Abstract
The LDL receptor-related protein 1 (LRP1) is a multifunctional cell surface receptor that is highly expressed on neurons. Neuronal LRP1 in vitro can mediate ligand endocytosis, as well as modulate signal transduction processes. However, little is known about its role in the intact nervous system. Here, we report that mice that lack LRP1 selectively in differentiated neurons develop severe behavioral and motor abnormalities, including hyperactivity, tremor, and dystonia. Since their central nervous systems appear histoanatomically normal, we suggest that this phenotype is likely attributable to abnormal neurotransmission. This conclusion is supported by studies of primary cultured neurons that show that LRP1 is present in close proximity to the N-methyl-d-aspartate (NMDA) receptor in dendritic synapses and can be coprecipitated with NMDA receptor subunits and the postsynaptic density protein PSD-95 from neuronal cell lysates. Moreover, treatment with NMDA, but not dopamine, reduces the interaction of LRP1 with PSD-95, indicating that LRP1 participates in transmitter-dependent postsynaptic responses. Together, these findings suggest that LRP1, like other ApoE receptors, can modulate synaptic transmission in the brain.
The LDL receptor-related protein 1 (LRP1) is a multifunctional member of the LDL receptor (LDLR) family of lipoprotein receptors. First recognized as an endocytic receptor, it mediates the cellular uptake of a great variety of ligands, e.g., chylomicron remnants (40) and protease-protease inhibitor complexes (reviewed in reference 19). Recent experimental studies have shown, however, that LRP1 exerts biological functions beyond cargo transport in several tissues (4, 12, 46, 48). Like other members of the LDL receptor family, LRP1 seems to have an important role in the regulation of intercellular signaling (2, 5, 29, 33-35). Loss of LRP1 in smooth muscle cells of the arterial wall, for instance, results in increased activation of the platelet-derived growth factor (PDGF) signaling pathway and disruption of vascular-wall architecture (4). In addition, LRP1 (also called CD91) of alveolar macrophages has been implicated in the control of the pulmonary inflammatory response to pathogens and damaged cells (12).
It is likely that further functions of LRP1 will continue to emerge with the study of this ubiquitously expressed protein in different tissues. Apart from liver, blood vessels, and lung, high levels of LRP1 are also found in the central nervous system (18), where it is present on both glial and neuronal cells throughout the brain and spinal cord (7, 21, 36). The in vivo functions of neuronal LRP1 have not been examined in detail, but several in vitro studies suggest that it plays an important role in different aspects of neuronal metabolism. LRP1 is found in the somatodendritic compartment of neurons (6), and it can mediate the endocytosis of extracellular ligands in these cells (31). LRP1 also interacts with the neuronally expressed amyloid precursor protein (APP) (23, 24) and regulates its proteolytical processing and the production of the Aβ peptide (38, 44), a process that is of central importance for the pathogenesis of Alzheimer's disease.
Moreover, LRP1 has been found to regulate calcium influx into neurons after stimulation with the glutamate receptor agonist N-methyl-d-aspartate (NMDA) (39). The molecular mechanism that underlies this effect has not yet been identified. However, the possibility that LRP1 might modulate the functions of neuronal synaptic proteins is in agreement with earlier results that showed that LRP1 can interact with the postsynaptic density protein PSD-95 (13) and might be part of a large postsynaptic density protein complex, where it would be able to modulate the conductance of neuronal ion channels.
Here, we show that endogenous PSD-95 can be coprecipitated with LRP1 from cultured primary neurons. Treatment of cells with NMDA reduces the amount of PSD-95 that is brought down with LRP1, indicating a function-dependent interaction of the two proteins. In addition, we demonstrate that NMDA receptor subunits also coprecipitate with neuronal LRP1 and that they colocalize with LRP1 in neurons.
To examine the functional significance of neuronal LRP1 in vivo we used the cre-loxP system to generate genetically modified mice that lack LRP1 expression in differentiated postmitotic neurons. Loss of neuronal LRP1 in these animals causes hyperactivity and motor dysfunction with prominent tremor and dystonia. In the absence of obvious morphological abnormalities, these symptoms are most likely due to a functional deficit in neurotransmission, which would be compatible with a role for LRP1 in modulating glutamatergic synapse function in the central nervous system.
MATERIALS AND METHODS
Preparation of rat embryonic cortical neurons.
Animals were maintained in accordance with National Institutes of Health and institutional animal care guidelines. Cortical neurons from embryonic day 18 rat embryos (Sprague-Dawley; Charles River, Wilmington, Mass.) were prepared and cultured as described previously (3).
Rat hippocampal neurons in glial cell coculture were generously provided by Iza Lesznicki (T. Sudhof Laboratory, University of Texas Southwestern Medical Center at Dallas).
Coimmunoprecipitation of proteins with LRP1.
Neuronal lysates for coimmunoprecipitation experiments were prepared with immunoprecipitation (IP) lysis buffer containing 20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM MgCl2, 1 mM CaCl2, and 1% Triton X-100 plus one tablet of Roche EDTA-free protease inhibitors per 10 ml of buffer and phosphatase inhibitor cocktails I and II (Sigma, St. Louis, Mo.) diluted 1:100 according to the manufacturer's instructions. Briefly, cultured rat embryonic cortical neurons were washed once with cold phosphate-buffered saline (PBS) and then scraped into 500 μl of IP lysis buffer per 100-mm-diameter dish. After 5 min of incubation at 37°C, the lysate was passed through a 22-gauge needle 10 times and then centrifuged at 20,000 × g at 4°C for 20 min. The supernatant was adjusted to a protein concentration of 1 μg per μl, and 1 ml of the final lysate was used for IP with 7 μl of LRP1 antiserum (18) or nonimmune control. Fifty microliters of 50% protein A-agarose (Sigma) in IP lysis buffer were added, and samples were rotated at 4°C for 12 h. The beads were recovered by centrifugation and washed once with IP lysis buffer and twice with IP lysis buffer with 0.1% Triton X-100. Finally, 50 μl of 2× sodium dodecyl sulfate (SDS) sample buffer containing 5% β-mercaptoethanol was added, and samples were heated to 95°C for 5 min. After centrifugation, the supernatants were separated by SDS-polyacrylamide gel electrophoresis and analyzed by Western blotting as described below.
Where applicable, 100 μM NMDA (Sigma) or 50 μM dopamine (Sigma) was added from a 1,000× stock solution in H2O 10 min prior to the preparation of cell lysates.
SDS-polyacrylamide gel electrophoresis and Western blot analysis were performed according to standard procedures on SDS-polyacrylamide gels. After electrophoresis and protein transfer to supported nitrocellulose membranes, immunoblot analysis was carried out with a rabbit polyclonal antibody against a carboxyl-terminal epitope of LRP1 (18), a mouse monoclonal PSD-95 antibody (Upstate, Charlottesville, Va.), or goat polyclonal antibodies against NR2A or NR2B (Santa Cruz, Santa Cruz, Calif.). After incubation with a horseradish peroxidase-conjugated secondary antibody, bound antibodies were visualized by enhanced chemiluminescence using SuperSignal CL-HRP Substrate (Pierce, Rockford, Ill.) according to the manufacturer's instructions.
For quantitative immunoblotting (LRP1), a radiolabeled secondary antibody (anti-rabbit whole antibody from donkey; 125I labeled; 106 cpm/ml; Amersham, Piscataway, N.J.) was employed. The radioactive signals of the LRP1 bands were quantified with a Fuji BAS1000 photoimager.
Immunocytochemistry.
Rat embryonic neurons cultured on coverslips were fixed with methanol (100%) at −20°C for 10 min. After being washed with Tris-buffered saline (TBS), samples were blocked with 10% donkey serum and 1% albumin in TBS for 1 h at room temperature. Then, incubation with the first antibodies (affinity-purified rabbit polyclonal α-LRP1 and goat polyclonal α-NR2A [Santa Cruz] diluted 1:100 in 1% albumin in TBS) was done overnight at 4°C. Alexa 488-labeled donkey anti-rabbit and Alexa 594-labeled donkey anti-goat antibodies (both from Molecular Probes, Eugene, Oreg.) were used at 1:100 in 1% albumin in TBS for the detection of bound primary antibody. Finally, the coverslips were mounted on slides in aqueous mounting medium (Molecular Probes) and examined by confocal laser scanning microscopy with a Leica TCS SP microscope. Photoshop software (Adobe) was used to merge exposures of the same frame obtained at different excitation wave lengths.
Synapsin Cre/LRPlox/lox mice.
Mice carrying a loxP-marked LRP1 allele were generated in our laboratory and were described previously (41). These mice were bred to animals transgenic for the viral Cre recombinase under the control of the synapsin I promoter. These mice have been characterized elsewhere (49). For experiments, age-matched 3- to 6-month-old mice were used.
Genotyping PCRs.
Genomic DNA from mouse tissues was prepared and examined by PCR according to standard protocols. The following PCR primers were used for the detection of the recombined and nonrecombined LRPlox allele, respectively: primer rec1, 5′-GGT GTG ACA TAG AGT TTT AAA GAG G-3′; primer rec2, 5′-GCA AGC TCT CCT GCT CAG ACC TGG A-3′; primer non-rec1, 5′-CAT ACC CTC TTC AAA CCC CTT CCT G-3′; primer non-rec2, 5′-GCA AGC TCT CCT GCT CAG ACC TGG A-]3′.
RNA preparation and Northern blot analysis.
Total RNA was prepared from adult mouse brains with 1 ml of RNA-STAT60 (Tel-Test, Friendswood, Tex.) per 100 mg of tissue according to the manufacturer's instructions. Twenty micrograms of total RNA was used for Northern blotting with the 2.7-kb KpnI/HindIII fragment of the human LRP1-cDNA serving as an LRP1-specific probe. Briefly, RNA samples were separated on a 1% agarose-5.5% formaldehyde gel and transferred to a Hybond N+ membrane (Amersham) by upward capillary transfer in 10× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate). Nucleic acids were cross-linked to the membrane by UV irradiation. RapidHyb buffer (Amersham) was used for prehybridization (30 min at 65°C) and hybridization (1 h at 65°C). For hybridization, 1 ng of probe was labeled with [32P]dCTP, employing the T7 QuickPrime kit (Amersham) according to the manufacturer's instructions. Probe bound to the membrane was detected by autoradiography.
Preparation of brain membranes and ligand blotting.
Brain membranes were prepared from adult synapsin Cre/LRPlox/lox and LRPlox/lox control mice as described previously (26). The protein concentration of the final supernatant was determined using a DC Protein Assay (Bio-Rad, Hercules, Calif.). Membrane proteins were analyzed by SDS-polyacrylamide gel electrophoresis and immunoblotting as described above.
Ligand blot analysis with 125I-labeled receptor-associated protein (RAP) was performed as described previously (45).
EMG.
Thin wire electrodes were inserted transcutaneously into the knee flexor muscles of SynCre/LRPlox/lox and LRPlox/lox control mice. Electromyograms (EMG) were recorded while the mice were suspended by their tails above the ground and freely moved their legs.
EEG.
Electroencephalographic (EEG) recordings were done from implanted cortical surface electrodes as described previously (43).
Hippocampal-slice preparation and electrophysiologal studies were done as described previously (47). Recombinant glutathione S-transferase (GST)-RAP was prepared as described previously (17).
Immunohistochemistry.
Anesthetized mice were perfused by cardiac puncture with warm phosphate-buffered saline, followed by 4% (wt/vol) paraformaldehyde in 0.1 M phosphate buffer. The brains were removed and postfixed for 2 h. They were incubated overnight in 25% (wt/vol) sucrose solution. Cryosections 30 μm thick were cut and used for free-floating immunohistochemistry. Samples were quenched in 10% methanol-3% H2O2 in PBS for 15 min at room temperature, permeabilized in 1% Triton X-100 in PBS for 15 min at room temperature, and blocked in 50% (vol/vol) normal goat serum in PBS for 8 h at 4°C. The sections were then incubated overnight with a C-terminal rabbit polyclonal LRP1 antibody. Bound immunoglobulin G was visualized with goat anti-rabbit antiserum and subsequent incubation with peroxidase-coupled rabbit anti-peroxidase antiserum (Sternberger, Lutherville, Md.) and detection with diaminobenzidine (peroxidase-antiperoxidase method).
In situ hybridization.
The 500-bp BsmI/HindIII fragment of the human LRP1 cDNA served as a template for the synthesis of RNA in situ hybridization probes. [α-33P]UTP (Amersham)-radiolabeled riboprobe was synthesized using the Riboprobe Systems kit (Promega, Madison, Wis.). To determine expression of LRP1 in neurons of synapsin Cre/LRPlox/lox mice, in situ hybridizations of brain sections were performed as described previously (30).
RESULTS
Targeted disruption of LRP1 in neurons.
LRP1 is a multifunctional receptor that is highly expressed in neurons and has diverse roles in endocytosis, as well as in the regulation of signal transduction processes. Several in vitro studies have indicated that LRP1 may play an important role in neuronal metabolism by modulating the extracellular environment through the endocytic uptake of extracellular proteins and possibly also by regulating NMDA receptor-dependent ion currents (31, 39). The in vivo role of neuronal LRP1, however, has been difficult to examine, partly because conventionally gene-targeted mice that lack LRP1 die early during embryogenesis (16). To circumvent this problem, we used tissue-specific gene disruption for the generation of mice that lack LRP1 in differentiated neurons. Mice harboring loxP recognition sites in the LRP1 gene (41) were bred to mice transgenic for the viral Cre recombinase under the control of the synapsin I promoter. This promoter is active in differentiated neurons throughout the central nervous system but inactive in neural progenitors and glial cells (49), resulting in the specific loss of neuronal LRP1 in animals that carry the transgene and are homozygous for the floxed LRP1 allele.
To assert that recombination of the loxP-marked LRP1 gene in synapsin Cre/LRPlox/lox mice occurs in and is restricted to neural tissues, we performed PCR analyses of genomic DNA from various organs with primer pairs specific for the recombined LRP1 allele. Figure 1A shows recombination in synapsin Cre/LRPlox/lox mice in the brain and in the spinal cord but not in other organs. Since neurons constitute only a fraction of all cell types in the brain and spinal cord, the nonrecombined allele is also detected.
FIG.1.
(A) Selective recombination of LRP1 in brain and spinal cord from SynCre/LRPlox/lox mice. Genomic DNA was prepared from the indicated tissues of SynCre/LRPlox/lox mice. PCR analysis with primers specific for the recombined or nonrecombined allele, respectively, was performed. (Left) Recombination is detected in brain and spinal cord only. (Right) The nonrecombined allele is present in all tissues. (B) Reduced expression of LRP1 in SynCre/LRPlox/lox neural tissue. (Top) Total brain RNA was prepared from two adult SynCre/LRPlox/lox and two age-matched LRPlox/lox mice. RNA samples were separated by agarose gel electrophoresis and analyzed by Northern blotting using an LRP1-specific probe. LRP1-mRNA in SynCre/LRPlox/lox mice (lanes 3 and 4) is reduced significantly in comparison to LRPlox/lox control animals (lanes 1 and 2). (Middle) Brain membrane preparations from adult SynCre/LRPlox/lox and LRPlox/lox mice were subjected to SDS-polyacrylamide gel electrophoresis and quantitative Western blotting with an LRP1 antibody. LRP1 protein levels are reduced by ∼50% in the brains of SynCre/LRPlox/lox mice (lanes 3 and 4) compared to LRPlox/lox mice (lanes 1 and 2). (Bottom) Ligand blotting with 125I-labeled RAP confirms reduced expression of LRP1 in the brains of adult SynCre/LRPlox/lox mice (lanes 3 and 4). RAP binds to the ligand binding domains of LRP1 and thus detects only the large 515-kDa subunit of the receptor. (C) LRP1 expression is lost in the majority of neurons from SynCre/LRPlox/lox mice. Brain sections from adult SynCre/LRPlox/lox (b, d, and f) and LRPlox/lox control (a, c, and e) mice were examined for LRP1 expression by immunohistochemistry (a to d) and by in situ hybridization (e to f). Immunohistochemical staining with a polyclonal anti-LRP1 antibody reveals complete loss of LRP1 expression in most, but not all, neocortical neurons in SynCre/LRPlox/lox (b) compared to LRPlox/lox (a) mice. Recombination of the loxP-marked LRP1 allele and subsequent loss of LRP1 expression is particularly pronounced in large pyramidal neurons (arrows in panel d). In situ hybridization with an LRP1-specific probe confirms the absence of LRP1 mRNA in the majority of SynCre/LRPlox/lox neurons (f).
LRP1 expression in SynCre/LRPlox/lox mice.
Having confirmed the recombination of loxP-marked LRP1 by the Cre recombinase in neural tissue, we next examined LRP1 expression levels in SynCre/LRPlox/lox mice. Northern blot analysis of total brain RNA and Western blotting experiments with brain membranes showed substantial overall reduction of LRP1 mRNA and the LRP1 protein. Quantitative immunoblots revealed that the LRP1 protein is reduced by ∼50% in SynCre/LRPlox/lox mice (Fig. 1B, lanes 3 and 4) compared to LRPlox/lox controls (Fig. 1B, lanes 1 and 2). Ligand blotting with radiolabeled RAP, which binds to the LRP1 extracellular domain, confirmed the results from Northern and Western blot analyses (Fig. 1B, bottom). The presence of residual RNA and protein was expected, since LRP1 is also expressed, albeit at lower levels, in glia. To ascertain the loss of neuronal LRP1, we performed immunohistochemical analyses (Fig. 1C, a to d) and in situ hybridizations (Fig. 1C, e to f) of brain sections from synapsin Cre/LRPlox/lox (Fig. 1C, b, d, and f) and control mice (Fig. 1C, a, c, and e). Comparison of images a to d shows that expression of LRP1 protein is absent in most, but not all, cortical neurons of SynCre/LRPlox/lox mice (pyramidal neurons are shown in image d). Images e and f show a corresponding loss of LRP1 mRNA expression. Similar results were obtained from other regions of the brain (data not shown).
Mice deficient in neuronal LRP1 exhibit behavioral and gross motor abnormalities.
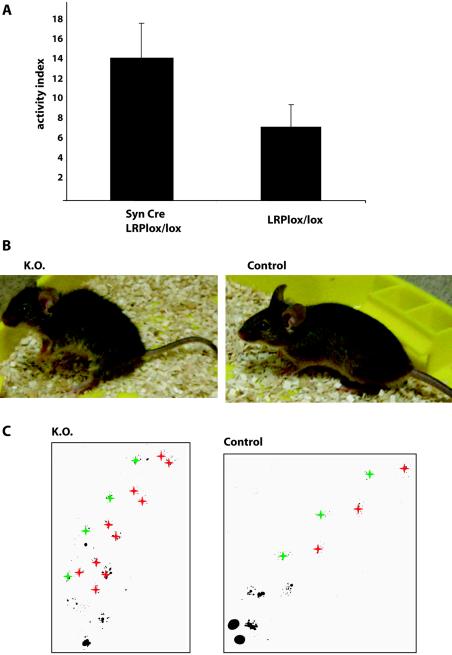
Synapsin Cre/LRPlox/lox mice are born at the expected Mendelian frequency from heterozygous breedings and show no abnormalities at birth. Beginning ∼3 weeks after birth, however, they gradually develop a complex phenotype consisting of behavioral and motor abnormalities. General hyperactivity is combined with increased voluntary movement (Fig. 2A) and a constant muscle tremor. Over time, dystonic posturing with increased thoracic kyphosis and increased plantar flexion of the feet occurs (Fig. 2B), with asymmetric involvement in some mice. Synapsin Cre/LRPlox/lox mice display a characteristic waddling gait, as revealed by their altered footprint patterns (Fig. 2C). Step width increases in relation to step length, and four tracks of prints become visible instead of two. Moreover, synapsin Cre/LRPlox/lox mice show pronounced hind limb weakness, evidenced by an inability to stand on their hind legs and mild lack of coordination.
FIG. 2.
(A) Increased voluntary motor activity and exploratory behavior of SynCre/LRPlox/lox mice. Ambulatory activity of adult SynCre/LRPlox/lox andLRPlox/lox mice in a new empty cage was measured as the number of times the midline of the cage was crossed. Over an observation time of 3 min for each animal, SynCre/LRPlox/lox mice exhibited greater voluntary motor activity than LRPlox/lox control animals. The error bars indicate standard deviations. (B and C) Disturbed motor function in SynCre/LRPlox/lox mice. (B) Adult SynCre/LRPlox/lox mice (K.O.) are smaller than their normal littermates, and their grooming appears less efficient. This 6-month-old animal shows typical features of older SynCre/LRPlox/lox mice: increased thoracic kyphosis, increased flexor tone of the tail, increased plantar flexion of the hind feet, and broadly based positioning of the hind legs. (C) Footprint analysis demonstrates that the gait pattern of SynCre/LRPlox/lox mice is disturbed. The ratio of step width to step length is increased. Tracks of left (green) and right (red) paws are emphasized.
Unlike wild-type mice, which regularly extend their legs when they are suspended by their tails, synapsin Cre/LRPlox/lox mice characteristically clasp their hind limbs, indicating motoneuronal disinhibition or motor excitation.
Although synapsin Cre/LRPlox/lox mice are initially of the same size and weight as wild-type mice, they gradually fall behind in their growth rate. Adult mice are lean, although they eat more than their wild-type counterparts. The reduced weight is likely caused by increased energy expenditure due to hyperactivity and constant tremor. Synapsin Cre/LRPlox/lox mice are hypoglycemic and hypoinsulinemic (Table 1) and die prematurely around 9 months of age.
TABLE 1.
Metabolic assessment of SynCre/LRPlox/lox micea
| Mouse | Food intake (g) | Water intake (ml) | Wt (g) | Plasma insulin (ng/ml) | Blood glucose (mg/dl) | TSH (mU/ml) | T4 (mU/ml) |
|---|---|---|---|---|---|---|---|
| LRPlox/lox | 3.9 ± 0.43 | 31 ± 3.6 | 20.4 ± 0.56 | 0.83 ± 0.3 | 173.2 ± 26 | 0.2 | 1.7 |
| SynCre/LRPlox/lox | 4.7 ± 0.38 | 41 ± 3.6 | 19.2 ± 1.31 | 0.19 ± 0.1 | 102.5 ± 23 | 0.3 | 1.8 |
Food and water intake of eight 2-month-old SynCre/LRPlox/lox and eight 2-month-old LRPlox/lox mice were measured over a 24-h period. In addition, body weight, plasma insulin, blood glucose, thyroid-stimulating hormone (TSH), and thyroid hormone T4 levels were determined. Despite increased food intake, SynCre/LRPlox/lox mice remained lean and their blood glucose and plasma insulin levels were lower than those in control animals. Thyroid function tests (TSH and T4 serum levels) were normal.
The fertility of synapsin Cre/LRPlox/lox mice is greatly reduced. Seizures are not part of the observed phenotype.
Brain morphology is normal in synapsin Cre/LRPlox/lox mice.
The brains of adult synapsin Cre/LRPlox/lox mice appear histologically normal (data not shown). As recombination in synapsin Cre transgenic mice takes place only in differentiated neurons and not in progenitors and progresses after birth, gross misplacement of neurons was not expected.
In addition, the absence of morphological alterations in synapsin Cre/LRPlox/lox mice suggests that there is no major neuronal loss caused by degeneration.
EEG and EMG studies.
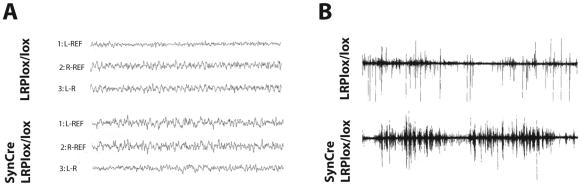
As no histological abnormalities could be detected in the nervous systems of SynCre/LRPlox/lox mice, we hypothesized that the phenotypic abnormalities might be caused by a functional deficit in neurotransmission. We therefore measured electroencephalographic potentials in synapsin Cre/LRPlox/lox mice to assess general cerebral electrical activity. Figure 3A shows EEG recordings from synapsin Cre/LRPlox/lox mice comparable to those of control mice with no obvious detectable abnormalities. By contrast, electromyographic studies revealed the consistent tremor but no abnormal neuromuscular transmission in synapsin Cre/LRPlox/lox mice (Fig. 3B). Moreover, microscopic analysis of α-bungarotoxin-stained neuromuscular endplates revealed normal morphology and numbers (data not shown). These findings suggest that the functional deficit in synapsin Cre/LRPlox/lox animals, which affects the generation and control of motor activity, is not caused by neuromuscular dysfunction but likely by a central nervous system defect. In addition, overall electrical activity in the brain is not altered. The defect thus must be restricted either to defined transmitter systems or to circumscribed regions of the central nervous system, where change in activity is not picked up by EEG recordings.
FIG. 3.
Electroencephalography and electromyography. (A) EEG. Electroencephalographic recordings from adult SynCre/LRPlox/lox mice reveal no obvious abnormalities compared to LRPlox/lox control animals. (B) EMG. Electrical muscle activity was recorded from the knee flexor muscles of SynCre/LRPlox/lox and LRPlox/lox control mice. Increased electrical activity with rhythmical bursts documenting tremor was recorded in the neuron-specific LRP1 knockout animals.
Coimmunoprecipitation with PSD-95 and NMDA receptor subunits NR2A and NR2B suggests postsynaptic localization of neuronal LRP1.
In order to further examine the role of neuronal LRP1 in the regulation of neurotransmission, we decided to determine the subcellular localization of the receptor and its potential coupling to neurotransmitter systems.
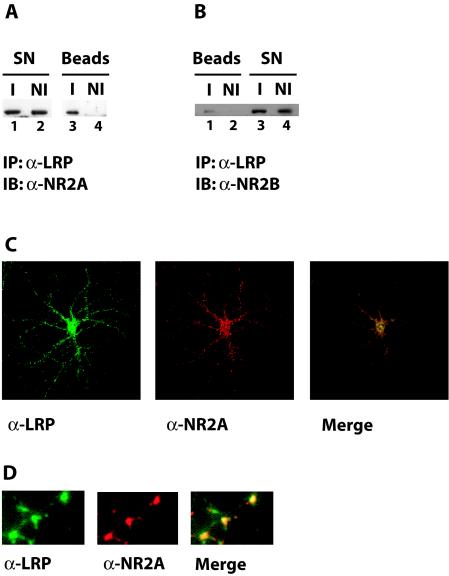
Results from a yeast two-hybrid screen had suggested an interaction of the cytoplasmic domain of LRP1 with the postsynaptic density protein PSD-95 (13). In order to confirm this finding, we examined whether the native proteins could be coprecipitated from primary cultures of rat embryonic neurons. Figure 4 shows that immunoprecipitation of LRP1 from neuronal lysates results in the coprecipitation of PSD-95 (lane 3), whereas no PSD-95 was detected in control immunoprecipitation experiments performed with nonimmune serum (lane 4). This not only confirms the interaction between LRP1 and PSD-95, it also suggests that LRP1 is present in the postsynaptic compartment.
FIG. 4.
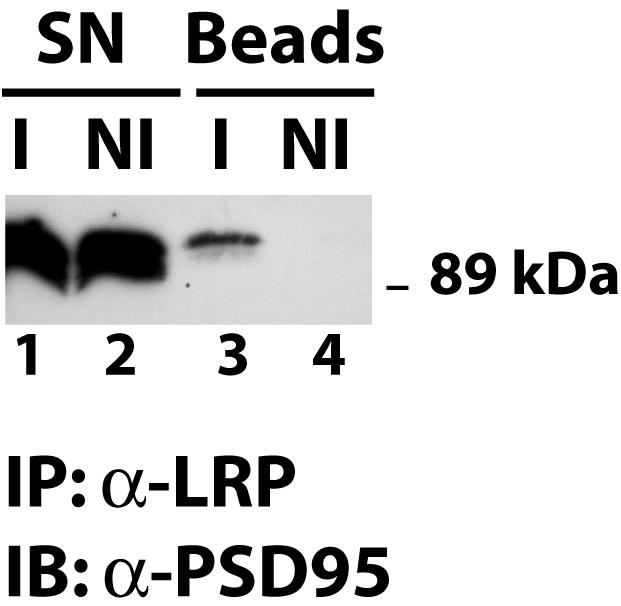
Coimmunoprecipitation of LRP1 and PSD-95. Whole-cell lysates (lanes 1 and 2) were prepared from cultured primary rat embryonic cortical neurons and used for immunoprecipitation with a polyclonal anti-LRP1 antibody (I) or with nonimmune control serum (NI). Immune complexes were recovered on protein A agarose beads and analyzed by SDS-polyacrylamide gel electrophoresis. Western blotting with a PSD-95 antibody revealed coprecipitation of PSD-95 with LRP1 (lane 3), whereas no PSD-95 was detected in the nonimmune control sample (lane 4). IB, immunoblotting; SN, supernatant.
PSD-95 is a scaffolding protein that takes part in the formation of high-molecular-weight protein complexes at postsynaptic density (20). Among the components of these complexes is the NMDA receptor, which mediates calcium influx into neurons in response to the neurotransmitter glutamate. As it has been reported that LRP1 might influence NMDA receptor function (39), we searched for evidence of coprecipitation of the NMDA receptor with LRP1. As can be seen in Fig. 5A and B, after precipitation with the LRP1 antibody, both NMDA receptor subunits NR2A (Fig. 5A, lane 3) and NR2B (Fig. 5B, lane 1) could be detected in the immunoprecipitate from neuronal lysates. No NR2A or NR2B protein was detected in the nonimmune controls (Fig. 5A, lane 4, and Fig. 5B, lane 2, respectively).
FIG. 5.
(A) Coimmunoprecipitation of LRP1 and NR2A. Whole-cell lysates were prepared from cultured primary rat embryonic cortical neurons and used for immunoprecipitation with a polyclonal anti-LRP1 antibody (I) or with nonimmune control serum (NI). Immune complexes were recovered on protein A agarose beads and analyzed by SDS-polyacrylamide gel electrophoresis. Western blotting with an antibody against the NMDA receptor subunit NR2A revealed coprecipitation of NR2A with LRP1 (lane 3). No NR2A was detected in the nonimmune control sample (lane 4). IB, immunoblotting; SN, supernatant. (B) Coimmunoprecipitation of LRP1 and NR2B. The experiment was conducted as described in the legend to Fig. 5A, except that Western blotting was performed with an antibody against the NMDA receptor subunit NR2B. Lane 1 shows coimmunoprecipitation of NR2B with LRP1. (C) Partial colocalization of LRP1 and NR2A. Rat hippocampal neurons in glial cell coculture were examined by indirect immunofluorescence. Costaining was performed with an affinity-purified LRP1 antibody and an antibody against the NMDA receptor subunit NR2A. Bound α-LRP1 was visualized with an Alexa 488-labeled secondary antibody (green fluorescence), and bound α-NR2A was visualized with an Alexa 594 conjugate (red fluorescence). Photographic overlay of images acquired by confocal laser scanning microscopy revealed partial colocalization of LRP1 and NR2A (yellow). (D) Higher magnification of a dendritic process from rat embryonic neurons stained as described for panel C.
Indirect immunofluorescence double staining confirms partial colocalization of LRP1 and NR2A.
Indirect immunofluorescence studies with antibodies against LRP1 and the NMDA receptor subunit NR2A showed that LRP1 is present on neuronal cell bodies and dendritic processes and confirmed its partial colocalization with the NMDA receptor subunit (Fig. 5C). Figure 5D shows the spatial proximity of LRP1 and NR2A on a dendritic process.
Together, these findings suggest that LRP1 might interact with the glutamatergic transmitter system, where it could modulate postsynaptic responses to NMDA receptor activation.
Pretreatment with NMDA reduces the coprecipitation of PSD-95 with LRP1.
We next examined whether postsynaptic LRP1 responded to NMDA treatment of neurons and tested whether the interaction with PSD-95 could be influenced by NMDA receptor activation. Interestingly, treatment of cultured neurons with 100 μM NMDA for 10 min prior to cell lysis and immunoprecipitation of LRP1 markedly reduced the amount of coprecipitated PSD-95 (Fig. 6, compare lane 6 to lane 4), indicating that the interaction and colocalization of the two proteins might be modulated by synaptic activity. Exposure of the neurons to 50 μM dopamine did not alter PSD-95 coprecipitation.
FIG. 6.
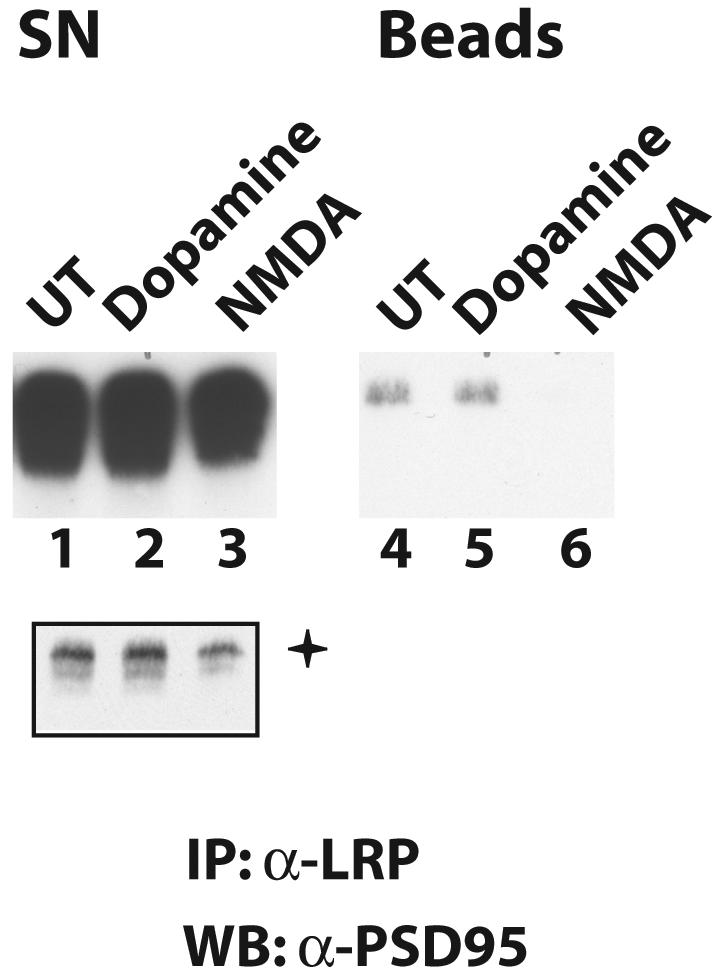
Treatment with NMDA reduces coprecipitation of PSD-95 with LRP1. Cultured rat embryonic neurons were either left untreated (UT) or treated with 100 μM NMDA or 50 μM dopamine for 10 min. Whole-cell lysates were then prepared and subjected to immunoprecipitation with an anti-LRP1 antibody. Immunoprecipitates were resolved by SDS-polyacrylamide gel electrophoresis, and immunoblotting was performed with an antibody against PSD-95. The amount of PSD-95 that coprecipitated with LRP1 was greatly reduced by NMDA treatment (lane 6), but dopamine had no effect (lane 5). +, shorter exposure of lanes 1 to 3. WB, Western blotting; SN, supernatant.
Notably, NMDA treatment also reduced PSD-95 levels in the IP supernatants (Fig. 6, lane 3). This is likely due to proteasomal degradation of PSD-95, which has been reported to occur after NMDA receptor activation (8) and which could be the mechanism of NMDA-dependent modulation of the LRP1-PSD-95 interaction.
As we found that LRP1 responded to NMDA treatment of neurons, we next examined whether we could detect modulation of NMDA receptor-dependent synaptic transmission by LRP1. For this purpose, we used hippocampal slices from SynCre/LRPlox/lox and control mice to measure long-term potentiation (LTP), a well-established parameter for assessing synaptic function in vitro. Furthermore, earlier studies had already implicated LRP1 in the regulation of hippocampal LTP (50).
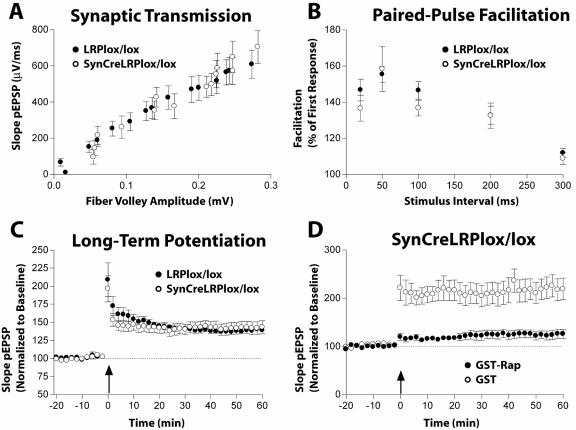
Normal hippocampal LTP in synapsin Cre/LRPlox/lox mice.
Measurements of synaptic function in hippocampal slices by a population field recording technique of excitatory postsynaptic potentials from area CA1 showed no abnormalities of synaptic transmission or short- and long-term potentiation in the hippocampus in SynCre/LRPlox/lox mice. Baseline synaptic transmission at Schaffer collateral synapses in SynCre/LRPlox/lox mice was not different from that in LRPlox/lox controls (Fig. 7A). Similarly, there was no abnormality in short-term synaptic plasticity as measured by paired-pulse facilitation in SynCre/LRPlox/lox animals (Fig. 7B). Long-term potentiation in CA1 at Schaffer collateral synapses was of approximately the same magnitude in SynCre/LRPlox/lox and control mice (Fig. 7C).
FIG. 7.
Synaptic transmission in hippocampal slices. (A) Synaptic transmission. Baseline transmission at Schaffer collateral synapses in the CA1 area was measured at 25°C in hippocampal slices and was comparable between adult SynCre/LRPlox/lox (open circles) and LRPlox/lox (closed circles) mice. (B) Short-term synaptic plasticity was determined by paired-pulse facilitation in SynCre/LRPlox/lox (open circles) and LRPlox/lox (closed circles) mice and was unaffected by the loss of LRP1. (C) Hippocampal LTP was induced by two trains of a 1-s 100-Hz stimulation separated by 20 s (arrow) in SynCre/LRPlox/lox (open circles) and LRPlox/lox (closed circles) mice. No significant difference was observed. (D) SynCre/LRPlox/lox mice were examined as described for panel C except that recombinant 10-μg/ml GST-RAP (closed circles) or 10-μg/ml GST (open circles) was added to the perfusion medium.
LRP1 has been implicated in hippocampal long-term potentiation because treatment of hippocampal slices with the receptor-associated protein RAP reduced the magnitude of LTP (50). Treatment of LRP1-deficient slices from SynCre/LRPlox/lox animals, however, also led to a reduction in LTP, suggesting that RAP acts through another LDLR family member (Fig. 7D), e.g., ApoER2 or VLDLR, which bind RAP and have been shown to play an important role in hippocampal LTP (47).
Taken together, our findings suggest a critical role for LRP1 in postsynaptic function in some neurons. Dysfunction of synaptic transmission occurs in the absence of histologically detectable abnormalities and may involve abnormal regulation of NMDA receptor function.
DISCUSSION
In the present study, we examined the in vivo functions of the LDLR receptor family member LRP1 in neurons. We found that it plays an important role in the regulation of motor activity in mice, possibly through the modulation of postsynaptic signals.
LRP1 is a ubiquitously expressed transmembrane protein that has a dual role in the endocytosis of diverse ligands and in the modulation of signal transduction processes (15, 19). Like other members of the family of LDL receptor-related proteins, LRP1 is highly expressed in the central nervous systems of mammals, predominantly in neurons and glial cells of the brain and the spinal cord (7, 21, 36). The function of neural LRP1 in vivo, however, has been difficult to examine, partly because of the lack of an LRP1-deficient model organism. Furthermore, mice with targeted disruption of the LRP1 gene die early during embryonic development, before differentiation of the nervous system (16).
To avoid the problem posed by early-embryonic lethality, we generated genetically modified mice that lose LPR1 expression in postmitotic differentiated neurons through cre-loxP-mediated disruption of the LRP1 gene. For this purpose, Cre recombinase was expressed from a transgene under the control of the synapsin I promoter in mice with loxP-marked LRP1 alleles. The synapsin I promoter is active in differentiated neurons (14), and Cre expression from the synapsin I Cre construct occurs as early as embryonic day 12.5 (49). Nevertheless, SynCre/LRPlox/lox mice appear normal at birth, and histological examination did not reveal any developmental defects in their nervous systems, suggesting that LRP1 on differentiated neurons is not critical for neural development. This does not exclude a role for LRP1 in neurodevelopment, since LRP1 expression in neural progenitors and glial cells is not altered in SynCre/LRPlox/lox mice (14, 49). While additional studies will be necessary to comprehensively assess the neurodevelopmental importance of LRP1, its functions in the adult nervous system can be examined in SynCre/LRPlox/lox mice without confounding influence from structural developmental defects.
LRP1 expression is lost in the majority of differentiated neurons of SynCre/LRPlox/lox mice (Fig. 1C, b and d), but recombination of the floxed LRP1 allele is not complete (Fig. 1A), with residual neuronal LRP1 expression remaining in SynCre/LRPlox/lox brains (Fig. 1C, d). Nevertheless the reduction of LRP1-expressing neurons is apparently critical in some areas of the nervous system, as SynCre/LRPlox/lox mice suffer from behavioral and motor abnormalities that ultimately lead to their premature death. Hyperactivity, tremor, and dystonia occurred in mutant mice without histologically detectable brain abnormalities, prompting us to hypothesize that loss of neuronal LRP1 may cause a functional deficit in neurotransmission without gross neurodegeneration.
What are the mechanisms by which LRP1 might participate in or modulate neuronal functioning? First, LRP1 is an endocytic receptor that can deliver a wide variety of extracellular ligands to cells (19). As reduced viability or significant loss of neurons was not observed in SynCre/LRPlox/lox mice, however, it seems unlikely that cargo transport by LRP1 has an essential nutritive function in the nervous system. On the other hand, it has been demonstrated that LRP1 is endocytotically active in neurons, and it could be a modulator of their extracellular microenvironment, e.g., in the synaptic cleft (31), although the exact mechanism by which this would influence synaptic transmission remains to be elucidated.
Second, there is a growing body of evidence for a role of LRP1 in modulating signal transduction processes (4, 35, 46, 48). LRP1 controls several signaling pathways in different tissues and likely has similar functions in the nervous system. In this respect, it is particularly intriguing that LRP1 was found to regulate NMDA-dependent calcium currents in vitro (39). A role for LRP1 in the modulation of ligand-dependent ion currents could explain the functional deficits in our SynCre/LRPlox/lox mice. In addition, it has been shown that LRP1 can interact with the scaffolding protein PSD-95 (13), which also interacts with NMDA receptors. In coimmunoprecipitation experiments with neuronal lysates, we showed that LRP1 does indeed interact with PSD-95 (Fig. 4) and that NMDA receptor subunits can be coprecipitated with LRP1 (Fig. 5A and B). Double immunofluorescence confirmed the colocalization of LRP1 and NMDA receptor subunits (Fig. 5C and D), indicating that LRP1 is present in postsynaptic densities in neurons, where it may modulate synaptic transmission. Treatment of neurons with NMDA reduced the coprecipitation of PSD-95 with LRP1 (Fig. 6). This shows that LRP1 is responsive to stimulation of the NMDA receptor system. The exact mechanism of the reduced LRP1-PSD-95 interaction remains to be elucidated, but several mechanisms are possible. First, rapid proteasomal degradation of PSD-95 after NMDA treatment of hippocampal neurons has been demonstrated (8), suggesting that the amount of PSD-95 that can interact with LRP1 is reduced. However, in our experiments, the total amount of PSD-95 in the lysate was only marginally reduced by NMDA treatment (Fig. 6, lane 3), while the amount of PSD-95 that was precipitated with LRP1 was dramatically diminished (Fig. 6, lane 6). This suggests that LRP1 interacts with the active pool of PSD-95 that is subject to regulation by proteasomal degradation in response to NMDA receptor activation.
A second possibility comes from the finding that the LRP1 intracellular domain can be released from the plasma membrane by a γ-secretase-dependent proteolytical cleavage step (35). If the proteolytic processing of LRP1 were increased by NMDA, as has recently been shown for the neuronal protein N-cadherin (32), interaction of the tail with PSD-95 might also be reduced. This could be due to a change in subcellular localization, or it could facilitate the degradation of PSD-95, as the free LRP1 tail is itself short-lived and subject to proteasomal degradation (35).
Third, posttranslational modification by phosphorylation in response to NMDA treatment could limit the interaction of PSD-95 and LRP1. Recent studies have shown that several kinases are capable of phosphorylating PSD-95, e.g., p38 (42), which is activated in response to NMDA receptor stimulation (22). Another candidate is cdk5, which can be modulated by calcium flux (10) and can also phosphorylate PSD-95 (37).
Finally, the phosphorylation status of LRP1 may itself regulate the interaction with PSD-95. LRP1 is phosphorylated in response to different stimuli, e.g., PDGF treatment of cells (5, 29) or PKA activation (28), although such a posttranslational modification of LRP1 in response to NMDA receptor activation remains be shown.
Independent of the precise mechanism by which NMDA affects the interaction of LRP1 and PSD-95, our results support previously published findings that suggested that LRP1 can modulate NMDA receptor signaling (1, 39). In addition, the phenotypic abnormalities of SynCre/LRPlox/lox mice are similar to the symptoms that can be observed after the treatment of rodents with NMDA receptor antagonists (11).
Another mouse model with a combination of hyperactivity and motor abnormalities similar to those of SynCre/LRPlox/lox animals was described recently. These animals lack the dopamine reuptake transporter and therefore suffer from greatly enhanced dopaminergic transmission (9). This is particularly intriguing, because activation of type D2 dopamine receptors has been shown to depress excitatory transmission by NMDA receptors in CA1 pyramidal neurons (25). This depression was dependent on PDGF receptor beta transactivation after dopaminergic stimulation (25). Since earlier studies have shown that in the arterial wall loss of LRP1 leads to increased activity in PDGF receptor beta-dependent signaling (4), the PDGF receptor-mediated cross talk between dopaminergic and glutamatergic neurotransmissions might be critically disturbed in SynCre/LRPlox/lox mice. Deregulated PDGF receptor activity might result in depression of NMDA receptor signaling in a way that is similar to dopaminergic hyperstimulation.
An area where this might occur is the prefrontal cortex or the nigrostriatal system, where interaction of dopaminergic and glutamatergic transmissions has been described and which are involved in the initiation and control of motor activity (27). By contrast, this regulatory pathway does not appear to play a role in hippocampal LTP, since electrophysiological analyses of hippocampal slices of SynCre/LRPlox/lox mice did not reveal any abnormalities (Fig. 7). A reason for this could be that there is enough LRP1 left to maintain normal function or LRP1 is not essential in this region because of functional redundancy with other lipoprotein receptors. In particular, ApoER2 has been shown to be necessary for normal LTP at Schaffer collateral synapses in the CA1 area of the hippocampus (47). This also explains the finding that treatment of hippocampal slices with the inhibitory receptor-associated protein (RAP) reduced long-term potentiation in this electrophysiological paradigm (50). This effect was initially ascribed to a blockade of LRP1. However, since LTP induction and maintenance were unchanged despite the loss of LRP1 expression in the majority of hippocampal CA1-CA3 neurons and because RAP induced the same LTP reduction in SynCre/LRPlox/lox mice (Fig. 7D), it is likely that this effect of RAP is caused by inhibition of one or more members of the LDL receptor family other than LRP1, such as ApoER2 and VLDLR.
In summary, we have uncovered a novel and essential role of LRP1 in the control of behavior and motor function in mice that involves regulation of postsynaptic signaling mechanisms through interaction with the NMDA receptors. These findings add to the expanding roles of members of the ancient and evolutionarily conserved LDL receptor gene family as regulators of intercellular communication and neurotransmission in the nervous system.
Acknowledgments
We thank Wen-Ling Niu and Huichuan Reyna for excellent technical assistance.
P.M. received an Emmy-Noether Fellowship from the Deutsche Forschungsgemeinschaft (DFG). A.R. was the recipient of a fellowship from the DFG. U.B. received fellowships from the Canadian Institutes of Health Research and the Human Frontier Science Program. This study was supported by grants from the NIH (HL20948, HL63762, and NS43408), the Alzheimer Association, the Perot Family Foundation, and the Wolfgang Paul Program of the Humboldt Foundation.
REFERENCES
- 1.Bacskai, B. J., M. Q. Xia, D. K. Strickland, G. W. Rebeck, and B. T. Hyman. 2000. The endocytic receptor protein LRP also mediates neuronal calcium signaling via N-methyl-d-aspartate receptors. Proc. Natl. Acad. Sci. USA 97:11551-11556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beffert, U., P. C. Stolt, and J. Herz. 2004. Functions of lipoprotein receptors in neurons. J. Lipid Res. 45:403-409. [DOI] [PubMed] [Google Scholar]
- 3.Bock, H. H., and J. Herz. 2003. Reelin activates Src family tyrosine kinases in neurons. Curr. Biol. 13:18-26. [DOI] [PubMed] [Google Scholar]
- 4.Boucher, P., M. Gotthardt, W. P. Li, R. G. Anderson, and J. Herz. 2003. LRP: role in vascular wall integrity and protection from atherosclerosis. Science 300:329-332. [DOI] [PubMed] [Google Scholar]
- 5.Boucher, P., P. Liu, M. Gotthardt, T. Hiesberger, R. G. Anderson, and J. Herz. 2002. Platelet-derived growth factor mediates tyrosine phosphorylation of the cytoplasmic domain of the low density lipoprotein receptor-related protein in caveolae. J. Biol. Chem. 277:15507-15513. [DOI] [PubMed] [Google Scholar]
- 6.Brown, M. D., G. A. Banker, I. M. Hussaini, S. L. Gonias, and S. R. VandenBerg. 1997. Low density lipoprotein receptor-related protein is expressed early and becomes restricted to a somatodendritic domain during neuronal differentiation in culture. Brain Res. 747:313-317. [DOI] [PubMed] [Google Scholar]
- 7.Bu, G., E. A. Maksymovitch, J. M. Nerbonne, and A. L. Schwartz. 1994. Expression and function of the low density lipoprotein receptor-related protein (LRP) in mammalian central neurons. J. Biol. Chem. 269:18521-18528. [PubMed] [Google Scholar]
- 8.Colledge, M., E. M. Snyder, R. A. Crozier, J. A. Soderling, Y. Jin, L. K. Langeberg, H. Lu, M. F. Bear, and J. D. Scott. 2003. Ubiquitination regulates PSD-95 degradation and AMPA receptor surface expression. Neuron 40:595-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cyr, M., J. M. Beaulieu, A. Laakso, T. D. Sotnikova, W. D. Yao, L. M. Bohn, R. R. Gainetdinov, and M. G. Caron. 2003. Sustained elevation of extracellular dopamine causes motor dysfunction and selective degeneration of striatal GABAergic neurons. Proc. Natl. Acad. Sci. USA 100:11035-11040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dhavan, R., P. L. Greer, M. A. Morabito, L. R. Orlando, and L. H. Tsai. 2002. The cyclin-dependent kinase 5 activators p35 and p39 interact with the alpha-subunit of Ca2+/calmodulin-dependent protein kinase II and alpha-actinin-1 in a calcium-dependent manner. J. Neurosci. 22:7879-7891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ford, L. M., A. B. Norman, and P. R. Sanberg. 1989. The topography of MK-801-induced locomotor patterns in rats. Physiol. Behav. 46:755-758. [DOI] [PubMed] [Google Scholar]
- 12.Gardai, S. J., Y. Q. Xiao, M. Dickinson, J. A. Nick, D. R. Voelker, K. E. Greene, and P. M. Henson. 2003. By binding SIRPα or calreticulin/CD91, lung collectins act as dual function surveillance molecules to suppress or enhance inflammation. Cell 115:13-23. [DOI] [PubMed] [Google Scholar]
- 13.Gotthardt, M., M. Trommsdorff, M. F. Nevitt, J. Shelton, J. A. Richardson, W. Stockinger, J. Nimpf, and J. Herz. 2000. Interactions of the low density lipoprotein receptor gene family with cytosolic adaptor and scaffold proteins suggest diverse biological functions in cellular communication and signal transduction. J. Biol. Chem. 275:25616-25624. [DOI] [PubMed] [Google Scholar]
- 14.Haas, C. A., and L. J. DeGennaro. 1988. Multiple synapsin I messenger RNAs are differentially regulated during neuronal development. J. Cell Biol. 106:195-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herz, J. 2003. LRP: a bright beacon at the blood-brain barrier. J. Clin. Investig. 112:1483-1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herz, J., D. E. Clouthier, and R. E. Hammer. 1992. LDL receptor-related protein internalizes and degrades uPA-PAI-1 complexes and is essential for embryo implantation. Cell 71:411-421. (Author's correction, 73:428, 1993.) [DOI] [PubMed] [Google Scholar]
- 17.Herz, J., J. L. Goldstein, D. K. Strickland, Y. K. Ho, and M. S. Brown. 1991. 39-kDa protein modulates binding of ligands to low density lipoprotein receptor-related protein/alpha 2-macroglobulin receptor. J. Biol. Chem. 266:21232-21238. [PubMed] [Google Scholar]
- 18.Herz, J., U. Hamann, S. Rogne, O. Myklebost, H. Gausepohl, and K. K. Stanley. 1988. Surface location and high affinity for calcium of a 500-kd liver membrane protein closely related to the LDL-receptor suggest a physiological role as lipoprotein receptor. EMBO J. 7:4119-4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herz, J., and D. K. Strickland. 2001. LRP: a multifunctional scavenger and signaling receptor. J. Clin. Investig. 108:779-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Husi, H., and S. G. Grant. 2001. Isolation of 2000-kDa complexes of N-methyl-d-aspartate receptor and postsynaptic density 95 from mouse brain. J. Neurochem. 77:281-291. [DOI] [PubMed] [Google Scholar]
- 21.Ishiguro, M., Y. Imai, and S. Kohsaka. 1995. Expression and distribution of low density lipoprotein receptor-related protein mRNA in the rat central nervous system. Brain Res. Mol. Brain Res. 33:37-46. [DOI] [PubMed] [Google Scholar]
- 22.Kawasaki, H., T. Morooka, S. Shimohama, J. Kimura, T. Hirano, Y. Gotoh, and E. Nishida. 1997. Activation and involvement of p38 mitogen-activated protein kinase in glutamate-induced apoptosis in rat cerebellar granule cells. J. Biol. Chem. 272:18518-18521. [DOI] [PubMed] [Google Scholar]
- 23.Kinoshita, A., C. M. Whelan, C. J. Smith, I. Mikhailenko, G. W. Rebeck, D. K. Strickland, and B. T. Hyman. 2001. Demonstration by fluorescence resonance energy transfer of two sites of interaction between the low-density lipoprotein receptor-related protein and the amyloid precursor protein: role of the intracellular adapter protein Fe65. J. Neurosci. 21:8354-8361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knauer, M. F., R. A. Orlando, and C. G. Glabe. 1996. Cell surface APP751 forms complexes with protease nexin 2 ligands and is internalized via the low density lipoprotein receptor-related protein (LRP). Brain Res. 740:6-14. [DOI] [PubMed] [Google Scholar]
- 25.Kotecha, S. A., J. N. Oak, M. F. Jackson, Y. Perez, B. A. Orser, H. H. Van Tol, and J. F. MacDonald. 2002. A D2 class dopamine receptor transactivates a receptor tyrosine kinase to inhibit NMDA receptor transmission. Neuron 35:1111-1122. [DOI] [PubMed] [Google Scholar]
- 26.Kowal, R. C., J. Herz, K. H. Weisgraber, R. W. Mahley, M. S. Brown, and J. L. Goldstein. 1990. Opposing effects of apolipoproteins E and C on lipoprotein binding to low density lipoprotein receptor-related protein. J. Biol. Chem. 265:10771-10779. [PubMed] [Google Scholar]
- 27.Laruelle, M., L. S. Kegeles, and A. Abi-Dargham. 2003. Glutamate, dopamine, and schizophrenia: from pathophysiology to treatment. Ann. N. Y. Acad. Sci. 1003:138-158. [DOI] [PubMed] [Google Scholar]
- 28.Li, Y., P. van Kerkhof, M. P. Marzolo, G. J. Strous, and G. Bu. 2001. Identification of a major cyclic AMP-dependent protein kinase A phosphorylation site within the cytoplasmic tail of the low-density lipoprotein receptor-related protein: implication for receptor-mediated endocytosis. Mol. Cell. Biol. 21:1185-1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loukinova, E., S. Ranganathan, S. Kuznetsov, N. Gorlatova, M. M. Migliorini, D. Loukinov, P. G. Ulery, I. Mikhailenko, D. A. Lawrence, and D. K. Strickland. 2002. Platelet-derived growth factor (PDGF)-induced tyrosine phosphorylation of the low density lipoprotein receptor-related protein (LRP). Evidence for integrated co-receptor function between LRP and the PDGF. J. Biol. Chem. 277:15499-15506. [DOI] [PubMed] [Google Scholar]
- 30.Lund, E. G., J. M. Guileyardo, and D. W. Russell. 1999. cDNA cloning of cholesterol 24-hydroxylase, a mediator of cholesterol homeostasis in the brain. Proc. Natl. Acad. Sci. USA 96:7238-7243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Makarova, A., I. Mikhailenko, T. H. Bugge, K. List, D. A. Lawrence, and D. K. Strickland. 2003. The low density lipoprotein receptor-related protein modulates protease activity in the brain by mediating the cellular internalization of both neuroserpin and neuroserpin-tissue-type plasminogen activator complexes. J. Biol. Chem. 278:50250-50258. [DOI] [PubMed] [Google Scholar]
- 32.Marambaud, P., P. H. Wen, A. Dutt, J. Shioi, A. Takashima, R. Siman, and N. K. Robakis. 2003. A CBP binding transcriptional repressor produced by the PS1/epsilon-cleavage of N-cadherin is inhibited by PS1 FAD mutations. Cell 114:635-645. [DOI] [PubMed] [Google Scholar]
- 33.May, P., H. H. Bock, J. Nimpf, and J. Herz. 2003. Differential glycosylation regulates processing of lipoprotein receptors by gamma-secretase. J. Biol. Chem. 278:37386-37392. [DOI] [PubMed] [Google Scholar]
- 34.May, P., and J. Herz. 2003. LDL receptor-related proteins in neurodevelopment. Traffic 4:291-301. [DOI] [PubMed] [Google Scholar]
- 35.May, P., Y. K. Reddy, and J. Herz. 2002. Proteolytic processing of low density lipoprotein receptor-related protein mediates regulated release of its intracellular domain. J. Biol. Chem. 277:18736-18743. [DOI] [PubMed] [Google Scholar]
- 36.Moestrup, S. K., J. Gliemann, and G. Pallesen. 1992. Distribution of the alpha 2-macroglobulin receptor/low density lipoprotein receptor-related protein in human tissues. Cell Tissue Res. 269:375-382. [DOI] [PubMed] [Google Scholar]
- 37.Morabito, M. A., M. Sheng, and L. H. Tsai. 2004. Cyclin-dependent kinase 5 phosphorylates the N-terminal domain of the postsynaptic density protein PSD-95 in neurons. J. Neurosci. 24:865-876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pietrzik, C. U., T. Busse, D. E. Merriam, S. Weggen, and E. H. Koo. 2002. The cytoplasmic domain of the LDL receptor-related protein regulates multiple steps in APP processing. EMBO J. 21:5691-5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qiu, Z., D. K. Strickland, B. T. Hyman, and G. W. Rebeck. 2002. Alpha 2-macroglobulin exposure reduces calcium responses to N-methyl-d-aspartate via low density lipoprotein receptor-related protein in cultured hippocampal neurons. J. Biol. Chem. 277:14458-14466. [DOI] [PubMed] [Google Scholar]
- 40.Rohlmann, A., M. Gotthardt, R. E. Hammer, and J. Herz. 1998. Inducible inactivation of hepatic LRP gene by cre-mediated recombination confirms role of LRP in clearance of chylomicron remnants. J. Clin. Investig. 101:689-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rohlmann, A., M. Gotthardt, T. E. Willnow, R. E. Hammer, and J. Herz. 1996. Sustained somatic gene inactivation by viral transfer of Cre recombinase. Nat. Biotechnol. 14:1562-1565. [DOI] [PubMed] [Google Scholar]
- 42.Sabio, G., S. Reuver, C. Feijoo, M. Hasegawa, G. M. Thomas, F. Centeno, S. Kuhlendahl, S. Leal-Ortiz, M. Goedert, C. Garner, and A. Cuenda. 2004. Stress- and mitogen-induced phosphorylation of the synapse-associated protein SAP90/PSD95 by activation of SAPK3/p38γ and ERK1/ERK2. Biochem. J. 380:19-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sutherland, M. L., S. H. Williams, R. Abedi, P. A. Overbeek, P. J. Pfaffinger, and J. L. Noebels. 1999. Overexpression of a Shaker-type potassium channel in mammalian central nervous system dysregulates native potassium channel gene expression. Proc. Natl. Acad. Sci. USA 96:2451-2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ulery, P. G., J. Beers, I. Mikhailenko, R. E. Tanzi, G. W. Rebeck, B. T. Hyman, and D. K. Strickland. 2000. Modulation of beta-amyloid precursor protein processing by the low density lipoprotein receptor-related protein (LRP). Evidence that LRP contributes to the pathogenesis of Alzheimer's disease. J. Biol. Chem. 275:7410-7415. [DOI] [PubMed] [Google Scholar]
- 45.van Vlijmen, B. J., A. Rohlmann, S. T. Page, A. Bensadoun, I. S. Bos, T. J. van Berkel, L. M. Havekes, and J. Herz. 1999. An extrahepatic receptor-associated protein-sensitive mechanism is involved in the metabolism of triglyceride-rich lipoproteins. J. Biol. Chem. 274:35219-35226. [DOI] [PubMed] [Google Scholar]
- 46.Wang, X., S. R. Lee, K. Arai, K. Tsuji, G. W. Rebeck, and E. H. Lo. 2003. Lipoprotein receptor-mediated induction of matrix metalloproteinase by tissue plasminogen activator. Nat. Med. 9:1313-1317. [DOI] [PubMed] [Google Scholar]
- 47.Weeber, E. J., U. Beffert, C. Jones, J. M. Christian, E. Forster, J. D. Sweatt, and J. Herz. 2002. Reelin and ApoE receptors cooperate to enhance hippocampal synaptic plasticity and learning. J. Biol. Chem. 277:39944-39952. [DOI] [PubMed] [Google Scholar]
- 48.Yepes, M., M. Sandkvist, E. G. Moore, T. H. Bugge, D. K. Strickland, and D. A. Lawrence. 2003. Tissue-type plasminogen activator induces opening of the blood-brain barrier via the LDL receptor-related protein. J. Clin. Investig. 112:1533-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu, Y., M. I. Romero, P. Ghosh, Z. Ye, P. Charnay, E. J. Rushing, J. D. Marth, and L. F. Parada. 2001. Ablation of NF1 function in neurons induces abnormal development of cerebral cortex and reactive gliosis in the brain. Genes Dev. 15:859-876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhuo, M., D. M. Holtzman, Y. Li, H. Osaka, J. DeMaro, M. Jacquin, and G. Bu. 2000. Role of tissue plasminogen activator receptor LRP in hippocampal long-term potentiation. J. Neurosci. 20:542-549. [DOI] [PMC free article] [PubMed] [Google Scholar]