Abstract
Background
An increased percentage of peripheral transitional B-cells producing IL-10 has been observed in patients tolerant to kidney allografts. In healthy volunteers, the balance between the CD40 and B-cell receptor (BCR) signalling modulated IL-10 production by B-cells, with stimulation via the BCR decreasing CD40-mediated-IL-10 production. In this study, we evaluate whether in tolerant kidney transplant patients the increased IL-10 production by B-cells was due to an altered CD40 and/or BCR signalling.
Methods
B-cells obtained from a new cohort of tolerant renal transplant recipients and those from age- and gender-matched healthy volunteers, were activated via CD40 and BCR, either alone or in combination.
Results
In tolerant patients we observed higher percentages of B-cells producing IL-10 after CD40 ligation and higher expression of CD40L on activated T-cells, compared to healthy controls. Furthermore, B-cells from tolerant recipients had reduced ERK signalling following BCR-mediated activation compared to healthy controls. In keeping with this, combining BCR signalling with CD40 ligation did not reduce IL-10 secretion as was observed in healthy control transitional B-cells.
Conclusion
Altogether our data suggests that the altered response of B-cells in tolerant recipients may contribute to long-term stable graft acceptance.
Introduction
A state of operational tolerance has been described in kidney transplant patients who deliberately decided to stop immunosuppression1. These patients displayed a stable graft function after complete drug withdrawal for longer than one year. In 2007, Brouard et al. performed the first study in kidney transplant patients aimed at identifying biomarkers of tolerance from peripheral blood samples, defining a “tolerant fingerprint” of 49 genes2. The same group reported later that tolerant recipients exhibited a significant increase in absolute numbers and frequency of total B-cells, particularly activated memory and early memory B-cells3. In the same year, two independent multicentre studies, the European IOT study4 and the USA ITN study5 reported and validated a B-cell signature in 11 and 19 kidney transplant tolerant patients, respectively. In both studies an over-expression of multiple B-cell related genes and maintenance of peripheral blood B-cell numbers and percentages in tolerant recipients were observed. Moreover, in the ITN study an up-regulation of CD20 mRNA in sediment cells from urine, and elevated numbers of peripheral blood naïve and transitional B-cells in tolerant participants, compared with those receiving immunosuppression, were also observed5. In 2012, another group reported that tolerant recipients exhibited similar numbers and percentages of circulating total B-cells, naive, memory and regulatory B-cells than healthy individuals, as well as preserved B-cell receptor repertoire. In addition, they demonstrated that tolerant recipients displayed a conserved capacity to activate CD40/STAT3 signalling pathway in regulatory B-cells6. In 2014, Brouard’s group showed that B-cells from tolerant recipients exhibited a defective expression of factors involved in the differentiation into plasma cells and the B-cells were more prone to cell death by apoptosis compared to patients with stable graft function7. Finally in 2015, the same group showed that tolerant recipient exhibit a higher number of Granzyme B+ B-cells compared to healthy volunteers and stable recipients 8. They showed that Granzyme B+ B-cells were able to inhibit CD25-CD4+Tcell responses through a pro-apoptotic mechanism8.
CD40 and BCR ligation on B-cells are key events in their function and T-cells contribute to both. The predominance of the BCR signalling alone favoures apoptosis9, whereas the predominance in the CD40 ligation favoures cell survival9 and IL-10 secretion10. The combination of both signals favoures cell activation, differentiation and antibody production11, however it has been reported that IL-10 production by CD27-B-cells is reduced when CD40 and BCR are triggered together compared to CD27-B-cells activated only through CD40-CD40L interaction10.1
In this study we hypothesised that altered BCR/CD40 signalling is linked to increased IL-10 production observed in tolerant patients. The B-cell responses from a cohort of tolerant renal transplant recipients were compared with age/gender-matched healthy volunteers. The data presented in our study demonstrated that B-cells from tolerant patients exhibited an imbalance in CD40/BCR signalling compared to healthy controls, suggesting that these differences may contribute their increased IL-10 production and to the long-term graft survival observed in tolerant patients.
Materials and Methods
Patients
Patient samples were donated from the Genetic Analysis & Monitoring of Biomarker of Immunological Tolerance (GAMBIT) study, approved by the Institute of Child Health/Great Ormond Street Hospital Research Ethics Committee 09/H0713/12. All experiments were performed in accordance with the approved guidelines and regulations. Samples were processed and analysed in a blinded fashion after informed consent was obtained from all subjects. Of patients from the GAMBIT study, the following ones have been used in this project:
Tolerant (n=16): Functionally stable kidney transplant recipients despite having stopped all their immunosuppression for longer than one year with a serum creatinine CRT < 160umol/l and < 10% rise in the last 12 months. ESRF causes have been summarised in SupplTable 1. Healthy control (n=11): Healthy volunteers were age and gender-matched to tolerant patients.
Patient characteristics are described in Table 1.
Table 1. Clinical data of tolerant kidney transplant recipients and healthy volunteers.
| Patient Data | Healthy Control | Tolerant |
| Number of patients | 11 | 16 |
| Age in years [mean (range)] | 48.0 (23-72) | 50.6 (22-77) |
| Gender (Male/Female) | (9/2) | (13/3) |
| Recipient age at Transpl. [mean (range)] | - | 32.1 (11-56) |
| Donor (Living/Deceased) | - | (9/7) |
| Time post Transpl. in years [mean (range)] | - | 18.4 (10-34) |
| Years immunosuppression free [mean (range)] | - | 6.0 (1-23) |
| HLA mismatches | ||
| Number of patients with: | ||
| -No mismatches | - | 6 |
| -HLA (A or B) mismatches | - | 2 |
| -HLA (A + B) mismatches | - | 1 |
| -HLA (A + DR) mismatches | - | 0 |
| -HLA (B + DR) mismatches | - | 0 |
| -HLA (A + B + DR) mismatches | - | 6 |
| -Missing data | - | 1 |
| Donor-specific antibodies (DSA) | ||
| Number of patients with: | ||
| -No DSA | - | 14 |
| -DSA Class I | - | 1 |
| -DSA Class II | - | 1 |
| -DSA Class I and II | - | 0 |
| Renal Function Parameters | ||
| Creatinine (mmols/L) [mean] | - | 117.2 ± 15.6 |
| eGFR (mL/min/1.73m2) [mean] | - | 60.9 ± 16.5 |
| Cell Count | ||
| Lymphocytes count x 109 [mean±SD] | 2.20 ± 0.55 | 2.30 ± 0.71 |
| B-cell count x109 [mean±SD] | 0.43 ± 0.24 | 0.46 ± 0.21 |
| Memory B-cell count x109 [mean±SD] | 0.12 ± 0.10 | 0.11 ± 0.07 |
| Naïve B-cell count x109 [mean±SD] | 0.34 ± 0.23 | 0.38 ± 0.16 |
| Transitional B-cell count x109 [mean±SD] | 0.03 ± 0.03 | 0.05 ± 0.02 |
DSA: donor-specific antibodies. eGFR: estimated glomerular filtration rate.
Sample Collection
PBMCs from patients and healthy controls were isolated from peripheral blood by Ficoll-Hypaque (PAA) density gradient centrifugation. Cells were re-suspended in 10% DMSO (Sigma-Aldrich)-AB human serum (BioWest) and frozen immediately at -80°C. After 24hrs, cells were transferred into liquid nitrogen where they were kept until use. Cell counts for each patient were obtained from their respective full blood count test (FBC; NHS). Serum samples were obtained after centrifugation of coagulated peripheral blood samples (2000RPM/20min/room temperature), stored at -80°C and kept until use.
B-cell phenotypic studies
PBMC samples were thawed from liquid nitrogen on the same day as the staining and 3x106 PBMCs were used to study B-cell phenotype. PBMCs were stained with Live/Dead (Invitrogen), anti-IgM-PercP-Cy5.5 (BD), anti-IgD-PE (BD), anti-CD19-AlexaFluor780, anti-CD20-AlexaFluor700, anti-CD27-APC, anti-CD24-FITC, anti-CD38-PECy7 (all eBioscience) for 30min at 4°C. Cells were acquired on LSRFortessa (BD). Data was acquired until at least 5,000 events in the B-cell subset were counted. All flow cytometry data was analysed using FlowJo (Tree Star, Inc. Ashland, OR 97520 USA) or DIVA software (BD).
B-cell functional assays in response to CD40L activation measured in kidney transplant patient and healthy control’s PBMCs
1x106 PBMCs from patient samples were cultured with or without 0.5x105 plate bound CD40L-transfected and non-transfected mouse L-fibroblast cells (X-ray irradiated for 30min 9,045 cGy) for 72hrs. After 3 days, expression of CD86-FITC and CD25-PE (all eBioscience) and intracellular IL-10-PE (BD) and TNF-α (eBioscience) were measured in CD20+B-cells. Supernatants were collected and stored at -80°C to subsequently measure cytokines by ELISA. Methods for intracellular staining, ELISA and experiments in B-cell subsets isolated from healthy leukocytes retained in filtering cones and patient samples can be found in the supplementary content.
T-cell activation
CD4+T-cells were isolated using CD4+T-cell Isolation Kit II, human (Miltenyi Biotec). Cells were activated with αCD3/CD28 beads (1:2 ratio) (Invitrogen) for 8 and 48hrs. After 8hrs, cells were stained with CD4-FITC (eBioscience) and CD40L-PE (eBioscience) for 30min at 4°C. Cytokines in the supernatants were measured using Cytometric Beads Array human Th1/Th2/Th17 kit (BD) For IL-2: the sensitivity of the kit was 2.6 pg/mL and the standard curve range was from 2.6 to 5000 pg/mL. For IL-4: the sensitivity of the kit was 4.9 pg/mL and the standard curve range was from 4.9 to 5000 pg/mL. Cells and beads were acquired on LSRFortessa (BD).
BCR activation measured on patient’s PBMCs
PBMCs from patients and healthy controls were thawed and rested overnight in media supplemented with IL-2 (100ng/ml, R&D) at 37°C 5% CO2. The next day, cells were stained with CD20-Pacific Blue (eBioscience), CD27-APC (eBioscience), CD24-PE (eBioscience), CD38-PE-Cy7 (eBioscience) and Live/Dead dye (Invitrogen) for 20min at 37°C 5% CO2. After the staining, 1x106 PBMCs were placed in FACS tubes, cells were washed, rested for 30min at 37°C 5% CO2 and activated with anti-IgM (20μg/ml)/anti-IgG (20μg/ml) (Southern Biotech) for 0, 10 and 30min and with PMA (0.1μM, SIGMA) for 10min at 37°C. Cells were fixed with BD Cytofix buffer (BD) for 10min at 37°C, permeabilized with BD Phosflow Perm Buffer III (BD) for 30min on ice and stained with anti-ERK1/2 (pT202/pY204)-AlexaFluor488 (BD) for 30min at 4°C. Cells were acquired on LSRFortessa (BD).
Detection of IgG1 serum levels
Serum IgG1 levels were measured with the Human Immunoglobulin Isotyping Magnetic Bead Panel Milliplex Map Kit (Millipore) in healthy volunteers and tolerant recipients.
Statistical analysis
All columns in graphs represent the mean and standard error of mean. Non-parametric Mann-Whitney test was used to analyse phenotypic flow cytometry data in patient and healthy control samples. Two-way Repeated Measurement (RM) ANOVA test with Sidak’s multiple comparisons was used to evaluate differences between non-activated and activated samples. We defined significance as **** P<0.0001, *** P<0.001, ** P<0.01 and * P<0.05. We performed data tables, statistical analysis results and graphs using Prism 6 (1992-2012 GraphPad Software, Inc La Jolla, CA 92037 USA).
Results
Tolerant kidney transplant recipients exhibit higher percentages of IL10+ B-cells after CD40L activation compared to healthy volunteers
Previous studies, from us and others, have established that the percentages of total B-cells in tolerant renal transplant patients were similar to healthy volunteers3,5,6. This was confirmed in a new cohort of tolerant recipients (Table.1) in whom similar percentages of total, memory, naïve and transitional B-cells between healthy controls and tolerant recipients were observed (Fig.1A and Fig.S1).
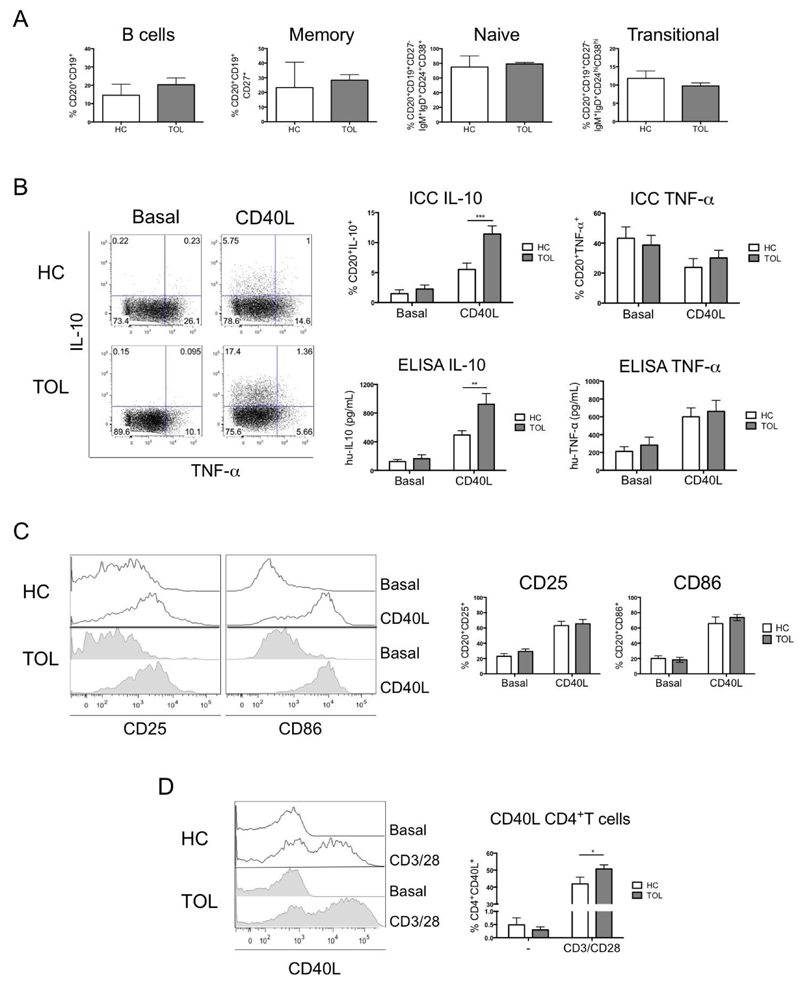
Fig. 1. Higher IL-10 production by B-cells from tolerant recipients was observed after CD40 ligation.
A) Comparison of the distribution of total, memory, naïve and transitional B-cells within PBMCs between healthy control (HC=11) and tolerant (Tol=16) patients. Total B-cells were identified as CD20+CD19+, memory B-cells as CD27+, naïve B-cells as CD27-IgD+IgM+CD24+CD38+ and transitional B-cells as CD27-IgD+IgM+CD24hiCD38hi by surface staining. A Mann-Whitney test was used. B) Production of IL-10 and TNF-α by intracellular staining and protein levels were measured after 3 days of activation with 0.5x105 non-transfected L-cells (Basal) or human-CD40L-transfected L-cells (CD40L) in 1.0x106 PBMCs from HC=8 and Tol=10 patients. Detection limit for IL-10 and TNF-α were 2 and 15.6pg/ml, respectively. C) The expression of CD25 and CD86 on CD20+ B-cells was measured after 3 days of activation with 0.5x105 non-transfected L-cells (Basal) or human-CD40L-transfected L-cells (CD40L) in 1.0x106 PBMCs from HC=11 and Tol=14 patients. D) Up-regulation of CD40L in CD4+T-cells from healthy HC=6 and Tol=7 patients were measured after 8hrs of activation with CD3/CD28 beads (1:2 ratio). Two-way RM ANOVA test with a Sidak’s multiple comparisons test was used. For all statistical tests **** P<0.0001, *** P<0.001, ** P<0.01 and * P<0.05 were considered significant.
IL-10 production has been shown to be the main regulatory mechanism for transitional B-cells in autoimmunity12, GVHD13, as well as transplantation5,7,14, therefore, the production of this cytokine was studied in the new cohort of tolerant patients. B-cells were stimulated via CD40 molecules by using CD40L-expressing L-cells, and as control non-transfected L-cells. CD40 stimulation of B-cells, in healthy volunteers sorted B-cell subsets isolated from peripheral blood cones, preferentially induced IL-10 production by transitional B-cells, while the TLR9 agonist CpG, used as positive control, led to IL-10 production by all the different B-cell subsets (Fig.S2). After confirming that CD40-ligation was inducing IL-10 mainly in transitional B-cells, the production of IL-10 was then compared in B-cells between healthy volunteers and tolerant patients. The percentage of IL-10+B-cells and the levels of IL-10 in the supernatants were significantly higher in samples from tolerant recipients compared to healthy volunteers, while no differences in the levels of TNF-α between the two groups was observed (Fig.1B). To confirm that the difference in IL-10 production between tolerant patients and healthy individuals was not due to differences in the susceptibility of B-cells to be activated, the expression of CD25 and CD86 molecules by B-cells after CD40-ligation was analysed. Similar up-regulation of both molecules was observed in the two cohorts of individuals (Fig.1C).
T-cells are key in the activation of B-cells and the engagement of CD40 expressed by B-cells by CD40L on T-cells is essential in inducing IL-10 production12. Therefore, we measured the expression of CD40L on CD4+T-cells from tolerant patients and healthy controls. We observed that the levels of expression of CD40L, while similar between the two cohorts of individuals before T-cell activation, were higher 8hrs-post activation with CD3/CD28 beads, in T-cells from tolerant patients, suggesting that T-cells from tolerant recipients have an advantage in engaging CD40 molecules on B-cells compared to healthy volunteers (Fig.1D).
B-cells from tolerant recipients have decreased BCR signalling compared to healthy volunteers.
We have shown so far that tolerant patients exhibited increased IL-10 production by B-cells and that CD40L expression is higher in T-cells compared to healthy volunteers. Another function of B-cells that may be relevant for the maintenance of a tolerant state, is their capacity to bind antigen via the BCR and then present it to T-cells15. At the same time, it has been shown previously that BCR engagement leads to the maturation and activation of B-cells via the MEK-ERK signalling pathway16.
Therefore, the phosphorylation of ERK was evaluated by stimulating B-cells with anti-IgG/anti-IgM (Fig.2A and B). Activation with PMA, that bypasses the BCR, was used as a positive control (Fig.2B). The results are presented as the differences in phosphorylation between samples activated via the BCR for 10min and non-activated (ΔERK) (Fig.2C). All B-cell subsets from tolerant recipients exhibited reduced ΔERK compared to healthy controls (Fig.2D), while no differences in ΔERK were observed after PMA activation (Fig.2E), suggesting that the reduction in ERK phosphorylation in the B-cells from tolerant recipients was specific to the BCR stimulation. CD40-mediated IL-10 production by CD27-B-cells was inhibited with simultaneous CD40 and BCR stimulation10. As predicted, IL-10 production was reduced in transitional B-cells from healthy individuals when B-cells were stimulated via CD40 and BCR compared to ligation of CD40 alone (Fig.2F and Fig.S3). In addition, we observed that the combination of CD40 and BCR stimulation resulted in down-regulation of ERK-phosphorylation compared to BCR stimulation alone (Fig.S4). Having shown that the effect on IL-10 release was mainly observed in the transitional population, IL-10 production by transitional B-cells in response to ligation of CD40 and BCR was compared between healthy individuals and tolerant patients. We found that, whereas IL-10 production following CD40 and BCR stimulation was decreased in transitional B-cells from healthy volunteers, it was maintained in transitional B-cells from tolerant recipients (Fig.2G). These results suggest that the reduced BCR signalling present in B-cells from tolerant recipients allowed sustained CD40-mediated IL-10 production after dual activation.
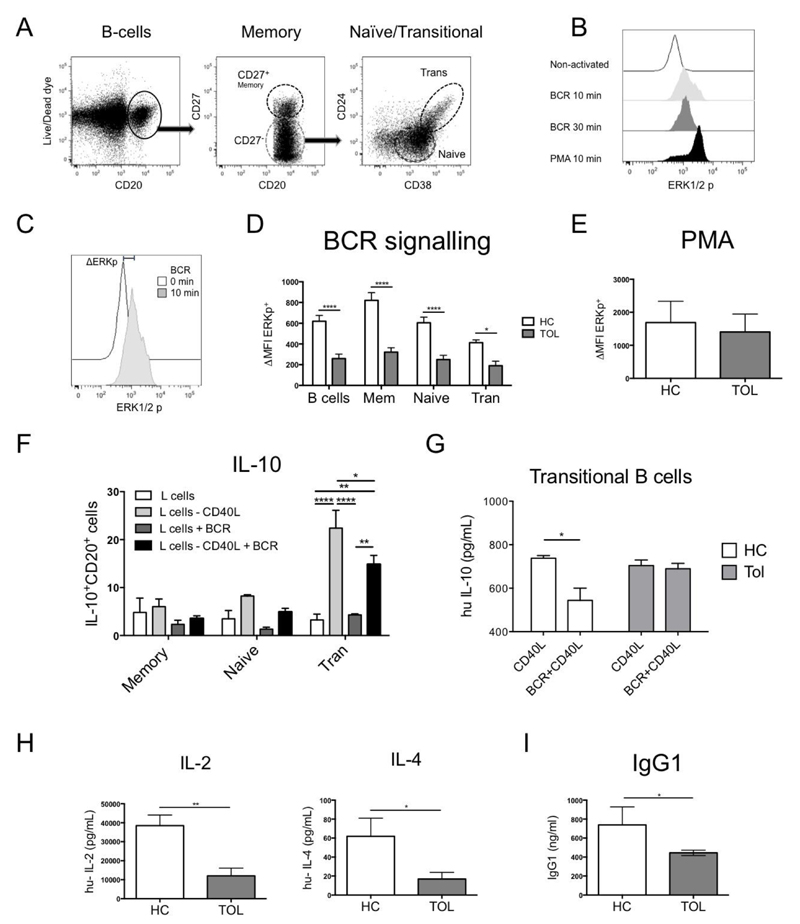
Fig. 2. B-cells from tolerant recipients display a reduced ERK signalling after BCR activation.
A) A phospho-flow B-cell panel was designed to identify p-ERK in B-cell subsets within PBMCs. B) A time course of p-ERK was measured in PBMCs after BCR-activation [anti-IgM (20μg/ml)/anti-IgG (20μg/ml)] during 0, 10 and 30min at 37°C, using PMA (0.1μM) for 10min as a positive control. C) Δp-ERK was defined as the difference between the p-ERK MFI from a non-activated and a 10min BCR-activated sample. D) The BCR signalling was measured in 1x106 PBMCs from healthy control (HC=9) and tolerant (Tol=9) patients after BCR-activation or after E) PMA (0.1μM) for 10min. F) IL-10 production after BCR/CD40 activation was measured by adding 0.5x105 non-transfected or CD40L-transfected L-cells in 0.5x106 non-activated or BCR-activated B-cell subsets from healthy leukocytes retained in filtering cones after 3 days of culture by intracellular staining. G) Levels of IL-10 were measured in the supernatants of 10x104 sorted transitional B-cells from 3 HC and 3 Tol recipients activated with 1x103 non-transfected or CD40L-transfected L-cells, with or without BCR activation for 3 days. H) IL-2 and IL-4 production from isolated CD4+T-cells of HC=6 and Tol=7 were measured after 48h of activation with CD3/CD28 beads (1:2 ratio). Detection limit for IL-2 and IL-4 were 2.6 and 4.9pg/ml, respectively. I) Levels of IgG1 were measured in the sera from HC and Tol patients. Mann-Whitney test and two-way RM ANOVA test with a Sidak’s multiple comparisons test were used, **** P<0.0001, *** P<0.001, ** P<0.01 and * P<0.05 were considered significant.
It has been described that mice deficient for Ras guanyl nucleotide exchange factor 3 (Rasgrp3−/−), exhibit reduced ERK phosphorylation after BCR activation and lower titters of IgG1 in their sera17, suggesting that reduction in the BCR-ERK signalling plays a role in the humoral response, skewing it to IgG subclasses with lower complement binding. Furthermore, IL-2 and IL-4 present during the class-switch also affect the production of IgG118,19. Therefore, the production of these two cytokines by CD4+T-cells from patients and healthy volunteers was measured after TCR activation. The analysis of these cytokines after 48hrs of culture revealed that the levels of IL-2 and IL-4 were lower in T-cells from tolerant patients compared to healthy controls (Fig.2H). In accordance with reduced BCR signalling and reduced levels of IL-2 and IL-4, lower levels of IgG1 were found in the serum of tolerant recipients compared to healthy volunteers (Fig.2I).
Discussion
Here we describe for the first time that tolerant patients exhibit higher percentages of IL-10-producing B-cells after CD40 activation and reduced BCR signalling compared to healthy controls. We also demonstrated that the reduction in BCR signalling favoured IL-10 production in the transitional B-cell population of tolerant recipients. Furthermore, the reduced ERK activation together with the reduced IL-2 and IL-4 production by T-cells may contribute to the low IgG1 levels observed in the serum (Fig.S5).
The percentages of B-cells and the expression of B-cell related genes has been previously compared between tolerant patients and either healthy individuals or other groups of patients under immunosuppressive therapy3–6. Newell et al. reported for the first time that following stimulation with PMA+Ionomycin, transitional B-cells from tolerant individuals expressed higher percentages of IL-10 relative to stable patients or healthy controls5. Although their differences were significant, they recognised that the overall percentages of IL-10 producing B-cells were extremely low, with many samples containing no IL-10-producing cells, and with a large overlap in IL-10 expression between groups, suggesting that PMA+Ionomycin may not have been the optimal stimulus to measure IL-10-producing B-cells within PBMCs. On the other hand, data from Silva et al. using specific CD40 activation, showed higher STAT-3 phosphorylation in regulatory B-cells from 5 tolerant patients compared to patients with chronic rejection, indicating that the IL-10 signalling pathway was activated in tolerant recipients6. More recently, Chesneau et al., demonstrated higher IL-10 production by B-cells from tolerant kidney transplant patients compared to healthy volunteers and stable recipient after a combination of anti-IgG/IgM/IgA, soluble CD40L, CpG and IL-27. However, the addition of CpG in the latter study complicated the interpretation of the results, as CpG induced IL-10 production by a different signalling pathway compared to CD40 and by different B-cell subsets (Fig.S2). Here we showed that CD40L activation induces IL-10 production preferentially by transitional B-cells and that IL-10 production was higher in transitional B-cells from tolerant patients after dual-BCR/CD40L activation compared to healthy volunteers. Since these activations targeted mainly the transitional B-cell population, it is difficult to compare the IL-10 production between tolerant recipients and patients under immunosuppressive treatment as the latter patients exhibited extremely low percentages of transitional B-cells (data not shown).
IL-10 is a well-known anti-inflammatory cytokine. The production of IL-10 by B-cells has been shown to inhibit T-cell proliferation13, cytokine production12 and promote regulatory T-cell differentiation20. Furthermore, IL-10 also attenuates the antigen presentation capacity of B-cells by down-regulating the expression of CD86 on their surface in mouse21 and human14. In human diseases, IL-10 production has been proposed as the main regulatory mechanism used by B-cells in lupus12, arthritis20. graft-versus-host disease (GVHD)13 and transplantation22. The production of IL-10 in tolerant recipients may indicate that in these patients it may contribute to the maintenance of a stable graft function in the absence of any immunosuppressive therapy.
Along with more IL-10 production, B-cells from tolerant recipients also exhibited a reduced BCR signalling. Mice with impaired BCR signalling exhibited lower titters of IgG1 in the sera compared to wild-type mice17 suggesting that reduced BCR signalling affects the Ig class-switch to IgG1. Furthermore, Chesneau et al. showed that B-cells from tolerant recipients exhibit a down-regulation of B-cell differentiation genes7. Accordingly, we observed in tolerant patients lower levels of IgG1 in the sera and lower secretion of the cytokines that contribute to the IgG class switching, IL-2 and IL-4 compared to healthy volunteers. More specifically, IL-4 has been shown to induce the production of the IgG subclasses IgG1, IgG2 and IgG3, while IL-2 promotes IgG1 and IgG3 release18,19. In addition, IL-2 produced by T-cells is required for ERK1/2-mediated differentiation of human naive B-cells into plasma cells23 and to enhance the effect of IL-10 for the induction of antibody production24. Furthermore, it has been shown that low levels of CD40 stimulation induced B-cell activation, whereas high levels of CD40 ligation inhibited Ig production11. Our results suggest that the imbalance in the BCR/CD40 signalling pathway and reduced IL-2 and IL-4 secretion by T-cells may have prevented the class-switch and further secretion of IgG1 in these patients. In the context of transplantation, reduced levels of IgG1 decreased complement dependent cytotoxicity and Fc-receptors binding, therefore impairment in the humoral response of these patients could have promoted the tolerant state by reducing antibody-dependent cellular cytotoxicity25.
Altogether our data suggest that the type of responses produced by B and T-cells in tolerant recipients may contribute to long-term stable graft function. Further studies are required to understand whether these responses were developed after transplant, as allograft-specific responses, or whether they are intrinsic or genetically determined in these patients.
Supplementary Materials
Acknowledgments
Funding: EN-L was funded by a scholarship from CONICYT Bicentennial Becas-Chile, Chile. The authors acknowledge financial support from the Medical Research Council (MRC) (grants G0801537/ID: 88245 and MRC Centre for Transplantation, – MRC grant no. MR/J006742/1) and Guy’s and St Thomas’ Charity (grants R080530 and R090782). The research was funded/supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy's and St Thomas' NHS Foundation Trust and King's College London. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. GL, and MPH-F have received funding from the European Union, Seventh Framework Programme [FP7/2007–2013], under grant agreement no HEALTH-F5–2010–260687: The ONE Study and PM and MPHF from FP7-HEALTH-2012-INNOVATION-1 project number 305147: BIO-DrIM.
Abbreviation
- BCR
B-cell receptor
- CD
Cluster of differentiation
- CD40L
CD40 ligand
- ERK1/2
Extracellular Signal-Regulated Kinases 1 and 2
- MEK
Mitogen-Activated Protein Kinase Kinase
- PMA
Phorbol-12-Myristate-13-Acetate
- TCR
T-cell receptor
Footnotes
Authorship
Author contribution: EN-L processed the samples used in this study, designed the experiments, performed the experiments, analysed the data and prepared the manuscript. PC and RM carried out Luminex analysis. PM, MR and YK processed GAMBIT samples. EN-L, GL, RIL and MH-F wrote the manuscript. GML, RIL, GL and MH-F obtained funding. MH-F directed the project. GAMBIT Consortium collaborators provided patient samples and detailed clinical information.
We would like to thank all GAMBIT patients and healthy volunteers for their participation in the project.
The authors declare no conflicts of interest.
References
- 1.Roussey-Kesler G, Giral M, Moreau A, et al. Clinical operational tolerance after kidney transplantation. Am J Transplant. 2006;6(4):736–746. doi: 10.1111/j.1600-6143.2006.01280.x. [DOI] [PubMed] [Google Scholar]
- 2.Brouard S, Mansfield E, Braud C, et al. Identification of a peripheral blood transcriptional biomarker panel associated with operational renal allograft tolerance. Proc Natl Acad Sci USA. 2007;104(39):15448–15453. doi: 10.1073/pnas.0705834104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pallier A, Hillion S, Danger R, et al. Patients with drug-free long-term graft function display increased numbers of peripheral B cells with a memory and inhibitory phenotype. Kidney Int. 2010;78(5):503–513. doi: 10.1038/ki.2010.162. [DOI] [PubMed] [Google Scholar]
- 4.Sagoo P, Perucha E, Sawitzki B, et al. Development of a cross-platform biomarker signature to detect renal transplant tolerance in humans. J Clin Invest. 2010;120(6):1848–1861. doi: 10.1172/JCI39922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Newell KA, Asare A, Kirk AD, et al. Identification of a B cell signature associated with renal transplant tolerance in humans. J Clin Invest. 2010;120(6):1836–1847. doi: 10.1172/JCI39933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silva HM, Takenaka MCS, Moraes-Vieira PMM, et al. Preserving the B-cell compartment favors operational tolerance in human renal transplantation. Mol Med. 2012;18:733–743. doi: 10.2119/molmed.2011.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chesneau M, Pallier A, Braza F, et al. Unique B cell differentiation profile in tolerant kidney transplant patients. Am J Transplant. 2014;14(1):144–155. doi: 10.1111/ajt.12508. [DOI] [PubMed] [Google Scholar]
- 8.Chesneau M, Michel L, Dugast E, et al. Tolerant Kidney Transplant Patients Produce B Cells with Regulatory Properties. J Am Soc Nephrol. 2015;26(10):2588–2598. doi: 10.1681/ASN.2014040404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsubata T, Wu J, Honjo T. B-cell apoptosis induced by antigen receptor crosslinking is blocked by a T-cell signal through CD40. Nature. 1993;364(6438):645–648. doi: 10.1038/364645a0. [DOI] [PubMed] [Google Scholar]
- 10.Duddy ME, Alter A, Bar-Or A. Distinct profiles of human B cell effector cytokines: a role in immune regulation? J Immunol. 2004;172(6):3422–3427. doi: 10.4049/jimmunol.172.6.3422. [DOI] [PubMed] [Google Scholar]
- 11.Miyashita T, McIlraith MJ, Grammer AC, et al. Bidirectional regulation of human B cell responses by CD40-CD40 ligand interactions. J Immunol. 1997;158(10):4620–4633. [PubMed] [Google Scholar]
- 12.Blair PA, NoreNa LY, Flores-Borja F, et al. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity. 2010;32(1):129–140. doi: 10.1016/j.immuni.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 13.Khoder A, Sarvaria A, Alsuliman A, et al. Regulatory B cells are enriched within the IgM memory and transitional subsets in healthy donors but are deficient in chronic GVHD. Blood. 2014;124(13):2034–2045. doi: 10.1182/blood-2014-04-571125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nova-Lamperti E, Fanelli G, Becker PD, et al. IL-10-produced by human transitional B-cells down-regulates CD86 expression on B-cells leading to inhibition of CD4(+)T-cell responses. Sci Rep. 2016;6:20044. doi: 10.1038/srep20044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noorchashm H, Reed AJ, Rostami SY, et al. B cell-mediated antigen presentation is required for the pathogenesis of acute cardiac allograft rejection. J Immunol. 2006;177(11):7715–7722. doi: 10.4049/jimmunol.177.11.7715. [DOI] [PubMed] [Google Scholar]
- 16.Richards JD, Davé SH, Chou CH, Mamchak AA, DeFranco AL. Inhibition of the MEK/ERK signaling pathway blocks a subset of B cell responses to antigen. J Immunol. 2001;166(6):3855–3864. doi: 10.4049/jimmunol.166.6.3855. [DOI] [PubMed] [Google Scholar]
- 17.Coughlin JJ, Stang SL, Dower NA, Stone JC. RasGRP1 and RasGRP3 regulate B cell proliferation by facilitating B cell receptor-Ras signaling. J Immunol. 2005;175(11):7179–7184. doi: 10.4049/jimmunol.175.11.7179. [DOI] [PubMed] [Google Scholar]
- 18.Flores-Romo L, Millsum MJ, Gillis S, Stubbs P, Sykes C, Gordon J. Immunoglobulin isotype production by cycling human B lymphocytes in response to recombinant cytokines and anti-IgM. Immunology. 1990;69(3):342–347. [PMC free article] [PubMed] [Google Scholar]
- 19.Splawski JB, Lipsky PE. Cytokine regulation of immunoglobulin secretion by neonatal lymphocytes. J Clin Invest. 1991;88(3):967–977. doi: 10.1172/JCI115400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flores-Borja F, Bosma A, Ng D, et al. CD19+CD24hiCD38hi B cells maintain regulatory T cells while limiting TH1 and TH17 differentiation. Science Translational Medicine. 2013;5(173):173ra23. doi: 10.1126/scitranslmed.3005407. [DOI] [PubMed] [Google Scholar]
- 21.Gillan V, Lawrence RA, Devaney E. B cells play a regulatory role in mice infected with the L3 of Brugia pahangi. International Immunology. 2005;17(4):373–382. doi: 10.1093/intimm/dxh217. [DOI] [PubMed] [Google Scholar]
- 22.Cherukuri A, Rothstein DM, Clark B, et al. Immunologic human renal allograft injury associates with an altered IL-10/TNF-α expression ratio in regulatory B cells. J Am Soc Nephrol. 2014;25(7):1575–1585. doi: 10.1681/ASN.2013080837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Le Gallou S, Caron G, Delaloy C, Rossille D, Tarte K, Fest T. IL-2 requirement for human plasma cell generation: coupling differentiation and proliferation by enhancing MAPK-ERK signaling. J Immunol. 2012;189(1):161–173. doi: 10.4049/jimmunol.1200301. [DOI] [PubMed] [Google Scholar]
- 24.Itoh K, Hirohata S. The role of IL-10 in human B cell activation, proliferation, and differentiation. J Immunol. 1995;154(9):4341–4350. [PubMed] [Google Scholar]
- 25.Arnold M-L, Ntokou I-S, Doxiadis IIN, Spriewald BM, Boletis JN, Iniotaki AG. Donor-specific HLA antibodies: evaluating the risk for graft loss in renal transplant recipients with isotype switch from complement fixing IgG1/IgG3 to noncomplement fixing IgG2/IgG4 anti-HLA alloantibodies. Transpl Int. 2014;27(3):253–261. doi: 10.1111/tri.12206. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.