to its distinct functional activity relative to nurr1 in CA1 pyramidal neurons.
DISCUSSION
We have demonstrated that inhibition of nor-1 expression in mice results in defective hippocampal axonal growth and postnatal neuronal cell death. These abnormalities arise during early postnatal hippocampal development and result in lasting changes in neuronal excitability in the adult hippocampus. Specifically, we have shown that mice lacking nor-1 display abnormal development of axonal projections of granule and mossy cells of the dentate gyrus and early postnatal death of pyramidal neurons in the CA1 field of Ammon's horn. The defects in hippocampal axonal growth and neuron survival are associated with a lowered seizure threshold of nor-1−/− mice relative to nor-1+/+ mice when challenged with the glutamatergic excitotoxin KA.
Guidance of outgrowing axons toward and within the hippocampus is directed by the combined action of long- and short-range repellant and attractive signals (37), which recognize specific receptor proteins at the growing tip of developing axons. While the signaling mechanisms that mediate outgrowth of hilar mossy cell axons of the dentate gyrus are poorly understood, several extracellular molecules known to mediate mossy fiber outgrowth from granule cells have recently been identified. These include the secreted semaphorin Sema3F and its receptor, neuropilin-2, limbic system-associated protein (29, 37), and polysialylated neural cell adhesion molecule (35). Repellant guidance by these factors is required for prevention of inappropriate layer termination of invading axons, and their absence results in ectopic layer termination of mossy fibers within the hippocampus. However, despite the availability of a growing body of information on the identity of these extracellular guidance cues, little information is known regarding the specific intracellular signaling pathways that mediate guided outgrowth and target selection by growing axons or the gene regulatory mechanisms that may control these pathways. Despite their decreased density, mossy fibers in nor-1−/− mice appear to respect their appropriate lamina-specific termination border with regard to the SL of CA3 and the CA3-CA1 border, suggesting that nor-1 may be responsible for regulating the expression of CA3 target-derived attractive guidance cues and/or intracellular signaling pathways within the growing axons of granule cells that promote neurite outgrowth and target selection.
We have also demonstrated that elimination of nor-1 results in selective loss of mossy cell axons that project to the suprapyramidal blade of the IML. However, the commissural mossy cell axons appear to cross the midline of the hippocampal commissure and appropriately terminate in the infrapyramidal layer of the IML. These findings suggest that nor-1 expression is not required for the elaboration of long-range guidance cues required for the guidance of extrinsic hippocampal commissural axons but plays an essential role in regulation of intrinsic signals responsible for guidance and target selection by commissural-associational fibers within the dentate gyrus. The molecular signals that mediate differential guidance and branching of fibers from mossy cells to the suprapyramidal and infrapyramidal blades of the IML remain elusive. Guidance of mossy cell axons is thought to be strongly influenced by as-yet-unidentified target-derived signals emanating from granule cells (9, 10, 12). Although the granule cell layer of the dentate gyrus was previously thought to be relatively homogeneous, emerging evidence supports the conclusion that the suprapyramidal and infrapyramidal layers exhibit significant structural and functional differences, including marked variation in dendritic architecture (6), lack of uniformity in the inhibitory circuitry, and greater contribution of the infrapyramidal blade to seizure activity (34). The identification of signaling pathways regulated by nor-1 should provide important new insights into the mechanisms underlying the structural and functional asymmetry of fiber pathways innervating the suprapyramidal and infrapyramidal layers of the dentate gyrus as well as the contribution of granule and mossy cell signaling to these pathways.
The developmental abnormalities observed in nor-1−/− mice show significant similarities to some aspects of hippocampal pathologies observed in human patients with TLE and experimental animal models of epilepsy. Histopathologically, human TLE associated with hippocampal sclerosis is characterized by neuronal death which is most severe in the CA1 region and in the hilus of the dentate gyrus (13, 38). Stimulation of rodents with KA or pilocarpine results in the development of acute severe seizures and hippocampal abnormalities that overlap with those observed in human TLE. These abnormalities include death of pyramidal neurons in the CA1 and/or CA3 fields of Ammon's horn, aberrant sprouting of mossy fiber axons of dentate granule cells to the inner molecular layer of the dentate gyrus, and retraction of axons from hilar mossy cells to the IML in the absence or presence of death of mossy cell soma (3, 19, 31). Although the hippocampal pathologies observed in the experimental animal models of epilepsy significantly overlap with those observed in human TLE, it remains unclear whether they are the cause or result of limbic seizure activity. The defects in axonal outgrowth from both mossy and granule cells and decreased survival of CA1 pyramidal cells observed during early postnatal development of nor-1−/− mice support the conclusion that underlying developmental defects in the hippocampus in these animals may contribute to later development of seizure susceptibility. The nor-1−/− mouse thus provides an excellent model system to dissect the gene regulatory mechanisms underlying axonal path finding in the dentate gyrus and pyramidal cell survival during postnatal neurogenesis whose aberrant activity may contribute activity-dependent synaptic plasticity associated with pathological seizure development.
The ability of NR4A nuclear receptors to interact with overlapping cis-acting DNA elements together with their overlapping expression patterns in the hippocampus and induction by seizure stimuli predict that members of this subfamily of nuclear receptors are likely to function redundantly to regulate activity-dependent changes in gene expression in the hippocampus. However, comparative analysis of the developmental expression patterns of the three nuclear receptors suggests that despite their structural relatedness and potential for interaction with overlapping target genes, nor-1 and nurr1 have distinct functions in the hippocampus during early postnatal development. Our observations that both nurr1 and nur77 show a similar induction pattern by KA in nor-1−/− mice and their inability to compensate for nor-1 loss in nor-1−/− mice add further support to the conclusion that the early developmental defects in nor-1−/− mice contribute to the lower threshold of seizure induction in nor-1-deficient mice.
The identification of nor-1 as an essential regulator of developmental pathways that ultimately control seizure susceptibility suggests that modulation of nor-1 activity may provide a novel therapeutic point of intervention to control epileptogenesis. In addition, the identification of nor-1-dependent target genes whose expression is disrupted in the hippocampus of nor-1−/− mice should facilitate the identification of novel molecular signaling pathways that mediate the establishment of hippocampal circuits associated with the development of epilepsy as well as uncover new approaches to clinical intervention.
Acknowledgments
We thank Jeffry Brace for technical assistance.
We acknowledge the Mental Retardation Research Center (HD 24064) behavioral core and NIH grant DK57743 to O.M.C.
REFERENCES
- 1.Andersen, P., T. W. Blackstad, and T. Lomo. 1966. Location and identification of excitatory synapses on hippocampal pyramidal cells. Exp. Brain Res. 1:236-248. [DOI] [PubMed] [Google Scholar]
- 2.Anonymous. 1999. A unified nomenclature system for the nuclear receptor superfamily. Cell 97:161-163. [DOI] [PubMed] [Google Scholar]
- 3.Bausch, S. B., and J. O. McNamara. 1999. Experimental partial epileptogenesis. Curr. Opin. Neurol. 12:203-209. [DOI] [PubMed] [Google Scholar]
- 4.Ben-Ari, Y. 1985. Limbic seizure and brain damage produced by kainic acid: mechanisms and relevance to human temporal lobe epilepsy. Neuroscience 14:375-403. [DOI] [PubMed] [Google Scholar]
- 5.Castillo, S. O., J. S. Baffi, M. Palkovits, D. S. Goldstein, I. J. Kopin, J. Witta, M. A. Magnuson, and V. M. Nikodem. 1998. Dopamine biosynthesis is selectively abolished in substantia nigra/ventral tegmental area but not in hypothalamic neurons in mice with targeted disruption of the Nurr1 gene. Mol. Cell. Neurosci. 11:36-46. [DOI] [PubMed] [Google Scholar]
- 6.Claiborne, B. J., D. G. Amaral, and W. M. Cowan. 1990. Quantitative, three-dimensional analysis of granule cell dendrites in the rat dentate gyrus. J. Comp. Neurol. 302:206-219. [DOI] [PubMed] [Google Scholar]
- 7.Crispino, M., G. Tocco, J. D. Feldman, H. R. Herschman, and M. Baudry. 1998. Nurr1 mRNA expression in neonatal and adult rat brain following kainic acid-induced seizure activity. Brain Res. Mol. Brain Res. 59:178-188. [DOI] [PubMed] [Google Scholar]
- 8.Davis, I. J., T. G. Hazel, R. H. Chen, J. Blenis, and L. F. Lau. 1993. Functional domains and phosphorylation of the orphan receptor Nur77. Mol. Endocrinol. 7:953-964. [DOI] [PubMed] [Google Scholar]
- 9.Deller, T., A. Drakew, and M. Frotscher. 1999. Different primary target cells are important for fiber lamination in the fascia dentata: a lesson from reeler mutant mice. Exp. Neurol. 156:239-253. [DOI] [PubMed] [Google Scholar]
- 10.Drakew, A., T. Deller, B. Heimrich, C. Gebhardt, D. Del Turco, A. Tielsch, E. Forster, J. Herz, and M. Frotscher. 2002. Dentate granule cells in reeler mutants and VLDLR and ApoER2 knockout mice. Exp. Neurol. 176:12-24. [DOI] [PubMed] [Google Scholar]
- 11.Fricke, R., and W. M. Cowan. 1978. An autoradiographic study of the commissural and ipsilateral hippocampo-dentate projections in the adult rat. J. Comp. Neurol. 181:253-269. [DOI] [PubMed] [Google Scholar]
- 12.Gebhardt, C., D. Del Turco, A. Drakew, A. Tielsch, J. Herz, M. Frotscher, and T. Deller. 2002. Abnormal positioning of granule cells alters afferent fiber distribution in the mouse fascia dentata: morphologic evidence from reeler, apolipoprotein E receptor 2-, and very low density lipoprotein receptor knockout mice. J. Comp. Neurol. 445:278-292. [DOI] [PubMed] [Google Scholar]
- 13.Gloor, P. 1991. Neurobiological substrates of ictal behavioral changes. Adv. Neurol. 55:1-34. [PubMed] [Google Scholar]
- 14.Hirata, Y., K. Kiuchi, H. C. Chen, J. Milbrandt, and G. Guroff. 1993. The phosphorylation and DNA binding of the DNA-binding domain of the orphan nuclear receptor NGFI-B. J. Biol. Chem. 268:24808-24812. [PubMed] [Google Scholar]
- 15.Hjorth-Simonsen, A., and S. Laurberg. 1977. Commissural connections of the dentate area in the rat. J. Comp. Neurol. 174:591-606. [DOI] [PubMed] [Google Scholar]
- 16.Honkaniemi, J., and F. R. Sharp. 1999. Prolonged expression of zinc finger immediate-early gene mRNAs and decreased protein synthesis following kainic acid induced seizures. Eur. J. Neurosci. 11:10-17. [DOI] [PubMed] [Google Scholar]
- 17.Law, S. W., O. M. Conneely, F. J. DeMayo, and B. W. O'Malley. 1992. Identification of a new brain-specific transcription factor, NURR1. Mol. Endocrinol. 6:2129-2135. [DOI] [PubMed] [Google Scholar]
- 18.Liu, Y., N. Fujise, and T. Kosaka. 1996. Distribution of calretinin immunoreactivity in the mouse dentate gyrus. I. General description. Exp. Brain Res. 108:389-403. [DOI] [PubMed] [Google Scholar]
- 19.Longo, B., L. Covolan, G. Chadi, and L. E. Mello. 2003. Sprouting of mossy fibers and the vacating of postsynaptic targets in the inner molecular layer of the dentate gyrus. Exp. Neurol. 181:57-67. [DOI] [PubMed] [Google Scholar]
- 20.Lothman, E. W., and R. C. Collins. 1981. Kainic acid induced limbic seizures: metabolic, behavioral, electroencephalographic and neuropathological correlates. Brain Res. 218:299-318. [DOI] [PubMed] [Google Scholar]
- 21.Maruyama, K., T. Tsukada, N. Ohkura, S. Bandoh, T. Hosono, and K. Yamaguchi. 1998. The NGFI-B subfamily of the nuclear receptor superfamily. Int. J. Oncol. 12:1237-1243. [DOI] [PubMed] [Google Scholar]
- 22.Morgan, J. I., and T. Curran. 1991. Proto-oncogene transcription factors and epilepsy. Trends Pharmacol. Sci. 12:343-349. [DOI] [PubMed] [Google Scholar]
- 23.Morrison, R. S., H. J. Wenzel, Y. Kinoshita, C. A. Robbins, L. A. Donehower, and P. A. Schwartzkroin. 1996. Loss of the p53 tumor suppressor gene protects neurons from kainate-induced cell death. J. Neurosci. 16:1337-1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23a.National Research Council. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, D.C.
- 24.O'Leary, D. D., R. A. Fricke, B. B. Stanfield, and W. M. Cowan. 1979. Changes in the associational afferents to the dentate gyrus in the absence of its commissural input. Anat. Embryol. (Berlin) 156:283-299. [DOI] [PubMed] [Google Scholar]
- 25.O'Leary, D. D., B. B. Stanfield, and W. M. Cowan. 1980. Evidence for the sprouting of the associational fibers to the dentate gyrus following removal of the commissural afferents in adult rats. Anat. Embryol. (Berlin) 159:151-161. [DOI] [PubMed] [Google Scholar]
- 26.Paulsen, R. F., K. Granas, H. Johnsen, V. Rolseth, and S. Sterri. 1995. Three related brain nuclear receptors, NGFI-B, Nurr1, and NOR-1, as transcriptional activators. J. Mol. Neurosci. 6:249-255. [DOI] [PubMed] [Google Scholar]
- 27.Pekarsky, Y., C. Hallas, A. Palamarchuk, A. Koval, F. Bullrich, Y. Hirata, R. Bichi, J. Letofsky, and C. M. Croce. 2001. Akt phosphorylates and regulates the orphan nuclear receptor Nur77. Proc. Natl. Acad. Sci. USA 98:3690-3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pena de Ortiz, S., and G. A. Jamieson, Jr. 1996. HZF-3, an immediate-early orphan receptor homologous to NURR1/NOT: induction upon membrane depolarization and seizures. Brain Res. Mol. Brain Res. 38:1-13. [DOI] [PubMed] [Google Scholar]
- 29.Pimenta, A. F., V. Zhukareva, M. F. Barbe, B. S. Reinoso, C. Grimley, W. Henzel, I. Fischer, and P. Levitt. 1995. The limbic system-associated membrane protein is an Ig superfamily member that mediates selective neuronal growth and axon targeting. Neuron 15:287-297. [DOI] [PubMed] [Google Scholar]
- 30.Ponnio, T., Q. Burton, F. A. Pereira, D. K. Wu, and O. M. Conneely. 2002. The nuclear receptor Nor-1 is essential for proliferation of the semicircular canals of the mouse inner ear. Mol. Cell. Biol. 22:935-945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ratzliff, A. H., V. Santhakumar, A. Howard, and I. Soltesz. 2002. Mossy cells in epilepsy: rigor mortis or vigor mortis? Trends Neurosci. 25:140-144. [DOI] [PubMed] [Google Scholar]
- 32.Saucedo-Cardenas, O., and O. M. Conneely. 1996. Comparative distribution of NURR1 and NUR77 nuclear receptors in the mouse central nervous system. J. Mol. Neurosci. 7:51-63. [DOI] [PubMed] [Google Scholar]
- 33.Saucedo-Cardenas, O., J. D. Quintana-Hau, W. D. Le, M. P. Smidt, J. J. Cox, F. De Mayo, J. P. Burbach, and O. M. Conneely. 1998. Nurr1 is essential for the induction of the dopaminergic phenotype and the survival of ventral mesencephalic late dopaminergic precursor neurons. Proc. Natl. Acad. Sci. USA 95:4013-4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scharfman, H. E., A. L. Sollas, K. L. Smith, M. B. Jackson, and J. H. Goodman. 2002. Structural and functional asymmetry in the normal and epileptic rat dentate gyrus. J. Comp. Neurol. 544:424-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seki, T., and U. Rutishauser. 1998. Removal of polysialic acid-neural cell adhesion molecule induces aberrant mossy fiber innervation and ectopic synaptogenesis in the hippocampus. J. Neurosci. 18:3757-3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seress, L., A. I. Gulyas, I. Ferrer, T. Tunon, E. Soriano, and T. F. Freund. 1993. Distribution, morphological features, and synaptic connections of parvalbumin- and calbindin D28k-immunoreactive neurons in the human hippocampal formation. J. Comp. Neurol. 337:208-230. [DOI] [PubMed] [Google Scholar]
- 37.Skutella, T., and R. Nitsch. 2001. New molecules for hippocampal development. Trends Neurosci. 24:107-113. [DOI] [PubMed] [Google Scholar]
- 38.Sloviter, R. S. 1994. On the relationship between neuropathology and pathophysiology in the epileptic hippocampus of humans and experimental animals. Hippocampus 4:250-253. [DOI] [PubMed] [Google Scholar]
- 39.Sutula, T., G. Cascino, J. Cavazos, I. Parada, and L. Ramirez. 1989. Mossy fiber synaptic reorganization in the epileptic human temporal lobe. Ann. Neurol. 26:321-330. [DOI] [PubMed] [Google Scholar]
- 40.Wang, Z., G. Benoit, J. Liu, S. Prasad, P. Aarnisalo, X. Liu, H. Xu, N. P. Walker, and T. Perlmann. 2003. Structure and function of Nurr1 identifies a class of ligand-independent nuclear receptors. Nature 423:555-560. [DOI] [PubMed] [Google Scholar]
- 41.Watanabe, Y., R. S. Johnson, L. S. Butler, D. K. Binder, B. M. Spiegelman, V. E. Papaioannou, and J. O. McNamara. 1996. Null mutation of c-fos impairs structural and functional plasticities in the kindling model of epilepsy. J. Neurosci. 16:3827-3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watson, M. A., and J. Milbrandt. 1989. The NGFI-B gene, a transcriptionally inducible member of the steroid receptor gene superfamily: genomic structure and expression in rat brain after seizure induction. Mol. Cell. Biol. 9:4213-4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilson, T. E., T. J. Fahrner, and J. Milbrandt. 1993. The orphan receptors NGFI-B and steroidogenic factor 1 establish monomer binding as a third paradigm of nuclear receptor-DNA interaction. Mol. Cell. Biol. 13:5794-5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xing, G., L. Zhang, T. Heynen, X. L. Li, M. A. Smith, S. R. Weiss, A. N. Feldman, S. Detera-Wadleigh, D. M. Chuang, and R. M. Post. 1997. Rat nurr1 is prominently expressed in perirhinal cortex, and differentially induced in the hippocampal dentate gyrus by electroconvulsive vs. kindled seizures. Brain Res. Mol. Brain Res. 47:251-261. [DOI] [PubMed] [Google Scholar]
- 45.Yang, D. D., C. Y. Kuan, A. J. Whitmarsh, M. Rincon, T. S. Zheng, R. J. Davis, P. Rakic, and R. A. Flavell. 1997. Absence of excitotoxicity-induced apoptosis in the hippocampus of mice lacking the Jnk3 gene. Nature 389:865-870. [DOI] [PubMed] [Google Scholar]
- 46.Zetterstrom, R. H., L. Solomin, L. Jansson, B. J. Hoffer, L. Olson, and T. Perlmann. 1997. Dopamine neuron agenesis in Nurr1-deficient mice. Science 276:248-250. [DOI] [PubMed] [Google Scholar]
- 47.Zetterstrom, R. H., L. Solomin, T. Mitsiadis, L. Olson, and T. Perlmann. 1996. Retinoid X receptor heterodimerization and developmental expression distinguish the orphan nuclear receptors NGFI-B, Nurr1, and Nor1. Mol. Endocrinol. 10:1656-1666. [DOI] [PubMed] [Google Scholar]
- 48.Zheng, D., L. S. Butler, and J. O. McNamara. 1998. Kindling and associated mossy fibre sprouting are not affected in mice deficient of NGFI-A/NGFI-B genes. Neuroscience 83:251-258. [DOI] [PubMed] [Google Scholar]