Abstract
Germinal center kinase (GCK), a member of the Ste20 family, selectively activates the Jun N-terminal kinase (JNK) group of mitogen-activated protein kinases. Here, we show that endogenous GCK is activated by polyinosine-polycytidine [poly(IC)] and lipopolysaccharides (LPS), lipid A, interleukin-1 (IL-1), and engagement of CD40, all agonists that require TRAF6 for JNK activation. RNA interference experiments indicate that GCK is required for the maximal activation of JNK by LPS, lipid A, poly(IC), and, to a lesser extent, IL-1 and engagement of CD40. GCK is ubiquitinated in situ and stabilized by inhibitors of the proteasome, indicating that GCK is subject to proteasomal turnover. GCK is constitutively active, and the kinase activity of GCK is required for GCK ubiquitination. Agonist activation of GCK involves the TRAF6-dependent transient stabilization of the GCK polypeptide rather than an increase in intrinsic kinase activity. Our results identify a physiologic function and unexpected mode of regulation for GCK.
Septic shock, a major cause of mortality among hospitalized patients, is triggered by the systemic presence of endotoxins produced by invading pathogens. Endotoxins trigger a coordinated wave of cellular signaling programs that marshal an organismal response to microbial challenges. Particularly potent endotoxins are lipopolysaccharides (LPS) produced by gram-negative bacteria. LPS-induced sepsis commences with the binding of LPS to toll-like receptor 4 (TLR-4) (1, 17).
The TLRs are a widely conserved family of receptor proteins that function to recognize specific subsets of pathogen-associated molecular patterns (PAMPs). PAMPs are a divergent group of molecular moieties, such as LPS, peptidoglycan, bacterial flagellin, DNA, and RNA, that are present in microbial and viral pathogens. The binding of PAMPs to target cell TLRs initiates innate immune responses by fostering the release of the proinflammatory cytokines tumor necrosis factor (TNF) and interleukin-1 (IL-1) as well as interferons and chemokines, such as IL-8 (1, 17, 33). At the cellular level, proinflammatory cytokines can promote apoptosis, lymphocyte development, leukocyte adhesion, and extravasation, the induction of chemokines and additional cytokines, and the secretion of additional inflammatory mediators, such as bioactive lipids. When left unchecked, this response becomes excessively magnified, resulting in septic shock.
Interestingly, the intracellular signal transduction pathways recruited by PAMPs, IL-1, CD40 ligand, and TNF are remarkably similar. Engagement of these receptors results in the binding of intracellular adapter proteins that transduce signals to intracellular effectors. These adapter proteins include members of the TNF receptor-associated factor (TRAF) family (1, 2, 5, 6, 7, 17, 34, 36). Biochemical and genetic studies indicate that TRAF2 is essential to TNF activation of NF-κB and activator protein 1 (AP-1) transcription factors, while TRAF6 is required for CD40, IL-1, and TLR activation of NF-κB and AP-1 (20, 21, 40).
AP-1 is a heterodimeric transcription factor consisting of c-Jun and either another member of the Jun family, a member of the Fos family, or a member of the activating transcription factor (ATF) family (13). The cell surface expression of integrins and integrin receptors, a process necessary for leukocyte adhesion and extravasation, requires in part AP-1, as does the induction by proinflammatory cytokines of chemokines and other chemoattractants that function to recruit myeloid cells to sites of inflammation (2, 15, 23).
AP-1 is activated by mitogen-activated protein kinases (MAPKs) either through the direct phosphorylation of AP-1 components (e.g., phosphorylation of c-Jun by members of the Jun N-terminal kinase [JNK] group of MAPKs) or through phosphorylation of transcription factors that function to induce AP-1 components. MAPKs themselves are regulated as part of three-tiered MAPK kinase kinase (MAP3K)→MAPK kinase (MKK)→MAPK pathways (19).
Biochemical and genetic evidence indicates that consistent with their roles as AP-1 regulators, the JNK and the related p38 pathways as well as their upstream MAP3Ks and MKKs are important to innate and acquired immunity. Thus, the JNKs and p38s are strongly activated by endotoxins, proinflammatory cytokines, and engagement of the T- and B-cell receptors. Disruption of jnk1 and jnk2 indicates a role for these kinases in the differentiation of splenic lymphocytes along the Th1 or Th2 lineage (17, 35). The MAP3Ks apoptosis signal-regulating kinase1, transforming growth factor β-activated kinase 1 (TAK1), and tumor progression locus 2 have been implicated in cytokine signaling to MAPKs (9, 33, 37). Moreover, as noted above, through AP-1, the JNKs and p38s are important to the induction and release of chemokines (e.g., IL-8 and monocyte chemoattractant protein 1) as well as the induction of inflammatory adhesion molecules necessary for leukocyte binding and extravasation (2, 19, 23, 38).
The biochemical basis of MAP3K regulation remains poorly understood. Germinal center kinase (GCK) is the founding member of the GCK group of kinases, which are distant relatives of Saccharomyces cerevisiae STE20, and is a candidate regulator of MAP3Ks (19). GCK is itself activated by TNF and, upon transient expression, potently and selectively activates the JNKs (16, 19, 28). We have found that GCK binds TRAF2 and binds and activates the MAP3Ks, MEKK1, and mixed-lineage kinase 3 (3, 41).
In this study, we examined the regulation and function of GCK. We find that endogenous GCK is activated by several proinflammatory stimuli that recruit JNK in a TRAF6-dependent manner, including LPS, lipid A (the core pyrogenic moiety of LPS), polyinosine-polycytidine [poly(IC)] (a PAMP that mimics viral RNA and signals through TLR3 [1, 17, 34]), IL-1, and engagement of CD40. Our RNA interference (RNAi) experiments indicate that GCK is required for the optimal activation of JNK by LPS and lipid A, and, to a lesser extent, by poly(IC), IL-1, and engagement of CD40.
We show that in resting cells, endogenous GCK is ubiquitinated and that treatment of resting cells with the ubiquitin (Ub) proteasome inhibitor lactacystin (11) increases endogenous GCK levels. Studies with recombinant GCK indicate that ubiquitination requires the kinase activity of GCK, and mutagenesis of two regulatory phosphoacceptor sites in the GCK kinase activation loop also abolishes GCK ubiquitination. GCK is exclusively ubiquitinated through Ub Lys48, even in the presence of TRAF6, an E3 ubiquitin ligase that can promote Lys63-linked ubiquitination of target proteins (8, 37).
Of particular note is our finding that the activation of GCK is accompanied by the transient stabilization of the GCK polypeptide, resulting in a stimulus-induced accumulation of Ub-GCK. This transient stabilization of GCK is not accompanied by an agonist-stimulated increase in intrinsic GCK kinase activity.
Finally, we find that endogenous GCK and TRAF6 associate, in intact cells, in an agonist-stimulated manner. Our RNAi experiments indicate that TRAF6 is required for poly(IC)- and LPS-stimulated GCK stabilization. GCK ubiquitination does not require TRAF6. Our results suggest that the binding of GCK to TRAF6 is sufficient to confer GCK stabilization. Thus, in resting cells, GCK is rapidly turning over, due to GCK kinase-dependent ubiquitination and degradation by the proteasome. Agonists such as poly(IC) and LPS, in a TRAF6-dependent manner, activate GCK by transiently stabilizing the ubiquitinated GCK polypeptide. GCK then participates in the recruitment of the JNK pathway.
MATERIALS AND METHODS
Cells, plasmids, transfection, and stimulation.
Jurkat human T-lymphoma cells and HL60 human promyelocytic leukemia cells (American Type Culture Collection) were cultured in RPMI medium containing 10% heat-inactivated, endotoxin-free fetal bovine serum. HL60 cells were differentiated to the macrophage lineage by treatment with 50 nM phorbol myristate acetate (Calbiochem) for 5 days. 293 cells were cultured in Dulbecco’s minimum essential medium containing 10% endotoxin-free fetal bovine serum. TNF, IL-1, and anti-CD40 were obtained from Calbiochem; phytohemagglutinin (PHA) and poly(IC) were obtained from Sigma-Aldrich; and LPS (ultrapure; from Escherichia coli O111:B4) and lipid A (from E. coli K12, D31m4) were obtained from List Biological Laboratories. Where indicated, cells were treated with lactacystin (1 μM) for 1 h, washed, and treated with agonists. Cells were stimulated with TNF (100 ng/ml), IL-1 (20 ng/ml), anti-CD40 (1 μg/ml), PHA (1 μg/ml), poly(IC) (100 nM), UVC (40 J/m2), LPS (1 μg/ml), or lipid A (1 μg/ml) for the times indicated in the figures. 293 cells were transfected by using Lipofectamine (Invitrogen) according to the manufacturer's instructions. Generally, 5 μg of GCK cDNA were transfected per 10-cm dish, except when changes in the stability of ectopically expressed GCK were under examination. In that instance, cells were transfected with 50 ng of GCK plasmid per 10-cm dish. cDNAs for GCK, TRAF6, MEKK1, p70 S6 kinase, wild-type (wt) Ub, and K63R Ub have been described (3, 25, 32, 41). Standard methods of mutagenesis were employed to generate K48R and K48R/K63R Ub).
RNAi.
RNA oligonucleotides were from Dharmacon and were deprotected according to the manufacturer's instructions. The double-stranded RNA (dsRNA) sense and antisense strands for human GCK RNAi were 5′-GGUGCAUAUGGGCGCCUGCdTdT-3′ and 5′-GCAGGCGCCCAUAUGCACCdTdT-3′, respectively. The dsRNA sense and antisense strands for the RNAi of human TRAF6 were 5′-CUGUGCUGCAUCAAUGGCAdTdT-3′ and 5′-UGCCAUUGAUGCAGCACAGdTdT-3′, respectively. The dsRNA sense and antisense strands for the RNAi of human MEKK1 were 5′-AGAGUUUCCCCAGUGCCUUdTdT-3′ and 5′-AAGGCACUGGGGAAACUCUdTdT-3′, respectively. The nonspecific RNAi control oligonucleotide was derived from green fluorescent protein (GFP). The sequence is available from Dharmacon. Jurkat cells were oligofected with 1 μg of each dsRNA.
Coimmunoprecipitation immunoblotting and kinase assays.
Preparation of cell extracts (10 to 15 mg of Jurkat cell extract protein for assays of endogenous proteins and 1 to 3 mg of cell extract protein for studies using transfected 293 cells), immunoprecipitation, and immunoblotting were performed as described previously (3, 41), with the following modifications. All lysis buffers included 10 μg of chymostatin/ml and 10 μg of antipain/ml in addition to the components described previously (3, 41). For assays involving ubiquitination, extracts were prepared with lysis buffer that also included 10 mM N-ethylmaleimide. JNK assays and GCK myelin basic protein (MBP) and autophosphorylation assays were performed as described previously (3, 16, 18, 41). Phosphospecific antibodies were from Cell Signaling Technologies, anti-GCK and anti-TRAF6 were from Santa Cruz Biotechnology, and antibodies to Ub were from BIOMOL International.
RT-PCR.
Jurkat cells were subjected to GCK RNAi as described above and treated with the agonists indicated for the times shown in the figures. RNAi efficacy was determined as described above and by examination for the suppression of stimulus-induced c-Jun phosphorylation (immunoblotting with a c-Jun phospho-Ser63/Ser73 phosphospecific antibody). Reverse transcriptase PCR (RT-PCR) was performed with human IL-8 primers (5′-ATGACTTCCAAGCTGGCCGTGGCT and 3′-TCTCAGCCCTCTTCAAAAACTTCTC) or primers for β-actin (5′-GTGGGGCGCCCCAGGCACCA and 3′-CTCCTTAATGTCACGCACGATTTC), total cellular RNA, and a Roche Titan One Tube kit. A starting amount of 2 μg of total RNA was used. PCR samples were run for 35 cycles (94°C for 50 sec, 60°C for 50 sec, 72°C for 90 sec) with a final extension at 72°C for 7 min.
RESULTS
Activation of GCK by endotoxins and proinflammatory stimuli.
Previous work has indicated that GCK can be activated in situ by TNF (16, 28). This recruitment of GCK by a proinflammatory cytokine coupled to TRAF proteins prompted us to investigate if GCK were broadly recruited by other agonists that signal through TRAFs. The acquisition of autophosphorylating activity closely follows GCK's kinase activity towards exogenous model substrates such as MBP (16, 27, 31, 41). Accordingly, measurement of autophosphorylation is a convenient assay for GCK activation. Figure 1A shows that, in addition to TNF, Jurkat human T-cell lymphoma GCK is activated by PHA, a lectin that functions to aggregate nonspecifically numerous cell surface glycoprotein receptors. GCK is also activated modestly by UVC radiation, which may also signal by triggering nonspecific receptor aggregation. Poly(IC) stimulates a particularly robust activation of GCK, suggesting that TLRs might couple especially strongly to GCK. The relative degrees of activation of JNK and GCK by these stimuli correspond reasonably well, although the poly(IC) activation of JNK seems to decline prior to that of GCK (Fig. 1A). Consistent with a role for GCK in PAMP signaling, LPS also vigorously activates Jurkat cell GCK (Fig. 1B).
FIG. 1.
Activation of GCK by proinflammatory stimuli. (A) Comparative activation of GCK by different agonists. Jurkat cells were treated with the indicated stimuli for the indicated times. In the top panel, GCK was immunoprecipitated and allowed to autophosphorylate (auto-P) as described in Materials and Methods. Products were separated by SDS-PAGE and subjected to autoradiography. In the bottom panel, JNKs were immunoprecipitated and assayed for phosphorylation of GST-c-Jun(1-135). (B) Activation of GCK by LPS. Jurkat cells were treated with LPS or poly(IC) as indicated. GCK was immunoprecipitated and assayed as described for panel A.
GCK is required for the activation of the JNK pathway by LPS, lipid A, and, to a lesser extent, poly(IC), IL-1, and engagement of CD40.
Both TLR3 and TLR4 couple to TRAF6 to recruit the ERK, JNK, and p38 MAPKs and the NF-κB pathway (1, 17, 21). Downstream elements that link TLRs and TRAF6 to specific MAPK pathways have yet to be identified. The transient expression of GCK strongly activates the JNKs without significantly activating the ERKs, p38s, or NF-κB (28, 29). Given that the results shown in Fig. 1 indicated strong GCK activation by TLR-coupled agonists, we asked next if GCKs were physiologically relevant to the activation of the JNKs by endotoxins and other agonists that couple to TRAF6. To answer this question, we used mammalian cell RNAi with small interfering RNA (siRNA) (10). Jurkat or HL60 promyelocytic leukemia cells were treated with a GCK-specific siRNA or a control oligonucleotide (corresponding to GFP); the GCK oligonucleotide reduced endogenous Jurkat cell gck to undetectable levels and HL60 cell GCK to near-undetectable levels (Fig. 2; Fig. S1 in the supplemental material). In Fig. S1, note that excess crude cell extract was used on the immunoblots to detect GCK and to monitor the efficacy of the silencing of gck. Under these conditions, in both cell types, the activation of JNK by either poly(IC), highly purified LPS, or lipid A (the fragment of LPS associated most significantly with pyrogenic activity) was substantially reduced (Fig. 2A; Fig. S1 in the supplemental material), indicating that GCK is required for maximal activation of JNK by agonists that couple to TLR-3 and TLR-4.
FIG.2.
Role of GCK in the recruitment of the JNK pathway by various agonists. (A) Efficient activation of JNK by LPS or lipid A requires GCK. As indicated, Jurkat or HL60 cells were treated with GCK siRNA overnight, followed by 1-h treatment with 1 μM lactacystin, washing, and treatment with LPS or lipid A as described in Materials and Methods. Cell extracts were prepared and subjected to SDS-PAGE and immunoblotting with the indicated antibodies. Total JNK and ERK immunoblots are loading controls. IB, immunoblot; p-JNK, immunoblot with antibodies selective for the phosphorylated, active form of JNK. (B) GCK is less important to JNK activation by IL-1 or engagement of CD40 and is not required for JNK, ERK, or p38 activation by IL-1, CD40, or T-cell receptor costimulation with anti-CD3/anti-CD28. Jurkat cells were treated with GCK siRNA, followed by treatment with the indicated agonists for the times shown, as described for panel A and in Materials and Methods. Cell extracts were prepared, and a portion was subjected to SDS-PAGE and immunoblotting with the indicated antibodies. GCK was immunoprecipitated from the remaining extract and assayed for phosphorylation of MBP. IP, immunoprecipitation; IB, immunoblot; p-JNK, p-p38, and p-ERK indicate immunoblots with the cognate phospho-specific, activation state antibody. (C) Efficient LPS stimulation of c-Jun phosphorylation at the JNK-specific phosphoacceptor sites (Ser63/Ser73) requires GCK. Jurkat cells were treated with GCK siRNA as indicated (16 h) and then treated with the indicated agonists. Cell extracts were prepared, and a portion was subjected to SDS-PAGE and immunoblotting with the indicated antibodies. The anti-ERK immunoblot is a loading control. p-Jun, immunoblot with anti-phospho-Ser63/Ser73-c-Jun. (D) Efficient LPS induction of IL-8 requires GCK. This experiment was performed in parallel to that shown in panel C. Total RNA was isolated from cells not used for results shown in panel C and subjected to RT-PCR with either IL-8 or β-actin primers as indicated. Efficacy of GCK RNAi is as indicated for panel C.
TLRs signal through TRAF6, and TRAF6 has been implicated in JNK activation by endotoxin, IL-1, and engagement of CD40 (1, 17, 21). Consistent with the observation that GCK is a selective JNK activator (28), we find that the silencing of gck also significantly reduces IL-1 and CD40 activation of Jurkat cell JNK, but not ERK or p38 (Fig. 2B). ERK and p38 activation by LPS and poly(IC) are also not affected by GCK RNAi (J. Zhong, unpublished observations). The effect of GCK RNAi on JNK activation by IL-1 and CD40 is less pronounced than its effect on JNK activation by LPS and lipid A or by poly(IC) (Fig. 2A; Fig. S1 in the supplemental material), and although GCK was activated upon T-cell receptor engagement by anti-CD3/CD28, the silencing of gck had at best a very modest effect on JNK activation upon T-cell receptor activation. It is likely, then, that IL-1, CD40, and the T-cell receptor couple to JNK through a more heterogeneous array of mechanisms to which the relative contribution of GCK is comparatively less important than the contribution of GCK to LPS activation of JNK is (7, 19, 37).
Consistent with a requirement for GCK for JNK activation by LPS, we also observed that the recruitment of JNK effectors by LPS required GCK. Thus, Jurkat cells were treated with anti-CD40, LPS, or poly(IC), and the phosphorylation of c-Jun at Ser63 and Ser73, the phosphoacceptor sites targeted by JNK (15, 19), was determined. From the results shown in Fig. 2C, it is evident that the silencing of gck substantially reduced LPS stimulation of c-Jun phosphorylation. Of note is the finding that CD40- and poly(IC)-dependent phosphorylation of c-Jun were not significantly reduced upon silencing of gck. The lack of an effect on poly(IC) signaling was unexpected, however, given that silencing of gck seemed to substantially reduce JNK activation (Fig. S1 in the supplemental material). It is possible that ERK may contribute to the phosphorylation of c-Jun Ser63 and Ser73. Indeed, ERK-dependent c-Jun phosphorylation at Ser63 and Ser73 has been observed in some instances (24).
We also assessed the role of GCK in the induction of IL-8 expression. IL-8 is expressed in response to proinflammatory stimuli and is a chemokine critical to the recruitment of myeloid cells to sites of infection. Induction of IL-8 mRNA involves transcriptional, posttranscriptional, and translational elements, all of which are mediated in part by MAPKs (14). Accordingly, as detected by RT-PCR (Fig. 2D), CD40, poly(IC), and LPS induction of Jurkat cell IL-8 is rapid, being first detectable at 3 min. The silencing of gck impairs significantly, but not completely, the LPS induction of IL-8. The induction of IL-8 mRNA by the other stimuli was not significantly affected upon silencing of gck. From the results shown in Fig. 2, we conclude that GCK exerts its primary impact on PAMP signaling pathways, especially that of LPS.
The GCK polypeptide contains three Pro-, Glu-, Ser-, and Thr-rich (PEST) domains (16). Proteins with PEST domains are frequently targets of degradation by the ubiquitin proteasome (12, 27, 29). Of note, we also observed that, coincident with activation of GCK's kinase activity by LPS, lipid A, IL-1, CD40 engagement, and T-cell receptor costimulation, there was an increase in GCK polypeptide levels (Fig. 2). Note that this increase is not apparent in Fig. S1 in the supplemental material, inasmuch as saturating levels of cell extract were used in that experiment in order to document the efficacy of GCK RNAi. Moreover, treatment with the specific proteasome inhibitor lactacystin (11) enhanced overall GCK levels and activation by poly(IC) and LPS JNK (Fig. 2A). Thus, GCK-dependent activation of JNK appears to be controlled in part by the ubiquitin proteasome system.
GCK is polyubiquitinated through Ub K48-linked chains in situ, ubiquitination requires the kinase activity of GCK, and poly(IC) activation of GCK involves transient stabilization.
The enhancement of poly(IC)-, LPS-, and lipid A-induced JNK activation by lactacystin suggested to us that GCK might itself be a target of the ubiquitin proteasome and that this system may contribute to GCK regulation.
We find that endogenous GCK is, in fact, ubiquitinated (Fig. 3). Thus, we pretreated Jurkat cells with vehicle or lactacystin. Cells were then treated with vehicle or poly(IC) for various times. Following these treatments, GCK was immunoprecipitated and either assayed for autophosphorylation or subjected to immunoblotting with either anti-Ub or low levels of anti-GCK antibodies. From the results shown in Fig. 3, it is evident that GCK is activated rapidly by poly(IC), with activation being detectable at 1 min, reaching a maximum by 10 min, and declining to the baseline by 20 min. Immunoprecipitates of resting-cell GCK contained Ub immunoreactivity. Poly(IC) stimulation triggered an increase in GCK ubiquitination, and the extent of Ub immunoreactivity increased and decreased precisely with the time course of poly(IC)-stimulation of GCK activity (Fig. 3). Of note was that in the absence of lactacystin, poly(IC) also stimulated an increase in total GCK levels in the cell. Moreover, GCK levels increased and then decreased with kinetics that mirrored precisely the GCK ubiquitination and kinase activity. Lactacystin promoted an increase in total resting cell GCK immunoreactivity, indicating that GCK levels are at least in part controlled by proteasomal degradation. In addition, lactacystin profoundly increased the extent and duration of poly(IC)-stimulated GCK levels and autophosphorylation (Fig. 3). Thus, GCK activation apparently involves a transient stabilization of the ubiquitinated GCK polypeptide. Resolution of the poly(IC) signal is accompanied by a reduction in total GCK polypeptide.
FIG. 3.
Endogenous GCK is ubiquitinated, and activation of GCK involves transient stabilization of the GCK polypeptide. Jurkat cells were treated with vehicle or lactacystin, followed by treatment with poly(IC) for the indicated times, as described in Materials and Methods. Crude cell extracts were prepared and normalized to contain equal amounts of protein (documented by the anti-ERK immunoblot loading control shown). GCK was immunoprecipitated, and the immunoprecipitates were split and subjected to either autophosphorylation assay, anti-GCK immunoblotting, or antiubiquitin blotting, as indicated.
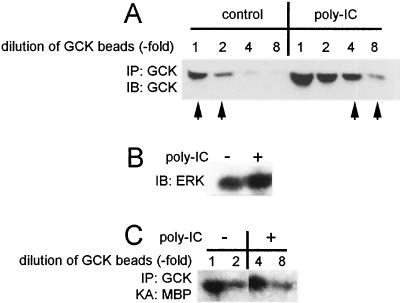
The stimulus-induced regulation of GCK levels compelled us to ask if GCK activation was accompanied by an increase in its intrinsic kinase activity or if GCK regulation was mediated entirely by the enhanced stabilization of the GCK polypeptide. Thus, we sought to determine the resting and poly(IC)-stimulated levels of GCK kinase activity under conditions in which the levels of GCK polypeptide being assayed were normalized to equal levels. Accordingly, we treated cells with vehicle or poly(IC) for 10 min. GCK was immunoprecipitated, and the immunoprecipitates from each treatment set were subjected to serial dilutions with blank protein G-agarose beads (Fig. 4A). Each set of beads was then either assayed for GCK immunoreactivity or for phosphorylation of MBP. From the results shown in Fig. 4A, it is evident that over the entire dilution set, poly(IC) triggered an increase in GCK polypeptide levels without a concomitant change in ERK levels in the crude extract (Fig. 4B). We then compared the GCK immunoreactivity in each dilution sample and identified GCK samples from control and poly(IC)-treated cells that contained equal levels of GCK immunoreactivity (Fig. 4A). From the results presented in Fig. 4C, it is clear that when samples from control and poly(IC)-treated cells were adjusted to contain equal levels of GCK polypeptide, these samples contained equal GCK kinase activity (compare the result for the control undiluted GCK beads to that for poly(IC)-stimulated GCK beads diluted 1:4, or the result for the control GCK beads diluted 1:2 to that for poly(IC)-stimulated GCK beads diluted 1:8). Thus, poly(IC) does not stimulate an increase in intrinsic GCK kinase activity. Instead, GCK appears to be constitutively active, and the levels of GCK polypeptide are enhanced by extracellular stimuli.
FIG. 4.
Activation of GCK involves an increase in GCK polypeptide levels without a concomitant increase in intrinsic GCK kinase activity. (A) poly(IC) induced increases in GCK polypeptide levels. Jurkat cells were treated with poly(IC) for 10 min as described in Materials and Methods. Crude extracts were prepared and normalized to contain equal amounts of protein, as documented by the anti-ERK immunoblot loading control shown in panel B. GCK was immunoprecipitated, and the beads were diluted as indicated with blank protein G-agarose beads. Equal levels of GCK immunoreactivity were detected in the undiluted control and the 1:4 diluted poly(IC) sample, as well as in the 1:2 diluted control sample and the 1:8 diluted poly(IC) sample (arrows). The GCK samples indicated with the arrows were subjected to in vitro kinase assays with MBP as a substrate. (B) Loading control, an anti-ERK immunoblot of crude cell extracts. (C) Kinase assay of GCK immunoprecipitates. IP, immunoprecipitate; IB, immunoblot; KA, kinase assay.
We next sought to dissect the nature and regulation of GCK ubiquitination. Many ubiquitination reactions require a triggering posttranslational modification of the target protein (27). Thus, for example, IκB must be phosphorylated (at Ser32 and Ser36 for IκBα or Ser19 and Ser23 for IκBβ) prior to ubiquitination (30). With this fact in mind, we wondered whether the kinase activity of GCK was required for its ubiquitination. To test this possibility, we expressed in 293 cells FLAG-tagged forms of either wt GCK or one of two kinase-dead GCK mutants, K44M (which alters the critical Lys residue in the ATP binding site) (16) or S169A/T173A (which changes two autophosphorylation phosphoacceptor sites in the kinase activation loop into nonphosphorylatable residues) (31), along with Myc-tagged Ub. Because we anticipated that only a small percentage of the recombinant, overexpressed GCK would be ubiquitinated, cells were treated with lactacystin or vehicle. Under these conditions, GCK ubiquitination was detected only in the presence of lactacystin, as the appearance of high-molecular-weight Myc immunoreactivity in the FLAG-GCK immunoprecipitates. From the results shown Fig. 5A, it is clear that only wt GCK immunoprecipitates contained detectable Myc Ub immunoreactivity. Thus, GCK ubiquitination requires the kinase activity of GCK. Insofar as endogenous GCK is apparently constitutively active (Fig. 4), this kinase activity may trigger the rapid Ub proteasome-dependent turnover of GCK.
FIG. 5.
Regulation and characteristics of GCK ubiquitination. (A) The kinase activity of GCK is required for GCK ubiquitination. 293 cells were transfected with FLAG-tagged wt, K44 M, or S169A/T173A GCK, plus Myc Ub as indicated. Cell extracts were prepared and normalized to contain equal levels of protein, as indicated by the anti-ERK immunoblot. GCK was immunoprecipitated, and the immunoprecipitates were subjected to SDS-PAGE and immunoblotting with anti-Myc, as indicated, to detect Myc-tagged Ub GCK. Expression of the constructs was documented by subjecting a portion of the cell extracts to SDS-PAGE and immunoblotting with the cognate epitope antibodies. (B) GCK is exclusively ubiquitinated with Lys48-linked poly(Ub) chains. 293 cells were transfected with GST-tagged wt GCK, FLAG-tagged TRAF6 plus either Myc-tagged wt, K63R, K48R, or K48R/K63R Ub, as indicated. Cell extracts were prepared and normalized to contain equal levels of protein, as indicated by the anti-ERK immunoblot. GST-GCK was isolated on glutathione-Sepharose and subjected to SDS-PAGE and immunoblotting with anti-Myc, as indicated, to detect Myc-tagged Ub GCK. Expressions of the various constructs were documented by subjecting a portion of the cell extracts to SDS-PAGE and immunoblotting with the cognate epitope antibodies. IP, immunoprecipitate or glutathione-agarose immobilization, as indicated; IB, immunoblot.
Two prominent forms of polyubiquitin chains are assembled onto target proteins. Proteins with poly(Ub) chains linked through Ub Lys48 are typically destined for proteasomal degradation (12, 27). A second, more recently identified form of polyubiquitin, linked through Ub Lys63, does not usually target a protein for the proteasome. Instead, this form of ubiquitination is thought to be involved in the regulation of protein-protein interactions (8, 13, 27, 37). For example, TRAF6 itself is an E3 Ub ligase and autoubiquitinates, assembling Lys63-linked poly(Ub) chains in a manner dependent upon its RING domain, a reaction that couples TRAF6 to elements upstream of NF-κB (37).
We found that although GCK interacts in intact cells with TRAF6, and, in response to poly(IC) or LPS, is activated in a TRAF6-dependent manner (as discussed below), GCK is ubiquitinated solely with K48-linked poly(Ub) chains, even when coexpressed with TRAF6. Thus, we expressed in 293 cells wt glutathione S-transferase (GST)-tagged GCK plus either wt, K48R, K63R, or K48R/K63R double mutant Ub (Myc-tagged) and either vector or FLAG-TRAF6 (Fig. 5B). The ubiquitination of GCK was detected as shown in Fig. 5A. It should be noted here that the GST-GCK was expressed from the EF-1α promoter, which expresses at much higher levels than the CMV promoter-driven FLAG-GCK construct used for Fig. 5A. Thus, we were able to detect ubiquitination of the GST GCK in the absence of lactacystin. We found that only expression with wt or K63R-Ub permitted the assembly of Myc-tagged Ub chains on the GST-GCK. Mutation of Ub Lys48 to Arg completely abolished GCK ubiquitination, indicating Lys48-linked polyubiquitination of GCK. Of particular note was that expression with TRAF6 did not lead to the appearance of detectable Lys63-linked Ub GCK (i.e., Myc Ub GCK detected in K48R Ub-transfected cells) (Fig. 5B).
Poly(IC) and LPS-stimulated GCK stabilization requires TRAF6, and poly(IC)-stimulated GCK stabilization also requires MEKK1.
TRAF6 is required for LPS activation of JNK (21), and we find that GCK is necessary for maximal JNK activation by LPS, lipid A, and poly(IC). Given that GCK activation by poly(IC) involves stimulus-induced transient stabilization, we next asked if LPS triggered a similar stabilization of GCK and if this stabilization, as well as that stimulated by poly(IC), required TRAF6. The results shown in Fig. 6 indicate that indeed, TRAF6 is required for LPS- and poly(IC)-induced stabilization and activation of endogenous GCK.
FIG. 6.
TRAF6 is required for resting cell and agonist-stimulated stabilization of GCK. Jurkat cells were treated with TRAF6 or control (GFP) siRNA as indicated. Cells were then treated with vehicle or lactacystin, followed by LPS (top panel) or poly(IC) (bottom panel) as indicated. Cell extracts were prepared and normalized to contain equal amounts of protein (the ERK immunoblot is an indication of protein normalization). GCK was immunoprecipitated and subjected to SDS-PAGE and immunoblotting with anti-Ub or anti-GCK as indicated. Cell extracts were subjected to immunoblotting with anti-TRAF6 to document the silencing of traf6. IB, immunoblot; IP, immunoprecipitate.
Thus, to silence Jurkat cell traf6, we treated the cells with TRAF6 siRNA. As a control, cells were treated with an irrelevant dsRNA sequence (corresponding to GFP). From the results shown in Fig. 6, it is clear that the silencing of traf6 resulted in a substantial decrease in resting- and stimulated-cell GCK polypeptide levels (compare lanes 10 to 12 and 16 to 18 to lanes 13 to 15; also compare lanes 1 to 3 and 7 to 9 to lanes 4 to 6). This decrease was partially reversed with lactacystin (compare lanes 13 to 15 to lanes 4 to 6; also compare lanes 1 to 3 and 7 to 9 to lanes 10 to 12 and 16 to 18). It is important to note that the TRAF6 RNAi was accomplished by incubating the cells overnight with TRAF6 siRNA. This step was followed by lactacystin treatment (1 h), washing, and then treatment with LPS or poly(IC). Under these circumstances, the lactacystin is only partially able to reverse the destabilization of GCK mediated by TRAF6 RNAi. Although Ub GCK levels also declined upon treatment with TRAF6 siRNA, these declines accompanied those of total GCK (in Fig. 6, compare lanes 10 to 12 and 16 to 18 to lanes 13 to 15; compare also lanes 1 to 3 and 7 to 9 to lanes 4 to 6), indicating a decrease in total GCK rather than a loss of GCK ubiquitination (a comparison of lanes 4 to 6 and 13 to 15 indicates that lactacystin treatment enabled the detection of trace Ub GCK under conditions of TRAF6 knockdown). ERK protein levels were unaffected by TRAF6 RNAi, and the control dsRNA had no effect on GCK levels (in Fig. 6, compare lanes 1 to 3 to 7 to 9; compare also lanes 10 to 12 to lanes 16 to 18). Thus, the resting-cell and agonist-stimulated stability of GCK is critically dependent upon the presence of TRAF6, and TRAF6 knockdown enhances the proteasomal degradation of GCK.
MEKK1 is a MAP3K that is relatively selective for the JNK pathway, although MEKK1 can also participate in ERK activation (19, 42). Recently, MEKK1 was shown to possess an E3 Ub ligase activity that was dependent upon a plant homeodomain motif in the MEKK1 amino terminal regulatory region. This E3 ligase activity assembles exclusively Ub K48-linked poly(Ub) chains (22, 39). Endogenous GCK binds endogenous MEKK1 tightly in situ, and GCK can activate MEKK1's MAP3K activity (3, 41). Because of the tight binding of GCK to MEKK1, coupled with the observations that (i) MEKK1 is an E3 ligase, and (ii) GCK is ubiquitinated with Lys48-linked poly(Ub) chains, we asked if silencing of mekk1 by RNAi might reduce the ubiquitination and degradation of GCK. To our surprise, we observed the opposite (Fig. 7). While the silencing of Jurkat cell mekk1 reduced the resting and poly(IC)-stimulated levels of endogenous Ub-GCK, this decline was accompanied by an almost complete loss of detectable total GCK immunoreactivity (Fig. 7, compare lanes 7 to 9 to 10 to 12; compare also lanes 1 to 3 to 4 to 6), a loss that was reversed upon treatment of the cells with lactacystin (Fig. 7, compare lanes 7 to 9 and 10 to 12 to lanes 4 to 6). Thus, it is unlikely that MEKK1 is an E3 ligase for GCK. Instead, MEKK1 protects GCK from proteasomal degradation.
FIG. 7.
MEKK1 is required for GCK stabilization. Jurkat cells were treated with MEKK1 or control (GFP minus MEKK1 RNAi) siRNA as indicated. Cells were then treated with vehicle or lactacystin, followed by poly(IC) as indicated. Cell extracts were prepared and normalized to contain equal amounts of protein (the ERK immunoblot is an indication of protein normalization). GCK was immunoprecipitated and subjected to SDS-PAGE and immunoblotting with anti-Ub or anti-GCK as indicated. Cell extracts were subjected to immunoblotting with anti-MEKK1 to document silencing of mekk1. IB, immunoblot; IP, immunoprecipitate.
The TRAF domains of TRAF6 are necessary for the stabilization of GCK in situ.
The results shown in Fig. 6 indicate that TRAF6 is necessary for the agonist-stimulated stabilization of GCK. We strongly doubt that TRAF6-mediated stabilization of GCK involves TRAF6-dependent ubiquitination of GCK, inasmuch as GCK is ubiquitinated solely through Lys48-linked poly(Ub) chains, even when TRAF6 is coexpressed. Accordingly, we sought to identify alternative mechanisms by which TRAF6 might modulate GCK polypeptide levels. It was previously shown that when transiently overexpressed, recombinant TRAF proteins bind recombinant GCK and that this interaction requires the TRAF proteins' TRAF domains (3, 19, 41). We first tested whether endogenous GCK and TRAF6 could interact in intact cells in an agonist-stimulated manner. Thus, Jurkat cells were treated with poly(IC). Cell extracts were prepared, and GCK and TRAF6 were immunoprecipitated. The GCK beads were then probed on immunoblots with anti-TRAF6. Likewise, the anti-TRAF6 immunoprecipitates were probed with anti-GCK. The results in Fig. 8A indicate that coincident with the poly(IC)-dependent accumulation of GCK, endogenous TRAF6 and GCK interacted in a poly(IC)-stimulated manner and could be coimmunoprecipitated. This finding is consistent with the idea that the agonist-dependent interaction of GCK and TRAF6 mediates GCK stabilization.
FIG. 8.
Endogenous GCK and TRAF6 interact in an agonist-dependent manner, and the binding of GCK to TRAF6 is likely sufficient to mediate GCK stabilization. (A) Binding of endogenous GCK to TRAF6. Jurkat cells were treated with poly(IC) for the indicated times. Endogenous GCK or TRAF6 were immunoprecipitated and subjected to SDS-PAGE and immunoblotting with the indicated antibodies. (B) Coexpression of GCK with wt or ΔRING TRAF6, but not ΔTRAF TRAF6 results in stabilization of the GCK polypeptide. 293 cells were transfected with 50 ng of GST GCK, 3 μg of Myc-tagged p70 S6 kinase 1 (S6K1) transfected as a loading control) and the indicated FLAG-tagged TRAF6 constructs (1 μg of wt TRAF6; 6 μg of each mutant TRAF6). Cell extracts were normalized to contain equal amounts of total protein (indicated by the anti-Myc blot showing equal levels of transfected Myc S6K1), and subjected to SDS-PAGE and immunoblotting with anti-GST, anti-FLAG, or anti-Myc, as indicated.
Inasmuch as GCK binds TRAF proteins through their TRAF domains (19, 41), we next asked if the expression of TRAF6 could stabilize coexpressed GCK in a manner that required the TRAF6 TRAF domain. Ectopically expressed GCK, when transfected at high levels, appears stable (Fig. 5). Thus, in order to detect changes in the stability of recombinant GCK, 293 cells were transfected with very low levels (50 ng/plate) of GST-GCK plus an excess of either vector, FLAG-tagged wt, ΔRING, or ΔTRAF TRAF6. From the results shown in Fig. 8B, it is evident that under the transfection conditions used, the level of GCK protein present in the transfected cells was substantially enhanced by wt TRAF6 and by ΔRING TRAF6, in spite of the fact that the RING domain is necessary for TRAF6's E3 ligase activity (8, 37). By contrast, the deletion of the TRAF domains, which eliminates GCK binding (41), specifically abolished TRAF6-dependent GCK stabilization. Levels of recombinant p70 S6 kinase (S6K1) were not affected by coexpression with TRAF6 (Fig. 8B). From this result, we conclude that the binding of GCK to the TRAF6 TRAF domain, and not the E3 ligase activity of TRAF6, is sufficient to confer stabilization of GCK.
DISCUSSION
The results presented herein document that GCK is required for the efficient activation of JNK by TRAF6-dependent stimuli, including PAMPs [poly(IC), LPS, lipid A] and, to a lesser degree, IL-1 and engagement of CD40. The requirement for GCK is most pronounced for LPS signaling, and extends to LPS stimulation of c-Jun phosphorylation and induction of IL-8. Moreover, our results show that GCK is activated by a novel mechanism. We find that GCK is constitutively active as a kinase, and that this kinase activity is necessary for GCK ubiquitination and, likely, its consequent rapid turnover by proteasomal degradation. In response to stimuli that recruit TRAF6 [e.g., poly(IC)], GCK binds TRAF6. This interaction transiently stabilizes the GCK polypeptide. The interaction between GCK and MEKK1 may also stabilize GCK. To our knowledge, GCK is the first signaling Ser/Thr protein kinase whose activation involves the transient stabilization of its ubiquitinated form.
The mechanism of GCK regulation differs significantly from that reported for GCK related (GCKR), another member of the GCK subgroup of the Ste20 family (32). GCKR is strongly activated by TNF and is required for TNF activation of JNK (31). Recent findings indicate that GCKR is activated by TRAF2 through a process that involves Lys63-linked ubiquitination, a reaction mediated by the E3 ubiquitin ligase activity of TRAF2 and by the E2 ubiquitin-conjugating proteins Uev1A and Ubc13 (32).
The reasons for the differences in GCK and GCKR regulation are unclear. Structurally, GCK and GCKR are strikingly similar. Both enzymes contain amino-terminal kinase domains followed by carboxy-terminal regulatory domains that include several PEST sequences as well as binding sites for polypeptides with SH3 domains. Moreover, both GCK and GCKR bind the TRAF domains of TRAF proteins (19, 31, 41). TRAF6, which links TLRs to JNK and is central to LPS, lipid A, and poly(IC) regulation of GCK, is, like TRAF2, an E3 ubiquitin ligase (8, 21, 37). The E3 ligase activity of TRAF6 is central to cytokine activation of NF-κB. Thus, in response to IL-1, TRAF6 autoubiquitinates, assembling Ub Lys63-linked chains (8, 37). Still, in spite of the facts that (i) endogenous GCK and TRAF6 bind in a ligand-dependent manner and (ii) TRAF6 is an E3 ubiquitin ligase, we can detect no K63-linked ubiquitination of GCK, even when GCK is coexpressed with TRAF6. Our observation that the deletion of the TRAF domain of TRAF6, but not the RING domain of TRAF6 (which mediates E3 ligase activity) (8, 37), inhibits the ability of TRAF6 to stabilize GCK, coupled with the earlier finding that GCK binds TRAF proteins through their TRAF domains, suggests that the binding of GCK and TRAF6 is sufficient to stabilize GCK in situ. It is possible that the GCKR polypeptide contains specific molecular determinants that drive TRAF2-dependent Lys63-linked polyubiquitination and that these determinants are not present on GCK. Consistent with this idea, transient expression studies using mutant Ub constructs indicate that, in contrast to the Lys48-linked ubiquitination of GCK, GCKR is exclusively ubiquitinated through Lys63-linked polyubiquitin chains (32).
Consequent to IL-1-dependent TRAF6 autoubiquitination, TRAF6 binds TAK1-associated binding protein 2, a regulatory subunit of TAK1. This binding brings TAK1 to the receptor signaling complex, triggering TAK1 activation and consequent phosphorylation of the IκB kinase complex (38). TAK1 is a MAP3K that can also recruit JNK (via phosphorylation of MKK4 and MKK7) and p38 (via phosphorylation of MKK-3 and MKK-6) (19). Moreover, TRAF6 is necessary for IL-1 as well as PAMP activation of JNK and NF-κB and is responsible for activating TAK1 in response to IL-1 (8, 21, 37). Furthermore, dTAK1, the Drosophila orthologue of TAK1, appears necessary for innate immunity (35). By contrast, we find that GCK, which does not detectably interact with TAK1 (J. M. Kyriakis, unpublished observations), is necessary for the PAMP [LPS and poly(IC)] activation of JNK.
The recruitment of TAK1 and TAK1-associated binding protein 2 by IL-1 raises questions as to the overall significance of GCK to cytokine-PAMP signaling. The RNAi knockdown of GCK to undetectable levels substantially reduces the LPS and poly(IC) activation of JNK but is less effective at diminishing JNK activation by IL-1 and engagement of CD40. Consistent with this observation, the LPS stimulation of c-Jun phosphorylation at Ser63 and Ser73 and the induction of IL-8 mRNA are also significantly reduced upon silencing of gck. By contrast, although poly(IC) activation of JNK is reduced upon silencing of gck, the phosphorylation of c-Jun and the induction of IL-8 mRNA are not detectably affected. It is entirely possible that TAK1 is more critical than GCK to p38 and NF-κB activation or that IL-1, poly(IC), and engagement of CD40 recruit multiple JNK activation pathways (e.g., TAK1) in which GCK is differentially important.
With this possibility in mind, the induction of IL-8 is noteworthy. The kinetics of this induction are quite rapid (Fig. 2D), and several studies suggest that cytokines (IL-1, TNF) induce IL-8 via transcriptional, posttranscriptional, and translational mechanisms (14). Previous work indicates that the posttranscriptional induction of IL-8 involves p38 MAPK-dependent transient stabilization of the mRNA (14). The lack of an effect of gck silencing on IL-1, CD40, or poly(IC) induction of IL-8 mRNA, coupled with the specificity of GCK for the JNK pathway, is consistent with this idea. On the other hand, the silencing of gck substantially reduces the rapid induction of IL-8 by LPS. It is possible that JNK may contribute to LPS-stimulated IL-8 mRNA stabilization. Indeed, while p38 plays a major role in agonist-induced mRNA stabilization, especially that of mRNAs containing 3′ AU-rich elements (14), JNK-dependent mRNA stabilization has been observed (4).
What might the biological function of GCK be? Our results plus the work of others indicate that GCK levels in the cell are regulated by multiple mechanisms. In human lymphoid tissue, B-cell GCK mRNA is strongly induced as cells migrate from the mantle zone to germinal centers (16). GCK is a very specific JNK pathway activator. ERK, p38 MAPK, and NF-κB are not activated by GCK even under conditions of overexpression (28, 31), and knockdown of GCK by RNAi fails to significantly reduce ERK or p38 activation by IL-1, engagement of CD40 (Fig. 2), or PAMPs (Zhong, unpublished). Consistent with these findings, our results indicate that GCK recruits two JNK-specific MAP3Ks, MEKK1 and mixed-lineage kinase 3 (3, 41). Our findings suggest that GCK is especially relevant to LPS stimulation of the JNK pathway. GCK itself is extremely low in abundance and is transiently stabilized upon agonist stimulation. It is possible that transcriptional and posttranslational regulation of GCK polypeptide levels allows GCK to modulate the intensity of JNK signaling, especially that triggered by endotoxin, relative to other pathways emanating from receptors of the innate immune system. Inasmuch as JNK has been implicated in inflammation-induced chemokine production and apoptosis during septic shock (23, 26, 38), it will be important to determine if GCK is involved in these processes as well.
Supplementary Material
Acknowledgments
We thank J. Kehrl for S169A/T173A GCK as well as wt and K63R ubiquitin plasmids. We are grateful to members of the Kyriakis lab for discussions.
This work was supported by USPHS grant R01-GM46577 from the National Institute of General Medical Sciences and by a basic science grant from the Arthritis Foundation (to J.M.K.).
Footnotes
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Akira, S. 2003. Mammalian Toll-like receptors. Curr. Opin. Immunol. 15:5-11. [DOI] [PubMed] [Google Scholar]
- 2.Baud, V., and M. Karin. 2001. Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol. 11:372-377. [DOI] [PubMed] [Google Scholar]
- 3.Chadee, D. N., T. Yuasa, and J. M. Kyriakis. 2002. Direct activation of mitogen-activated protein kinase kinase kinase MEKK1 by the STE20p homologue GCK and the adapter protein TRAF2. Mol. Cell. Biol. 22:737-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen, C. Y., F. Del Gatto-Konczak, Z. Wu, and M. Karin. 1998. Stabilization of interleukin-2 mRNA by the c-Jun NH2-terminal kinase pathway. Science 280:1945-1949. [DOI] [PubMed] [Google Scholar]
- 5.Chen, G., and D. V. Goeddel. 2002. TNF-R1 signaling: a beautiful pathway. Science 296:1634-1635. [DOI] [PubMed] [Google Scholar]
- 6.Chung, J. Y., Y. C. Park, H. Ye, and H. Wu. 2002. All TRAFs are not created equal: common and distinct molecular mechanisms of TRAF-mediated signal transduction. J. Cell Sci. 115:679-688. [DOI] [PubMed] [Google Scholar]
- 7.Dempsey, P. W., S. E. Doyle, J. Q. He, and G. Cheng. 2003. The signaling adaptors and pathways activated by TNF superfamily. Cytokine Growth Factor Rev. 14:193-209. [DOI] [PubMed] [Google Scholar]
- 8.Deng, L., C. Wang, E. Spencer, L. Yang, A. Braun, J. You, C. Slaughter, C. Pickart, and Z. Chen. 2000. Activation of the IκB kinase complex by TRAF6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell 103:351-361. [DOI] [PubMed] [Google Scholar]
- 9.Dumitru, C. D., J. D. Ceci, C. Tsatsanis, D. Kontoyiannis, K. Stamakis, J.-H. Lin, C. Patriotis, N. A. Jenkins, N. G. Copeland, G. Kollias, and P. N. Tsichlis. 2000. TNF-α induction by LPS is regulated posttranscriptionally via a TPL2/ERK-dependent pathway. Cell 103:1071-1083. [DOI] [PubMed] [Google Scholar]
- 10.Elbashir, S. M., J. Harborth, W. Lendeckel, A. Yalcin, K. Weber, and T. Tuschl. 2001. Duplexes of 21 nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411:494-498. [DOI] [PubMed] [Google Scholar]
- 11.Fenteany, G., R. F. Standaert, W. S. Lane, S. Choi, E. J. Corey, and S. L. Schreiber. 1995. Inhibition of proteasome activities and subunit-specific amino-terminal threonine modification by lactacystin. Science 268:726-731. [DOI] [PubMed] [Google Scholar]
- 12.Hochstrasser, M. 1996. Ubiquitin-dependent protein degradation. Annu. Rev. Genet. 30:405-439. [DOI] [PubMed] [Google Scholar]
- 13.Hofmann, R. M., and C. M. Pickart. 1999. Noncanonical MMS2-encoded ubiquitin-conjugating enzyme functions in assembly of novel polyubiquitin chains for DNA repair. Cell 96:645-653. [DOI] [PubMed] [Google Scholar]
- 14.Holtmann, H., R. Winzen, P. Holland, S. Eikemeier, E. Hoffmann, D. Wallach, N. L. Malinin, J. A. Cooper, K. Resch, and M. Kracht. 1999. Induction of interleukin-8 synthesis integrates effects on transcription and mRNA degradation from at least three different cytokine- or stress-activated signal transduction pathways. Mol. Cell. Biol. 19:6742-6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karin, M., Z.-G. Liu, and E. Zandi. 1997. AP-1 function and regulation. Curr. Opin. Cell Biol. 9:240-246. [DOI] [PubMed] [Google Scholar]
- 16.Katz, P., G. Whalen, and J. H. Kehrl. 1994. Differential expression of a novel protein kinase in human B lymphocytes: preferential localization in the germinal center. J. Biol. Chem. 269:16802-16809. [PubMed] [Google Scholar]
- 17.Kopp, E., and R. Medzhitov. 2003. Recognition of microbial infection by Toll-like receptors. Curr. Opin. Immunol. 15:396-401. [DOI] [PubMed] [Google Scholar]
- 18.Kyriakis, J. M., P. Banerjee, E. Nikolakaki, T. Dai, E. A. Rubie, M. F. Ahmad, J. Avruch, and J. R. Woodgett. 1994. The stress-activated protein kinase subfamily of c-Jun kinases. Nature 369:156-160. [DOI] [PubMed] [Google Scholar]
- 19.Kyriakis, J. M., and J. Avruch. 2001. Mammalian mitogen-activated protein kinase pathways activated by stress and inflammation. Physiol. Rev. 81:807-869. [DOI] [PubMed] [Google Scholar]
- 20.Lee, S. Y., A. Reichlin, A. Santana, K. A. Sokol, M. C. Nussenzweig, and Y. Choi. 1997. TRAF2 is essential for JNK but not NF-κB activation and regulates lymphocyte proliferation and survival. Immunity 7:703-713. [DOI] [PubMed] [Google Scholar]
- 21.Lomaga, M. A., W.-C. Yeh, I. Sarosi, G. S. Duncan, C. Furlonger, A. Ho, S. Morony, C. Capparelli, G. Van, S. Kaufman, A. van der Heiden, A. Itie, A. Wakeham, W. Khoo, T. Sasaki, Z. Cao, J. M. Penninger, C. J. Paige, D. L. Lacey, C. R. Dunstan, W. J. Boyle, D. V. Goeddel, and T. W. Mak. 1999. TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes Dev. 13:1015-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu, Z., S. Xu, C. Joazeiro, M. H. Cobb, and T. Hunter. 2002. The PHD domain of MEKK1 acts as an E3 ubiquitin ligase and mediates ubiquitination and degradation of ERK1/2. Mol. Cell 9:945-956. [DOI] [PubMed] [Google Scholar]
- 23.Manning, A. M., and R. J. Davis. 2003. Targeting JNK for therapeutic benefit: from junk to gold? Nat. Rev. Drug Discov. 2:554-565. [DOI] [PubMed] [Google Scholar]
- 24.Morton, S., R. J. Davis, A. McLaren, and P. Cohen. 2003. A reinvestigation of the multisite phosphorylation of the transcription factor c-Jun. EMBO J. 22:3876-3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nishitoh, H., M. Saitoh, Y. Mochida, K. Takeda, H. Nakano, M. Rothe, K. Miyazono, and H. Ichijo. 1998. ASK1 is essential for JNK/SAPK activation by TRAF2. Mol. Cell 2:389-395. [DOI] [PubMed] [Google Scholar]
- 26.Park, J. M., H. Brady, M. G. Ruocco, H. Sun, D. Williams, S. J. Lee, T. Kato, Jr., N. Richards, K. Chan, F. Mercurio, M. Karin, and S. A. Wasserman. 2004. Targeting of TAK1 by the NF-κB protein Relish regulates the JNK-mediated immune response in Drosophila. Genes Dev. 18:584-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pickart, C. 2001. Mechanisms underlying ubiquitination. Annu. Rev. Biochem. 70:503-533. [DOI] [PubMed] [Google Scholar]
- 28.Pombo, C. M., J. H. Kehrl, I. Sánchez, P. Katz, J. Avruch, L. I. Zon, J. R. Woodgett, T. Force, and J. M. Kyriakis. 1995. Activation of the SAPK pathway by the human STE20 homologue germinal centre kinase. Nature 377:750-754. [DOI] [PubMed] [Google Scholar]
- 29.Rechsteiner, M., and S. W. Rogers. 1996. PEST sequences and regulation by proteolysis. Trends Biochem. Sci. 21:267-271. [PubMed] [Google Scholar]
- 30.Rothwarf, D. M., and M. Karin. 1999. The NF-κB activation pathway: a paradigm in information transfer from membrane to nucleus. Sci. STKE 5:RE1. [Online.] http://www.stke.org/cgi/content/full/OC_sigtrans;1999/5/re1. [DOI] [PubMed]
- 31.Shi, C.-S., and J. H. Kehrl. 1997. Activation of stress-activated protein kinase/c-Jun N-terminal kinase, but not NF-κB, by the tumor necrosis factor (TNF) receptor 1 through a TNF receptor-associated factor 2- and germinal center kinase related-dependent pathway. J. Biol. Chem. 272:32102-32107. [DOI] [PubMed] [Google Scholar]
- 32.Shi, C.-S., and J. H. Kehrl. 2003. Tumor necrosis factor (TNF)-induced germinal center kinase-related (GCKR) and stress-activated protein kinase (SAPK) activation depends upon the E2/E3 complex Ubc13-Uev1A/TNF receptor-associated factor 2 (TRAF2). J. Biol. Chem. 278:15429-15434. [DOI] [PubMed] [Google Scholar]
- 33.Tobiume, K., A. Matsuzawa, T. Takahashi, H. Nishitoh, K. Morita, K. Takeda, O. Minowa, K. Miyazono, T. Noda, and H. Ichijo. 2001. ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep. 2:222-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vaidya, S. A., and G. Cheng. 2003. Toll-like receptor and innate antiviral responses. Curr. Opin. Immunol. 15:402-407. [DOI] [PubMed] [Google Scholar]
- 35.Vidal, S., R. S. Khush, F. Leulier, P. Tzou, M. Nakamura, and B. Lemaitre. 2001. Mutations in the Drosophila dTAK1 gene reveal a conserved function for MAPKKKs in the control of rel/NF-κB-dependent innate immune responses. Genes Dev. 15:1900-1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wajant, H., K. Pfizenmaier, and P. Scheruich. 2003. Tumor necrosis factor signaling. Cell Death Differ. 10:45-65. [DOI] [PubMed] [Google Scholar]
- 37.Wang, C., L. Deng, M. Hong, G. R. Akkaraju, J.-I. Inoue, and Z. J. Chen. 2001. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature 412:346-351. [DOI] [PubMed] [Google Scholar]
- 38.Weston, C. R., and R. J. Davis. 2002. The JNK signal transduction pathway. Curr. Opin. Genet. Dev. 12:14-21. [DOI] [PubMed] [Google Scholar]
- 39.Witowsky, J. A., and G. L. Johnson. 2003. Ubiquitylation of MEKK1 inhibits its phosphorylation of MKK1 and MKK4 and activation of the ERK1/2 and JNK pathways. J. Biol. Chem. 278:1403-1406. [DOI] [PubMed] [Google Scholar]
- 40.Yeh, W.-C., A. Shahinian, D. Speiser, J. Kraunus, F. Billia, A. Wakeham, J. L. de la Pompa, D. Ferrick, B. Hum, N. Iscove, P. Ohashi, M. Rothe, D. V. Goeddel, and T. W. Mak. 1997. Early lethality, functional NF-κB activation, and increased sensitivity to TNF-induced cell death in TRAF2-deficient mice. Immunity 7:715-725. [DOI] [PubMed] [Google Scholar]
- 41.Yuasa, T., S. Ohno, J. H. Kehrl, and J. M. Kyriakis. 1998. Tumor necrosis factor signaling to stress-activated protein kinase (SAPK)/Jun NH2-terminal kinase (JNK) and p38. J. Biol. Chem. 273:22681-22692. [DOI] [PubMed] [Google Scholar]
- 42.Yujiri, T., S. Sather, G. R. Fanger, and G. L. Johnson. 1998. Role of MEKK1 in cell survival and activation of JNK and ERK pathways defined by targeted gene disruption. Science 282:1911-1914. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.