Abstract
Thyroid hormone (T3) has long been known to be important for vertebrate development and adult organ function. Whereas thyroid hormone receptor (TR) knockout and transgenic studies of mice have implicated TR involvement in mammalian development, the underlying molecular bases for the resulting phenotypes remain to be determined in vivo, especially considering that T3 is known to have both genomic, i.e., through TRs, and nongenomic effects on cells. Amphibian metamorphosis is an excellent model for studying the role of TR in vertebrate development because of its total dependence on T3. Here we investigated the role of TR in metamorphosis by developing a dominant positive mutant thyroid hormone receptor (dpTR). In the frog oocyte transcription system, dpTR bound a T3-responsive promoter and activated the promoter independently of T3. Transgenic expression of dpTR under the control of a heat shock-inducible promoter in premetamorphic tadpoles led to precocious metamorphic transformations. Molecular analyses showed that dpTR induced metamorphosis by specifically binding to known T3 target genes, leading to increased local histone acetylation and gene activation, similar to T3-bound TR during natural metamorphosis. Our experiments indicated that the metamorphic role of T3 is through genomic action of the hormone, at least on the developmental parameters tested. They further provide the first example where TR is shown to mediate directly and sufficiently these developmental effects of T3 in individual organs by regulating target gene expression in these organs.
Thyroid hormone receptors (TRs) are dual-function transcriptional regulators (60). On positive hormone response elements in the absence of thyroid hormone (T3), TRs repress T3 response genes, and in the presence of ligand, TRs activate these genes. The dual effects of TR are accomplished by recruiting mutually exclusive sets of coregulators to target promoters (39, 63). Corepressor complexes comprising nuclear corepressor (N-CoR) or silencing mediator of retinoid and thyroid hormone receptors (SMRT) with histone deacetylase 3, TBL1/TBLR1, and GPS2 associate with unliganded TR and deacetylate histones (18, 27, 28, 32, 53, 61, 62). The presence of T3 induces a conformational change in TR, promoting the release of corepressor complexes and recruitment of coactivator complexes that increase histone acetylation (5, 26, 35, 39, 41, 63). Despite the wealth of knowledge of the molecular mechanisms of TR action in vitro, relatively little is known of the role of TR and associated mechanisms in vivo during vertebrate development.
TR genes comprise two genetic loci, α and β, each with multiple splice variants, and knockout studies of various subsets of TR gene products in mice reveal a large diversity of developmental effects (10-12, 15, 16, 57). Some phenotypes may be explained based on differential tissue expression of TRα and TRβ. For example, TR expression in the ear is predominantly TRβ, and TRβ but not TRα knockouts affect hearing. However, other phenotypes cannot be explained based on differential expression alone. Equal amounts of TRα and TRβ are expressed in the intestine, yet only TRα knockouts have a dramatic effect on intestine development. Also, double knockouts are less severe than mutations or treatments that eliminate T3, which leaves open the possibility that some of the developmental roles of T3 may be due to nongenomic effects of T3, which have been well documented (8). In addition, the molecular bases of these various developmental effects have not been clarified, and the resolution of these questions requires an in vivo model system.
Frog metamorphosis provides an attractive model to address the developmental roles of TR at the molecular level. The ease and speed of making transgenic tadpoles allow rapid evaluation of effects of transgenes on tadpole development, and the molecular mechanisms of these effects can be readily assessed because of the large size of the free-living tadpoles. More importantly, thyroid hormone physiology in frogs exists in a simplified state compared to that in mice, where maternal T3 is always present. In frogs, T3 is absent from premetamorphic tadpoles (56), and postembryonic developmental events that take place during metamorphosis are completely dependent upon T3. In addition, metamorphosis can be induced precociously by the addition of exogenous T3 (45). Thus, all TRs are in the unliganded state before metamorphosis, and at the climax of metamorphosis or upon addition of T3 to the rearing water, the TRs become ligand bound. Previous work with frogs has validated in vitro cell culture models of TR function in development, in that TR is constitutively bound to target promoters in different tissues in vivo (43). In addition, corepressors N-CoR, SMRT, and TBLR1 are present on the promoters of T3 response genes TRβ and basic leucine zipper transcription factor (TH/bZIP) in premetamorphic tadpoles (42, 53). Furthermore, transgenesis using a dominant negative TR has been used to show that T3-induced activation or derepression is necessary for gene regulation and morphological changes during metamorphosis (3, 7, 44).
In the present study, we addressed whether TR activation is sufficient for the changes in gene regulation and morphology in metamorphosis. We developed a dominant positive TR (F-dpTR), which has a FLAG tag and VP16 activation domain fused to the N terminus of TR and the receptor-interacting domain (RID) of Xenopus N-CoR fused to the C terminus. We showed that this mutant receptor strongly activates positively regulated promoters in the absence of ligand. In tadpoles transgenic for the F-dpTR under heat shock control, metamorphic changes were induced after heat shock, even in the absence of T3, suggesting that TR is sufficient to mediate these metamorphic effects of thyroid hormone.
MATERIALS AND METHODS
Molecular cloning.
A series of 11 PCRs was used to make the dominant positive constructs. In reaction 1, a FLAG-tagged activation domain of VP16 (herpes simplex virus type 1; amino acids [aa] 413 to 490) was created by PCR amplification using the primers DRB 42 and DRB 43 (see Table 1 for primer sequences) from the template CGI-DBEcR (gift from David Johns, Johns Hopkins University). These primers removed the natural XbaI site from VP16. Full-length (aa 2 to 419) (reaction 2) and two dominant negative (aa 2 to 400) (reactions 3 and 4) Xenopus TRαA (xTRαA) minus the initial methionine and stop codon were amplified by using primers DRB 44 and 45, DRB 44 and 46, and DRB 44 and 49b from the template pSP64[poly(A)]-TRαA (59). Two RIDs (aa 1680 to 2357 and 1988 to 2357) (reactions 5 and 6) from Xenopus N-CoR were amplified from pSP64-N-CoR-4 with primers DRB 47 and 48 and DRB 50b and 48 (42). The FLAG-tagged VP16 activation domain was attached to the TRs by mixing PCR products from reactions 1 and 2 with primers DRB 42 and 45 to make F-VP-TR (reaction 7), PCR products from reactions 1 and 3 with primers DRB 42 and 46 (reaction 8), and PCR products from reactions 1 and 4 with primers DRB 42 and 49b (reaction 9). To attach the RIDs to the FLAG-VP16-dnTRs, PCRs were performed using products from reactions 5 and 8 with primers DRB 42 and 48 to make F-dpTR-l (reaction 10) and products from reactions 6 and 9 with primers DRB 42 and 48 to make F-dpTR (reaction 11). PCR products from reactions 7, 10, and 11 were cloned into pSP64RI (S. Sato, National Institute of Diabetes and Digestive and Kidney Diseases) by using EcoRI for making mRNA, and the F-dpTR from reaction 11 was subcloned by using AgeI and EcoRI into pCGHG, a double promoter transgenesis vector (13). The constructs F-TR-l and F-TR-s were made by digesting F-dpTR-l and F-dpTR with EagI, which removed the fragment containing VP16, and religating.
TABLE 1.
Primers for cloning
| Primer | Direction | Primer sequence (5′-3′) |
|---|---|---|
| DRB 42 | Forward | CATCATGAATTCACCGGTATGGACTACAAGGATGACGACGACAAGCGGCCGGCCCCCCCGACCGATGTCAGC |
| DRB 43 | Reverse | TCTTCTAGAGGTACCTAGAAGCTTCGGCCGCCCACCGTACTCGTCAATTCC |
| DRB 44 | Forward | CTTCTAGGTACCTCTAGAAGAATATCAGACCAGAATCTCAGCGGGCTG |
| DRB 45 | Reverse | CATCATCTCGAGGAATTCTCACCGCGGTCAAACTTCCTGGTCCTCAAAG |
| DRB 46 | Reverse | CAACATCCGGCCTCAAATTAACGGGGCACTCGACCTTCATGTG |
| DRB 47 | Forward | CACATGAAGGTCGAGTGCCCCGTTAATTTGAGGCCGGATGTTG |
| DRB 48 | Reverse | CATCATCTCGAGGAATTCTCACCGCGGTGATTGGTTCCCTTGTGGTAC |
| DRB 49b | Reverse | CATAGCGTGACTTTGTGTGCGCGGGGCACTCGACCTTCATGTG |
| DRB 50b | Forward | CACATGAAGGTCGAGTGCCCCGCGCACACAAAGTCACGCTATG |
Animals.
Adult frogs were purchased (Nasco, Fort Atkinson, Wis.) for oocyte assay and transgenesis. Transgenesis by restriction enzyme-mediated integrations was carried out as described previously (3, 31). Wild-type and transgenic tadpoles were reared at room temperature in 1 mM methimazole to block endogenous T3 synthesis (4, 6). The tadpoles were fed daily, and water was changed every other day. Premetamorphic tadpoles (stage 45 to 54) (36) were heat shocked at 33°C for 5 min on day 0 and then heat shocked for 1 h at 33°C on days 1 through 10. For comparison, some wild-type tadpoles at stage 52 were treated with 5 nM T3 for 3 days (47). Tadpoles were not fed during the treatment period. Animals were photographed under bright field and fluorescence with a green fluorescent protein (GFP) filter, and the images were merged to show GFP expression in transgenic tadpoles harboring the double promoter transgenesis vector (13).
Transcription assay of the oocyte.
Oocytes were prepared according to established procedures (52, 58). Wild-type and mutant TR and retinoid X receptor (RXR) mRNAs for oocyte injections were synthesized with mMESSAGE mMACHINE (Ambion) from the mutant TR templates (the present study) and pSP64[poly(A)]-TRαA and pSP64[poly(A)]-RXR (59). For the luciferase assay, 23 nl of water or a 50/50 mixture of 50 ng of RXR mRNA/μl and 50 ng of the indicated TR mRNAs/μl was injected into the oocyte cytoplasm. Then, 13.4 nl of a 66/33 mixture of 25 ng of pGL-TRE luciferase (TRE-luc)/μl (1) and 5 ng of phRG-thymidine kinase (TK)/μl (Promega) was injected into the nucleus. After overnight incubation at 18°C, six oocytes were lysed together by pipetting in 90 μl of 1× lysis buffer from the dual-luciferase assay kit (Promega), and 7 μl of oocyte lysate was used for each assay with 37 μl of substrate and 37 μl of Stop and Glo buffer according to the manufacturer's instructions. Assays were performed in triplicate. Western blotting, immunoprecipitation, and chromatin immunoprecipitation (ChIP) from oocytes were performed as previously described (52, 53).
Histology and TUNEL assay.
Tadpole intestines were fixed in 4% paraformaldehyde for 2 h at room temperature, cryoprotected in 0.5 M sucrose-60% phosphate-buffered saline (PBS), and embedded in Tissue-Tek OCT medium. Cryosections 10 μm in size were made and stained with methyl green-pyronine Y (Muto) or processed for the terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) assay. For TUNEL, sections on slides were refixed in 4% paraformaldehyde for 15 min at room temperature and then rinsed in PBS. The sections were permeabilized with 20 μg of protease K/ml in 10 nM Tris, pH 8, for 10 min at room temperature. After sections were rinsed in PBS, endogenous peroxidase activity was quenched for 20 min with 3% H2O2 in 90% methanol. The TUNEL reaction with and without terminal deoxynucleotidyl transferase (TdT) was carried out at 37°C for 90 min in TdT buffer (Oncogene), 0.016 mM biotin-14-dATP (Invitrogen), and 0.3 U of TdT/ml (Invitrogen). Then, the sections were rinsed in PBS, blocked for 10 min with 1% bovine serum albumin, and incubated with ABC Elite Ready-To-Use solution (Vector Labs). After 30 min, antibody binding was detected with a DAB staining kit (Vector Labs).
Protein and RNA extraction, Western blotting, and RT-PCR.
Tadpole organs were crushed in lysis buffer (10 mM Tris-HCl [pH 7.5], 150 mM NaCl, 5 mM EDTA, 1% Triton X-100, complete mini EDTA-free protease inhibitor tablet [Roche], 1 mM dithiothreitol) and then forced through a syringe after an equal volume of 2× sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer was added. The protein lysate was boiled, run on an SDS-PAGE gel, and subjected to anti-N-CoR and anti-FLAG (Stratagene) Western blotting (52, 53). RNA from tails and intestines was isolated with TRIzol reagent (Invitrogen). Reverse transcription (RT)-PCR was carried out as described previously using the same primers (3).
In vivo chromatin immunoprecipitation.
Isolation of chromatin from tadpole organs was performed as described previously (43, 53). Immunoprecipitation (IP) was carried out by using anti-FLAG (Stratagene) and anti-acetyl histone H4 (Upstate) antibodies. Preclearing and IP for anti-FLAG antibodies were done with protein G Sepharose beads (Amersham Biosciences) rather than protein A Sepharose beads. After reverse cross-linking, DNA was purified with a PCR purification kit (QIAGEN). A quantitative PCR was carried out to analyze the ChIP DNA samples; each sample was run in duplicate on an ABI 7000 (Applied Biosystems) using gene-specific primers and FAM (6-carboxyfluorescein)-labeled Taq-man probes (Applied Biosystems) (Table 2). To ensure the validity of the quantitative PCR, the following control experiments were done. For each assay, six twofold serial dilutions from a large batch of ChIP input DNA mixed from tails and intestines prepared especially for the purpose of serving as standards were used for quantification of the experimental samples. The calculated standard curves ranged in slope from −3.30 to −3.70, where theoretical amplification has a slope of −3.32. Also included in each run was a no-template control where pure water was added to control for PCR product contamination. In addition, a sample of 0.1 ng of tadpole genomic DNA/μl was used to assess the consistency of the standard curve calculated from the serial dilutions between runs. The coefficient of variation for the control samples was less than 5% for each primer probe set used. Results from the control sample and experimental ChIP samples fell within the range of the standard curve. Over 10 side-by-side comparisons of results from radioactive gel electrophoresis and quantitative PCR using the same ChIP DNA samples gave the same qualitative conclusions (data not shown).
TABLE 2.
Primers and probes for quantitative PCR
| Gene region | Forward primer (5′-3′) | Reverse primer (5′-3′) | FAM-labeled probe (5′-3′) |
|---|---|---|---|
| TRβA TRE | CCCCTATCCTTGTTCGTCCTC | GCGCTGGGCTGTCCT | CCTAGGCAGGTCATTTC |
| TH/bZIP TREs | GGACGCACTAGGGTTAAGTAAGG | TCTCCCAACCCTACAGAGTTCAA | ATGAGGCTGAGCATTCA |
| TRβA exon 5 | CCCCGAAAGTGAAACTCTAACGT | AAACCACTCCAAGTCCTCCATTTT | CCTTGTCACTGCCATCTC |
RESULTS
Construction of dominant positive TR.
Because no known TR mutants are dominant positive, we designed mutant TRs to enable high levels of gene induction without ligand and maintain native promoter binding. As a first step, we wanted to mutate TR to block endogenous corepressor binding. Unfortunately, mutations within the TR ligand-binding domain and hinge region that inhibit interaction with corepressors also reduce natural heterodimerization with the endogenous TR binding partner, 9-cis-retinoid X receptor (RXR), which would ensure native promoter binding affinity and specificity (33, 64). An alternative way to block TR-mediated repression is to use dominant negative forms of N-CoR (9). However, this strategy requires highly overexpressed amounts of dominant negative N-CoR in vivo, is not specific for TR, and is undesirable because two proteins would be needed for subsequent transgenesis. We decided to fuse the RID of Xenopus N-CoR to the C terminus of TR. The N-CoR and TRα moieties are expected to bind intramolecularly, thus preventing gene repression due to the binding of native corepressor complexes to the TRα moiety in the absence of T3. Because helix 12 of TR reduces corepressor binding (34), we fused the RID of Xenopus N-CoR to a dominant negative TRα (dnTR) lacking the C-terminal helix 12 to promote the intramolecular association. Two different fragments containing the RID (21, 55) of Xenopus N-CoR were made and used to generate two dnTR fusion proteins (Fig. 1A). The long version, F-TR-l, had an N-terminal extension from the RID in hopes that it would be able to adopt the proper TR interaction conformation. For the shorter version, F-TR-s, we estimated the minimal number of amino acids required for the N-CoR binding surface of TR to fit the corresponding corepressor nuclear receptor binding motif of N-CoR RID I. The N-terminal FLAG tag was added to wild-type and mutant TRs for ease of detection.
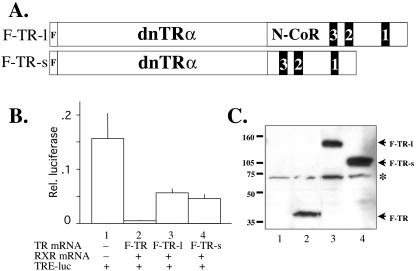
FIG. 1.
The fusion of the RID of N-CoR to a dominant negative TR leads to partial derepression. (A) Diagram of TR fusion proteins. The fusion constructs F-TR-l and F-TR-s include a long and short fragment of Xenopus N-CoR containing the RID. The black boxes labeled 3, 2, and 1 represent the corepressor nuclear receptor binding motifs (I/LXXI/VI) of N-CoR that directly bind TR (21). The dominant negative Xenopus TRα (dnTRα) part of the fusion protein corresponds to Xenopus TRα without the terminal helix 12 (3, 44). Both fusion proteins also contain an N-terminal FLAG tag. (B) The fusion proteins have reduced abilities to repress T3 target genes in the frog oocyte transcription system. The plasmids for the dual luciferase reporter system, where the firefly luciferase was driven by the T3-inducible Xenopus TRβA promoter (TRE-luc) and the Renilla luciferase was driven by the T3-independent TK promoter, were injected into the oocyte nucleus. The mRNAs for RXR and FLAG-tagged wild-type TRα (F-TR), F-TR-l, or F-TR-s were injected into the cytoplasm. After overnight incubation in the absence of T3, the oocytes were harvested for luciferase assay, and the relative expression of the reporter firefly luciferase over the control Renilla luciferase was determined. The experiment was repeated with similar results. (C) Western blot showing the expression of the receptor proteins in the frog oocyte. Protein extracts from luciferase assay from lanes 1 to 4 as shown in panel B were separated by SDS-PAGE, and FLAG-tagged wild-type and mutant TRs were detected by anti-FLAG Western blotting. Molecular weight markers are shown, and the asterisk indicates a cross-reacting band.
To test the effectiveness of the N-CoR moieties to inhibit repression by the TR moiety, we used the constructs F-TR-l and F-TR-s in the oocyte transcription assay, where we can study gene regulation by TR in the context of chromatin (58). We expressed RXR together with FLAG-tagged wild-type TR (F-TR), F-TR-l, or F-TR-s by microinjecting their mRNAs into the oocyte cytoplasm. As a reporter, we microinjected into the oocyte nucleus the plasmid containing the firefly luciferase under the control of the T3-inducible promoter of the Xenopus TRβA gene (40) together with a control plasmid containing the Renilla luciferase under the control of the T3-independent TK promoter. The luciferase assay of the oocyte extract after overnight incubation showed that as expected, F-TR repressed basal activity in the absence of ligand (Fig. 1B, lanes 1 and 2). On the other hand, both F-TR-l and F-TR-s were fourfold less effective in repressing the promoter (Fig. 1B, lanes 3 and 4). Under the experimental conditions, similar or slightly higher levels of mutant TR were expressed (Fig. 1C), indicating that the N-CoR moiety in F-TR-l and F-TR-s fusion proteins was able to reverse much of the repression caused by the TR moiety of the fusion protein.
To achieve gene activation in the absence of ligand, we fused the VP16 activation domain (hereafter VP16) to the N terminus of wild-type TR, F-TR-l, and F-TR-s to make F-VP-TR, F-dpTR-l, and F-dpTR (Fig. 2A). The ability of these fusion proteins to activate gene expression in the absence of T3 compared to liganded wild-type receptor was tested in the oocyte transcription system. Again, F-TR in the absence of ligand repressed transcription and in the presence of T3 activated transcription above basal levels (Fig. 2B, lanes 1 to 3). All three VP16 fusion proteins induced gene activation in the absence of ligand (Fig. 2B, lanes 4 to 6). Because the expression level of the mutants was higher than that of F-TR (Fig. 2C), quantitative comparisons between wild-type and mutant TRs are not possible. Nevertheless, gene upregulation by mutant TRs was well above basal transcription levels, and the N-CoR moiety in F-dpTR-l and F-dpTR increased the activation ability compared to the F-VP-TR fusion protein. As F-dpTR appeared to be the most active TR, we chose it for the rest of the studies.
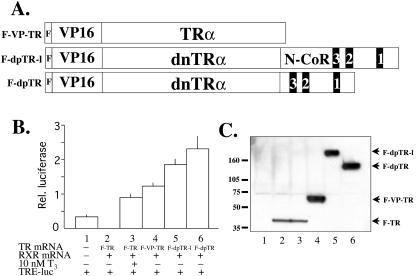
FIG. 2.
The transactivation domain of VP16 fused to wild-type TR, F-TR-l, and F-TR-s induces gene activation in the absence of T3. (A) Diagram of TR fusion proteins. The activation domain of VP16 was inserted between the FLAG tag and the N terminus of wild-type Xenopus TRα, F-TR-l, and F-TR-s (Fig. 1) to produce F-VP-TR, F-dpTR-l, and F-dpTR, respectively. (B) The VP16 fusion proteins activate transcription in Xenopus oocytes. The oocytes were injected as described in the legend to Fig. 1, except with the indicated receptor mRNAs. Luciferase activity was measured after overnight incubation in the presence or absence of 10 nM T3. Note that all TR fusion proteins not only failed to repress the promoter but also induced transcription above basal levels in the absence of T3. The experiment was repeated with similar results. (C) Western blot showing the expression of the receptor proteins in the frog oocyte. Protein extracts from the luciferase assay from lanes 1 to 6 in panel B were separated by SDS-PAGE and detected by anti-FLAG Western blotting. For unknown reasons, the VP16 fusion proteins were expressed at higher levels than the FLAG-tagged wild-type TR when the same amount of mRNA was injected, but this result does not affect the conclusion that they activate the promoter in the absence of T3. Molecular weight markers are shown.
The results from the transcription assays suggested that endogenous corepressors were at least partially excluded from binding to mutant TR containing the N-CoR moieties. To directly address this, we performed a coimmunoprecipitation experiment using anti-FLAG antibody on lysates from oocytes injected with F-TR and RXR or F-dpTR and RXR incubated with or without T3. As expected, N-CoR was coimmunoprecipitated with F-TR only in the absence of T3 (Fig. 3A, lanes 2 and 3). In contrast, there was little N-CoR coimmunoprecipitated with F-dpTR in the presence or absence of T3 (Fig. 3A, lanes 4 and 5), corroborating the transcription data that N-CoR is largely excluded from functioning with the mutant receptors.
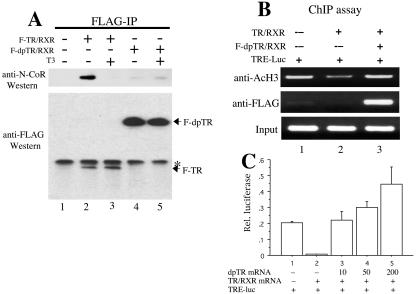
FIG. 3.
F-dpTR has drastically reduced ability to associate with N-CoR in vivo, correlating with its failure to induce histone deacetylation in the absence of T3, and competes with wild-type TR for transcription regulation. (A) Coimmunoprecipitation revealed dramatically weakened interaction between N-CoR and F-dpTR. Xenopus oocytes were injected with RXR and F-TR or F-dpTR and treated with or without T3. After overnight incubation, anti-FLAG antibodies were added to oocyte lysates to immunoprecipitate the receptor complexes. Immunoprecipitates were run on SDS-PAGE and blotted with anti-N-CoR or anti-FLAG antibodies. Note that N-CoR associated with wild-type TR in the absence but not the presence of T3 (lanes 2 and 3), whereas little N-CoR bound to F-dpTR independently of T3 (lanes 4 and 5). The weak signals in both lane 4 and lane 5 were due to residual binding of endogenous N-CoR to the TR moiety or immunoprecipitation background. Lane 1 is a no-injection control, and the asterisk indicates a cross-reacting band. (B) Coexpression of F-dpTR prevents histone deacetylation caused by wild-type TR in oocytes. Oocytes were injected with the reporter DNA and the indicated receptor mRNAs as described in the legend to Fig. 1 and incubated overnight in the absence of T3. ChIP was carried out on oocyte homogenates with antibodies against acetylated H3 or FLAG, and the ChIP DNA was detected by using a primer set flanking the TRE region in the reporter DNA. Note that the F-dpTR was bound to the TRE and prevented histone deacetylation (lane 3) as seen with wild-type TR alone (lane 2). (C) F-dpTR induces transcription even in the presence of unliganded wild-type TR. Oocytes were injected with reporter plasmids and the indicated receptor mRNAs as described in the legend to Fig. 1. Wild-type TR and RXR were injected at a concentration of 50 ng/μl, and dpTR was injected at 10, 50, and 200 ng/μl. After an overnight incubation in the absence of T3, the relative luciferase activity was measured. Note that the mutant receptor can completely counter the repression by wild-type TR and at higher mutant TR concentrations induce transcription above basal levels.
Because tadpoles, unlike oocytes, express endogenous TR, a final test for the feasibility of using F-dpTR in transgenesis was to determine whether the mutant TRs could function in a dominant positive fashion. We coinjected mRNAs encoding wild-type TR without FLAG tag and F-dpTR together with RXR and the reporter DNA into oocytes. After overnight incubation, we assayed luciferase activity and found that F-dpTR was able to activate the promoter in oocytes even in the presence of unliganded wild-type TR (Fig. 3C). In addition, we subjected some of the oocytes to a ChIP assay with anti-acetylated histone H3 or anti-FLAG antibodies. PCR analysis of the precipitated DNA showed that the region encompassing the thyroid hormone response element (TRE) of the reporter plasmid had a basal level of acetylated histones that was reduced in the presence of wild-type TR (Fig. 3B, lanes 1 and 2). However, when F-dpTR was coinjected with wild-type TR, the level of histone acetylation was restored (Fig. 3B, lane 3). The anti-FLAG ChIP assay showed that F-dpTR was bound to the TRE region when coexpressed with TR (Fig. 3B, lane 3). These results indicate that F-dpTR can bind T3-regulated promoters in vivo, even in the presence of wild-type TR, and function as a dominant positive transcriptional activator.
Transgenic expression of F-dpTR induces metamorphic events.
To investigate whether TR is sufficient to mediate the metamorphic effects of T3, we introduced F-dpTR into developing animals through transgenesis. We used a double promoter transgenesis construct (13). First, to prevent potential deleterious effects on embryonic development, we placed F-dpTR under the control of a heat shock-inducible promoter, which has little basal expression in the absence of heat shock (13). The other promoter in the transgenesis construct was the γ-crystalline promoter driving the expression of GFP in the eye, allowing easy sorting of the transgenic and nontransgenic sibling tadpoles when they are observed under UV light (13).
Frog transgenesis was performed by restriction enzyme-mediated integration, and sibling transgenic and wild-type tadpoles were reared in methimazole to block synthesis of endogenous T3. In the presence of methimazole, tadpoles stop developing at the limb bud stage but continue growing. Wild-type and transgenic tadpoles were heat shocked at 33°C for 1 h per day for 4 to 10 days. During heat shock treatments, which induced expression of the transgene, the transgenic tadpoles stopped growing and began transforming, similar to metamorphic changes that are normally induced by T3 treatment of premetamorphic tadpoles. For example, gill resorption and limb bud outgrowth and differentiation were observed in transgenic tadpoles, similar to T3-treated wild-type animals, whereas wild-type tadpoles without T3 treatment retained the larval phenotype regardless of heat shock treatments (Fig. 4). Transgenic tadpoles survived about 10 days of heat shock, and thus further metamorphic progress, such as tail shortening or forelimb emergence, was not observed. We observed no deleterious effects on wild-type tadpoles after weeks of heat shock, indicating that lethality in heat-shocked transgenic animals was due to incomplete and/or precocious tissue morphogenesis. This result is also similar to that observed during T3-induced metamorphosis, where partially transformed tadpoles die before tail resorption takes place. Roughly 50% of the transgenic tadpoles (11 of 19) began to metamorphose after heat shock, a result most likely due to differences in transgene expression levels, as seen in other studies (3, 44).
FIG. 4.
Expression of F-dpTR in transgenic tadpoles initiates metamorphosis. Wild-type tadpoles (wt) and sibling tadpoles transgenic for F-dpTR (Tg) under the control of a heat shock-inducible promoter were reared together in methimazole to block endogenous T3 synthesis and were heat shocked daily for 8 days. For comparison, wild-type tadpoles were treated with 5 nM T3 for 3 days. At day 0, tadpoles were the same size and at the same stage (stage 52 to 54). Note that after 8 days of heat shock, transgenic and wild-type animals without T3 treatment were morphologically distinct, as highlighted by the gills (white brackets) and the hind limbs (white arrowheads), and the morphology of the transgenic animal looked similar to that of the T3-treated wild-type tadpoles. The color differences among animals are due to photography and do not affect the conclusion. This experiment was repeated more than 10 times with similar results.
In addition to external morphological changes in response to heat shock in the absence of T3, we examined internal metamorphic changes. We focused on the intestine because it is one of the best-studied organs and because intestinal remodeling involves apoptosis of larval epithelial cells and proliferation of adult precursor epithelial cells (22, 48). First, we examined cell death using the TUNEL assay in heat-shocked wild-type and transgenic tadpoles as well as wild-type tadpoles treated with T3. After 4 days of heat shock, the intestinal epithelium of wild-type animals showed no TUNEL labeling, whereas the intestinal epithelium of F-dpTR transgenic animals but not the muscle or connective tissue exhibited numerous apoptotic nuclei, as in T3-treated wild-type tadpoles (Fig. 5). Second, histological changes reflecting cell proliferation were examined by using methyl green-pyronine Y staining (24). After 10 days of heat shock, the intestines of wild-type animals were larval in character, i.e., the epithelium had a thin muscle layer and little connective tissue (Fig. 6A). In heat-shocked transgenic animals, remodeling was evident, as reflected by the thicker muscle and connective tissue layers and by nests of proliferating adult precursor cells (Fig. 6B).
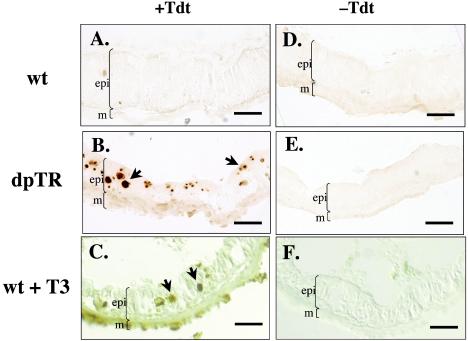
FIG. 5.
Expression of F-dpTR causes larval intestinal epithelial cells to undergo apoptosis. Wild-type and F-dpTR transgenic tadpoles were reared in methimazole to block metamorphosis and then heat shocked. A TUNEL assay to detect apoptotic cells was carried out on cross sections of intestine after 4 days of heat shock. For comparison, wild-type tadpoles were treated with 5 nM T3 for 3 days before the TUNEL assay. Wild-type tadpoles without T3 treatment showed no TUNEL labeling (A), whereas the intestine of F-dpTR transgenic and T3-treated tadpoles had many stained cells, indicating cell death (B and C, black arrows). Lack of staining in the sections performed without terminal TdT revealed the specificity of the reaction (D to F). Brackets indicate boundaries of muscles (m) and larval intestinal epithelium (epi) facing the intestinal lumen (not marked is a thin layer of connective tissue present between the muscles and epithelium). The color differences among sections were due to photography but do not affect the conclusion. This experiment was repeated three times with similar results. Bars, 25 μm.
FIG. 6.
Expression of F-dpTR initiated the development of the adult intestine. Wild-type and sibling transgenic tadpoles were reared in methimazole to block metamorphosis and then heat shocked for 10 days. For comparison, wild-type tadpoles were treated with 5 nM T3 for 3 days. The intestines were isolated, and sections were stained with methyl green-pyronine Y. Note that wild-type intestine remained typical of tadpole intestine, as seen by the presence of a thin muscle layer, little connective tissue, and no adult intestinal precursor cells (A). Histology of the F-dpTR and T3-treated wild-type intestines revealed increases in muscle layer thickness, proliferation of connective tissue, and appearance of adult epithelial precursor cells (B and C). Staining intensity differed among the sections due to staining conditions but does not affect the conclusions. This experiment was repeated four times with similar results. Bars, 25 μm.
F-dnTR induces metamorphosis by activating the T3-dependent gene regulation pathway through direct binding to endogenous T3 target genes.
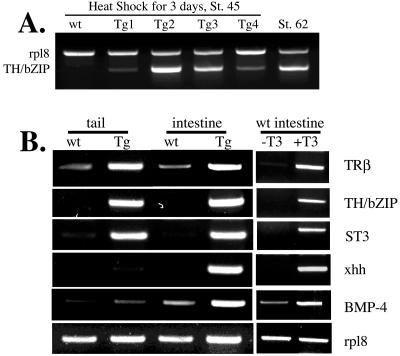
To characterize the molecular nature of the metamorphosis induced by F-dpTR, we examined the expression of genes regulated during natural and T3-induced metamorphosis. First, we isolated RNA from whole bodies of early premetamorphic tadpoles (stage 45) after 3 days of heat shock and analyzed the expression of TH/bZIP, a direct, ubiquitous T3-induced gene (25). As expected, it was not expressed in heat-shocked, wild-type premetamorphic tadpoles but was expressed during metamorphosis (stage 62) (Fig. 7A). TH/bZIP was also expressed in all four premetamorphic transgenic tadpoles after heat shock (Fig. 7A). Interestingly, each of the four transgenic animals from this experiment exhibited a different level of TH/bZIP induction, consistent with variation in the ability to elicit a phenotypic response by heat shock in transgenic tadpoles. Because all animals in this study were F0 generated from the transgenic procedure directly, individual transgenic animals likely differ in transgene copy number and insertion site.
FIG. 7.
Transgenic expression of F-dpTR leads to the activation of known T3-regulated genes in the absence of T3. (A) The T3 response gene, TH/bZIP, is upregulated in whole bodies of early premetamorphic tadpoles (stage 45). Total RNA was extracted from whole bodies of individual stage (St.) 45 wild-type (wt) and transgenic (Tg) tadpoles after 3 days of heat shock and subjected to RT-PCR. No expression was detected in wild-type tadpoles, whereas variable levels of upregulation were observed in four different F-dpTR transgenic tadpoles, and this range of expression encompassed that seen in the tails of wild-type tadpoles at the climax of metamorphosis (stage 62). The gene rpl8 was used as an RNA-loading control. (B) F-dpTR regulates gene expression as in T3-treated wild-type tadpoles. Wild-type and F-dpTR transgenic tadpoles blocked at stage 52 by methimazole were heat shocked for 4 days, and RT-PCR analysis was carried out on RNA isolated from tails and intestines for the expression of T3 response genes, with the T3-independent gene rpl8 as an internal control. For comparison, the same analysis was carried out on intestines from wild-type tadpoles treated with 5 nM T3 for 3 days. The following genes were analyzed: TRβ and TH/bZIP, ubiquitously expressed, direct T3 response genes; stromelysin-3 (ST3), a fibroblast-specific, direct T3 response gene; sonic hedgehog (xhh), an intestinal, epithelium-specific, direct T3 response gene; and bone morphogenic protein 4 (BMP-4), a fibroblast-specific, late upregulated gene. The experiments were repeated with similar results for six transgenic tadpoles showing a phenotype similar to that seen in Fig. 4.
To examine gene regulation in more detail, RNA from tails and intestines of tadpoles whose metamorphosis was blocked with methimazole was analyzed for several genes with varying tissue expression profiles and T3 dependence (Fig. 7B). The direct T3 response genes (i.e., genes containing TREs), TRβ and TH/bZIP (14, 40), were upregulated in heat-shocked transgenic tadpoles but not in wild-type animals. Another direct T3 response gene, stromelysin-3 (37), and the late response gene, bone morphogenic protein 4 (BMP-4) (23), which are expressed in fibroblasts, were upregulated in tails and intestines only in transgenic tadpoles subjected to heat shock treatments. Furthermore, the intestine-specific, direct T3 response gene, sonic hedgehog (xhh) (49), was induced by the F-dpTR transgene after heat shock. Interestingly, F-dpTR did not induce xhh in the tail, as observed during T3 treatment (49). These results are in perfect agreement with those observed during natural and T3-induced metamorphosis (Fig. 7B) (14, 23, 37, 40, 49), indicating that F-dpTR specifically induced the endogenous T3-dependent gene regulation pathway to initiate metamorphosis in transgenic animals.
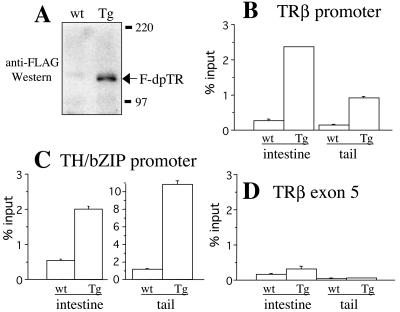
One advantage of using frog metamorphosis is the ability to study directly the mechanisms of nuclear receptor function at the molecular level in vivo during development by using a ChIP assay. First, we confirmed the expression of the transgene after heat shock in transgenic tadpoles by using anti-FLAG Western blotting, which recognizes the FLAG tag of F-dpTR (Fig. 8A). Next, we used a ChIP assay with the anti-FLAG antibodies to determine whether F-dpTR regulates T3 response genes through direct binding to known TREs. For this purpose, we focused on two T3-regulated promoters, TRβ and TH/bZIP (14, 40), using nuclei isolated from the tails and intestines of heat-shocked wild-type and transgenic tadpoles. Quantitative PCR analysis showed that the transgenic F-dpTR bound to the TREs in both tissues at both promoters in the transgenic animals and that only low levels of nonspecific binding were observed in wild-type animals, as expected (Fig. 8B and C). Furthermore, this binding by F-dpTR was specific to the TREs of the two T3-dependent genes in the transgenic but not the wild-type animals, because in the transgenic animals, F-dpTR did not bind to TRβ exon 5, which lacks a TRE (Fig. 8D).
FIG. 8.
ChIP assay using quantitative PCR shows that F-dpTR binds specifically to TRE of T3 target promoters in transgenic animals. After 4 days of heat shock, proteins from the head region were isolated from wild-type and transgenic tadpoles for Western blotting, and nuclei from tails and intestines were isolated for the ChIP assay. (A) Western blotting revealed the expression of F-dpTR in transgenic but not wild-type tadpoles after heat shock. The proteins were separated by SDS-PAGE and detected with anti-FLAG antibody. (B to D) ChIP from isolated nuclei with anti-FLAG antibodies followed by quantitative PCR shows that F-dpTR binds specifically to TRE of T3 target promoters in transgenic animals. Quantitative PCR was carried out on purified ChIP DNA. The anti-FLAG ChIP showed background levels of 0.5% or less of input DNA in wild-type (wt) tadpoles, compared to 1 to 10% in transgenic (Tg) tadpoles for the T3 response genes TRβ and TH/bZIP (B and C). Less than 0.5% of input DNA was immunoprecipitated in both wild-type and transgenic tadpoles for TRβ exon 5 in the coding region (D), demonstrating the specificity of F-dpTR binding to chromatin targets in vivo. These experiments were repeated two to four times with similar results.
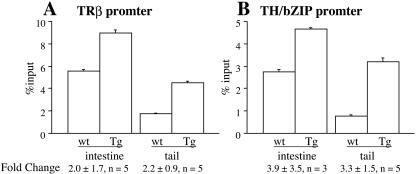
Because T3-induced transcription of target genes is associated with increases in local histone acetylation, we examined histone acetylation levels at the TRE regions of TRβ and TH/bZIP. A ChIP assay with anti-acetylated histone H4 showed that histone acetylation increased by two- to fourfold, although for unknown reasons, there was considerable variation in the intestine (Fig. 9). Thus, F-dpTR may also utilize increased histone acetylation to activate target gene expression, similar to T3-bound TR.
FIG. 9.
Histone acetylation levels at T3 target promoters in tails and intestines increase after heat shock in dpTR transgenic tadpoles. Wild-type and transgenic tadpoles were heat shocked for between 4 and 10 days before chromatin was isolated from intestines and tails for anti-acetyl-H4 ChIP. Acetylation levels were higher at the TRβ (A) and TH/bZIP (B) promoters in both the intestines and the tails. The error bars are from duplicate PCR samples of a representative ChIP experiment. The values below the bars represent the average ± standard error for the increase (n-fold) in acetylation levels between wild-type and dpTR transgenic tadpoles from three to five independent ChIP experiments.
DISCUSSION
Thyroid hormone regulation of postembryonic development occurs in all vertebrates, and thyroid hormone deficiency has profound effects during human gestation, leading to cretinism, a disease characterized by short stature and mental retardation (20). Natural TR mutations are the basis for most incidences of resistance to thyroid hormones syndrome, where patients exhibit a wide variety of abnormalities (17). Furthermore, these diseases and the high occurrence of hypothyroidism due to various etiologies have led to broad-based research on the molecular mechanisms of TR in transducing the T3 signal. In vitro cell culture studies have formed the basis for our molecular understanding of TR function (5, 26, 35, 39, 41, 63). Genetic studies of mice, comprising knock-ins of mutant TRs and knockouts of TR isoforms, reveal multiple developmental roles for TRs (10). However, due to the lack of a model system amenable to molecular studies of gene regulation in development, knowledge of the role of TR in development is limited. Similar developmental events dependent on thyroid hormone occur both in mammals and in frogs (50), such as intestine remodeling during weaning and metamorphosis (38, 48, 54). Therefore, molecular studies of free-living tadpoles undergoing T3-dependent metamorphosis are valuable additions to our basic knowledge of the role of TR in vertebrate development.
Frog metamorphosis is entirely dependent upon T3 and involves cell proliferation and apoptosis in nearly every organ in the tadpole (45). In the absence of T3, tadpoles continue to grow to a large size and fail to undergo any morphological changes. In addition, if premetamorphic tadpoles are treated with exogenous T3 before endogenous T3 production, precocious metamorphosis ensues. These effects of T3 are believed to be mediated by TR. Recent molecular and genetic studies in vivo have provided some support for this idea. A dominant negative form of TR with a deletion of the C-terminal activation domain, when overexpressed in transgenic tadpoles, blocks T3-induced metamorphosis and fails to release corepressors in the presence of ligand, thereby demonstrating that TR function is necessary for transformation (3). These studies, however, leave one important question unanswered, i.e., whether TR is sufficient to mediate all the developmental effects of T3, especially considering the well-documented, nongenomic effects of T3 (8).
Our present data from a dominant positive TR suggest that the genomic action of TR is sufficient for metamorphosis, at least for all the changes analyzed in this study. Transgenic tadpoles bearing F-dpTR under heat shock-inducible control undergo external and internal morphological changes indistinguishable from those of wild-type tadpoles treated with T3 (45). In addition, T3 response genes with or without TRE-containing promoters expressed ubiquitously or in specific tissues all responded to heat shock induction of F-dpTR, a result similar to that seen when T3 is added to wild-type animals (46). Furthermore, these actions of F-dpTR took place via direct, in vivo binding to chromatin, i.e., F-dpTR bound to TRE-containing promoters and failed to bind coding regions. Similar to tadpoles undergoing precocious induction by T3, transgenic tadpoles expressing F-dpTR died before completing metamorphosis. These identical morphological and gene expression changes observed in F-dpTR transgenic animals and T3-treated premetamorphic tadpoles suggest that TR activation is sufficient for these events and that TR is the sole mediator of the T3 signal in these processes.
As mentioned above, T3 is known to affect postembryonic development in mammals. Genetic analyses of transgenic and knockout mice have resulted in various phenotypes that are not readily explainable at the molecular level based on knowledge from cell culture studies (10-12, 15, 16, 57). For example, mice lacking TRα, TRβ, or both have fewer developmental defects than those lacking T3. In addition, mice with dominant negative TRβ (dnTRβ), which mimics resistance to thyroid hormone syndrome in humans, and dnTRα show different phenotypes (19, 29, 30, 51, 65), and in some cases, a number of known T3 response genes in different tissues were found to have different expression levels in mice with dnTRβ and dnTRα and in wild-type mice (29, 30). However, the results from mice with dnTRα and dnTRβ were quite different and could not be explained simply based on the regulation mechanisms obtained from in vitro or cell culture studies. A number of potential mechanisms could explain the different phenotypes and differences in gene expression found in the various TR knockout and mutant animals. These include potential nongenomic effects of T3, different expression profiles and levels of different receptors, distinct isoform-dependent in vivo signaling pathways, and/or indirect effects through altered circulating T3 titer.
Given the similarity between postembryonic development in mammals and frog metamorphosis (2, 45, 50), our results here suggest that the effects of T3 during mammalian postembryonic development are also mediated by TR and that the nongenomic action of T3 is not likely to have a major impact during this period of mammalian development. On the other hand, it is unclear whether the various developmental phenotypes observed in TR knockout and transgenic mice are caused directly or indirectly by TR-mediated gene regulation in the affected tissues and/or organs. The methodologies and reagents reported here should be useful for future studies to address such issues, especially in probing the role of tissue-tissue interactions in TR-dependent developmental events. For example, epithelium-mesenchyme interactions are of critical importance in organogenesis and postembryonic development. The use of dpTR in concert with tissue-specific promoters allows the opportunity to “activate” one tissue layer of interest a time, thereby allowing one to determine whether cell-cell interaction plays a role in the corresponding developmental process.
Acknowledgments
We thank David Johns for the gift of plasmid.
REFERENCES
- 1.Amano, T., K. Leu, K. Yoshizato, and Y.-B. Shi. 2002. Thyroid hormone regulation of a transcriptional coactivator in Xenopus laevis: implication for a role in postembryonic tissue remodeling. Dev. Dyn. 223:526-535. [DOI] [PubMed] [Google Scholar]
- 2.Atkinson, B. G. 1994. Metamorphosis: model systems for studying gene expression in postembryonic development. Dev. Genet. 15:313-319. [Google Scholar]
- 3.Buchholz, D. R., S.-C. V. Hsia, L. Fu, and Y.-B. Shi. 2003. A dominant negative thyroid hormone receptor blocks amphibian metamorphosis by retaining corepressors at target genes. Mol. Cell. Biol. 23:6750-6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buckbinder, L., and D. D. Brown. 1993. Expression of the Xenopus laevis prolactin and thyrotropin genes during metamorphosis. Proc. Natl. Acad. Sci. USA 90:3820-3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, J. D., and H. Li. 1998. Coactivation and corepression in transcriptional regulation by steroid/nuclear hormone receptors. Crit. Rev. Eukaryot. Gene Expr. 8:169-190. [DOI] [PubMed] [Google Scholar]
- 6.Cooper, D. S., J. D. Kieffer, V. Saxe, H. Mover, F. Maloof, and E. C. Ridgway. 1984. Methimazole pharmacology in the rat: studies using a newly developed radioimmunoassay for methimazole. Endocrinology 114:786-793. [DOI] [PubMed] [Google Scholar]
- 7.Das, B., A. M. Schreiber, H. Huang, and D. D. Brown. 2002. Multiple thyroid hormone-induced muscle growth and death programs during metamorphosis in Xenopus laevis. Proc. Natl. Acad. Sci. USA 99:12230-12235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis, P. J., and F. B. Davis. 1996. Nongenomic actions of thyroid hormone. Thyroid 6:497-504. [DOI] [PubMed] [Google Scholar]
- 9.Feng, X., Y. Jiang, P. Meltzer, and P. M. Yen. 2001. Transgenic targeting of a dominant negative corepressor to liver blocks basal repression by thyroid hormone receptor and increases cell proliferation. J. Biol. Chem. 276:15066-15072. [DOI] [PubMed] [Google Scholar]
- 10.Flamant, F., and J. Samarut. 2003. Thyroid hormone receptors: lessons from knockout and knock-in mutant mice. Trends Endocrinol. Metab. 14:85-90. [DOI] [PubMed] [Google Scholar]
- 11.Forrest, D., E. Hanebuth, R. J. Smeyne, N. Everds, C. L. Stewart, J. M. Wehner, and T. Curran. 1996. Recessive resistance to thyroid hormone in mice lacking thyroid hormone receptor beta: evidence for tissue-specific modulation of receptor function. EMBO J. 15:3006-3015. [PMC free article] [PubMed] [Google Scholar]
- 12.Fraichard, A., O. Chassande, M. Plateroti, J. P. Roux, J. Trouillas, C. Dehay, C. Legrand, K. Gauthier, M. Kedinger, L. Malaval, B. Rousset, and J. Samarut. 1997. The T3R alpha gene encoding a thyroid hormone receptor is essential for post-natal development and thyroid hormone production. EMBO J. 16:4412-4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fu, L., D. R. Buchholz, and Y.-B. Shi. 2002. Novel double promoter approach for identification of transgenic animals: a tool for in vivo analysis of gene function and development of gene-based therapies. Mol. Reprod. Dev. 62:470-476. [DOI] [PubMed] [Google Scholar]
- 14.Furlow, J. D., and D. D. Brown. 1999. In vitro and in vivo analysis of the regulation of a transcription factor gene by thyroid hormone during Xenopus laevis metamorphosis. Mol. Endocrinol. 13:2076-2089. [DOI] [PubMed] [Google Scholar]
- 15.Gauthier, K., O. Chassande, M. Plateroti, J. P. Roux, C. Legrand, B. Pain, B. Rousset, R. E. Weiss, J. Trouillas, and J. Samarut. 1999. Different functions for the thyroid hormone receptors TRa and TRb in the control of thyroid hormone production and post-natal development. EMBO J. 18:623-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gothe, S., Z. Wang, L. Ng, J. M. Kindblom, A. C. Barros, C. Ohlsson, B. Vennstrom, and D. Forrest. 1999. Mice devoid of all known thyroid hormone receptors are viable but exhibit disorders of the pituitary-thyroid axis, growth, and bone maturation. EMBO J. 13:1329-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greenspan, F. S., and J. D. Baxter. 1994. Basic and clinical endocrinology, 4th ed. Appleton and Lange, Norwalk, Conn.
- 18.Guenther, M. G., W. S. Lane, W. Fischle, E. Verdin, M. A. Lazar, and R. Shiekhattar. 2000. A core SMRT corepressor complex containing HDAC3 and TBL1, a WD40-repeat protein linked to deafness. Genes Dev. 14:1048-1057. [PMC free article] [PubMed] [Google Scholar]
- 19.Hashimoto, K., F. H. Curty, P. P. Borges, C. E. Lee, E. D. Abel, J. K. Elmquist, R. N. Cohen, and F. E. Wondisford. 2001. An unliganded thyroid hormone receptor causes severe neurological dysfunction. Proc. Natl. Acad. Sci. USA 98:3998-4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hetzel, B. S. 1989. The story of iodine deficiency: an international challenge in nutrition. Oxford University Press, Oxford, United Kingdom.
- 21.Hu, X., and M. A. Lazar. 1999. The CoRNR motif controls the recruitment of corepressors by nuclear hormone receptors. Nature 402:93-96. [DOI] [PubMed] [Google Scholar]
- 22.Ishizuya-Oka, A., and S. Ueda. 1996. Apoptosis and cell proliferation in the Xenopus small intestine during metamorphosis. Cell Tissue Res. 286:467-476. [DOI] [PubMed] [Google Scholar]
- 23.Ishizuya-Oka, A., S. Ueda, T. Amano, K. Shimizu, N. Ueno, and K. Yoshizato. 2001. Thyroid-hormone-dependent and fibroblast-specific expression of BMP-4 correlates with adult epithelial development during amphibian intestinal remodeling. Cell Tissue Res. 303:187-195. [DOI] [PubMed] [Google Scholar]
- 24.Ishizuya-Oka, A., S. Ueda, S. Damjanovski, Q. Li, V. Liang, and Y.-B. Shi. 1997. Anteroposterior gradient of epithelial transformation during amphibian intestinal remodelling: immunohistochemical detection of intestinal fatty acid binding protein. Dev. Biol. 192:149-161. [DOI] [PubMed] [Google Scholar]
- 25.Ishizuya-Oka, A., S. Ueda, and Y.-B. Shi. 1997. Temporal and spatial regulation of a putative transcriptional repressor implicates it as playing a role in thyroid hormone-dependent organ transformation. Dev. Genet. 20:329-337. [DOI] [PubMed] [Google Scholar]
- 26.Ito, M., and R. G. Roeder. 2001. The TRAP/SMCC/mediator complex and thyroid hormone receptor function. Trends Endocrinol. Metab. 12:127-134. [DOI] [PubMed] [Google Scholar]
- 27.Jepsen, K., and M. G. Rosenfeld. 2002. Biological roles and mechanistic actions of co-repressor complexes. J. Cell Sci. 115:689-698. [DOI] [PubMed] [Google Scholar]
- 28.Jones, P. L., and Y.-B. Shi. 2003. N-CoR-HDAC corepressor complexes: roles in transcriptional regulation by nuclear hormone receptors. Curr. Top. Microbiol. Immunol. 274:237-268. [DOI] [PubMed] [Google Scholar]
- 29.Kaneshige, M., H. Suzuki, K. Kaneshige, J. Cheng, H. Wimbrow, C. Barlow, M. C. Willingham, and S.-Y. Cheng. 2001. A targeted dominant negative mutation of the thyroid hormone alpha1 receptor causes increased mortality, infertility, and dwarfism in mice. Proc. Natl. Acad. Sci. USA 98:15095-15100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaneshige, M., K. Kaneshige, X.-G. Zhu, A. Dace, L. Garrett, T. A. Carter, R. Kazlauskaite, D. G. Pankratz, A. Wynshaw-Boris, S. Refetoff, B. D. Weintraub, M. C. Willingham, C. Barlow, and S.-Y. Cheng. 2000. Mice with a targeted mutation in the thyroid hormone beta receptor gene exhibit impaired growth and resistance to thyroid hormone. Proc. Natl. Acad. Sci. USA 97:13209-13214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kroll, K., and E. Amaya. 1996. Transgenic Xenopus embryos from sperm nuclear transplantations reveal FGF signaling requirements during gastrulation. Development 122:3173-3183. [DOI] [PubMed] [Google Scholar]
- 32.Li, J., J. Wang, J. Wang, Z. Nawaz, J. M. Liu, J. Qin, and J. Wong. 2000. Both corepressor proteins SMRT and N-CoR exist in large protein complexes containing HDAC3. EMBO J. 19:4342-4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mangelsdorf, D. J., and R. M. Evans. 1995. The RXR heterodimers and orphan receptors. Cell 83:841-850. [DOI] [PubMed] [Google Scholar]
- 34.Marimuthu, A., W. Feng, T. Tagami, H. Nguyen, J. L. Jameson, R. J. Fletterick, J. D. Baxter, and B. L. West. 2002. TR surfaces and conformations required to bind nuclear receptor corepressor. Mol. Endocrinol. 16:271-286. [DOI] [PubMed] [Google Scholar]
- 35.McKenna, N. J., R. B. Lanz, and B. W. O'Malley. 1999. Nuclear receptor coregulators: cellular and molecular biology. Endocr. Rev. 20:321-344. [DOI] [PubMed] [Google Scholar]
- 36.Nieuwkoop, P. D., and J. Faber. 1994. Normal table of Xenopus laevis (Daudin). Garland Publishing, Inc., New York, N.Y.
- 37.Patterton, D., W. P. Hayes, and Y.-B. Shi. 1995. Transcriptional activation of the matrix metalloproteinase gene stromelysin-3 coincides with thyroid hormone-induced cell death during frog metamorphosis. Dev. Biol. 167:252-262. [DOI] [PubMed] [Google Scholar]
- 38.Plateroti, M., O. Chassande, A. Fraichard, K. Gauthier, J.-N. Freund, J. Samarut, and M. Kedinger. 1999. Involvement of T3Ra- and b-receptor subtypes in mediation of T3 functions during postnatal murine intestinal development. Gastroenterology 116:1367-1378. [DOI] [PubMed] [Google Scholar]
- 39.Rachez, C., and L. P. Freedman. 2001. Mediator complexes and transcription. Curr. Opin. Cell Biol. 13:274-280. [DOI] [PubMed] [Google Scholar]
- 40.Ranjan, M., J. Wong, and Y.-B. Shi. 1994. Transcriptional repression of Xenopus TRb gene is mediated by a thyroid hormone response element located near the start site. J. Biol. Chem. 269:24699-24705. [PubMed] [Google Scholar]
- 41.Rosenfeld, M. G., and C. K. Glass. 2001. Coregulator codes of transcriptional regulation by nuclear receptors. J. Biol. Chem. 276:36865-36868. [DOI] [PubMed] [Google Scholar]
- 42.Sachs, L. M., P. L. Jones, E. Havis, N. Rouse, B. A. Demeneix, and Y.-B. Shi. 2002. Nuclear receptor corepressor recruitment by unliganded thyroid hormone receptor in gene repression during Xenopus laevis development. Mol. Cell. Biol. 22:8527-8538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sachs, L. M., and Y.-B. Shi. 2000. Targeted chromatin binding and histone acetylation in vivo by thyroid hormone receptor during amphibian development. Proc. Natl. Acad. Sci. USA 97:13138-13143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schreiber, A. M., B. Das, H. Huang, N. Marsh-Armstrong, and D. D. Brown. 2001. Diverse developmental programs of Xenopus laevis metamorphosis are inhibited by a dominant negative thyroid hormone receptor. Proc. Natl. Acad. Sci. USA 98:10739-10744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shi, Y.-B. 1999. Amphibian metamorphosis: from morphology to molecular biology. John Wiley and Sons, Inc., New York, N.Y.
- 46.Shi, Y.-B. 1996. Thyroid hormone-regulated early and late genes during amphibian metamorphosis, p. 505-538. In L. I. Gilbert, J. R. Tata, and B. G. Atkinson (ed.), Metamorphosis: postembryonic reprogramming of gene expression in amphibian and insect cells. Academic Press, San Diego, Calif.
- 47.Shi, Y.-B., and W. P. Hayes. 1994. Thyroid hormone-dependent regulation of the intestinal fatty acid-binding protein gene during amphibian metamorphosis. Dev. Biol. 161:48-58. [DOI] [PubMed] [Google Scholar]
- 48.Shi, Y.-B., and A. Ishizuya-Oka. 1996. Biphasic intestinal development in amphibians: embryogenesis and remodeling during metamorphosis. Curr. Top. Dev. Biol. 32:205-235. [DOI] [PubMed] [Google Scholar]
- 49.Stolow, M. A., and Y.-B. Shi. 1995. Xenopus sonic hedgehog as a potential morphogen during embryogenesis and thyroid hormone-dependent metamorphosis. Nucleic Acids Res. 23:2555-2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tata, J. R. 1993. Gene expression during metamorphosis: an ideal model for post-embryonic development. Bioessays 15:239-248. [DOI] [PubMed] [Google Scholar]
- 51.Tinnikov, A., K. Nordstrom, P. Thoren, J. M. Kindblom, S. Malin, B. Rozell, M. Adams, O. Rajanayagam, S. Pettersson, C. Ohlsson, K. Chatterjee, and B. Vennstrom. 2002. Retardation of post-natal development caused by a negatively acting thyroid hormone receptor alpha1. EMBO J. 21:5079-5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tomita, A., D. R. Buchholz, K. Obata, and Y.-B. Shi. 2003. Fusion protein of retinoic acid receptor alpha with promyelocytic leukemia protein or promyelocytic leukemia zinc finger protein recruits N-CoR-TBLR1 corepressor complex to repress transcription in vivo. J. Biol. Chem. 278:30788-30795. [DOI] [PubMed] [Google Scholar]
- 53.Tomita, A., D. R. Buchholz, and Y.-B. Shi. 2004. Recruitment of N-CoR/SMRT-TBLR1 corepressor complexes by unliganded thyroid hormone receptor for gene repression during frog development. Mol. Cell. Biol. 24:3337-3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Trier, J. S., and P. C. Moxey. 1979. Morphogenesis of the small intestine during fetal development. Ciba Found. Symp. 70:3-20. [DOI] [PubMed] [Google Scholar]
- 55.Webb, P., C. M. Anderson, C. Valentine, P. Nguyen, A. Marimuthu, B. L. West, J. D. Baxter, and P. J. Kushner. 2000. The nuclear receptor corepressor (N-CoR) contains three isoleucine motifs (I/LXXII) that serve as receptor interaction domains (IDs). Mol. Endocrinol. 14:1976-1985. [DOI] [PubMed] [Google Scholar]
- 56.White, B. A., and C. S. Nicoll. 1981. Hormonal control of amphibian metamorphosis, p. 363-396. In L. I. Gilbert and E. Frieden (ed.), Metamorphosis, 2nd ed. Plenum Press, New York, N.Y.
- 57.Wikstrom, L., C. Johansson, C. Salto, C. Barlow, A. C. Barros, F. Bass, D. Forrest, P. Thoren, and B. Vennstrom. 1998. Abnormal heart rate and body temperature in mice lacking thyroid hormone receptor alpha 1. EMBO J. 17:455-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wong, J., and Y.-B. Shi. 1995. Coordinated regulation of and transcription activation by Xenopus thyroid hormone and retinoid X receptors. J. Biol. Chem. 270:18479-18483. [DOI] [PubMed] [Google Scholar]
- 59.Wong, J., Y.-B. Shi, and A. P. Wolffe. 1995. A role for nucleosome assembly in both silencing and activation of the Xenopus TRbA gene by the thyroid hormone receptor. Genes Dev. 9:2696-2711. [DOI] [PubMed] [Google Scholar]
- 60.Yen, P. M. 2001. Physiological and molecular basis of thyroid hormone action. Physiol. Rev. 81:1097-1142. [DOI] [PubMed] [Google Scholar]
- 61.Yoon, H., D. Chan, Z. Huang, J. Li, J. Fondell, J. Qin, and J. M. Wong. 2003. Purification and functional characterization of the human N-CoR complex: the roles of HDAC3, TBL1 and TBLR1. EMBO J. 22:1336-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang, J., M. Kalkum, B. T. Chait, and R. G. Roeder. 2002. The N-CoR-HDAC3 nuclear receptor corepressor complex inhibits the JNK pathway through the integral subunit GPS2. Mol. Cell 9:611-623. [DOI] [PubMed] [Google Scholar]
- 63.Zhang, J., and M. A. Lazar. 2000. The mechanism of action of thyroid hormones. Annu. Rev. Physiol. 62:439-466. [DOI] [PubMed] [Google Scholar]
- 64.Zhang, J., I. Zamir, and M. A. Lazar. 1997. Differential recognition of liganded and unliganded thyroid hormone receptor by retinoid X receptor regulates transcriptional repression. Mol. Cell. Biol. 17:6887-6897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang, X.-Y., M. Kaneshige, Y. Kamiya, K. Kaneshige, P. McPhie, and S.-Y. Cheng. 2002. Differential expression of thyroid hormone receptor isoforms dictates the dominant negative activity of mutant beta receptor. Mol. Endocrinol. 16:2077-2092. [DOI] [PubMed] [Google Scholar]