Abstract
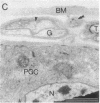
Female Heliothis moths normally produce their species-specific male attractant (sex pheromone blend) during scotophase, and this production is stimulated by pheromone biosynthesis activating neuropeptide (PBAN), presumably carried in the hemolymph. Several lines of evidence indicate that the central nervous system plays another critical role in this regulation. Pheromone biosynthesis was induced during photophase by electrical stimulation of the ventral nerve cord or the peripheral nerves projecting from the terminal abdominal ganglion to the pheromone gland in the tip of the abdomen. Electron microscopy further revealed that axonal branches innervate the gland tissue. Nerve branches associated with pheromone gland cells are enwrapped in glia and contain dense-core vesicles, suggesting that the innervation of the gland might be neurosecretory. Finally, the biogenic monoamine octopamine was nearly as effective as purified Heliothis zea PBAN in stimulating pheromone biosynthesis when injected into intact females during mid-photophase. Furthermore, both octopamine and PBAN stimulated significant increases in the pheromone content of the glands in isolated abdomens lacking a ventral nerve cord but only when abdomens were treated at the onset of scotophase. These data suggest that the regulation of sex pheromone production in Heliothis is more complex than previously thought. Activation of the gland appears to be governed by both neural and hormonal mechanisms, and these control mechanisms depend on photoperiodic cues.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altner H., Thies G. A functional unit consisting of an eversible gland with neurosecretory innervation and a proprioreceptor derived from a complex sensillum in an insect. Z Zellforsch Mikrosk Anat. 1973 Dec 21;145(4):503–519. doi: 10.1007/BF00306721. [DOI] [PubMed] [Google Scholar]
- Bacon J. P., Altman J. S. A silver intensification method for cobalt-filled neurones in wholemount preparations. Brain Res. 1977 Dec 16;138(2):359–363. doi: 10.1016/0006-8993(77)90753-3. [DOI] [PubMed] [Google Scholar]
- Berry M. S., Pentreath V. W. Criteria for distinguishing between monosynaptic and polysynaptic transmission. Brain Res. 1976 Mar 19;105(1):1–20. doi: 10.1016/0006-8993(76)90919-7. [DOI] [PubMed] [Google Scholar]
- Christensen T. A., Sherman T. G., McCaman R. E., Carlson A. D. Presence of octopamine in firefly photomotor neurons. Neuroscience. 1983 May;9(1):183–189. doi: 10.1016/0306-4522(83)90055-6. [DOI] [PubMed] [Google Scholar]
- Cusson M., McNeil J. N. Involvement of juvenile hormone in the regulation of pheromone release activities in a moth. Science. 1989 Jan 13;243(4888):210–212. doi: 10.1126/science.243.4888.210. [DOI] [PubMed] [Google Scholar]
- Evans P. D. Multiple receptor types for octopamine in the locust. J Physiol. 1981 Sep;318:99–122. doi: 10.1113/jphysiol.1981.sp013853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans P. D., O'Shea M. An octopaminergic neurone modulates neuromuscular transmission in the locust. Nature. 1977 Nov 17;270(5634):257–259. doi: 10.1038/270257a0. [DOI] [PubMed] [Google Scholar]
- Franzl S., Locke M., Huie P. Lenticles: innervated secretory structures that are expressed at every other larval moult. Tissue Cell. 1984;16(2):251–268. doi: 10.1016/0040-8166(84)90048-x. [DOI] [PubMed] [Google Scholar]
- Gordon M. J., Huang X., Pentoney S. L., Jr, Zare R. N. Capillary electrophoresis. Science. 1988 Oct 14;242(4876):224–228. doi: 10.1126/science.242.4876.224. [DOI] [PubMed] [Google Scholar]
- Holman G. M., Nachman R. J., Wright M. S. Insect neuropeptides. Annu Rev Entomol. 1990;35:201–217. doi: 10.1146/annurev.en.35.010190.001221. [DOI] [PubMed] [Google Scholar]
- King D. S., Fields C. G., Fields G. B. A cleavage method which minimizes side reactions following Fmoc solid phase peptide synthesis. Int J Pept Protein Res. 1990 Sep;36(3):255–266. doi: 10.1111/j.1399-3011.1990.tb00976.x. [DOI] [PubMed] [Google Scholar]
- Kitamura A., Nagasawa H., Kataoka H., Inoue T., Matsumoto S., Ando T., Suzuki A. Amino acid sequence of pheromone-biosynthesis-activating neuropeptide (PBAN) of the silkworm, Bombyx mori. Biochem Biophys Res Commun. 1989 Aug 30;163(1):520–526. doi: 10.1016/0006-291x(89)92168-2. [DOI] [PubMed] [Google Scholar]
- Levine R. B., Truman J. W. Dendritic reorganization of abdominal motoneurons during metamorphosis of the moth, Manduca sexta. J Neurosci. 1985 Sep;5(9):2424–2431. doi: 10.1523/JNEUROSCI.05-09-02424.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lázár G. Application of cobalt-filling technique to show retinal projections in the frog. Neuroscience. 1978;3(8):725–736. doi: 10.1016/0306-4522(78)90068-4. [DOI] [PubMed] [Google Scholar]
- Martinez T., Fabriás G., Camps F. Sex pheromone biosynthetic pathway in Spodoptera littoralis and its activation by a neurohormone. J Biol Chem. 1990 Jan 25;265(3):1381–1387. [PubMed] [Google Scholar]
- Nathanson J. A. Octopamine receptors, adenosine 3',5'-monophosphate, and neural control of firefly flashing. Science. 1979 Jan 5;203(4375):65–68. doi: 10.1126/science.214856. [DOI] [PubMed] [Google Scholar]
- Oland L. A., Tolbert L. P. Glial patterns during early development of antennal lobes of Manduca sexta: a comparison between normal lobes and lobes deprived of antennal axons. J Comp Neurol. 1987 Jan 8;255(2):196–207. doi: 10.1002/cne.902550204. [DOI] [PubMed] [Google Scholar]
- Orchard I., Carlisle J. A., Loughton B. G., Gole J. W., Downer R. G. In vitro studies on the effects of octopamine on locust fat body. Gen Comp Endocrinol. 1982 Sep;48(1):7–13. doi: 10.1016/0016-6480(82)90031-4. [DOI] [PubMed] [Google Scholar]
- Orchard I., Lange A. B. Evidence for octopaminergic modulation of an insect visceral muscle. J Neurobiol. 1985 May;16(3):171–181. doi: 10.1002/neu.480160303. [DOI] [PubMed] [Google Scholar]
- Orchard I., Loughton B. G. Is octopamine a transmitter mediating hormone release in insects? J Neurobiol. 1981 Mar;12(2):143–153. doi: 10.1002/neu.480120204. [DOI] [PubMed] [Google Scholar]
- Penzlin H. Neuropeptides--occurrence and functions in insects. Naturwissenschaften. 1989 Jun;76(6):243–252. doi: 10.1007/BF00368633. [DOI] [PubMed] [Google Scholar]
- Percy J. E. Ultrastructure of sex-pheromone gland cells and cuticle before and during release of pheromone in female eastern spruce budworm, Choristoneura fumiferana (Clem.) (Lepidoptera: Tortricidae). Can J Zool. 1974 Jun;52(6):695–705. doi: 10.1139/z74-094. [DOI] [PubMed] [Google Scholar]
- Rafaeli A., Soroker V. Cyclic AMP mediation of the hormonal stimulation of 14C-acetate incorporation by Heliothis armigera pheromone glands in vitro. Mol Cell Endocrinol. 1989 Aug;65(1-2):43–48. doi: 10.1016/0303-7207(89)90163-9. [DOI] [PubMed] [Google Scholar]
- Raina A. K., Jaffe H., Kempe T. G., Keim P., Blacher R. W., Fales H. M., Riley C. T., Klun J. A., Ridgway R. L., Hayes D. K. Identification of a neuropeptide hormone that regulates sex pheromone production in female moths. Science. 1989 May 19;244(4906):796–798. doi: 10.1126/science.244.4906.796. [DOI] [PubMed] [Google Scholar]
- Raina A. K., Klun J. A. Brain factor control of sex pheromone production in the female corn earworm moth. Science. 1984 Aug 3;225(4661):531–533. doi: 10.1126/science.225.4661.531. [DOI] [PubMed] [Google Scholar]
- Tang J. D., Charlton R. E., Jurenka R. A., Wolf W. A., Phelan P. L., Sreng L., Roelofs W. L. Regulation of pheromone biosynthesis by a brain hormone in two moth species. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1806–1810. doi: 10.1073/pnas.86.6.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teal P. E., Tumlinson J. H., Oberlander H. Neural regulation of sex pheromone biosynthesis in Heliothis moths. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2488–2492. doi: 10.1073/pnas.86.7.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorn R. S., Truman J. W. Sex-specific neuronal respecification during the metamorphosis of the genital segments of the tobacco hornworm moth Manduca sexta. J Comp Neurol. 1989 Jun 22;284(4):489–503. doi: 10.1002/cne.902840402. [DOI] [PubMed] [Google Scholar]