Abstract
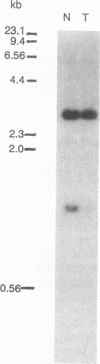
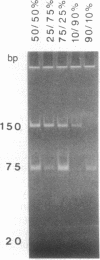
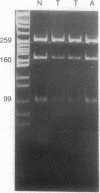
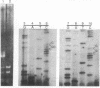
Loss of heterozygosity affecting chromosome 17p has been detected at high frequencies in a variety of human tumors, including cancers of the colon, breast, lung, and brain. One presumed target of these losses is p53, a tumor suppressor gene located on 17p. To our knowledge, loss of heterozygosity has not yet been reported at any locus, including p53, in human esophageal cancer. Moreover, current methods of detecting loss of heterozygosity depend on the availability of large amounts of high molecular weight DNA, making the study of small biopsy specimens or paraffin-embedded tissues problematic. We examined 52 primary human esophageal neoplasms for loss of heterozygosity affecting the p53 gene by using the polymerase chain reaction. Loss of one allele was detected in 52% of informative cases and was more common in squamous carcinomas than in adenocarcinomas. Southern blot analysis was used to confirm polymerase chain reaction-derived data. The identification of allelic loss in approximately half of the tumors analyzed supports the hypothesis that inactivation of p53 is involved in the pathogenesis of esophageal cancer.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ara S., Lee P. S., Hansen M. F., Saya H. Codon 72 polymorphism of the TP53 gene. Nucleic Acids Res. 1990 Aug 25;18(16):4961–4961. doi: 10.1093/nar/18.16.4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker S. J., Fearon E. R., Nigro J. M., Hamilton S. R., Preisinger A. C., Jessup J. M., vanTuinen P., Ledbetter D. H., Barker D. F., Nakamura Y. Chromosome 17 deletions and p53 gene mutations in colorectal carcinomas. Science. 1989 Apr 14;244(4901):217–221. doi: 10.1126/science.2649981. [DOI] [PubMed] [Google Scholar]
- Baker S. J., Preisinger A. C., Jessup J. M., Paraskeva C., Markowitz S., Willson J. K., Hamilton S., Vogelstein B. p53 gene mutations occur in combination with 17p allelic deletions as late events in colorectal tumorigenesis. Cancer Res. 1990 Dec 1;50(23):7717–7722. [PubMed] [Google Scholar]
- Bird A. P. CpG-rich islands and the function of DNA methylation. Nature. 1986 May 15;321(6067):209–213. doi: 10.1038/321209a0. [DOI] [PubMed] [Google Scholar]
- Cheng J., Haas M. Frequent mutations in the p53 tumor suppressor gene in human leukemia T-cell lines. Mol Cell Biol. 1990 Oct;10(10):5502–5509. doi: 10.1128/mcb.10.10.5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devilee P., van den Broek M., Kuipers-Dijkshoorn N., Kolluri R., Khan P. M., Pearson P. L., Cornelisse C. J. At least four different chromosomal regions are involved in loss of heterozygosity in human breast carcinoma. Genomics. 1989 Oct;5(3):554–560. doi: 10.1016/0888-7543(89)90023-2. [DOI] [PubMed] [Google Scholar]
- Franceschi S., Talamini R., Barra S., Barón A. E., Negri E., Bidoli E., Serraino D., La Vecchia C. Smoking and drinking in relation to cancers of the oral cavity, pharynx, larynx, and esophagus in northern Italy. Cancer Res. 1990 Oct 15;50(20):6502–6507. [PubMed] [Google Scholar]
- Friedman P. N., Kern S. E., Vogelstein B., Prives C. Wild-type, but not mutant, human p53 proteins inhibit the replication activities of simian virus 40 large tumor antigen. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9275–9279. doi: 10.1073/pnas.87.23.9275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg D., Michael-Michalovitz D., Ginsberg D., Oren M. Induction of growth arrest by a temperature-sensitive p53 mutant is correlated with increased nuclear localization and decreased stability of the protein. Mol Cell Biol. 1991 Jan;11(1):582–585. doi: 10.1128/mcb.11.1.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goelz S. E., Hamilton S. R., Vogelstein B. Purification of DNA from formaldehyde fixed and paraffin embedded human tissue. Biochem Biophys Res Commun. 1985 Jul 16;130(1):118–126. doi: 10.1016/0006-291x(85)90390-0. [DOI] [PubMed] [Google Scholar]
- Gyllensten U. B., Erlich H. A. Generation of single-stranded DNA by the polymerase chain reaction and its application to direct sequencing of the HLA-DQA locus. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7652–7656. doi: 10.1073/pnas.85.20.7652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hameeteman W., Tytgat G. N., Houthoff H. J., van den Tweel J. G. Barrett's esophagus: development of dysplasia and adenocarcinoma. Gastroenterology. 1989 May;96(5 Pt 1):1249–1256. doi: 10.1016/s0016-5085(89)80011-3. [DOI] [PubMed] [Google Scholar]
- Hamilton S. R., Smith R. R., Cameron J. L. Prevalence and characteristics of Barrett esophagus in patients with adenocarcinoma of the esophagus or esophagogastric junction. Hum Pathol. 1988 Aug;19(8):942–948. doi: 10.1016/s0046-8177(88)80010-8. [DOI] [PubMed] [Google Scholar]
- Hamilton S. R., Smith R. R. The relationship between columnar epithelial dysplasia and invasive adenocarcinoma arising in Barrett's esophagus. Am J Clin Pathol. 1987 Mar;87(3):301–312. doi: 10.1093/ajcp/87.3.301. [DOI] [PubMed] [Google Scholar]
- Hinds P. W., Finlay C. A., Quartin R. S., Baker S. J., Fearon E. R., Vogelstein B., Levine A. J. Mutant p53 DNA clones from human colon carcinomas cooperate with ras in transforming primary rat cells: a comparison of the "hot spot" mutant phenotypes. Cell Growth Differ. 1990 Dec;1(12):571–580. [PubMed] [Google Scholar]
- Hollstein M. C., Smits A. M., Galiana C., Yamasaki H., Bos J. L., Mandard A., Partensky C., Montesano R. Amplification of epidermal growth factor receptor gene but no evidence of ras mutations in primary human esophageal cancers. Cancer Res. 1988 Sep 15;48(18):5119–5123. [PubMed] [Google Scholar]
- James C. D., Carlbom E., Dumanski J. P., Hansen M., Nordenskjold M., Collins V. P., Cavenee W. K. Clonal genomic alterations in glioma malignancy stages. Cancer Res. 1988 Oct 1;48(19):5546–5551. [PubMed] [Google Scholar]
- Jeffreys A. J., Wilson V., Thein S. L. Hypervariable 'minisatellite' regions in human DNA. Nature. 1985 Mar 7;314(6006):67–73. doi: 10.1038/314067a0. [DOI] [PubMed] [Google Scholar]
- Jiang W., Kahn S. M., Guillem J. G., Lu S. H., Weinstein I. B. Rapid detection of ras oncogenes in human tumors: applications to colon, esophageal, and gastric cancer. Oncogene. 1989 Jul;4(7):923–928. [PubMed] [Google Scholar]
- Johnson P., Gray D., Mowat M., Benchimol S. Expression of wild-type p53 is not compatible with continued growth of p53-negative tumor cells. Mol Cell Biol. 1991 Jan;11(1):1–11. doi: 10.1128/mcb.11.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraiss S., Quaiser A., Oren M., Montenarh M. Oligomerization of oncoprotein p53. J Virol. 1988 Dec;62(12):4737–4744. doi: 10.1128/jvi.62.12.4737-4744.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok S., Higuchi R. Avoiding false positives with PCR. Nature. 1989 May 18;339(6221):237–238. doi: 10.1038/339237a0. [DOI] [PubMed] [Google Scholar]
- Lee J. H., Kavanagh J. J., Wildrick D. M., Wharton J. T., Blick M. Frequent loss of heterozygosity on chromosomes 6q, 11, and 17 in human ovarian carcinomas. Cancer Res. 1990 May 1;50(9):2724–2728. [PubMed] [Google Scholar]
- Litt M., Luty J. A. A hypervariable microsatellite revealed by in vitro amplification of a dinucleotide repeat within the cardiac muscle actin gene. Am J Hum Genet. 1989 Mar;44(3):397–401. [PMC free article] [PubMed] [Google Scholar]
- Lu S. H., Hsieh L. L., Luo F. C., Weinstein I. B. Amplification of the EGF receptor and c-myc genes in human esophageal cancers. Int J Cancer. 1988 Oct 15;42(4):502–505. doi: 10.1002/ijc.2910420406. [DOI] [PubMed] [Google Scholar]
- Malkin D., Li F. P., Strong L. C., Fraumeni J. F., Jr, Nelson C. E., Kim D. H., Kassel J., Gryka M. A., Bischoff F. Z., Tainsky M. A. Germ line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science. 1990 Nov 30;250(4985):1233–1238. doi: 10.1126/science.1978757. [DOI] [PubMed] [Google Scholar]
- Meltzer S. J., Mane S. M., Wood P. K., Resau J. H., Newkirk C., Terzakis J. A., Korelitz B. I., Weinstein W. M., Needleman S. W. Activation of c-Ki-ras in human gastrointestinal dysplasias determined by direct sequencing of polymerase chain reaction products. Cancer Res. 1990 Jun 15;50(12):3627–3630. [PubMed] [Google Scholar]
- Menon A. G., Anderson K. M., Riccardi V. M., Chung R. Y., Whaley J. M., Yandell D. W., Farmer G. E., Freiman R. N., Lee J. K., Li F. P. Chromosome 17p deletions and p53 gene mutations associated with the formation of malignant neurofibrosarcomas in von Recklinghausen neurofibromatosis. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5435–5439. doi: 10.1073/pnas.87.14.5435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner J., Medcalf E. A., Cook A. C. Tumor suppressor p53: analysis of wild-type and mutant p53 complexes. Mol Cell Biol. 1991 Jan;11(1):12–19. doi: 10.1128/mcb.11.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y., Ballard L., Leppert M., O'Connell P., Lathrop G. M., Lalouel J. M., White R. Isolation and mapping of a polymorphic DNA sequence (pYNZ22) on chromosome 17p [D17S30]. Nucleic Acids Res. 1988 Jun 24;16(12):5707–5707. doi: 10.1093/nar/16.12.5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y., Leppert M., O'Connell P., Wolff R., Holm T., Culver M., Martin C., Fujimoto E., Hoff M., Kumlin E. Variable number of tandem repeat (VNTR) markers for human gene mapping. Science. 1987 Mar 27;235(4796):1616–1622. doi: 10.1126/science.3029872. [DOI] [PubMed] [Google Scholar]
- Neubauer A., Neubauer B., Liu E. Polymerase chain reaction based assay to detect allelic loss in human DNA: loss of beta-interferon gene in chronic myelogenous leukemia. Nucleic Acids Res. 1990 Feb 25;18(4):993–998. doi: 10.1093/nar/18.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigro J. M., Baker S. J., Preisinger A. C., Jessup J. M., Hostetter R., Cleary K., Bigner S. H., Davidson N., Baylin S., Devilee P. Mutations in the p53 gene occur in diverse human tumour types. Nature. 1989 Dec 7;342(6250):705–708. doi: 10.1038/342705a0. [DOI] [PubMed] [Google Scholar]
- Sato T., Tanigami A., Yamakawa K., Akiyama F., Kasumi F., Sakamoto G., Nakamura Y. Allelotype of breast cancer: cumulative allele losses promote tumor progression in primary breast cancer. Cancer Res. 1990 Nov 15;50(22):7184–7189. [PubMed] [Google Scholar]
- Srivastava S., Zou Z. Q., Pirollo K., Blattner W., Chang E. H. Germ-line transmission of a mutated p53 gene in a cancer-prone family with Li-Fraumeni syndrome. Nature. 1990 Dec 20;348(6303):747–749. doi: 10.1038/348747a0. [DOI] [PubMed] [Google Scholar]
- Stratton M. R., Moss S., Warren W., Patterson H., Clark J., Fisher C., Fletcher C. D., Ball A., Thomas M., Gusterson B. A. Mutation of the p53 gene in human soft tissue sarcomas: association with abnormalities of the RB1 gene. Oncogene. 1990 Sep;5(9):1297–1301. [PubMed] [Google Scholar]
- Takahashi T., Nau M. M., Chiba I., Birrer M. J., Rosenberg R. K., Vinocour M., Levitt M., Pass H., Gazdar A. F., Minna J. D. p53: a frequent target for genetic abnormalities in lung cancer. Science. 1989 Oct 27;246(4929):491–494. doi: 10.1126/science.2554494. [DOI] [PubMed] [Google Scholar]
- Tsai Y. C., Nichols P. W., Hiti A. L., Williams Z., Skinner D. G., Jones P. A. Allelic losses of chromosomes 9, 11, and 17 in human bladder cancer. Cancer Res. 1990 Jan 1;50(1):44–47. [PubMed] [Google Scholar]
- Tsuda T., Nakatani H., Matsumura T., Yoshida K., Tahara E., Nishihira T., Sakamoto H., Yoshida T., Terada M., Sugimura T. Amplification of the hst-1 gene in human esophageal carcinomas. Jpn J Cancer Res. 1988 May;79(5):584–588. doi: 10.1111/j.1349-7006.1988.tb00026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsumi M., Sakamoto H., Yoshida T., Kakizoe T., Koiso K., Sugimura T., Terada M. Coamplification of the hst-1 and int-2 genes in human cancers. Jpn J Cancer Res. 1988 Apr;79(4):428–432. doi: 10.1111/j.1349-7006.1988.tb01609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor T., Du Toit R., Jordaan A. M., Bester A. J., van Helden P. D. No evidence for point mutations in codons 12, 13, and 61 of the ras gene in a high-incidence area for esophageal and gastric cancers. Cancer Res. 1990 Aug 15;50(16):4911–4914. [PubMed] [Google Scholar]
- Vogelstein B. Cancer. A deadly inheritance. Nature. 1990 Dec 20;348(6303):681–682. doi: 10.1038/348681a0. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Fearon E. R., Hamilton S. R., Kern S. E., Preisinger A. C., Leppert M., Nakamura Y., White R., Smits A. M., Bos J. L. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988 Sep 1;319(9):525–532. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- Wagata T., Ishizaki K., Imamura M., Shimada Y., Ikenaga M., Tobe T. Deletion of 17p and amplification of the int-2 gene in esophageal carcinomas. Cancer Res. 1991 Apr 15;51(8):2113–2117. [PubMed] [Google Scholar]
- Yamamoto T., Kamata N., Kawano H., Shimizu S., Kuroki T., Toyoshima K., Rikimaru K., Nomura N., Ishizaki R., Pastan I. High incidence of amplification of the epidermal growth factor receptor gene in human squamous carcinoma cell lines. Cancer Res. 1986 Jan;46(1):414–416. [PubMed] [Google Scholar]
- Yokota J., Wada M., Shimosato Y., Terada M., Sugimura T. Loss of heterozygosity on chromosomes 3, 13, and 17 in small-cell carcinoma and on chromosome 3 in adenocarcinoma of the lung. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9252–9256. doi: 10.1073/pnas.84.24.9252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Calle-Martín O., Fabregat V., Romero M., Soler J., Vives J., Yagüe J. AccII polymorphism of the p53 gene. Nucleic Acids Res. 1990 Aug 25;18(16):4963–4963. [PMC free article] [PubMed] [Google Scholar]