Abstract
Background
Spatial modelling of STH and schistosomiasis epidemiology is now commonplace. Spatial epidemiological studies help inform decisions regarding the number of people at risk as well as the geographic areas that need to be targeted with mass drug administration; however, limited attention has been given to propagated uncertainties, their interpretation, and consequences for the mapped values. Using currently published literature on the spatial epidemiology of helminth infections we identified: (1) the main uncertainty sources, their definition and quantification and (2) how uncertainty is informative for STH programme managers and scientists working in this domain.
Methodology/Principal Findings
We performed a systematic literature search using the Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) protocol. We searched Web of Knowledge and PubMed using a combination of uncertainty, geographic and disease terms. A total of 73 papers fulfilled the inclusion criteria for the systematic review. Only 9% of the studies did not address any element of uncertainty, while 91% of studies quantified uncertainty in the predicted morbidity indicators and 23% of studies mapped it. In addition, 57% of the studies quantified uncertainty in the regression coefficients but only 7% incorporated it in the regression response variable (morbidity indicator). Fifty percent of the studies discussed uncertainty in the covariates but did not quantify it. Uncertainty was mostly defined as precision, and quantified using credible intervals by means of Bayesian approaches.
Conclusion/Significance
None of the studies considered adequately all sources of uncertainties. We highlighted the need for uncertainty in the morbidity indicator and predictor variable to be incorporated into the modelling framework. Study design and spatial support require further attention and uncertainty associated with Earth observation data should be quantified. Finally, more attention should be given to mapping and interpreting uncertainty, since they are relevant to inform decisions regarding the number of people at risk as well as the geographic areas that need to be targeted with mass drug administration.
Author Summary
In recent years spatial modelling studies of schistosome and soil-transmitted helminth infections have become commonplace; however there is no standard framework for uncertainty evaluation and reporting. In this study we aim to identify faults in existing studies and propose a framework for evaluation and reporting. We conducted a systematic review of the literature to identify the gaps in knowledge in relation to how uncertainty is dealt with in existing studies addressing the spatial modelling of helminth infections. It was found that none of the studies considered adequately all sources of uncertainty. Uncertainty in the response variables and covariates should be incorporated into the modelling framework. More attention should be given to mapping and interpreting uncertainty, and to quantify the different sources of uncertainty present in the observed covariates (environmental variables), measured response variable (morbidity indicators), used model and uncertainty representation and interpretation of the predicted morbidity indicators.
Introduction
Helminth infections from as soil-transmitted helminths (STHs) and schistosomes are among the most prevalent neglected tropical diseases (NTDs) affecting human populations living in countries where clean water, sanitation, and hygiene (WASH) are limited. STHs and schistosomes, affect more than 1.7 billion and 252 million [1,2] people worldwide respectively. The majority of these infections are concentrated in sub-Saharan [3,4] and North Africa, Asia, and central and Andean regions of Latin America [1]. STH and schistosome infections influence directly the nutrition status, educational development, individual productivity, physical and mental development in human populations [5]. The World Health Organization (WHO), the World Bank and other agencies defined control and elimination targets in the poorest populations [6]. Although the global burden of NTDs declined by 27% from 1990 to 2010 in upper-middle income countries [6], low and lower middle income countries still need attention. Besides, according to the Global Burden of Disease Study 2010 [1], STHs due to intestinal nematode infections, and schistosomiasis, caused the largest number of cases reported in 2010. In order to improve population health and accomplish WHO targets, the 2012 London declaration for Neglected Tropical Diseases and the 2013 World Health Assembly resolution highlighted the importance of mass drug administration (MDA) with benzimidazoles [7,8] to communities at risk.
To identify communities at risk, indirect indicators of morbidity such as prevalence of infection and intensity of infection can be measured via surveying at-risk populations [9]. Communities at risk can then be categorized into disease prevalence classes (e.g. low, moderate, high) based on WHO guidelines [10]. In the absence of empirical data on infection at unsampled communities, one way to identify communities at risk is to study the role of the environment (physical and biological) to characterize potential habitats of parasites and intermediate hosts, as well as to understand the ecology and epidemiology of infections. Statistical modelling of the spatial distribution of helminth infections provides empirical relationships between infections and risk factors, which can then be used to predict the level of infection prevalence at unsampled locations [9,11–13]. In the statistical model, prevalence or another morbidity indicator, is treated as the response variable.
Although statistical modelling of helminth infections is useful to effectively and efficiently manage surveillance, control and prevention of the infection [14], the mapped outputs should be interpreted with care because these can be weakened by several sources of uncertain information [15]. Sources of uncertainty that need to be accounted for in the modelling process include differences in variable selection criteria, statistical methods used, selected spatial and temporal scales of analysis [16], sampling design, sensitivity and specificity of diagnostic techniques as well as the quality of the spatial data used.
Uncertainty has been the subject of extensive discussion in Geographic Information Science (GIScience) [17–32] and related subjects [33–43]. Uncertainty may relate to (1) a state of mind and our perception of the world or (2) statements about the world or observations on natural phenomena [17,18,22,32] and is relevant in terms of specifications and representations, measurement and the transformations, processing and modelling performed on raw data to turn them into usable information [17,22]. In order to address uncertainty, a more formal approach is required [17,18]. Here we conceptualize uncertainty as imperfection, which is further categorized as inaccuracy or imprecision.
Imprecision may arise because the phenomenon is vague (i.e., the phenomenon is not clearly defined), ambiguous (i.e., different definitions can be applied to the phenomenon) [23,32] or due to the granularity of the observation [17]. In the spatial setting granularity relates to the resolution or spatial support (area or volume) of the observation and affects our ability to discern objects [17,44]. Imprecision may also arise due to natural variability, measurement error and model variability and may be described statistically, for example by the variance or standard deviation [32,45,46]. In this context, model variability may arise due to uncertain data, stochastic processes within the model or variability between competing models. The reader may be familiar with the narrow statistical definition of precision as the inverse of the variance [47], whereas the imprecision that is applied here encompasses a wider set of concepts [17,18]. Put another way, in this conceptualization, variance is not the only measure of precision.
Accuracy is a measure of closeness between the observed phenomenon and reference observations, considered representative of the reality [17,45,48]. Accuracy assessment is often referred to as validation [20,49]. Common measures of accuracy include the root mean square error (RMSE) for continuous data [45,48], the overall accuracy (OA) for categorical data [27,28,50] and the area under the receiver operator characteristic curve (AUC) for binary data [45]. Bias relates to accuracy and refers to systematic differences between the observations and reference data.
Accounting for uncertainty in disease mapping is important for the assessment of the applicability and validity of the predicted morbidity indicators [15]. Furthermore, it will allow a complete risk assessment and the identification of potential sources of bias [51]. Ignoring uncertainty can lead to incorrect predictions, thus wrong estimates of disease burden, which can result in misleading public health advocacy and decisions regarding disease control. Consideration of information about uncertainty is critical for control programs, health care workers, populations at risk, and other involved users who attempt to reduce prevalence and incidence of helminth infections across the affected areas [51,52]. For example, control programs need accurate information to decide about drug distribution strategies and the frequency of treatment of the target populations. Decision makers can use information about uncertainty to target more resources (e.g., data acquisition) or to focus investigative efforts on low or highly uncertain risk areas [53,54].
This paper is a systematic review that aims at the identification of the gaps in knowledge of the different components of uncertainty associated with mapping and modelling helminth infections. It also aims at providing a basis for a complete uncertainty communication, by evaluating the impact of uncertainty on the predicted morbidity indicators. This paper starts by investigating how uncertainty is informative for decision makers, public health scientists and the affected community. It then identifies main sources of uncertainty in helminth infection mapping studies, and how uncertainties have been defined and quantified. Regarding the sources of uncertainty, their definition and quantification, the focus will be put on sources relating to Earth Observation. The significance of this paper is that it contributes to inform control programs and health workers about the importance of uncertainty in mapping and modeling helminth infections, by putting special attention on relevant sources of uncertainty, and analyzing their real influence on the predicted morbidity indicator values used to guide mass drug administration strategies and their cost effectiveness.
Methods
Search strategy
An online search was performed using two search engines, the Web of Knowledge (Core collection and MEDLINE) and PubMed. Only articles published in English were considered. The date range was 1 January 1980 to 24 October 2016. The search strategy aimed at the identification of primary research studies that have looked into establishing the geographical limits of STH and schistosomiasis present only in humans; therefore the search strategy combined variations of three terms: spatial, helminth infection, and uncertainty terms. The full list of terms used in the systematic review is shown in Table 1. Six searches were performed by combining the three terms in each search engine, using the keywords described in Table 2.
Table 1. Classification of search terms.
| Uncertainty term (UT) | Spatial term (ST) | Disease term (UT) |
|---|---|---|
| Uncertainty, uncertain, uncertainties. | Geographic, geographical, geography | helminth(s), helminthiasis, soil-transmitted helminths, soil-transmitted helminthiasis, neglected tropical diseases. |
| Vagueness, vague | Spatial, geospatial | Schistosome, Schistosoma, schistosomiasis. |
| Imprecision, precision, precise, imprecise | Remote sensing, remotely sensed | Hookworm(s) |
| Accuracy, inaccuracy, accurate, inaccurate | Trichuris trichiura | |
| Fuzzy, fuzziness | Ascaris lumbricoides | |
| Error(s) | ||
| Bias |
Table 2. Keywords used in the literature search,* indicates wildcard.
| Uncertainty term | Spatial term | Disease term | |||
|---|---|---|---|---|---|
| 1 | TS = uncertain* | 3 | TS = geogra* OR TS = spatial OR TS = geo$spatial OR TS = "remote* sens*" | 4 | TI = schistosom* |
| 2 | TS = vague* OR TS = *precision OR TS = *precise OR TS = *accura* OR TS = fuzz* OR TS = error* OR TS = bias | 5 | TI = hookworm* OR TI = "trichuris trichiura" OR TI = "ascaris lumbricoides" | ||
| 6 | TI = helminth* OR TI = "soil$transmitted helminth*" OR TS = “neglected tropical disease*” | ||||
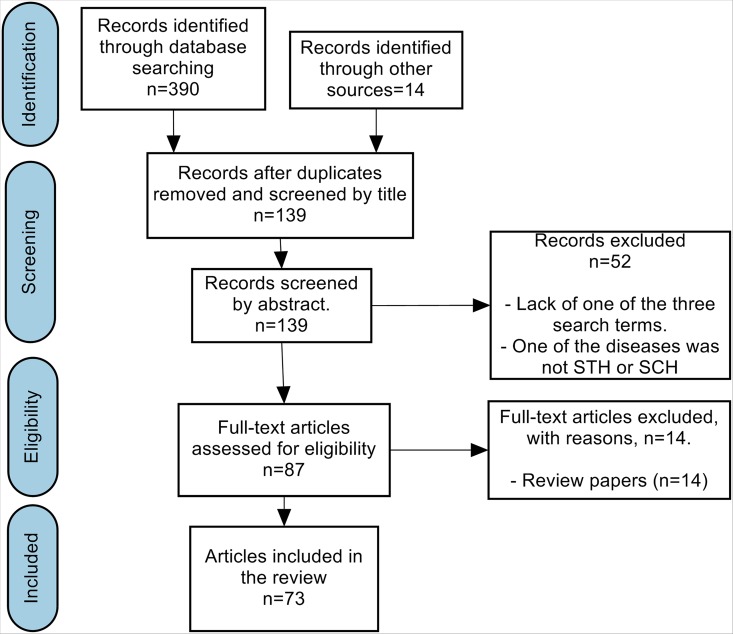
After removing duplicates, the abstracts of 139 papers were read. Papers written in languages other than English (11 papers) were automatically excluded. Review papers (14 papers) were also excluded. Further criteria were then applied to select the final papers to read, but also to make the reading process more efficient. The inclusion criteria considered were (i) the presence of the three spatial, uncertainty and helminth infection search terms in the abstracts and (ii) also articles related to only STH and schistosomiasis helminth infections. The papers were classified into schistosomiasis and soil transmitted helminth studies. The selection of the papers, data acquisition and analysis was undertaken by the first author. The PRISMA flow diagram is given in Fig 1.
Fig 1. PRISMA flow diagram.
Data collection process
Data collection from each paper focused on addressing three main research questions. (1) How is uncertainty informative for decision making in the public health context? (2) What are the different uncertainty sources reported in the reviewed studies? (3) How were uncertainty and its sources defined and quantified in the studies? Papers addressing these questions were enumerated.
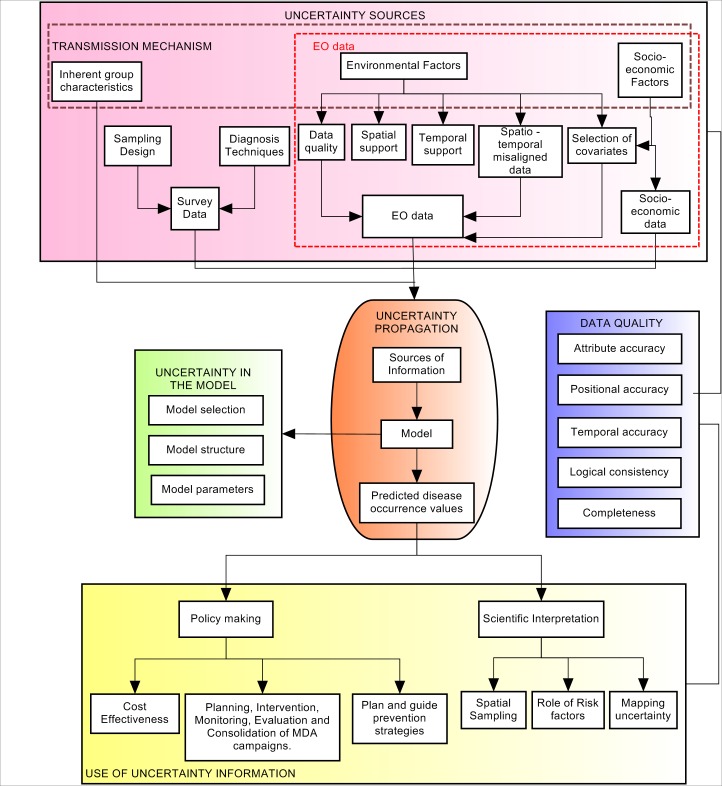
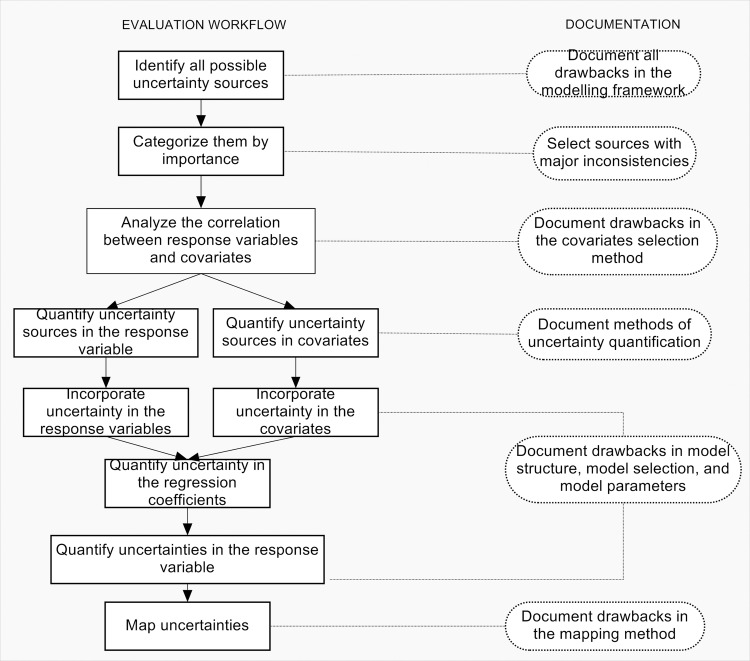
Fig 2 illustrates the relevant three uncertainty stages that drive the final mapping and modelling of STH and schistosomiasis infections. The first stage (pink box) describes the origin of uncertainty coming from data sources, including uncertainties in the response variable and covariates. The second stage (orange box) shows how uncertainty from the pink box propagates through the predictive model (green box). The green box incorporates uncertainties derived from the selection of the predictive model, considering that there could be different ways to model the same helminth infection. It also includes uncertainties in model structure, which refers to all possible limitations and assumptions in the selected model, such as: the lack of understanding about the interaction between the environment, helminth infections and human populations, as well as the assumptions of stationarity and spatial isotropy [9]. Finally, the green box includes uncertainties in the methods used to estimate the model parameters. The third stage (yellow box), shows how uncertainty in the predicted morbidity indicator is addressed, firstly in policy and decision making settings and secondly in a scientific setting. This stage aims to understand how information on uncertainty is used practically and how is it defined and quantified. The blue box represents different elements of data quality that relate to the sources of information (pink box), and the predicted morbidity indicators (yellow box), which due to its wide field of study and importance was separated into a different box.
Fig 2. Uncertainty propagation through the process chain of mapping and modelling helminth infections.
Pink box: uncertainty from information data sources. Orange box: uncertainty from the predictive model. Yellow box: uncertainty in the predictions.
Uncertainty use in helminth infection mapping for morbidity control (uncertainty interpretation)
Two approaches were considered to describe the possible usage of uncertainty in helminth infections modelling. The first approach indicates that uncertainty could be used in policy making in order to support public health institutions, governments and national or international organizations involved in the control and prevention of STH and schistosome infections. Three foci of attention for policy making were considered: (1) plan and guide prevention strategies, (2) plan the intervention, monitoring, evaluation and consolidation of MDA campaigns, (3) evaluate cost-effectiveness of control programmes. The second approach proposes to use uncertainty to support scientific interpretation by looking at the influence of different information sources on the modelling process, and decide about new improvements or conclusions that need to be considered. Three foci of attention for scientific research were considered: (1) spatial sampling, (2) the role of risk factors (covariates in the statistical model), (3) the mapping of uncertainty. An overview of the different foci of attention of uncertainty information is explained in Table 3.
Table 3. Description of communication of uncertainty.
| Uncertainty informs about | Description | |
|---|---|---|
| Policy Making | Planning, Intervention, Monitoring, Evaluation and Consolidation of MDA campaigns. | • Plan spatial targeting and the frequency of deworming campaigns to estimate required drug supplies. • Guide interventions towards high risk populations. • Monitoring: Maintain success and long term sustainability of control programs. • Evaluation: compare and choice more efficient strategies to control the disease. • Consolidate control and move towards disease elimination. |
| Cost effectiveness | • Inform about the cost associated with the health benefit acquired by implementing a specific control strategy. • Ensure the resources are distributed efficiently by channel funds to high risk populations. |
|
| Plan and guide prevention Strategies | • Plan and guide hygiene education and infrastructure programs in water sanitation and hygiene, as well as implement environmental educational health awareness programs. • Control intermediate host or parasite sources to prevent transmission to definitive hosts. |
|
| Scientific Interpretation | Sampling | • Define uncertain risk areas where further data collection is required. • Guarantee the safety of local citizens from future infection resurgence by determining appropriate surveys and monitoring strategies. |
| Role of risk factors | • Investigate the effect of environmental risk factors on transmission of parasites. • Guide control efforts in the absence of epidemiological information. |
|
| Mapping Uncertainties | • Spatial representation of uncertainty as a necessary resource for decision making. | |
Uncertainty sources in modelling and mapping helminth infections (uncertainty in the data)
Sources of uncertainty shown in the red box in Fig 2 were classified into four: (1) survey, (2) Earth observation, and (3) socio-economic data, (4) inherent group characteristics. Survey data encompassed uncertainties in the response variable, while Earth observation and socio-economic data were uncertainty sources coming from the covariates. Survey data contained uncertainty from the sampling design and diagnostic technique. Sampling design refers to the type of survey used, sample manipulation, sample size selection, incomplete sample coverage, logistic limitations, survey registration method, adjustment for confounding and the measured morbidity indicator. Uncertainty in the diagnostic technique arises due to the lack of sensitivity and specificity in the methods used to detect helminth parasites eggs in the stool or urine of affected individuals. Uncertainties derived from Earth observation data arise due to spatio-temporal misaligned data, incorrect selection of significant environmental and socio-economic variables, as well as selection of spatial and temporal support of analysis which do not fit the study purpose. The term misaligned data refers to the combination of multiple datasets that may be defined on different or non-aligned spatial units [55], whereas the support refers to size, shape and orientation of the spatial units [56]. The term scale can have multiple meanings in geographical information science (GIScience) [44]; here we consider scale in terms of the support of the data and the extent of the study domain [45]. Data quality refers to the evaluation in terms of fitness-for-use for a given application [11]. This evaluation addresses the completeness, logical consistency, time, attribute and positional accuracy of spatial data [57–60]. Different measurements of the same variable may even have different qualities according to the sensitivity, specificity and accuracy of the instrument or measurement technique.
Scale is a major concern in spatial epidemiology [11,45,61–63]. Different environmental and socio-economic risk factors may be relevant according to the scale of the analysis [11,64]. For a given extent the choice of support may affect the patterns identified in the data [65,66] as well as the relationship between the response variable and covariates. This is known as the modifiable areal unit problem (MAUP) in GIScience [11,44]. Different datasets may be misaligned and need to be brought to a common grid prior to analysis [66,67]. Hence it may be necessary to aggregate, disaggregate or interpolate data prior to analysis [11,68]. All of these operations may be applied in time and space and all have an associated uncertainty. Issues about the selection of significant environmental and socio-economic variables referred to: (1) the exclusion of some socio-economic and climatic factors, which due to logistics or lack of reliable information have not been included in the modelling process; (2) the uncertain choice of covariates produced by the lack of knowledge about the influence of risk factors depending on the spatial support of analysis, the spatial support of the data and other aspects of data quality. Sources of uncertainty derived from inherent group characteristics refer to the heterogeneous distribution of parasites in the population, and the influence of polyparasitism (infection due to multiple parasites also termed coinfections) on the risk of infection.
Uncertainty definition and quantification in helminth infections mapping
As mentioned in the introduction, uncertainty was conceptualized as imperfection and further categorized as accuracy and imprecision [17,18]. Accuracy may be evaluated by comparison with a reference dataset [17,18,27,28,45,48,50] and different quantitative measures may be used depending on the type of data. Continuous data may be evaluated using the root mean square error (RMSE) or mean absolute error (MAE), which are both measures of the average error. Bias can be evaluated using the mean error. Categorical data are typically evaluated using a confusion matrix with summary measures including the overall accuracy, user’s and producer’s accuracy and kappa statistic. Binary data may be evaluated using the area under the receiver operator curve (ROC) (AUC). Measures of accuracy are summarized in Table 4.
Table 4. Measures of uncertainty corresponding to different types of data.
| Categories of imperfection | Types of data | Measures of uncertainty | Abbreviation |
|---|---|---|---|
| Imprecision | Continuous data | Standard deviation | SD |
| Credible intervals | CrI | ||
| Confidence Intervals | CI | ||
| Categorical data (Vagueness) | Fuzzy sets | ||
| Rough sets | |||
| Inaccuracy | Continuous data | Root mean square error | RMSE |
| Mean absolute error | MAE | ||
| Residual mean square | RME | ||
| Mean error (bias) | ME | ||
| Categorical data | Overall accuracy | OA | |
| User’s accuracy | UA | ||
| Producer’s accuracy | PA | ||
| Kappa statistic | K | ||
| Binary data | Area under the receiver operator characteristic curve | AUC |
Evaluation of imprecision depends on the nature of the phenomena and data being studied. Where these are well defined, imprecision may be defined statistically [21,32] and applied in both Bayesian and frequentist settings. The error variance is the usual measure here, although this is commonly expressed as the standard deviation or standard error [32] or as an interval–such as the 95% confidence interval (frequentist) or credible/credibility interval (Bayesian) [69]. Vagueness may be evaluated using fuzzy set or rough set theory [21,32]. Table 4 shows the elements and measures of uncertainty conceptualized as imperfection.
Results
Search strategy
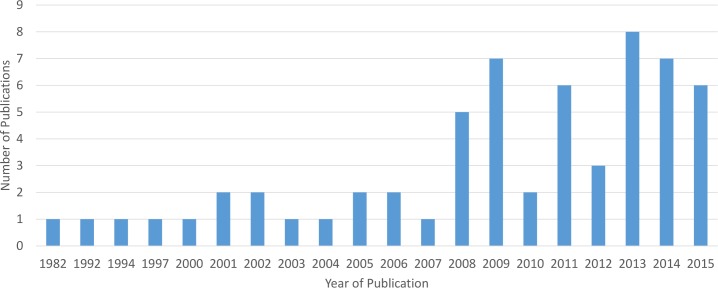
The total number of papers found in each search is shown in Table 5. Table 6 shows the resulting number of read and discarded papers presented per infection. In total 73 papers were selected, from which 14 were review papers. While the identified review papers were not included in this review we examined their reference lists; this yielded another 14 valuable references that had not been identified by our original search. Finally 73 primary research papers were included in our systematic review. Our results demonstrate that the annual number of publications on mapping and modelling STH and schistosome infections was constant until the year 2007 and steadily increased since then; since 2008 a total of 49 (67% of the total) papers were published (Fig 3).
Table 5. Results of the search performed in the Web of Knowledge and PubMed, using the search terms and the corresponding keywords given in Table 1 and Table 2 respectively.
| UT | ST | DT | Results Web of Science | Results PubMed |
|---|---|---|---|---|
| 1 | 3 | 4 | 24 | 23 |
| 2 | 3 | 4 | 72 | 65 |
| 1 | 3 | 5 | 0 | 5 |
| 2 | 3 | 5 | 7 | 18 |
| 1 | 3 | 6 | 19 | 13 |
| 2 | 3 | 6 | 52 | 90 |
Table 6. Total number of read and discarded papers presented per infection.
| Read papers | Discarded papers | |
|---|---|---|
| Schistosomes | 47 | 26 |
| STH | 26 | 26 |
Fig 3. Year of publication of studies included in this review.
Data collection process
Uncertainty use in helminth infection mapping for morbidity control
For policy making 47 (64%) studies used uncertainty information, in planning, intervention, monitoring, evaluation and consolidation of MDA campaigns (Table 7). This was followed by 15 (21%) studies that focused on increasing cost effectiveness of these programmes. Five studies (7%) used uncertainty in disease maps to inform about prevention strategies such as to plan and guide hygiene education and infrastructure WASH programmes. For scientific interpretation, only seven studies (10%) used uncertainty to improve spatial sampling, eight studies (11%) used it to investigate the role of environmental and socio-economic risk factors on the infections, and 17 (23%) papers mapped uncertainty.
Table 7. Use of information on uncertainty in the public health context.
| Uncertainty informs about | Papers SCH | Papers STH | Total | |
|---|---|---|---|---|
| Policy Making | Cost effectiveness | [66,71,77,81,98,99,103,112,130,148] | [87,88,107,108,149] | 15 |
| Planning, intervention, monitoring, evaluation and consolidation of MDA campaigns. | [16,53,65,66,71,74–77,79–81,93,96–102,104,105,111,119,125,130–132,138,147,148,150–155] | [87,90–92,107–109,129,140,156,157] | 47 | |
| Plan and guide prevention strategies | [79,130,154] | [108,140] | 5 | |
| Scientific Interpretation | Sampling | [71,75,80,106,119,152] | [82] | 7 |
| Role of risk factors | [54,78,89,94,95,155] | [84,85] | 8 | |
| Mapping uncertainty | [66,70–72,74,75,77,79,80,98,105,111,119,130,131] | [108,109] | 17 | |
Uncertainty sources in modelling and mapping helminth infections
Table 8 shows that, from the total number of reviewed papers, sampling design was the most highlighted source of uncertainty, with a total of 42 (58%) papers acknowledging it. The second and third most highlighted sources of uncertainty were diagnostic techniques, with a total of 29 (40%) papers acknowledging it, and selection of significant environmental and socio-economic variables, acknowledged by 22 (30%) papers. The last highlighted uncertainty source was related to spatial support, with 19 (26%) papers acknowledging it. The least highlighted uncertainty sources were: inherent group characteristics, use of data with insufficient quality, temporal support, and spatio-temporal misalignment, with 15 (20%), 15, 7 (10%) and 5 (7%) papers acknowledging them respectively. From the category sampling design, the most highlighted sources of uncertainty were: incomplete sample coverage and sample size, with respectively 16 (37%) and 22 (51%) papers acknowledging them respectively (Table 9). Heterogeneity and polyparasitism were acknowledged by nine (12%) and six papers (8%) respectively
Table 8. Uncertainty sources in modelling and mapping helminth infections.
| Uncertainty sources | Papers using different measures of uncertainty | Papers highlighting the importance of uncertainty sources | Total | |||
|---|---|---|---|---|---|---|
| Papers SCH | Papers STH | |||||
| Input data | Survey Data | Sampling design | ROC (AUC) [71] | [66,71–74,76,78–81,91,93,96,97,99–101,103–105,111,125,130–133,138,147] | [86–88,90,92,107,109,110,129,140,156,158–160] | 42 |
| Credible intervals [101] | ||||||
| Diagnostic Techniques | Credible intervals [76,87] | [65,66,76,78,79,81,95–97,101,104–106,111,112,130–132,138,148,154,155] | [86,87,107,108,140,149,157] | 29 | ||
| EO data | Spatial support | [71,76,77,81,95,97,103,106,111,130,131,147,154] | [84,85,108,109,156,159] | 19 | ||
| Temporal support | [73,106] | [84–86,88,109] | 7 | |||
| Data quality | [16,74,77,79,89,91,93,95,99,106,119] | [88,90,129,140] | 15 | |||
| Spatio-temporal misaligned data | [103,154] | [119,129,140] | 5 | |||
| Selection of significant environmental and socio-economic risk factors | Credible Intervals: | [71,76,79,81,94,101,104,111,125,130,131,133,147,150,151,154] | [86,87,107,108,140,156] | 22 | ||
| Socio-economic data | ||||||
| SCH: [53,54,65,66,71,73,76,89,93–106,111,112,119,130,147,148,155] | ||||||
| STH: [84,85,87,88,90,107–110,140,156,157] | ||||||
| Confidence Intervals: | ||||||
| SCH: [73,106,138] | ||||||
| STH: [84–86,159] | ||||||
| Inherent group characteristics | Heterogeneity | ROC (AUC) [99] | [66,76,94,99,104,148] | [107,140,160] | 9 | |
| Polyparasitism | [66,111,112,148] | [110,129] | 6 | |||
Table 9. Categories of sources of uncertainty and papers included in this review grouped into categories.
| Categories | Uncertainty sources | Papers focusing in schistosomiasis | Papers focusing on STH | TOTAL |
|---|---|---|---|---|
| Sampling Design | Type of survey | [97,100,101,125,160] | [156] | 6 |
| Samples manipulation | [138] | 1 | ||
| Sample size | [66,72–74,80,100,103–105,111,125,130–132] | [86,87,107,109,110,140,156,158] | 22 | |
| Sample coverage | [76,80,93,99,105,111,130,147] | [87,88,90,92,107,129,140,159] | 16 | |
| Logistics | [78,81,99,131,133] | [86,92] | 6 | |
| Survey registration method | [71,91,103] | 3 | ||
| Adjust for confounders | [101] | 1 | ||
| Selection of the measure of risk | [125] | [140,160] | 3 | |
| Diagnostic Techniques | Sensitivity and specificity of diagnostic methods | [65,66,76,78,79,81,95–97,101,104–106,111,112,130–132,138,148,154,155] | [86,87,107,108,140,149,157] | 29 |
| Spatial support | Spatial aggregation and disaggregation | [71,76,77,81,95,97,103,106,111,130,131,147,154] | [84,85,108,109,156,159] | 19 |
| Temporal support | Temporal aggregation and disaggregation | [73,106] | [84–86,88,109] | 7 |
| Data quality | Position accuracy, logical consistency, time accuracy, completeness, attribute accuracy (pre-processing) | [16,74,77,79,89,91,93,95,99,106,119] | [88,90,129,140] | 15 |
| Spatio-temporal misaligned EO data | Spatial and temporal misaligned EO data. | [103,154] | [119,129,140] | 5 |
| Selection of environmental and socio-economic variables | Environmental: Distance to water bodies, land surface temperature, soil moisture, vegetation cover, Rainfall. | [71,76,79,81,94,101,104,111,125,130,131,133,147,150,151,154] | [86,87,107,108,140,156] | 22 |
| Socio-Economic: poverty, clean water, sanitation and hygiene, urbanization, land use. | ||||
| Inherent group characteristics | Heterogeneity | [66,76,94,99,104,148] | [107,140,160] | 9 |
| Polyparasitism | [66,111,112,148] | [110,129] | 6 |
Regarding uncertainty relating to the model, model structure was the most highlighted source of uncertainty, with 19 (26%) papers acknowledging it, followed by, uncertainty in model selection and uncertainty in model parameters with 3 (4%) papers each (Table 10).
Table 10. Model sources of uncertainty.
Uncertainty definition and quantification in helminth infections mapping
Four ways to define uncertainty were found: accuracy, imprecision, bias and vagueness. Sixty-one (83%) papers expressed uncertainty in the modelled results using measures of imprecision and credible intervals were the most frequently used measure of imprecision (Table 11). Thirty-nine (53%) papers defined uncertainty by means of accuracy, using mostly the area under the curve of the receiver operating characteristic and the percentage of correctly predicted morbidity indicators. Bias and vagueness were the least used measure of uncertainty with only five (7%) and one (1%) papers quantifying uncertainty in their results by means of mean error and fuzzy sets respectively.
Table 11. Uncertainty definition and quantification.
| Uncertainty definition | Uncertainty quantification | Model + parameters | Total | Parameters | ||
|---|---|---|---|---|---|---|
| Papers SCH | Papers STH | Papers SCH | Papers STH | |||
| Accuracy | Residual mean square. | [77] | 1 | |||
| Mean absolute error. | [66,97,101,150] | [65,108–110] | 8 | |||
| Percentage of locations that were predicted within a 95% confidence/credible interval. | [66,89,98,101,105,112,130,148,161] | [107,109,110] | 12 | |||
| Receiving operating characteristics (AUC). | [71,76,81,93,98–100,111,119,125,147,162] | [87,90,108,140,157] | 18 | [71,99] | ||
| Point-wise standard error. | [80] | 1 | ||||
| Log likelihood ratio. | [151] | 1 | ||||
| Root mean square error. | [70,72,162] | 3 | ||||
| Kappa statistic. | [74] | [82] | 2 | |||
| Precision | Bayesian approaches (Credible Intervals). | [53,54,65,66,71,73,76,89,93–106,111,112,119,130,147,148,155] | [84,85,87,88,90,107–110,140,156,157] | 42 | [53,54,65,66,71,73,76,89,93–106,111,112,119,130,147,148,155] | [84,85,87,88,90,107–110,140,156,157] |
| Standard deviation. | [70,75,131,153] | 4 | ||||
| Standard deviational ellipse. | [79] | 1 | ||||
| Frequentist approaches (Confidence intervals, R squared). | [16,53,54,66,70–81,89,91,93,95,96,106,110,130,138,154,161] | [82,84–88,90,92,94,159] | 38 | [53,54,66,70–81,89,91,93,95,96,106,110,130,138] | [82,84–88,90,92,94,159] | |
| Ranking statistic based on maximum likelihood. | [16] | 1 | ||||
| Bias | Residual, mean error | [65,66,70,103] | [108] | 5 | ||
| Vagueness | Fuzzy theory | [163] | 1 | |||
A total of 57 (78%) studies evaluated regression coefficient parameters by means of precision, and quantified them using Bayesian approaches (57%), and frequentist approaches (52%). This overlap arose because several authors first used frequentist non-spatial approaches to identify the significant covariates [54,60,65,66,70–96] and then applied these covariates in a Bayesian geostatistical model [2,4,95,97–112]. Two papers (3%) quantified the uncertainty arising due to questionnaires data, as well as the uncertainty arising due to combining age-groups in the predictions [71,101]. Regarding diagnostic techniques, two studies (3%) addressed diagnostic uncertainty by modelling sensitivity and specificity as random variables, specified as beta distributions, and quantified as posterior credible intervals [76,87].
Discussion
Currently, decisions about helminth control programs and their cost-effectiveness are made under uncertainty. To assist decisions about investment and allocation of disease control resources such as mass drug administration, maps depicting the geographical limits of risk are being used as decision support tools. Modern disease mapping utilizes advanced modelling frameworks to determine the endemicity of infection. There is a concern about the validity of spatial modelling frameworks in that, if spatial uncertainty is not adequately taken into account, this could result in erroneous conclusions and decisions about the spatial distribution of these diseases [51].
Uncertainty use in helminth infections mapping for morbidity control
Most of the studies used information on uncertainty to guide MDA campaigns and evaluate their cost effectiveness. Information on uncertainty was also used to evaluate the role of risk factors in mapping helminth infections. Nevertheless, prevention strategies, improvements in sampling design, and mapping of uncertainty have not yet been addressed [113–116]. We advise to use information on uncertainty not only to inform about MDA campaigns, but also to inform about prevention strategies such as improving sanitation and hygiene education [117] or delineating potential transmission sites [116]. Transmission control is important for its public health relevance, since potential disease transmission sites could guide direct intervention measures at the place of infection [62,116]. Likewise, mapping of uncertainty is also recommended, since it is known to be an important tool for public health decision making, especially to determine the geographical distribution of areas for which information is lacking [112]. Mapping could be used as a tool to improve the sampling strategy and modelling efforts. Maps of uncertainty could also support communication of uncertainty to the affected communities. A complete exploration and judgement of uncertainty information would enhance the assessment of the risk of getting these infections, and would allow to understand potential impacts on human health [51].
While most studies identified and discussed different sources of uncertainty, this was mainly limited to a qualitative discussion, rather than a quantitative one [118] (Table 11). For instance, 38 (52%) papers highlighted qualitatively the importance of sampling design in mapping helminth infections, but only two studies (3%) have quantified their possible effects on the accuracy of the predicted morbidity indicator. An example is given by Clements et al [119], where uncertainties in the predictions were used to identify areas requiring further data collection before programme implementation. The lack of a quantitative assessment limits the utility of the findings in both policy/decision making setting and a scientific setting [51,118,120,121]. Communication of uncertainty will never be complete without an extensive quantification of uncertainties in all possible information sources [51,120,122], where model assumptions, selection of covariates and acquisition of survey data are clearly explained, either within the publication or as supplementary information.
Uncertainty sources in modelling and mapping helminth infections
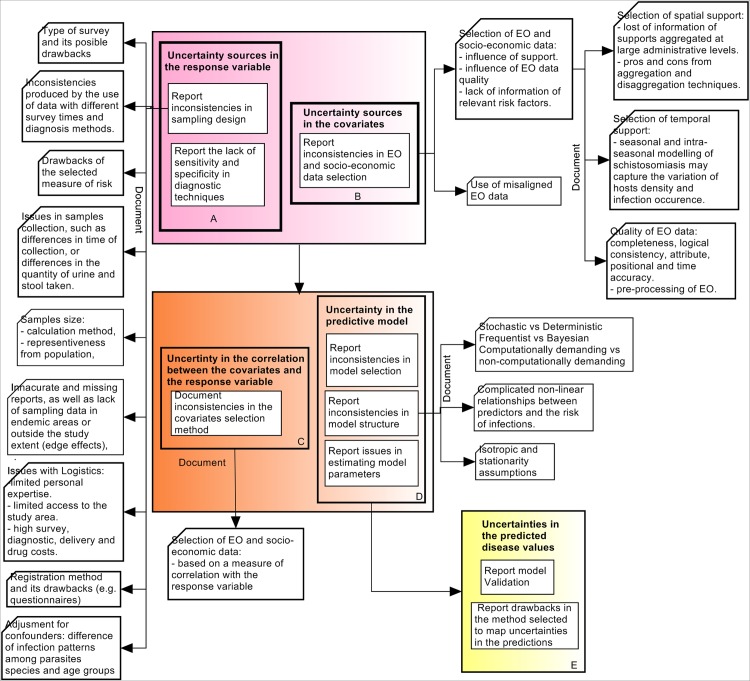
Fig 4 shows the three uncertainty stages previously described in Fig 2, where these stages encompass specific uncertainty components, which need to be considered for a complete uncertainty communication. Each of these components is analyzed in the next sections.
Fig 4. Stages of uncertainty analysis when mapping STH and schistosome helminth infections.
Colour coding as for Fig 2.
Uncertainty in the response variable (morbidity indicator)
This uncertainty belongs to the first uncertainty stage (uncertainty coming from different data sources) and is described in Box A from Fig 4. This type of uncertainty exists as a function of the measurement [46] or data collection. Uncertainty in the response variable depends on the survey data quality, generated based on the sampling design, and the used diagnostic approach (Fig 2). A total of 68% of the papers mentioned the importance of sampling design as the main source of uncertainty, supporting the idea that significantly biased results may be produced due to an inappropriate sampling design [123]. When mapping helminth infections, it is suggested to document the sample size calculation method, together with the analysis of a certain target group selection. Other sources of uncertainty in sampling design are related to the type of survey, type of morbidity indicator and the use of misaligned survey data. For instance, Chammartin et al. [97] argued that cross sectional studies might not capture well the focal pattern of schistosomiasis, since their information is based on an specific point in time. Likewise, prevalence as the most frequently used morbidity indicator, underestimates morbidity values [76,124–128] and was considered a biased and poor indicator of risk [123,125]. Also, combining data from different sources of information, with different survey times and diagnosis methods may result in inaccurate estimates [66,71,100,101,129]. This is why it is suggested to document all possible drawbacks in the selected type of survey and measure of risk, and document all problems when using misaligned survey data.
Data collection also influenced the results when there was a lack of spatial and laboratory sampled data in areas where the presence of infection was suspected to be high [66,72–74,80,100,103–105,111,125,130–132]. This could be due to inaccurate and missing reports [131], lack of people’s participation [132] and limited access to geographical areas [81]. All these potential causes should be reported as well as issues regarding high costs of the survey, diagnosis, delivery of drugs, type of registration resource and limited training and expertise of field personnel, which might also influence the quality of the results [78,81,99,131,133–135]. For instance, the use of questionnaires might underestimate prevalence data, since their discriminatory performance differs among regions, and these are not always completely returned by surveyed people [71,103,136,137]. Finally, issues related to diagnostic technique, sample manipulation [135,138], and lack of stratification due to confounders [101,126,139] are also important to be considered and should also be reported and analyzed.
Uncertainty in the covariates (EO data)
This uncertainty is also part of the first uncertainty stage and is represented in Box B of Fig 4. Main sources of uncertainty in the covariates were related to the selection of significant environmental and socio-economic risk factors, the type of environmental data, and also to the selection of the spatial support of analysis. The importance of including risk factors such as sewage system, water supply and other climatic, demographic and socio-economic variables were the most highlighted issues (Table 8). Soares Magalhães et al [140] found that including WASH indicators as random variables in the model contributed to improved definition of the areas to target for integrated helminth control and improvement of WASH risk factors. The selection of EO data depends on the selected spatial support, defined based on the research objective and analysis method used [141,142], but also on the quality of EO data itself. In addition Walz et al. [4] argued that the relevance of environmental variables are expected to vary between different landscapes and ecological regions, having an impact on the predicted morbidity indicators. Likewise, socio-economic and ecological processes that govern schistosomiasis transmission operate and vary across different scales of observation [143,144]. Since statistical correlation can vary according to the extent of the studied area and the scale of aggregation [116,145], quantitative methods to select the optimal support of analysis, such as aggregation and disaggregation process should be documented. Clear guidance on the selection of the optimal support of EO data does not exist [11], and this remains an open topic of research. Nevertheless the choices made as well as an applied aggregation or disaggregation should be documented. Although few studies highlighted the relevance of data quality, temporal support and extent, and spatio-temporal misaligned data (Table 9), these sources of uncertainty cannot be ignored. Data quality elements (i.e completeness, logical consistency, temporal accuracy, spatial accuracy, and attribute accuracy [58]) relate to the identification of uncertainty sources, and have been shown to influence the predicted disease risk [11]. EO quality elements should also be addressed and analyzed, as well as possible inconsistencies in their pre-processing. Attention should also be put to the selection of the temporal support of analysis [146], which need to be defined depending on the study objective and the host and vectors epidemiology and ecology. Finally, both temporal and spatial supports need to be adjusted into a common temporal and spatial grid since different spatial and temporal supports, could lead to erroneous conclusions in the predictions [56].
According to our analysis, although uncertainty in the covariates has been highlighted by most studies, almost none of them have quantified their impact on the disease risk predictions, and just a few have incorporated uncertainty in the response variable. Uncertainty quantification and documentation is suggested in order to completely inform about uncertainty and help decision makers and public health scientists to undertake independent uncertainty assessments [121] and better communicate uncertainty [51,120].
Uncertainty in the EO data selection, predictive model and predicted disease values
Spatial prediction of parasitic disease risk patterns are explained by the statistical relationships between environmental and socio-economic covariates, individuals, and observed risk of infection [9]. Setting initial candidate environmental and socio-economic covariates and their inclusion in the predictive model is one of the first steps for geostatistical modelling of helminth infections. Thus the methods used for this selection should be explained and documented explicitly such that the statistical method itself and the measure used for covariates inclusion are clearly interpreted in the mapping process (Box C from Fig 4). The selection of the predictive model, its possible limitations (when estimating model parameters, predicting morbidity indicators, or handling non-linear relations between response variables and covariates) and assumptions made, should also be reported and justified, explaining step by step the reasoning behind the use of the specific model (Box D from Fig 4). Boxes C and D in Fig 4 relate to the green box (uncertainty in the predictive model) in Fig 2, whereas Box E relates to the model output (yellow Box from Fig 2).
The mean predicted values are often aggregated to different administrative supports, without considering the uncertainty in the predictions [147]. This could lead to a biased estimate of treatment needs [144,147]. Uncertainty can and should be incorporated into the aggregation process, yielding measures of precision (e.g., credible intervals) in the aggregated predictions. Where feasible, we advise validation of the predicted aggregated morbidity indicators (Box E in Fig 4) against empirical observations [147]. This will facilitate a more appropriate spatial target of intervention and prevention strategies.
Conclusions
Acknowledging and incorporating uncertainty in mapping and modelling helminth infections is a step-by-step process, which should be considered formally when developing geographical models of helminth infection. Geographical models aim at informing, not only about MDA campaigns and their cost-effectiveness, but also prevention strategies, where it is necessary to define transmission areas and plan and guide hygiene education and infrastructure programs in water sanitation and hygiene. A quantitative and qualitative analysis of uncertainty is necessary for a complete assessment of risk, to understand potential impacts on human health, and to allow a complete uncertainty communication to public health managers. Five components of uncertainty analysis were recognized: (1) uncertainty in the response variable, (2) uncertainty in the covariates, (3) uncertainty in the relationship between them, (4) uncertainty in the predictive model, and (5) the propagated uncertainty on the results. Our conclusions are shown diagrammatically in Fig 5, which aims at providing a framework for a full uncertainty evaluation when undertaking spatial modeling of helminth infections for policy formulation. Uncertainty analysis should start by identifying possible sources of uncertainty in the studies and categorize them such that at least the most important ones can be incorporated into the predictive model. Sampling design and EO data have been acknowledged as the major sources of uncertainty and should be given primary attention in the modelling process. In particular, sampling design, diagnosis, selection of significant risk factors, and selection of an adequate spatial support of analysis. Next, uncertainties in the response variable and covariates should be quantified and incorporated into the model. Methods used to define the relationship between covariates and response variables should also be documented, as well as the selection of the predictive model and its limitations. Finally, uncertainties in the parameters and response variables should be quantified, and uncertainty mapping should be performed as a valuable element for uncertainty communication and policy formulation.
Fig 5. Framework for the evaluation and utilization of uncertainty in mapping soil transmitted helminth infections and schistosomiasis.
Supporting Information
(DOC)
(DOCX)
(DOCX)
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors have indicated that no explicit funding was received for this work. ALAN’s doctoral research is funded by the University of Twente. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hotez PJ, Alvarado M, Basáñez M- G, Bolliger I, Bourne R, Boussinesq M, Brooker SJ, Brown AS, Buckle G, Budke CM, Carabin H, Coffeng LE, Fèvre EM, Fürst T, Halasa YA, Jasrasaria R, Johns NE, Keiser J, King CH, Lozano R, Murdoch ME, O'Hanlon S, Pion SDS, Pullan RL, Ramaiah KD, Roberts T, Shepard DS, Smith JL, Stolk WA, Undurraga EA, Utzinger J, Wang M, Murray CJL, Naghavi M (2014) The Global Burden of Disease Study 2010: Interpretation and Implications for the Neglected Tropical Diseases. PLoS Negl Trop Dis 8: e2865 10.1371/journal.pntd.0002865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pullan RL, Smith JL, Jasrasaria R, Brooker SJ (2014) Global numbers of infection and disease burden of soil transmitted helminth infections in 2010. Parasit Vectors 7: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J (2006) Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis 6: 411–425. 10.1016/S1473-3099(06)70521-7 [DOI] [PubMed] [Google Scholar]
- 4.Walz Y, Wegmann M, Dech S, Raso G, Utzinger J (2015) Risk profiling of schistosomiasis using remote sensing: approaches, challenges and outlook. Parasit Vectors 8: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor‐Robinson DC, Maayan N, Soares‐Weiser K, Donegan S, Garner P (2015) Deworming drugs for soil‐transmitted intestinal worms in children: effects on nutritional indicators, haemoglobin, and school performance. Cochrane Libr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stolk WA, Kulik MC, le Rutte EA, Jacobson J, Richardus JH, de Vlas SJ, Houweling TAJ (2016) Between-Country Inequalities in the Neglected Tropical Disease Burden in 1990 and 2010, with Projections for 2020. PLoS Negl Trop Dis 10: e0004560 10.1371/journal.pntd.0004560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCarty TR, Turkeltaub JA, Hotez PJ (2014) Global progress towards eliminating gastrointestinal helminth infections. Curr Opin Gastroenterol 30: 18–24. 10.1097/MOG.0000000000000025 [DOI] [PubMed] [Google Scholar]
- 8.Keenan JD, Hotez PJ, Amza A, Stoller NE, Gaynor BD, Porco TC, Lietman TM (2013) Elimination and Eradication of Neglected Tropical Diseases with Mass Drug Administrations: A Survey of Experts. PLoS Negl Trop Dis 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soares Magalhães RJ, Clements ACA, Patil AP, Gething PW, Brooker S (2011) The Applications of Model-Based Geostatistics in Helminth Epidemiology and Control. Adv Parasitol 74: 267–296. 10.1016/B978-0-12-385897-9.00005-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montresor A, Crompton DW, Hall A, Bundy D, Savioli L (1998) Guidelines for the evaluation of soil-transmitted helminthiasis and schistosomiasis at community level. Geneva: World Health Organization; pp. 1–49. [Google Scholar]
- 11.Hamm NAS, Soares Magalhães RJ, Clements ACA (2015) Earth Observation, Spatial Data Quality, and Neglected Tropical Diseases. PLoS Negl Trop Dis 9: e0004164 10.1371/journal.pntd.0004164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cadavid Restrepo AM, Yang YR, McManus DP, Gray DJ, Giraudoux P, Barnes TS, Williams GM, Soares Magalhães RJ, Hamm NAS, Clements ACA (2016) The landscape epidemiology of echinococcoses. Infect Dis Poverty 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weiss DJ, Mappin B, Dalrymple U, Bhatt S, Cameron E, Hay SI, Gething PW (2015) Re-examining environmental correlates of Plasmodium falciparum malaria endemicity: a data-intensive variable selection approach. Malar J 14: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stensgaard A, Jorgensen A, Kabatareine N, Malone J, Kristensen T (2005) Modeling the distribution of Schistosoma mansoni and host snails in Uganda using satellite sensor data and Geographical Information Systems. Parassitologia 47: 115–125. [PubMed] [Google Scholar]
- 15.Manyangadze T, Chimbari MJ, Gebreslasie M, Mukaratirwa S (2015) Application of geo-spatial technology in schistosomiasis modelling in Africa: a review. Geospat Health 10: 99–110. [DOI] [PubMed] [Google Scholar]
- 16.Duarte HdO, Droguett EL, Moura MdC, de Souza Gomes EC, Barbosa C, Barbosa V, Araujo M (2014) An Ecological Model for Quantitative Risk Assessment for Schistosomiasis: The Case of a Patchy Environment in the Coastal Tropical Area of Northeastern Brazil. Risk Anal 34: 831–846. 10.1111/risa.12139 [DOI] [PubMed] [Google Scholar]
- 17.Worboys M, Duckham M (2004) GIS: A Computing Perspective. Boca Raton: CRC press. [Google Scholar]
- 18.Duckham M, Mason K, Stell J, Worboys M (2001) A formal approach to imperfection in geographic information. Comput Environ Urban Syst 25: 89–103. [Google Scholar]
- 19.Zhang J, Goodchild MF (2002) Uncertainty in Geographical Information. New York: CRC Press. [Google Scholar]
- 20.Foody GM, Atkinson PM, editors (2002) Uncertainty in Remote Sensing and GIS. Chichester: John Wiley [Google Scholar]
- 21.Tavana M, Liu W, Elmore P, Petry FE, Bourgeois BS (2016) A practical taxonomy of methods and literature for managing uncertain spatial data in geographic information systems. Measurement 81: 123–162. [Google Scholar]
- 22.Longley PA, Goodchild MF, Maguire DJ, Rhind DW (2015) Geographic Information Science and Systems Chichester: John Wiley & Sons. [Google Scholar]
- 23.Foody GM (2003) Uncertainty, knowledge discovery and data mining in GIS. Prog Phys Geogr 27: 113–121. [Google Scholar]
- 24.Stein A, Hamm NAS, Ye Q (2009) Handling uncertainties in image mining for remote sensing studies. Int J Remote Sens 30: 5365–5382. [Google Scholar]
- 25.Comber A, Fisher P, Wadsworth R (2005) What is land cover? Environ Plann B Plann Des 32: 199–209. [Google Scholar]
- 26.Fisher P, Comber A, Wadsworth R (2006) Approaches to uncertainty in spatial data In: Devillers R, Jeansoulin R, editors. Fundamentals of Spatial Data Quality. London: ISTE; pp. 43–59. [Google Scholar]
- 27.Congalton RG, Green K (2009) Assessing the Accuracy of Remotely Sensed Data: Principles and Practices. Boca Raton: CRC Press. [Google Scholar]
- 28.Congalton RG (2010) How to Assess the Accuracy of Maps Generated from Remotely Sensed Data In: Bossler JD, Campbell JB, McMaster RB, Rizos C, editors. Manual of Geospatial Science and Technology. Second ed. London: CRC Press; pp. 403–421. [Google Scholar]
- 29.Congalton RG, Gu J, Yadav K, Thenkabail P, Ozdogan M (2014) Global land cover mapping: a review and uncertainty analysis. Remote Sensing 6: 12070–12093. [Google Scholar]
- 30.Masuoka E, Roy D, Wolfe R, Morisette J, Sinno S, Teague M, Saleous N, Devadiga S, Justice C, Nickeson J (2011) MODIS Land Data Products: Generation, Quality Assurance and Validation In: Ramachandran B, Justice CO, Abrams MJ, editors. Land Remote Sensing and Global Environmental Change: Springer; New York: pp. 509–531. [Google Scholar]
- 31.Maling DH (1989) Measurements from Maps. New York: Pergamon Press. [Google Scholar]
- 32.Fisher PF (1999) Models of Uncertainty in Spatial Data In: Longley P, Goodchild M, Maguire D, Rhind D, editors. Geographical Information Systems: Principles, Techniques, Management and Applications. New York: Wiley and Sons; pp. 191–205. [Google Scholar]
- 33.Barry S, Elith J (2006) Error and uncertainty in habitat models. J Appl Ecol 43: 413–423. [Google Scholar]
- 34.Rocchini D, Foody GM, Nagendra H, Ricotta C, Anand M, He KS, Amici V, Kleinschmit B, Förster M, Schmidtlein S, Feilhauer H, Ghisla A, Metz M, Neteler M (2013) Uncertainty in ecosystem mapping by remote sensing. Comput Geosci 50: 128–135. [Google Scholar]
- 35.Lechner AM, Langford WT, Bekessy SA, Jones SD (2012) Are landscape ecologists addressing uncertainty in their remote sensing data? Landsc Ecol 27: 1249–1261. [Google Scholar]
- 36.Regan HM, Colyvan M, Burgman MA (2002) A taxonomy and treatment of uncertainty for ecology and conservation biology. Ecol Appl 12: 618–628. [Google Scholar]
- 37.Helton JC, Johnson JD, Oberkampf WL (2004) An exploration of alternative approaches to the representation of uncertainty in model predictions. Reliab Eng Syst Saf 85: 39–71. [Google Scholar]
- 38.Raj R, Hamm NAS, van der Tol C, Stein A (2014) Variance-based sensitivity analysis of BIOME-BGC for gross and net primary production. Ecol Model 292: 26–36. [Google Scholar]
- 39.Gething PW, Patil AP, Hay SI (2010) Quantifying Aggregated Uncertainty in Plasmodium falciparum Malaria Prevalence and Populations at Risk via Efficient Space-Time Geostatistical Joint Simulation. PLoS Comput Biol 6: e1000724 10.1371/journal.pcbi.1000724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O'Hagan A, Oakley JE (2004) Probability is perfect, but we can't elicit it perfectly. Reliab Eng Syst Saf 85: 239–248. [Google Scholar]
- 41.Hamm NAS, Finley AO, Schaap M, Stein A (2015) A spatially varying coefficient model for mapping PM10 air quality at the European scale. Atmos Environ 102: 393–405. [Google Scholar]
- 42.Rougier J, Sparks S, Hall L (2014) Risk and Uncertainty Assessment for Natural Hazards. Cambridge: Cambridge University Press. [Google Scholar]
- 43.Beven K, Hall J (2014) Applied Uncertainty Analysis for Flood Risk Management: World Scientific. [Google Scholar]
- 44.Dungan JL, Perry JN, Dale MRT, Legendre P, Citron-Pousty S, Fortin MJ, Jakomulska A, Miriti M, Rosenberg MS (2002) A balanced view of scale in spatial statistical analysis. Ecography 25: 626–640. [Google Scholar]
- 45.Atkinson P, Graham A (2006) Issues of Scale and Uncertainty in the Global Remote Sensing of Disease. Adv Parasitol 62: 79–118. 10.1016/S0065-308X(05)62003-9 [DOI] [PubMed] [Google Scholar]
- 46.Dungan JL (2002) Toward a Comprehensive View of Uncertainty in Remote Sensing Analysis In: Atkinson PM, Foody GM, editors. Uncertainty in Remote Sensing and GIS. Chichester: John Wiley & Sons Ltd; pp. 25–35. [Google Scholar]
- 47.Gelman A, Carlin JB, Stern HS, Rubin DB (1995) Bayesian Data Analysis. London: Chapman & Hall. [Google Scholar]
- 48.Atkinson P, Foody G (2002) Uncertainty in Remote Sensing and GIS: An Overview In: Foody G, Atkinson P, editors. Uncertainty in Remote Sensing and GIS. Chichester: John Wiley; pp. 1–35. [Google Scholar]
- 49.Morisette JT, Privette JL, Justice CO (2002) A framework for the validation of MODIS Land products. Remote Sens Environ 83: 77–96. [Google Scholar]
- 50.Foody GM (2002) Status of land cover classification accuracy assessment. Remote Sens Environ 80: 185–201. [Google Scholar]
- 51.Burns CJ, Wright JM, Pierson JB, Bateson TF, Burstyn I, Goldstein DA, Klaunig JE, Luben TJ, Mihlan G, Ritter L (2014) Evaluating Uncertainty to Strengthen Epidemiologic Data for Use in Human Health Risk Assessments. Environ Health Perspect 122: 1160–1165. 10.1289/ehp.1308062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bhatt S, Weiss DJ, Cameron E, Bisanzio D, Mappin B, Dalrymple U, Battle KE, Moyes CL, Henry A, Eckhoff PA, Wenger EA, Briet O, Penny MA, Smith TA, Bennett A, Yukich J, Eisele TP, Griffin JT, Fergus CA, Lynch M, Lindgren F, Cohen JM, Murray CLJ, Smith DL, Hay SI, Cibulskis RE, Gething PW (2015) The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 526: 207–211. 10.1038/nature15535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Clements AC, Moyeed R, Brooker S (2006) Bayesian geostatistical prediction of the intensity of infection with Schistosoma mansoni in East Africa. Parasitology 133: 711–719. 10.1017/S0031182006001181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Raso G, Li Y, Zhao Z, Balen J, Williams GM, McManus DP (2009) Spatial Distribution of Human Schistosoma japonicum Infections in the Dongting Lake Region, China. PLoS One 4: e6947 10.1371/journal.pone.0006947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mugglin AS, Carlin BP (1998) Hierarchical modeling in geographic information systems: Population interpolation over incompatible zones. J Agric Biol Environ Stat 3: 117–130. [Google Scholar]
- 56.Gotway CA, Young LJ (2002) Combining Incompatible Spatial Data. J Am Stat Assoc 97: 632–648. [Google Scholar]
- 57.Shi W (2009) Principles of Modeling Uncertainties in Spatial Data and Spatial Analyses. Boca Raton: Taylor & Francis. [Google Scholar]
- 58.ISO (2013) ISO 19157: Geographic Information—Data Quality International Organization for Standarization (ISO).
- 59.Bossler JD, Campbell JB, McMaster R, Rizos C (2010) Spatial Data Quality Manual of Geospatial Science and Technology. London: CRC Press; pp. 593–610. [Google Scholar]
- 60.Yang X, Blower J, Bastin L, Lush V, Zabala A, Masó J, Cornford D, Díaz P, Lumsden J (2013) An integrated view of data quality in Earth observation. Philos Transact A Math Phys Eng Sci 371: 20120072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pleydell DR, Yang YR, Danson FM, Raoul F, Craig PS, McManus DP, Vuitton DA, Wang Q, Giraudoux P (2008) Landscape composition and spatial prediction of alveolar echinococcosis in southern Ningxia, China. PLoS Negl Trop Dis 2: e287 10.1371/journal.pntd.0000287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Walz Y, Wegmann M, Leutner B, Dech S, Vounatsou P, N'Goran EK, Raso G, Utzinger J (2015) Use of an ecologically relevant modelling approach to improve remote sensing-based schistosomiasis risk profiling. Geospat Health 10: 271–279. [DOI] [PubMed] [Google Scholar]
- 63.Young LJ, Gotway CA, Yang J, Kearney G, DuClos C (2008) Assessing the association between environmental impacts and health outcomes: A case study from Florida. Stat Med 27: 3998–4015. 10.1002/sim.3249 [DOI] [PubMed] [Google Scholar]
- 64.Simoonga C, Utzinger J, Brooker S, Vounatsou P, Appleton C, Stensgaard A-S, Olsen A, Kristensen TK (2009) Remote sensing, geographical information system and spatial analysis for schistosomiasis epidemiology and ecology in Africa. Parasitology 136: 1683–1693. 10.1017/S0031182009006222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schur N, Huerlimann E, Garba A, Traore MS, Ndir O, Ratard RC, Tchuente L-AT, Kristensen TK, Utzinger J, Vounatsou P (2011) Geostatistical Model-Based Estimates of Schistosomiasis Prevalence among Individuals Aged < = 20 Years in West Africa. PLoS Negl Trop Dis 5: e1194 10.1371/journal.pntd.0001194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schur N, Huerlimann E, Stensgaard A-S, Chimfwembe K, Mushinge G, Simoonga C, Kabatereine NB, Kristensen TK, Utzinger J, Vounatsou P (2013) Spatially explicit Schistosoma infection risk in eastern Africa using Bayesian geostatistical modelling. Acta Trop 128: 365–377. 10.1016/j.actatropica.2011.10.006 [DOI] [PubMed] [Google Scholar]
- 67.Gething PW, Patil AP, Smith DL, Guerra CA, Elyazar IRF, Johnston GL, Tatem AJ, Hay SI (2011) A new world malaria map: Plasmodium falciparum endemicity in 2010. Malar J 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Raj R, Hamm NAS, Kant Y (2013) Analysing the effect of different aggregation approaches on remotely sensed data. Int J Remote Sens 34: 4900–4916. [Google Scholar]
- 69.Willink R, Lira I (2005) A united interpretation of different uncertainty intervals. Measurement 38: 61–66. [Google Scholar]
- 70.Chen Z, Zhou X-N, Yang K, Wang X-H, Yao Z-Q, Wang T-P, Yang G-J, Yang Y-J, Zhang S-Q, Wang J, Jia T-W, Wu X-H (2007) Strategy formulation for schistosomiasis japonica control in different environmental settings supported by spatial analysis: a case study from China. Geospat Health 1: 223–231. 10.4081/gh.2007.270 [DOI] [PubMed] [Google Scholar]
- 71.Clements ACA, Brooker S, Nyandindi U, Fenwick A, Blair L (2008) Bayesian spatial analysis of a national urinary schistosomiasis questionnaire to assist geographic targeting of schistosomiasis control in Tanzania, East Africa. Int J Parasitol 38: 401–415. 10.1016/j.ijpara.2007.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fonseca F, Freitas C, Dutra L, Guimaraes R, Carvalho O (2014) Spatial modeling of the schistosomiasis mansoni in Minas Gerais State, Brazil using spatial regression. Acta Trop 133: 56–63. 10.1016/j.actatropica.2014.01.015 [DOI] [PubMed] [Google Scholar]
- 73.Malone JB, Yilma JM, McCarroll JC, Erko B, Mukaratirwa S, Zhou XY (2001) Satellite climatology and the environmental risk of Schistosoma mansoni in Ethiopia and east Africa. Acta Trop 79: 59–72. [DOI] [PubMed] [Google Scholar]
- 74.Martins-Bede FT, Dutra LV, Freitas CC, Guimardes RJPS, Amaral RS, Drummond SC, Carvalho OS (2010) Schistosomiasis risk mapping in the state of Minas Gerais, Brazil, using a decision tree approach, remote sensing data and sociological indicators. Mem Inst Oswaldo Cruz 105: 541–548. [DOI] [PubMed] [Google Scholar]
- 75.Martins-Bede FT, Freitas CC, Dutra LV, Sandri SA, Fonseca FR, Drummond IN, Souza Guimaraes RJdP, Amaral RS, Carvalho OS (2009) Risk Mapping of Schistosomiasis in Minas Gerais, Brazil, Using MODIS and Socioeconomic Spatial Data. IEEE Trans Geosci Remote Sens 47: 3899–3908. [Google Scholar]
- 76.Soares Magalhães RJ, Salamat MS, Leonardo L, Gray DJ, Carabin H, Halton K, McManus DP, Williams GM, Rivera P, Saniel O (2014) Geographical distribution of human Schistosoma japonicum infection in the Philippines: tools to support disease control and further elimination. Int J Parasitol 44: 977–984. 10.1016/j.ijpara.2014.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Souza Guimaraes RJdP, Freitas CC, Dutra LV, Carvalho Scholte RG, Martins-Bede FT, Fonseca FR, Amaral RS, Drummonds SC, Felgueiras CA, Oliveira GC, Carvalho OS (2010) A geoprocessing approach for studying and controlling schistosomiasis in the state of Minas Gerais, Brazil. Mem Inst Oswaldo Cruz 105: 524–531. [DOI] [PubMed] [Google Scholar]
- 78.Spear RC (2012) Internal versus external determinants of Schistosoma japonicum transmission in irrigated agricultural villages. J R Soc Interface 9: 272–282. 10.1098/rsif.2011.0285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang K, Li W, Sun L-P, Huang Y-X, Zhang J-F, Wu F, Hang D-R, Steinmann P, Liang Y-S (2013) Spatio-temporal analysis to identify determinants of Oncomelania hupensis infection with Schistosoma japonicum in Jiangsu province, China. Parasit Vectors 6: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang ZJ, Davies TM, Gao J, Wang Z, Jiang Q- W (2013) Identification of high-risk regions for schistosomiasis in the Guichi region of China: an adaptive kernel density estimation-based approach. Parasitology 140: 868–875. 10.1017/S0031182013000048 [DOI] [PubMed] [Google Scholar]
- 81.Zhang ZJ, Carpenter TE, Lynn HS, Chen Y, Bivand R, Clark AB, Hui FM, Peng WX, Zhou YB, Zhao GM, Jiang QW (2009) Location of active transmission sites of Schistosoma japonicum in lake and marshland regions in China. Parasitology 136: 737–746. 10.1017/S0031182009005885 [DOI] [PubMed] [Google Scholar]
- 82.Brooker S, Singhasivanon P, Waikagul J, Supavej S, Kojima S, Takeuchi T, Luong TV, Looareesuwan S (2003) Mapping Soil-Transmitted Helminths in Southeast Asia and Implications for Parasite Control. Southeast Asian J Trop Med Public Health 34: 24–36. [PubMed] [Google Scholar]
- 83.Fortes BdPMD, Ortiz Valencia LI, Ribeiro SdV, Medronho RdA (2004) Geostatistical modeling of Ascaris lumbricoides infection. Cad Saude Publica 20: 727–734. [DOI] [PubMed] [Google Scholar]
- 84.Saathoff E, Olsen A, Kvalsvig JD, Appleton CC, Sharp B, Kleinschmidt I (2005) Ecological Covariates of Ascaris lumbricoides Infection in Schoolchildren from Rural KwaZulu‐Natal, South Africa. Trop Med Int Health 10: 412–422. 10.1111/j.1365-3156.2005.01406.x [DOI] [PubMed] [Google Scholar]
- 85.Saathoff E, Olsen A, Sharp B, Kvalsvig JD, Appleton CC, Kleinschmidt I (2005) Ecologic Covariates of Hookworm Infection and Reinfection in Rural Kwazulu-natal/South Africa: A Geographic Information System–Based Study. Am J Trop Med Hyg 72: 384–391. [PubMed] [Google Scholar]
- 86.Schuele SA, Clowes P, Kroidl I, Kowuor DO, Nsojo A, Mangu C, Riess H, Geldmacher C, Laubender RP, Mhina S, Maboko L, Loescher T, Hoelscher M, Saathoff E (2014) Ascaris lumbricoides Infection and Its Relation to Environmental Factors in the Mbeya Region of Tanzania, a Cross-Sectional, Population-Based Study. PLoS One 9: e92032 10.1371/journal.pone.0092032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Soares Magalhães RJ, Salamat MS, Leonardo L, Gray DJ, Carabin H, Halton K, McManus DP, Williams GM, Rivera P, Saniel O, Hernandez L, Yakob L, McGarvey ST, Clements ACA (2015) Mapping the Risk of Soil-Transmitted Helminthic Infections in the Philippines. PLoS Negl Trop Dis 9: e0003915–e0003915. 10.1371/journal.pntd.0003915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sturrock HJW, Picon D, Sabasio A, Oguttu D, Robinson E, Lado M, Rumunu J, Brooker S, Kolaczinski JH (2009) Integrated Mapping of Neglected Tropical Diseases: Epidemiological Findings and Control Implications for Northern Bahr-el-Ghazal State, Southern Sudan. PLoS Negl Trop Dis 3: e537 10.1371/journal.pntd.0000537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Beck-Woerner C, Raso G, Vounatsou P, N’Goran EK, Rigo G, Parlow E, Utzinger J (2007) Bayesian Spatial Risk Prediction of Schistosoma mansoni Infection in Western Côte d’Ivoire Using a Remotely-Sensed Digital Elevation Model. Am J Trop Med Hyg 76: 956–963. [PubMed] [Google Scholar]
- 90.Clements ACA, Deville MA, Ndayishimiye O, Brooker S, Fenwick A (2010) Spatial co-distribution of neglected tropical diseases in the East African Great Lakes region: revisiting the justification for integrated control. Trop Med Int Health 15: 198–207. 10.1111/j.1365-3156.2009.02440.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ekpo UF, Huerlimann E, Schur N, Oluwole AS, Abe EM, Mafe MA, Nebe OJ, Isiyaku S, Olamiju F, Kadiri M, Poopola TOS, Braide EI, Saka Y, Mafiana CF, Kristensen TK, Utzinger J, Vounatsou P (2013) Mapping and prediction of schistosomiasis in Nigeria using compiled survey data and Bayesian geospatial modelling. Geospat Health 7: 355–366. 10.4081/gh.2013.92 [DOI] [PubMed] [Google Scholar]
- 92.Dorkenoo AM, Bronzan RN, Ayena KD, Anthony G, Agbo YM, Sognikin KSE, Dogbe KS, Amza A, Sodahlon Y, Mathieu E (2012) Nationwide integrated mapping of three neglected tropical diseases in Togo: countrywide implementation of a novel approach. Trop Med Int Health 17: 896–903. 10.1111/j.1365-3156.2012.03004.x [DOI] [PubMed] [Google Scholar]
- 93.Hodges MH, Magalhaes RJS, Paye J, Koroma JB, Sonnie M, Clements A, Zhang Y (2012) Combined Spatial Prediction of Schistosomiasis and Soil-Transmitted Helminthiasis in Sierra Leone: A Tool for Integrated Disease Control. PLoS Negl Trop Dis 6: e1694 10.1371/journal.pntd.0001694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pullan RL, Bethony JM, Geiger SM, Cundill B, Correa-Oliveira R, Quinnell RJ, Brooker S (2008) Human Helminth Co-Infection: Analysis of Spatial Patterns and Risk Factors in a Brazilian Community. PLoS Negl Trop Dis 2: e352 10.1371/journal.pntd.0000352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Raso G, Matthys B, N'goran E, Tanner M, Vounatsou P, Utzinger J (2005) Spatial risk prediction and mapping of Schistosoma mansoni infections among schoolchildren living in western Côte d'Ivoire. Parasitology 131: 97–108. [DOI] [PubMed] [Google Scholar]
- 96.Raso G, Vounatsou P, McManus DP, Utzinger J (2007) Bayesian risk maps for Schistosoma mansoni and hookworm mono-infections in a setting where both parasites co-exist. Geospat Health 2: 85–96. 10.4081/gh.2007.257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chammartin F, Houngbedji CA, Huerlimann E, Yapi RB, Silue KD, Soro G, Kouame FN, N'Goran EK, Utzinger J, Raso G, Vounatsou P (2014) Bayesian Risk Mapping and Model-Based Estimation of Schistosoma haematobium-Schistosoma mansoni Co-distribution in Cote d'Ivoire. PLoS Negl Trop Dis 8: e3407 10.1371/journal.pntd.0003407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Clements ACA, Garba A, Sacko M, Toure S, Dembele R, Landoure A, Bosque-Oliva E, Gabrielli AF, Fenwick A (2008) Mapping the Probability of Schistosomiasis and Associated Uncertainty, West Africa. Emerging Infect Dis 14: 1629–1632. 10.3201/eid1410.080366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Clements ACA, Firth S, Dembele R, Garba A, Toure S, Sacko M, Landoure A, Bosque-Oliva E, Barnett AG, Brooker S, Fenwick A (2009) Use of Bayesian geostatistical prediction to estimate local variations in Schistosoma haematobium infection in western Africa. Bull WHO 87: 921–929. 10.2471/BLT.08.058933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Clements ACA, Bosque-Oliva E, Sacko M, Landoure A, Dembele R, Traore M, Coulibaly G, Gabrielli AF, Fenwick A, Brooker S (2009) A Comparative Study of the Spatial Distribution of Schistosomiasis in Mali in 1984–1989 and 2004–2006. PLoS Negl Trop Dis 3: e431 10.1371/journal.pntd.0000431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schur N, Utzinger J, Vounatsou P (2011) Modelling age-heterogeneous Schistosoma haematobium and S.mansoni survey data via alignment factors. Parasit Vectors 4: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Spear RC, Hubbard A, Liang S, Seto E (2002) Disease Transmission Models for Public Health Decision Making: Toward an Approach for Designing Intervention Strategies for Schistosomiasis japonica. Environ Health Perspect 110: 907–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sturrock HJW, Pullan RL, Kihara JH, Mwandawiro C, Brooker SJ (2013) The Use of Bivariate Spatial Modeling of Questionnaire and Parasitology Data to Predict the Distribution of Schistosoma haematobium in Coastal Kenya. PLoS Negl Trop Dis 7: e2016 10.1371/journal.pntd.0002016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tarafder MR, Balolong E, Carabin H, Belisle P, Tallo V, Joseph L, Alday P, Gonzales RO, Riley S, Olveda R, McGarvey ST (2006) A cross-sectional study of the prevalence of intensity of infection with Schistosoma japonicum in 50 irrigated and rain-fed villages in Samar Province, the Philippines. BMC Public Health 6: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang X-H, Zhou X-N, Vounatsou P, Chen Z, Utzinger J, Yang K, Steinmann P, Wu X-H (2008) Bayesian Spatio-Temporal Modeling of Schistosoma japonicum Prevalence Data in the Absence of a Diagnostic 'Gold' Standard. PLoS Negl Trop Dis 2: e250 10.1371/journal.pntd.0000250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Woodhall DM, Wiegand RE, Wellman M, Matey E, Abudho B, Karanja DMS, Mwinzi PMN, Montgomery SP, Secor WE (2013) Use of Geospatial Modeling to Predict Schistosoma mansoni Prevalence in Nyanza Province, Kenya. PLoS One 8: e71635 10.1371/journal.pone.0071635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chammartin F, Scholte RGC, Malone JB, Bavia ME, Nieto P, Utzinger J, Vounatsou P (2013) Modelling the geographical distribution of soil-transmitted helminth infections in Bolivia. Parasit Vectors 6: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pullan RL, Gething PW, Smith JL, Mwandawiro CS, Sturrock HJW, Gitonga CW, Hay SI, Brooker S (2011) Spatial Modelling of Soil-Transmitted Helminth Infections in Kenya: A Disease Control Planning Tool. PLoS Negl Trop Dis 5: e958 10.1371/journal.pntd.0000958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Scholte RGC, Schur N, Bavia ME, Carvalho EM, Chammartin F, Utzinger J, Vounatsou P (2013) Spatial analysis and risk mapping of soil-transmitted helminth infections in Brazil, using Bayesian geostatistical models. Geospat Health 8: 97–110. 10.4081/gh.2013.58 [DOI] [PubMed] [Google Scholar]
- 110.Schur N, Gosoniu L, Raso G, Utzinger J, Vounatsou P (2011) Modelling the geographical distribution of co‐infection risk from single‐disease surveys. Stat Med 30: 1761–1776. 10.1002/sim.4243 [DOI] [PubMed] [Google Scholar]
- 111.Soares Magalhães RJ, Biritwum N-K, Gyapong JO, Brooker S, Zhang Y, Blair L, Fenwick A, Clements A (2011) Mapping Helminth Co-Infection and Co-Intensity: Geostatistical Prediction in Ghana. PLoS Negl Trop Dis 5: e1200 10.1371/journal.pntd.0001200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Raso G, Vounatsou P, Singer BH, Eliézer K, Tanner M, Utzinger J (2006) An integrated approach for risk profiling and spatial prediction of Schistosoma mansoni–hookworm coinfection. Proc Natl Acad Sci USA 103: 6934–6939. 10.1073/pnas.0601559103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ziegelbauer K, Speich B, Mäusezahl D, Bos R, Keiser J, Utzinger J (2012) Effect of Sanitation on Soil-Transmitted Helminth Infection: Systematic Review and Meta-Analysis. PLoS Med 9: e1001162 10.1371/journal.pmed.1001162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Singer BH, de Castro MC (2007) Bridges to sustainable tropical health. Proc Natl Acad Sci USA 104: 16038–16043. 10.1073/pnas.0700900104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Utzinger J, Bergquist R, Shu-Hua X, Singer BH, Tanner M (2003) Sustainable schistosomiasis control—the way forward. Lancet 362: 1932–1934. 10.1016/S0140-6736(03)14968-9 [DOI] [PubMed] [Google Scholar]
- 116.Walz Y, Wegmann M, Dech S, Vounatsou P, Poda J-N, N'Goran EK, Utzinger J, Raso G (2015) Modeling and Validation of Environmental Suitability for Schistosomiasis Transmission Using Remote Sensing. PLoS Negl Trop Dis 9: e0004217 10.1371/journal.pntd.0004217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hotez PJ, Bundy DA, Beegle K, Brooker S, Drake L, de Silva N, Montresor A, Engels D, Jukes M, Chitsulo L (2006) Helminth Infections: Soil-transmitted Helminth Infections and Schistosomiasis In: Jamison DT BJ, Measham AR, et al. , editor. Disease Control Priorities in Developing Countries. Washington (DC): World Bank; pp. 467–482. [Google Scholar]
- 118.Jurek AM, Maldonado G, Greenland S, Church TR (2006) Exposure-measurement error is frequently ignored when interpreting epidemiologic study results. Eur J Epidemiol 21: 871–876. 10.1007/s10654-006-9083-0 [DOI] [PubMed] [Google Scholar]
- 119.Clements ACA, Lwambo NJS, Blair L, Nyandindi U, Kaatano G, Kinung'hi S, Webster JP, Fenwick A, Brooker S (2006) Bayesian spatial analysis and disease mapping: tools to enhance planning and implementation of a schistosomiasis control programme in Tanzania. Trop Med Int Health 11: 490–503. 10.1111/j.1365-3156.2006.01594.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Stürmer T, Glynn RJ, Rothman KJ, Avorn J, Schneeweiss S (2007) Adjustments for Unmeasured Confounders in Pharmacoepidemiologic Database Studies Using External Information. Med Care 45: S158–S165. 10.1097/MLR.0b013e318070c045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jurek AM, Maldonado G, Greenland S, Church TR (2007) Uncertainty analysis: an example of its application to estimating a survey proportion. J Epidemiol Community Health 61: 650–654. 10.1136/jech.2006.053660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Eskenazi B, Quiros-Alcala L, Lipsit JM, Wu LD, Kruger P, Ntimbane T, Nawn JB, Bornman MSR, Seto E (2014) mSpray: A mobile phone technology to improve malaria control efforts and monitor human exposure to malaria control pesticides in Limpopo, South Africa. Environ Int 68: 219–226. 10.1016/j.envint.2014.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rothman KJ (2012) Epidemiology: an introduction New York: Oxford University Press. [Google Scholar]
- 124.Brooker S (2007) Spatial epidemiology of human schistosomiasis in Africa: risk models, transmission dynamics and control. Trans R Soc Trop Med Hyg 101: 1–8. 10.1016/j.trstmh.2006.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.McCreesh N, Nikulin G, Booth M (2015) Predicting the effects of climate change on Schistosoma mansoni transmission in eastern Africa. Parasit Vectors 8: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bundy DA, Hall A, Medley G, Savioli L (1992) Evaluating Measures to Control Intestinal Parasitic Infections. World Health Stat Q 45: 168–179. [PubMed] [Google Scholar]
- 127.Brooker S, Clements ACA, Bundy DAP (2006) Global Epidemiology, Ecology and Control of Soil-Transmitted Helminth Infections. Adv Parasitol 62: 221–261. 10.1016/S0065-308X(05)62007-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sikaala CH, Chinula D, Chanda J, Hamainza B, Mwenda M, Mukali I, Kamuliwo M, Lobo NF, Seyoum A, Killeen GF (2014) A cost-effective, community-based, mosquito-trapping scheme that captures spatial and temporal heterogeneities of malaria transmission in rural Zambia. Malar J 13: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Booth M, Bundy DAP (1992) Comparative prevalences of Ascaris lumbricoides, Trichuris trichiura and hookworm infections and the prospects for combined control. Parasitology 105: 151–157. [DOI] [PubMed] [Google Scholar]
- 130.Scholte RGC, Gosoniu L, Malone JB, Chammartin F, Utzinger J, Vounatsou P (2014) Predictive risk mapping of schistosomiasis in Brazil using Bayesian geostatistical models. Acta Trop 132: 57–63. 10.1016/j.actatropica.2013.12.007 [DOI] [PubMed] [Google Scholar]
- 131.Hu Y, Bergquist R, Lynn H, Gao F, Wang Q, Zhang S, Li R, Sun L, Xia C, Xiong C, Zhang Z, Jiang Q (2015) Sandwich mapping of schistosomiasis risk in Anhui Province, China. Geospat Health 10: 111–116. [DOI] [PubMed] [Google Scholar]
- 132.Scott D, Senker K, England EC (1982) Epidemiology of human Schistosoma-haematobium infection around Volta Lake, Ghana, 1973–75 Bull WHO 60: 89–100. [PMC free article] [PubMed] [Google Scholar]
- 133.Abdel-Rahman MS, El-Bahy MM, El-Bahy NM, Malone JB (1997) Development and Validation of a Satellites Based Geographic Information System (GIS) Model for Epidemiology of Schistosoma Risk Assessment on Snail Level in Kafr El-Sheikh Governorate. J Egypt Soc Parasitol 27: 299–316. [PubMed] [Google Scholar]
- 134.Butterworth AE (1990) Studies on Human Schistosomiasis—Chemotherapy, Immunity and Morbidity. Ann Parasitol Hum Comp 65: 53–57. [DOI] [PubMed] [Google Scholar]
- 135.Boatin BA, Wurapa FK, Ulrich AM (1985) The Prevalence and Distribution of Schistosomiasis in Zambia. Cent Afr J Med 31: 170–176. [PubMed] [Google Scholar]
- 136.Brooker S, Kabatereine NB, Gyapong JO, Stothard JR, Utzinger J (2009) Rapid mapping of schistosomiasis and other neglected tropical diseases in the context of integrated control programmes in Africa. Parasitology 136: 1707–1718. 10.1017/S0031182009005940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Utzinger J, Raso G, Brooker S, de Savigny D, Tanner M, Ornbjerg N, Singer BH, N'Goran EK (2009) Schistosomiasis and neglected tropical diseases: towards integrated and sustainable control and a word of caution. Parasitology 136: 1859–1874. 10.1017/S0031182009991600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Krauth SJ, Coulibaly JT, Knopp S, Traore M, N'Goran EK, Utzinger J (2012) An In-Depth Analysis of a Piece of Shit: Distribution of Schistosoma mansoni and Hookworm Eggs in Human Stool. PLoS Negl Trop Dis 6: e1969 10.1371/journal.pntd.0001969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Brooker S, Michael E (2000) The potential of geographical information systems and remote sensing in the epidemiology and control of human helminth infections. Adv Parasitol 47: 245–288. [DOI] [PubMed] [Google Scholar]
- 140.Soares Magalhães RJ, Barnett AG, Clements ACA (2011) Geographical analysis of the role of water supply and sanitation in the risk of helminth infections of children in West Africa. Proc Natl Acad Sci U S A 108: 20084–20089. 10.1073/pnas.1106784108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nijland W, Addink E, De Jong S, Van der Meer F (2009) Optimizing spatial image support for quantitative mapping of natural vegetation. Remote Sens Environ 113: 771–780. [Google Scholar]
- 142.Atkinson PM, Aplin P (2004) Spatial variation in land cover and choice of spatial resolution for remote sensing. Int J Remote Sens 25: 3687–3702. [Google Scholar]
- 143.Levin SA (1992) The problem of pattern and scale in ecology: the Robert H. MacArthur award lecture. Ecology 73: 1943–1967. [Google Scholar]
- 144.Schur N, Vounatsou P, Utzinger J (2012) Determining treatment needs at different spatial scales using geostatistical model-based risk estimates of schistosomiasis. PLoS Negl Trop Dis 6: e1773 10.1371/journal.pntd.0001773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Marshall RJ (1991) A Review of Methods for the Statistical Analysis of Spatial Patterns of Disease. J Roy Stat Soc Ser A (Stat Soc): 421–441. [Google Scholar]
- 146.Rd Jong, Sd Bruin (2012) Linear trends in seasonal vegetation time series and the modifiable temporal unit problem. Biogeosciences 9: 71–77. [Google Scholar]
- 147.Kabore A, Biritwum N-K, Downs PW, Magalhaes RJS, Zhang Y, Ottesen EA (2013) Predictive vs. Empiric Assessment of Schistosomiasis: Implications for Treatment Projections in Ghana. PLoS Negl Trop Dis 7: e2051 10.1371/journal.pntd.0002051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Vounatsou P, Raso G, Tanner M, N'Goran EK, Utzinger J (2009) Bayesian geostatistical modelling for mapping schistosomiasis transmission. Parasitology 136: 1695–1705. 10.1017/S003118200900599X [DOI] [PubMed] [Google Scholar]
- 149.Clasen T, Boisson S, Routray P, Cumming O, Jenkins M, Ensink JHJ, Bell M, Freeman MC, Peppin S, Schmidt W-P (2012) The effect of improved rural sanitation on diarrhoea and helminth infection: design of a cluster-randomized trial in Orissa, India. Emerg Themes Epidemiol 9: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Medina DC, Findley SE, Doumbia S (2008) State-Space Forecasting of Schistosoma haematobium Time-Series in Niono, Mali. PLoS Negl Trop Dis 2: e276 10.1371/journal.pntd.0000276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gao F-h, Abe EM, Li S-z, Zhang L-j, He J-c, Zhang S-q, Wang T-p, Zhou X-n, Gao J (2014) Fine scale Spatial-temporal cluster analysis for the infection risk of Schistosomiasis japonica using space-time scan statistics. Parasit Vectors 7: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Nihei N, Komagata O, Kobayashi M, Saitoh Y, Mochizuki K-i, Nakamura S (2009) Spatial Analysis and Remote Sensing for Monitoring Systems of Oncomelania nosophora Following the Eradication of Schistosomiasis Japonica in Yamanashi Prefecture, Japan. Jpn J Infect Dis 62: 125–132. [PubMed] [Google Scholar]
- 153.Seto E, Liang S, Qiu D, Gu X, Spear RC (2001) A Protocol for Geographically Randomized Snail Surveys in Schistosomiasis Fieldwork Using the Global Positioning System. Am J Trop Med Hyg 64: 98–99. [DOI] [PubMed] [Google Scholar]
- 154.Seto E, Xu B, Liang S, Gong P, Wu WP, Davis G, Qiu DC, Gu XG, Spear R (2002) The Use of Remote Sensing for Predictive Modeling of Schistosomiasis in China. Photogramm Eng Remote Sensing 68: 167–174. [Google Scholar]
- 155.Raso G, Vounatsou P, Gosoniu L, Tanner M, N'Goran EK, Utzinger J (2006) Risk factors and spatial patterns of hookworm infection among schoolchildren in a rural area of western Côte d'Ivoire. Int J Parasitol 36: 201–210. 10.1016/j.ijpara.2005.09.003 [DOI] [PubMed] [Google Scholar]
- 156.Karagiannis-Voules D- A, Biedermann P, Ekpo UF, Garba A, Langer E, Mathieu E, Midzi N, Mwinzi P, Polderman AM, Raso G (2015) Spatial and temporal distribution of soil-transmitted helminth infection in sub-Saharan Africa: a systematic review and geostatistical meta-analysis. Lancet Infect Dis 15: 74–84. 10.1016/S1473-3099(14)71004-7 [DOI] [PubMed] [Google Scholar]
- 157.Brooker S, Clements AC (2009) Spatial heterogeneity of parasite co-infection: Determinants and geostatistical prediction at regional scales. Int J Parasitol 39: 591–597. 10.1016/j.ijpara.2008.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bisht D, Verma AK, Bharadwaj HHD (2011) Intestinal parasitic infestation among children in a semi-urban Indian population. Trop Parasitol 1: 104–107. 10.4103/2229-5070.86946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Brooker S, Donnelly CA, Guyatt HL (2000) Estimating the number of helminthic infections in the Republic of Cameroon from data on infection prevalence in schoolchildren. Bull WHO 78: 1456–1465. [PMC free article] [PubMed] [Google Scholar]
- 160.Chan M, Medley G, Jamison D, Bundy D (1994) The evaluation of potential global morbidity attributable to intestinal nematode infections. Parasitology 109: 373–387. [DOI] [PubMed] [Google Scholar]
- 161.Abdel-Rahman MS, El-Bahy MM, Malone JB, Thompson RA, El-Bahy NM (2001) Geographic information systems as a tool for control program management for schistosomiasis in Egypt. Acta Trop 79: 49–57. [DOI] [PubMed] [Google Scholar]
- 162.Brooker S, Hay SI, Issae W, Hall A, Kihamia CM, Lwambo NJ, Wint W, Rogers DJ, Bundy DA (2001) Predicting the distribution of urinary schistosomiasis in Tanzania using satellite sensor data. Trop Med Int Health 6: 998–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Liu Z, Li C, Tang L, Zhou X, Ma L, Liu C. Prediction of oncomelania hupensis (vector of schistosomiasis) distribution based on remote sensing data and fuzzy information theory; 2015. IEEE. pp. 4408–4411.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.